Optimization and Quality Control of Automated Quantitative Mineralogy Analysis for Acid Rock Drainage Prediction
Abstract
:1. Introduction
1.1. Background
1.1.1. Previous Study
1.1.2. Deposit Geology and Mineralogy
2. Methods
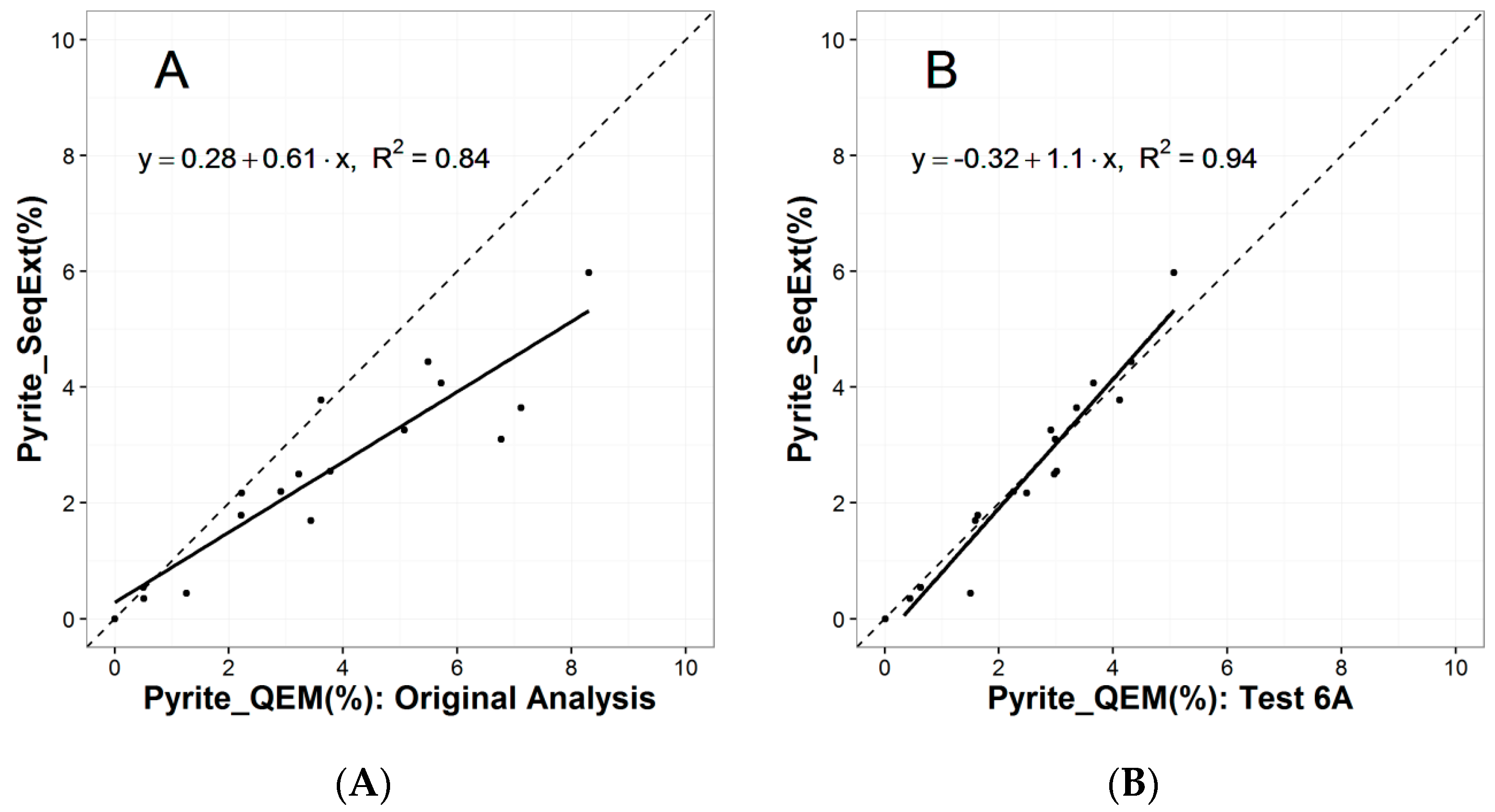
2.1. Mineralogical Analyses by QEMSCAN®
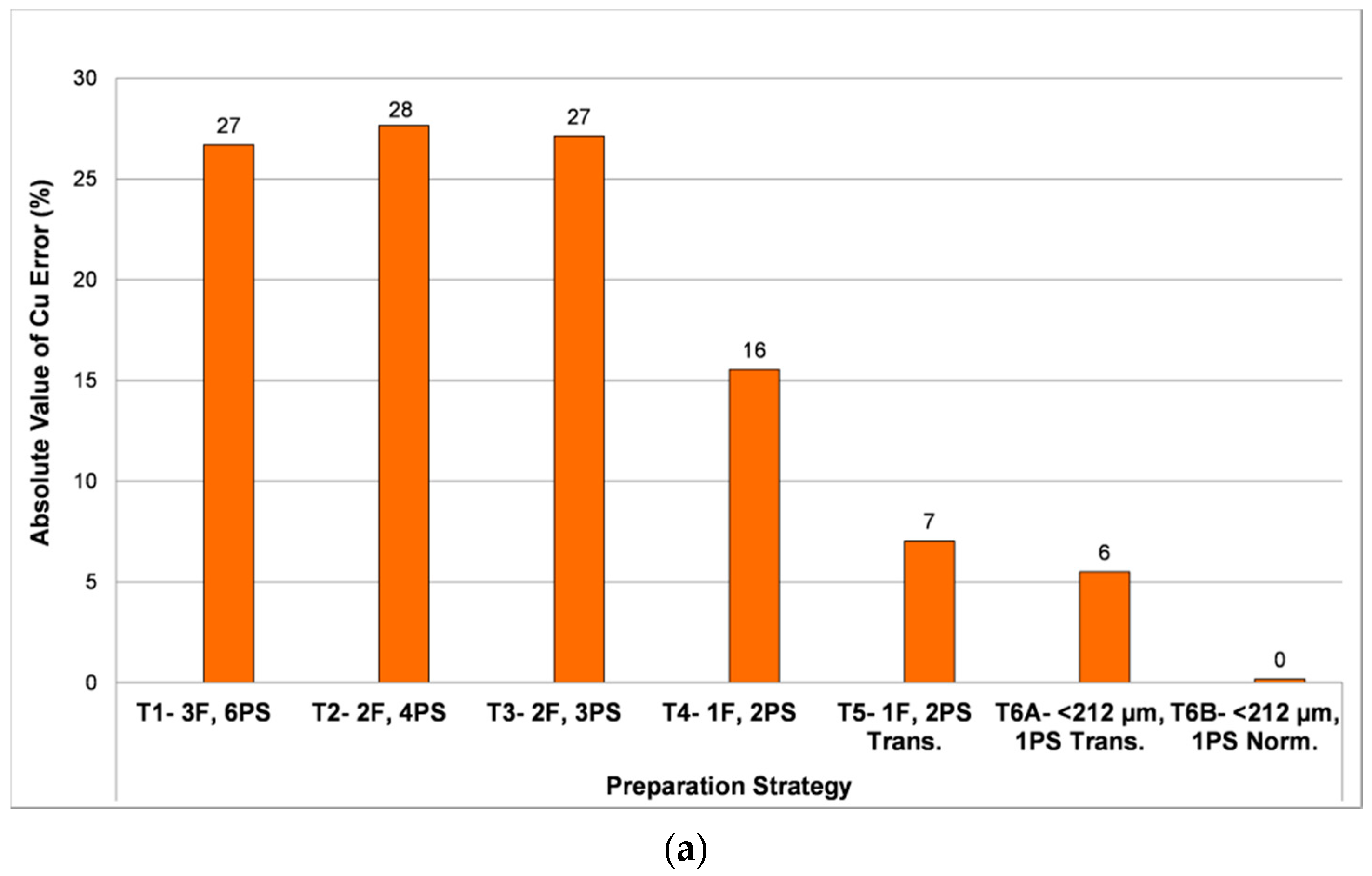
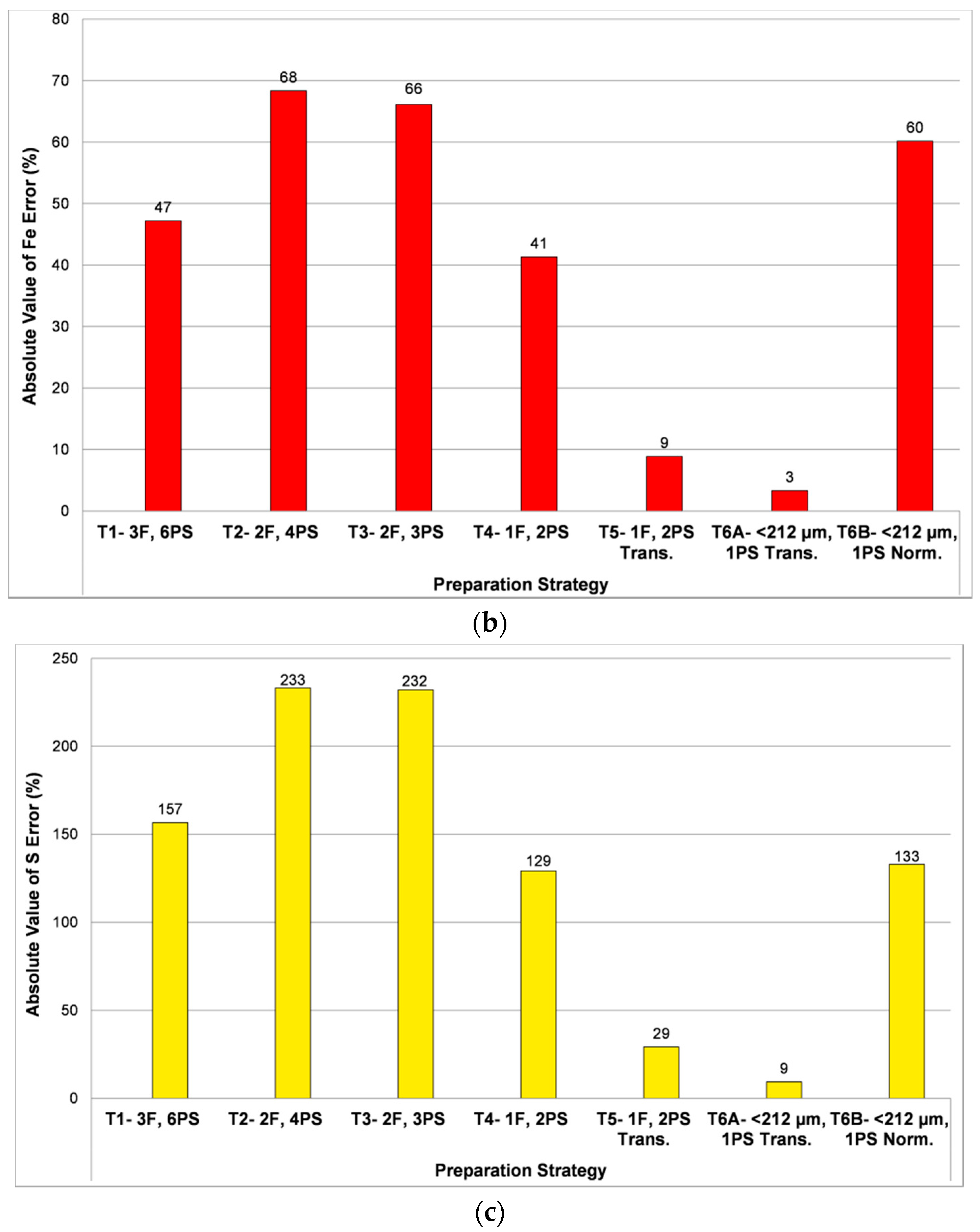
- Tests 1 to 3: Evaluate effect of sieving the samples into several particle size fractions. More replicates are analyzed for the coarser fractions, because in these cases fewer particles are exposed at the surface of each 3 cm diameter polished section. The number of sections and size fractions are systematically reduced from Tests 1 to 3 in order to observe the effect on overall results.
- Test 4: The sample is not divided into size fractions, but a duplicate polished section is analyzed in order to evaluate the effect of increasing the number of particles with respect to the original analyses.
- Test 5: The use of transverse sections is investigated, where the original section is cut vertically and the two halves generated are turned 90° and mounted in a new resin-encapsulated polished section, exposing the vertical profile of the original section. It is hypothesized that this may reduce or eliminate the effect of particle segregation in the original section (e.g., Kwitko-Ribeiro [42]; Blaskovich [16]; Grant et al. [43]).
- Test 6A and 6B: The effect of particle size reduction through controlled mechanical pulverization is evaluated. The objective is to reduce the range of particle sizes and therefore the competition between particles as they settle towards the bottom of the sample mold during resin curing.
2.2. Sequential Extraction Analyses
3. Results and Discussion
3.1. Mineralogical Analyses
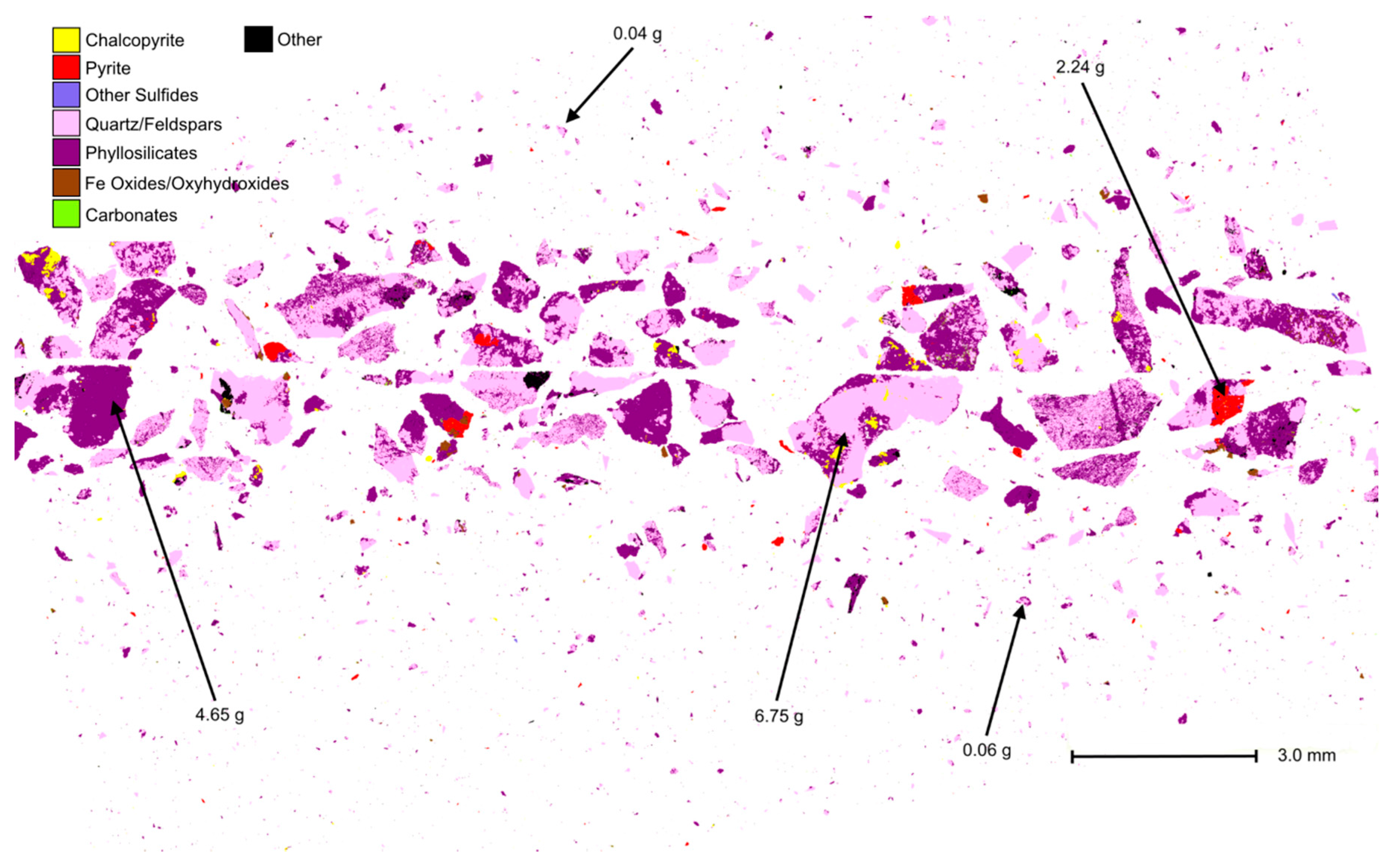
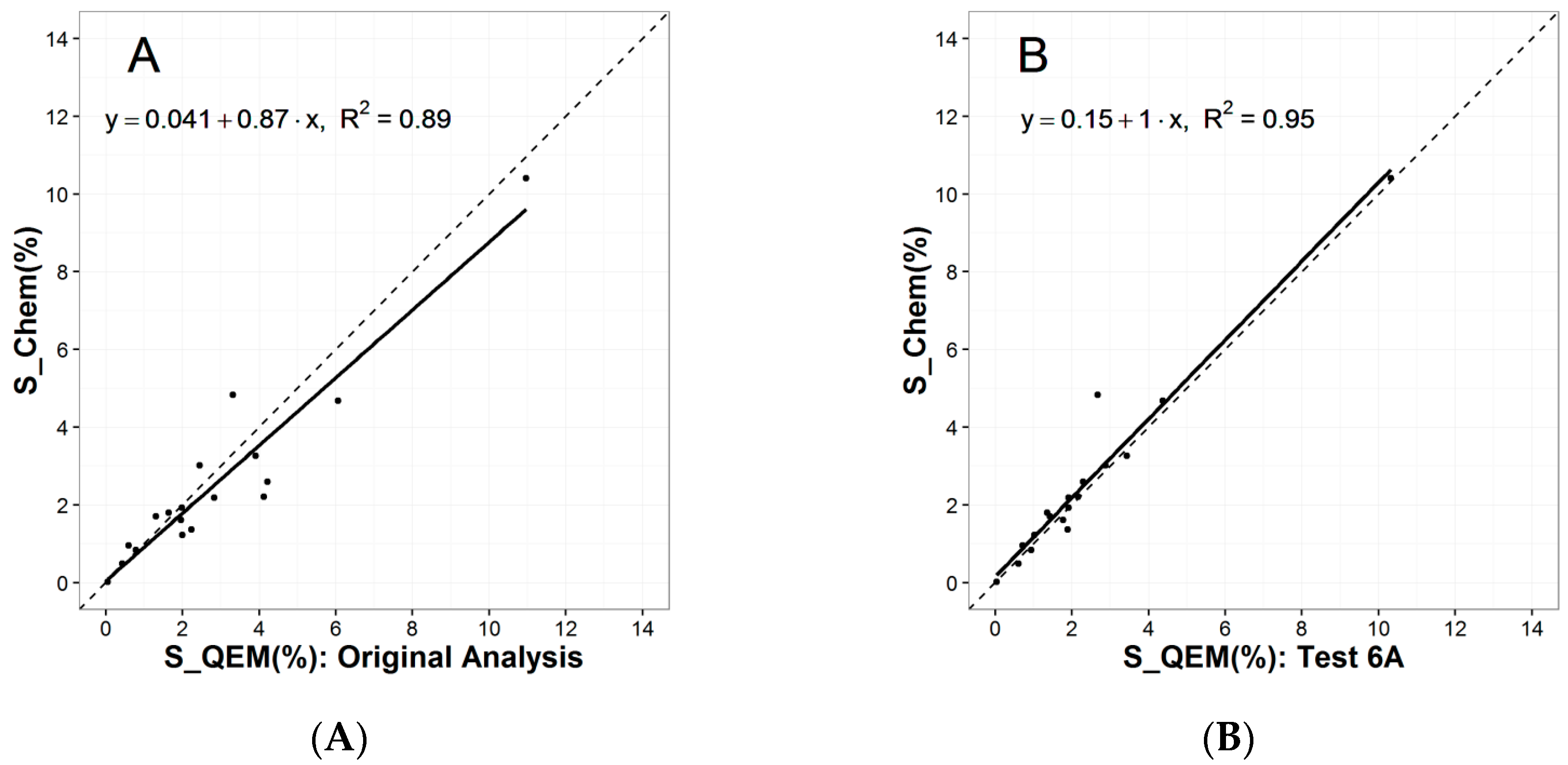
3.1.1. Particle Segregation Study
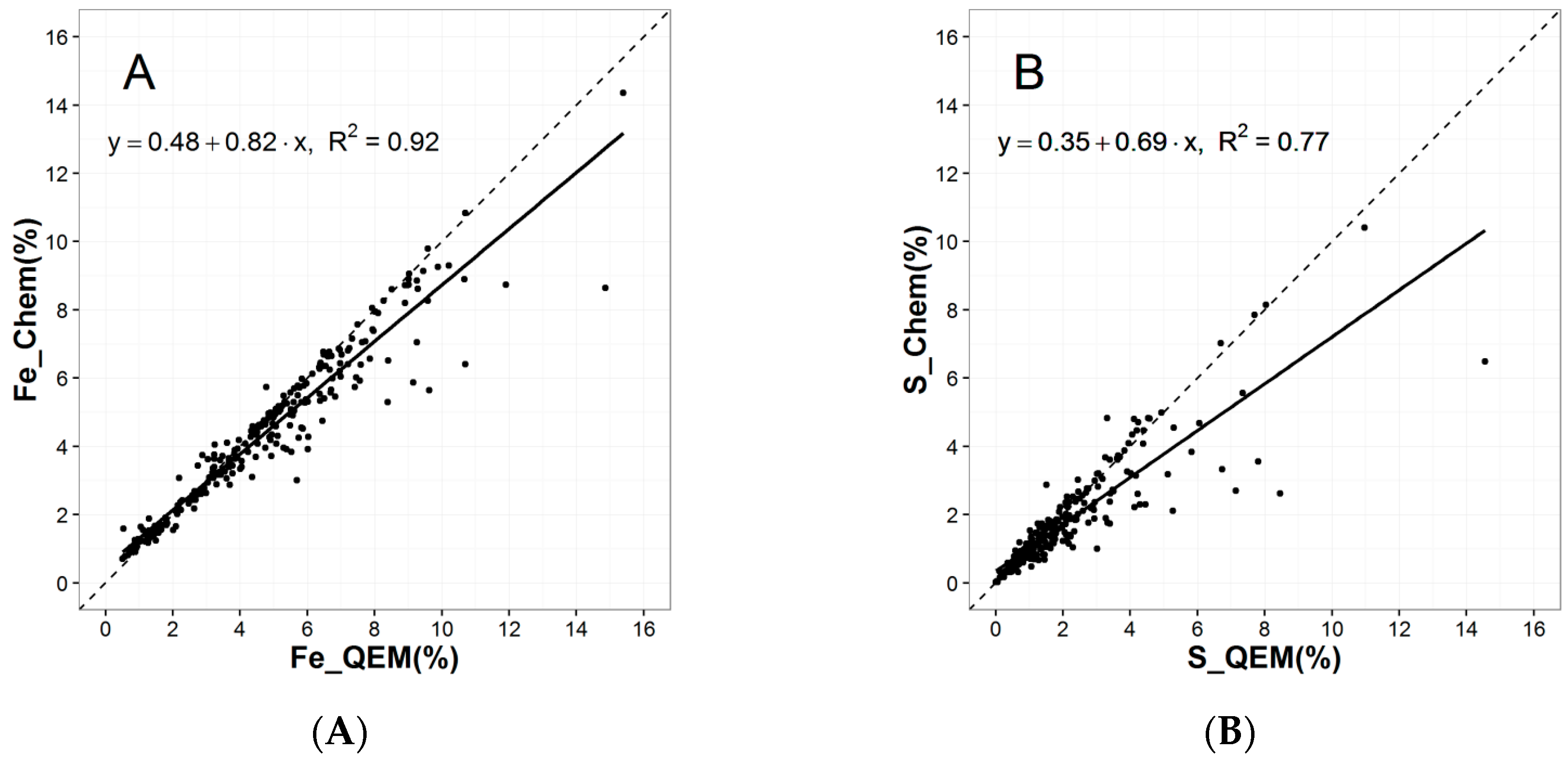
- %Element_QS = The calculated elemental percentage based upon QEMSCAN® mineralogical results.
- %Element_Chem = The measured elemental percentage from traditional chemical assays.
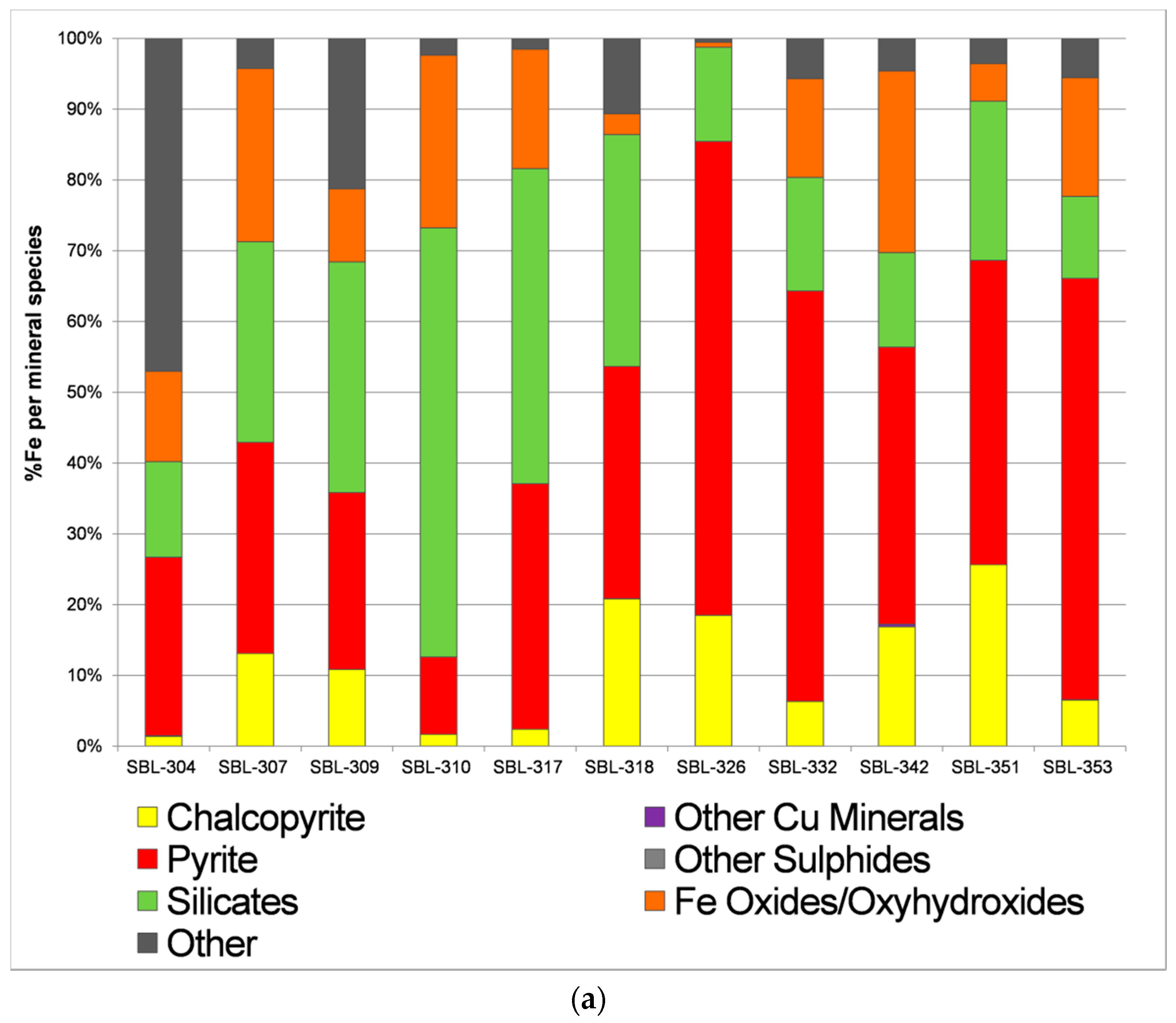
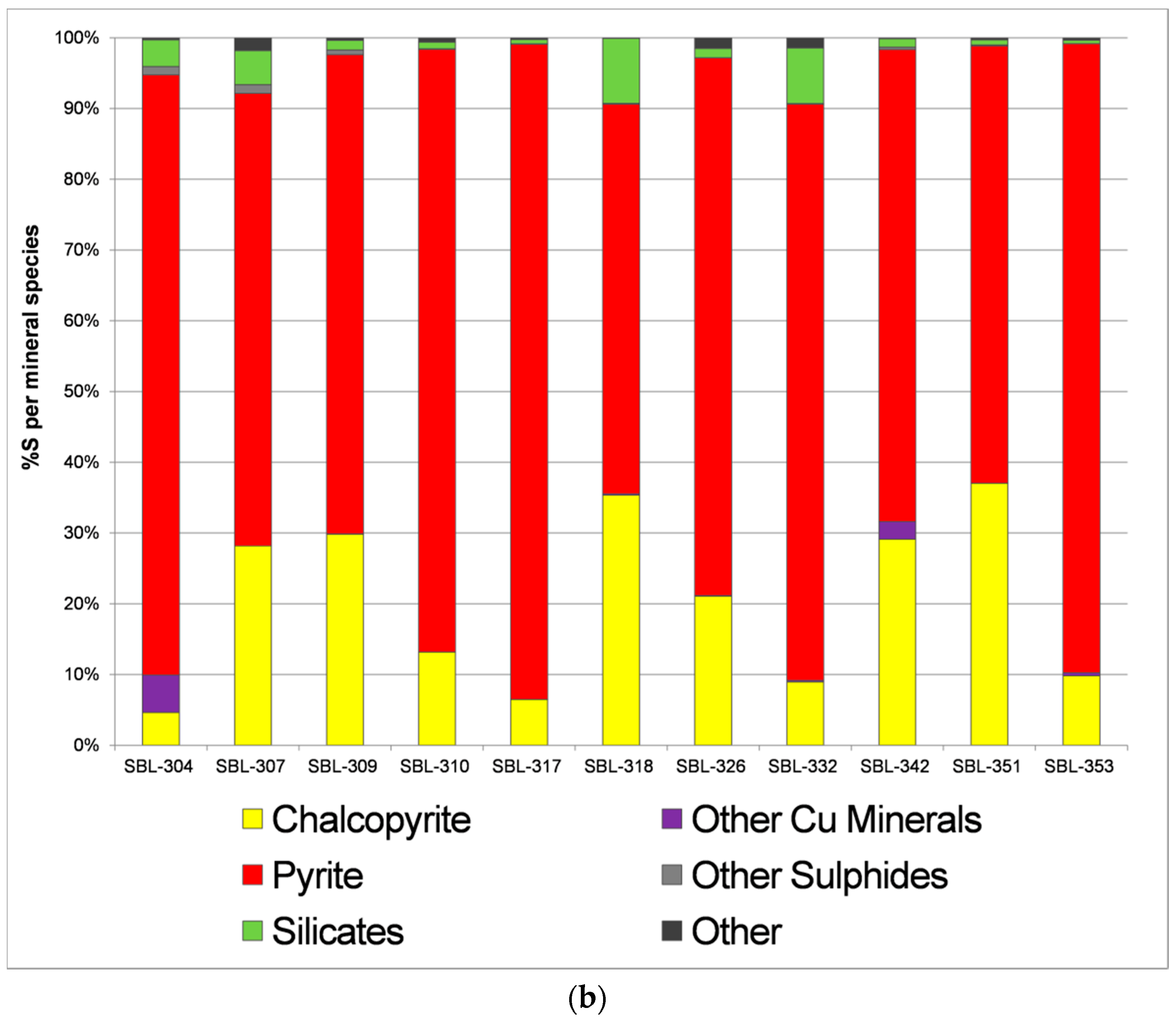
- Calculate %galena (using %Pb from the chemical assay)
- Calculate %molybdenite (using %Mo)
- Calculate %tennantite (using %As)
- Calculate %sphalerite (%Zn_total − %Zn_tennantite)
- Calculate %chalcopyrite (%Cu_total − %Cu_tennantite)
- Calculate %pyrite (%S_total − %S in the minerals from 1 to 5 above)
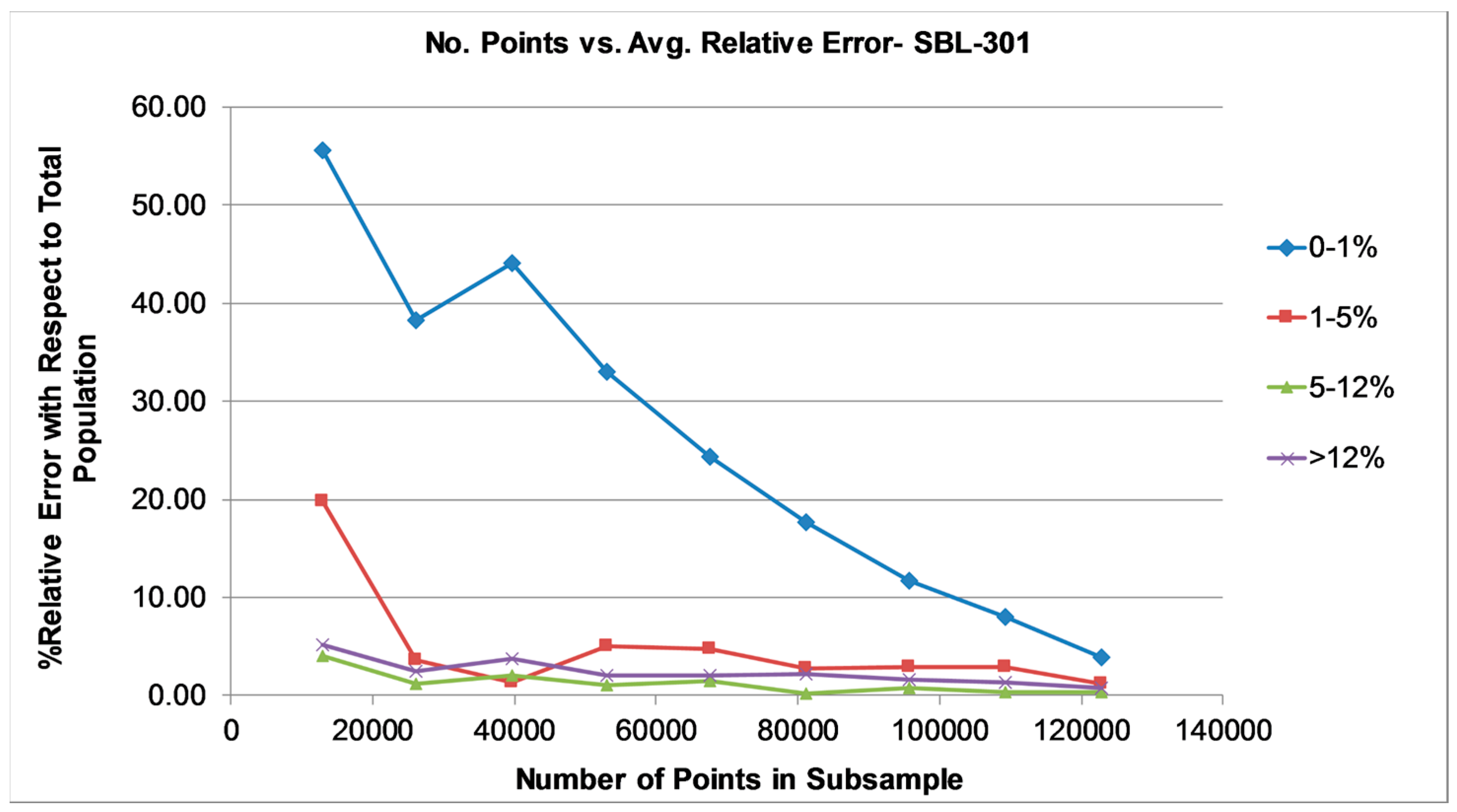
3.1.2. Particle Number Determination
- >0% < 1%
- ≥1% < 5%
- ≥5% < 12%
- ≥12%
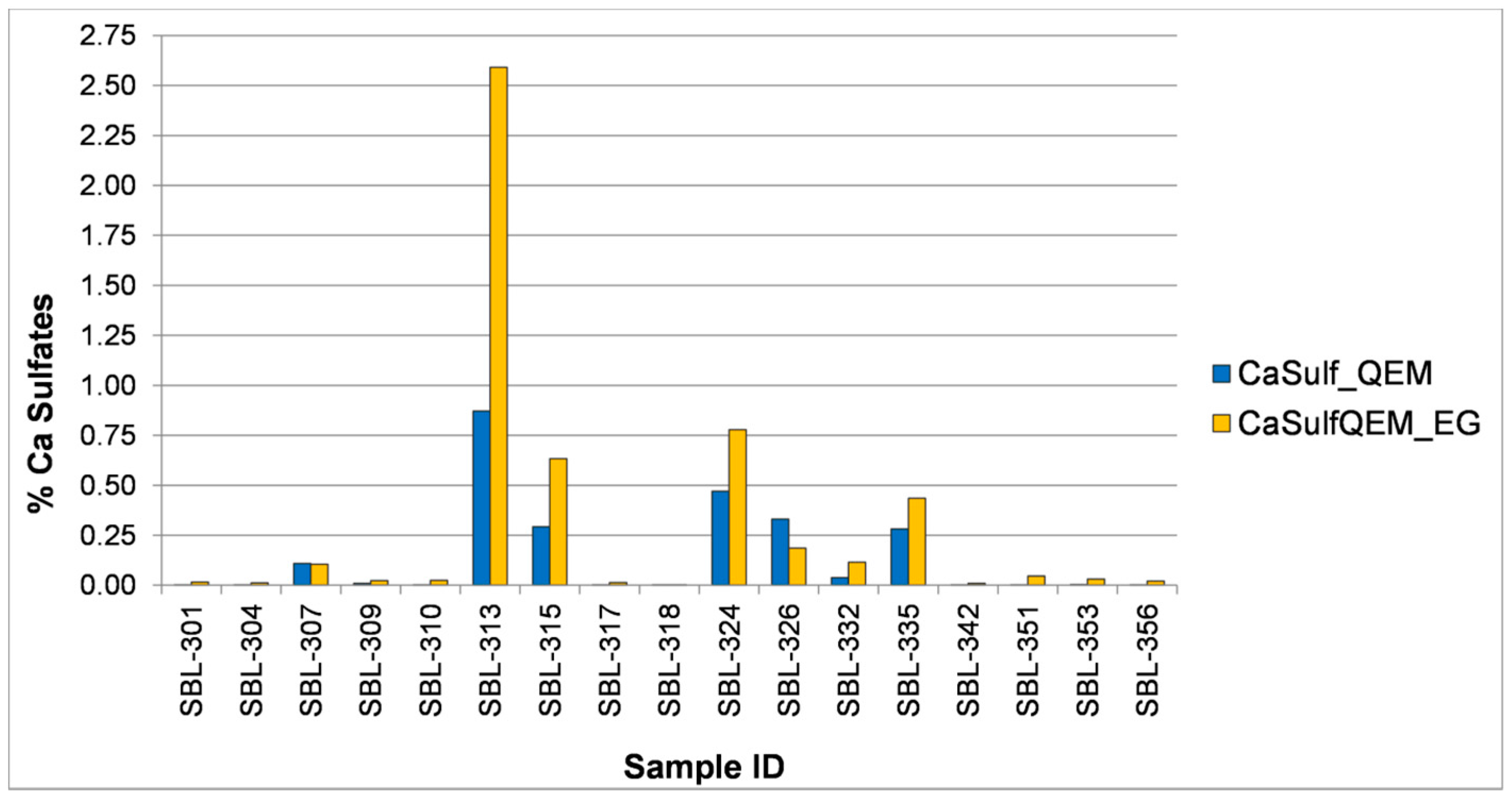
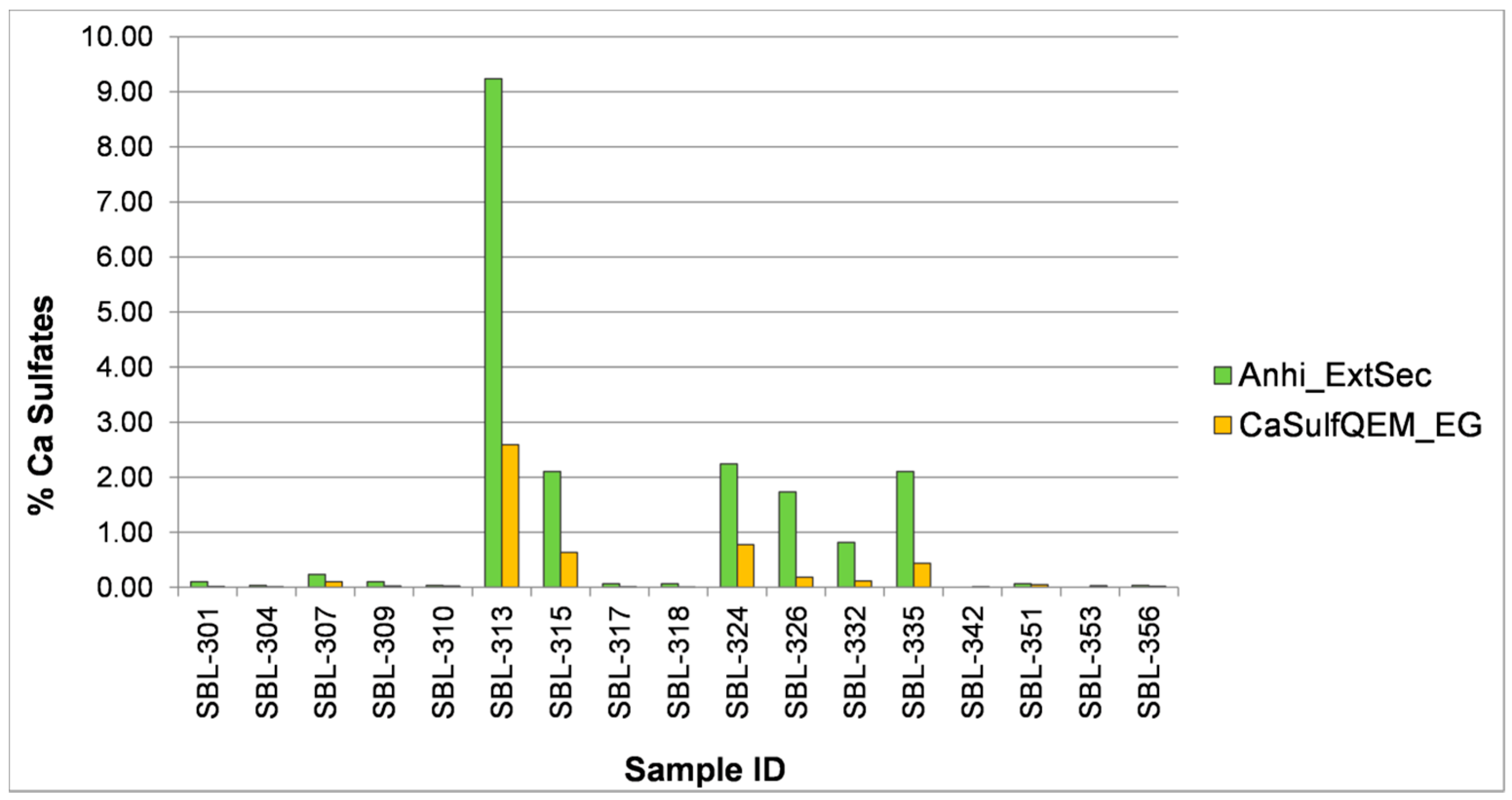
3.1.3. Ethylene Glycol Polishing Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Price, W. Prediction Manual for Drainage Chemistry from Sulphidic Geologic Materials; Mend Report; Mining and Mineral Sciences Laboratories: Smithers, BC, Canada, 2009; pp. 1–579. [Google Scholar]
- Dold, B. Submarine tailings disposal—A review. Minerals 2014, 4, 642–666. [Google Scholar] [CrossRef]
- Smuda, J.; Dold, B.; Spangenberg, J.E.; Pfeifer, H.R. Geochemistry of fresh alkaline porphyry copper tailings: Implications on sources and mobility of elements during transport and early stages of deposition. Chem. Geol. 2008, 256, 62–76. [Google Scholar] [CrossRef]
- Dold, B.; Fontboté, L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J. Geochem. Explor. 2001, 74, 3–55. [Google Scholar] [CrossRef]
- Plumlee, G.S. The environmental geology of mineral deposits. In The Environmental Geochemistry of Mineral Deposits; Part A, Processes, Techniques and Health Issues; Plumlee, G.S., Logsdon, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 71–116. [Google Scholar]
- Dold, B. Basic concepts of environmental geochemistry of sulfide mine-waste. In UNESCO-SEG-SGA Latin American Metallogency Course; Ponificia Universidad Católica del Perú: Lima, Perú, 2005; p. 36. [Google Scholar]
- Dold, B. Evolution of Acid Mine drainage formation in sulphidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Dold, B.; Weibel, L. Biogeometallurgical pre-mining characterization of ore deposits: An approach to increase sustainability in the mining process. Environ. Sci. Pollut. Res. 2013, 20, 7777–7786. [Google Scholar] [CrossRef] [PubMed]
- Dold, B. Basic concepts in environmental geochemistry of mine waste management. In Waste Managemente; Kumar, S., Ed.; InTech: Rijeka, Croatia, 2010; pp. 173–198. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Dold, B.; Spangenberg, J.E. Sulfur speciation and stable isotope trends of water-soluble sulfates in mine tailings profiles. Environ. Sci. Technol. 2005, 39, 5650–5656. [Google Scholar] [CrossRef] [PubMed]
- Smuda, J.; Dold, B.; Spangenberg, J.E. Element cycling during the transition from alkaline to acidic environment in an active porphyry copper tailings impoundment, Chuquicamata, Chile. J. Geochem. Explor. 2014, 140, 23–40. [Google Scholar] [CrossRef]
- Nordstrom, D.K. What was the groundwater quality before mining in a mineralized region? Lessons from the Questa Project. Geosci. J. 2008, 12, 139–149. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.; Bradshaw, D. Evaluating waste rock mineralogy and microtexture during kinetic testing for improved acid rock drainage prediction. Miner. Eng. 2013, 52, 111–124. [Google Scholar] [CrossRef]
- Becker, M.; Dyantyi, N.; Broadhurst, J.L.; Harrison, S.T.L.; Franzidis, J. A mineralogical approach to evaluating laboratory scale acid rock drainage characterisation tests. Miner. Eng. 2015, 80, 33–36. [Google Scholar] [CrossRef]
- Blaskovich, R.J. Characterizing Waste Rock Using Automated Quantitative Electron Microscopy. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Buckwalter-Davis, M. Automated Mineral Analysis of Mine Waste. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2013. [Google Scholar]
- Camm, G.S.; Glass, H.J.; Bryce, D.W.; Butcher, A.R. Characterisation of a mining-related arsenic-contaminated site, Cornwall, UK. J. Geochem. Explor. 2004, 82, 1–15. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J. An overview of the advantages and disadvantages of the determination of gold mineralogy by automated mineralogy. Miner. Eng. 2007, 20, 506–517. [Google Scholar] [CrossRef]
- Pirrie, D.; Rollinson, G.K.; Power, M.R. Role of automated mineral analysis in the characterisation of mining-related contaminated land. Geosci. South West Engl. 2009, 12, 162–170. [Google Scholar]
- Redwan, M.; Rammlmair, D. Understanding micro-environment development in mine tailings using MLA and image analysis. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; pp. 589–596.
- Dold, B.; Weibel, L.; Cruz, J. New modified humidity cells test for acid rock drainage prediction in porphyry copper deposits. In 2nd International Seminar on Environmental Issues in the Mining Industry; Gecamin Ltd.: Santiago, Chile, 2011. [Google Scholar]
- Weibel, L.; Dold, B.; Cruz, J. Application and Limitation of Standard Humidity Cell Tests at the Andina Porphyry Copper Mine, CODELCO, Chile. In Proceedings of the 11th SGA Biennial Meeting, Antofagasta, Chile, 26–29 September 2011.
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Becker, M.; Harris, P.J.; Wiese, J.G.; Bradshaw, D.J. Mineralogical characterisation of naturally floatable gangue in Merensky Reef ore flotation. Int. J. Miner. Process. 2009, 93, 246–255. [Google Scholar] [CrossRef]
- Coetzee, L.L.; Theron, S.J.; Martin, G.J.; van der Merwe, J.D.; Stanek, T.A. Modern gold deportments and its application to industry. Miner. Eng. 2011, 24, 565–575. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Mercer, W.; Gunning, C.; Prout, S. Quantitative characterization of the REE minerals by QEMSCAN from the Nechalacho Heavy Rare Earth Deposit, Thor Lake Project, NWT, Canada. In Proceedings of the 43rd Annual Canadian Mineral Processors Conference, Ottawa, ON, Canada, 18–20 January 2011; pp. 381–398.
- Smythe, D.M.; Lombard, A.; Coetzee, L.L. Rare Earth Element deportment studies utilising QEMSCAN technology. Miner. Eng. 2013, 52, 52–61. [Google Scholar] [CrossRef]
- Clark, A.H. Are Outsize Porphyry Copper Deposits either Anatomically or Environmentally Distinctive; Society of Economic Geology: Littleton, CO, USA, 1993; pp. 213–283. [Google Scholar]
- Cooke, D.R.; Hollings, P.; Walshe, J.L. Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Econ. Geol. 2005, 100, 801–818. [Google Scholar] [CrossRef]
- Deckart, K.; Clark, A.H.; Aguilar, A.C.; Vargas, R.R.; Bertens, A.N.; Mortensen, J.K.; Fanning, M. Magmatic and hydrothermal chronology of the Giant Río Blanco porphyry copper deposit, central Chile: Implications of an integrated U-Pb and 40Ar/39Ar database. Econ. Geol. 2005, 100, 905–934. [Google Scholar] [CrossRef]
- Deckart, K.; Clark, A.H.; Cuadra, P.; Fanning, M. Refinement of the time-space evolution of the giant Mio-Pliocene Río Blanco-Los Bronces porphyry Cu-Mo cluster, Central Chile: New U-Pb (SHRIMP II) and Re-Os geochronology and 40Ar/39Ar thermochronology data. Miner. Depos. 2013, 48, 57–79. [Google Scholar] [CrossRef]
- Deckart, K.; Silva, W.; Spröhnle, C.; Vela, I. Timing and duration of hydrothermal activity at the Los Bronces porphyry cluster: An update. Miner. Depos. 2014, 49, 535–546. [Google Scholar] [CrossRef]
- Gustafson, L.B.; Hunt, J.P. The porphyry copper desposit at El Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Lowell, D.J.; Guilbert, J.M. Lateral and Vertical alteration-mineralization zoning in porphyry Ore Deposits. Econ. Geol. 1970, 65, 373–408. [Google Scholar] [CrossRef]
- Rose, A.W. Zonal relations of wallrock alteration and sulfide distribution at porphyry copper deposits. Econ. Geol. 1970, 65, 920–936. [Google Scholar] [CrossRef]
- Serrano, L.; Vargas, R.; Stambuk, V. The late Miocene to early Pliocene rio Blanco-Los bronces copper deposit, Central Chilean Andes. In Andean Copper Deposits: New Discoveries, Mineralization, Styles and Metallogeny; Camus, F., Sillitoe, R.H., Petersen, R., Eds.; Society of Economic Geology: Littleton, CO, USA, 1996; pp. 119–130. [Google Scholar]
- Miller, P.R.; Reid, A.F.; Zuiderwyk, M.A. QEM*SEM image analysis in the determination of modal assays, mineral associations and mineral liberation. In Proceedings of the 14th International Mineral Processing Congress, Toronto, ON, Canada, 17–23 October 1982.
- Gottlieb, P.; Wilkie, G.; Sutherland, D.; Ho-Tun, E. Using quantitative electron microscopy for process mineralogy applications. J. Miner. 2000, 52, 24–27. [Google Scholar] [CrossRef]
- Haberlah, D.; Owen, M.; Botha, P.W.S.K.; Gottlieb, P. SEM-EDS-based protocol for subsurface drilling material identification and petrological classification. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; pp. 265–273.
- Jackson, B.R.; Reid, A.F.; Zuiderwyk, M.A. Rapid production of high quality polished sections for automated image analysis of minerals. Proc. Australas. Inst. Min. Metall. 1984, 289, 93–97. [Google Scholar]
- Kwitko-Ribeiro, R. New sample preparation developments to minimize mineral segregation in process mineralogy. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; pp. 411–417.
- Grant, D.C.; Goudie, D.J.; Shaffer, M.; Sylvester, P. A single-step trans-vertical epoxy preparation method for maximising throughput of iron-ore samples via SEM-MLA analysis. Trans. Inst. Min. Metall. Sect. B 2016, 125, 57–62. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Pearson: Harlow, UK, 1992. [Google Scholar]
- Mermillod-Blondin, R.; Benzaazoua, M.; Kongolo, M.; de Donato, P.; Bussière, B.; Marion, P. Development and calibration of a quantitative, automated mineralogical assessment method based on SEM-EDS and image analysis: Application for fine tailings. J. Miner. Mater. Charact. Eng. 2011, 10, 1111–1130. [Google Scholar] [CrossRef]
- Petersen, L.; Minkkinen, P.; Esbensen, K.H. Representative sampling for reliable data analysis: Theory of Sampling. Chemom. Intell. Lab. Syst. 2005, 77, 261–277. [Google Scholar] [CrossRef]
- Pitard, F. Pierre Gy’s Sampling Theory and Sampling Practice. Heterogeneity, Sampling Correctness, and Statistical Process Control; CRC Press, Inc.: Boca Raton, FL, USA, 1993; Volume 37. [Google Scholar]
- Goodall, W.R.; Leatham, J.D.; Scales, P.J. A new method for determination of preg-robbing in gold ores. Miner. Eng. 2005, 18, 1135–1141. [Google Scholar] [CrossRef]
- Poole, A.B.; Sims, I. Concrete Petrography: A Handbook of Investigative Techniques, 2nd ed.; CRC Press, Inc.: Boca Raton, FL, USA, 2016. [Google Scholar]
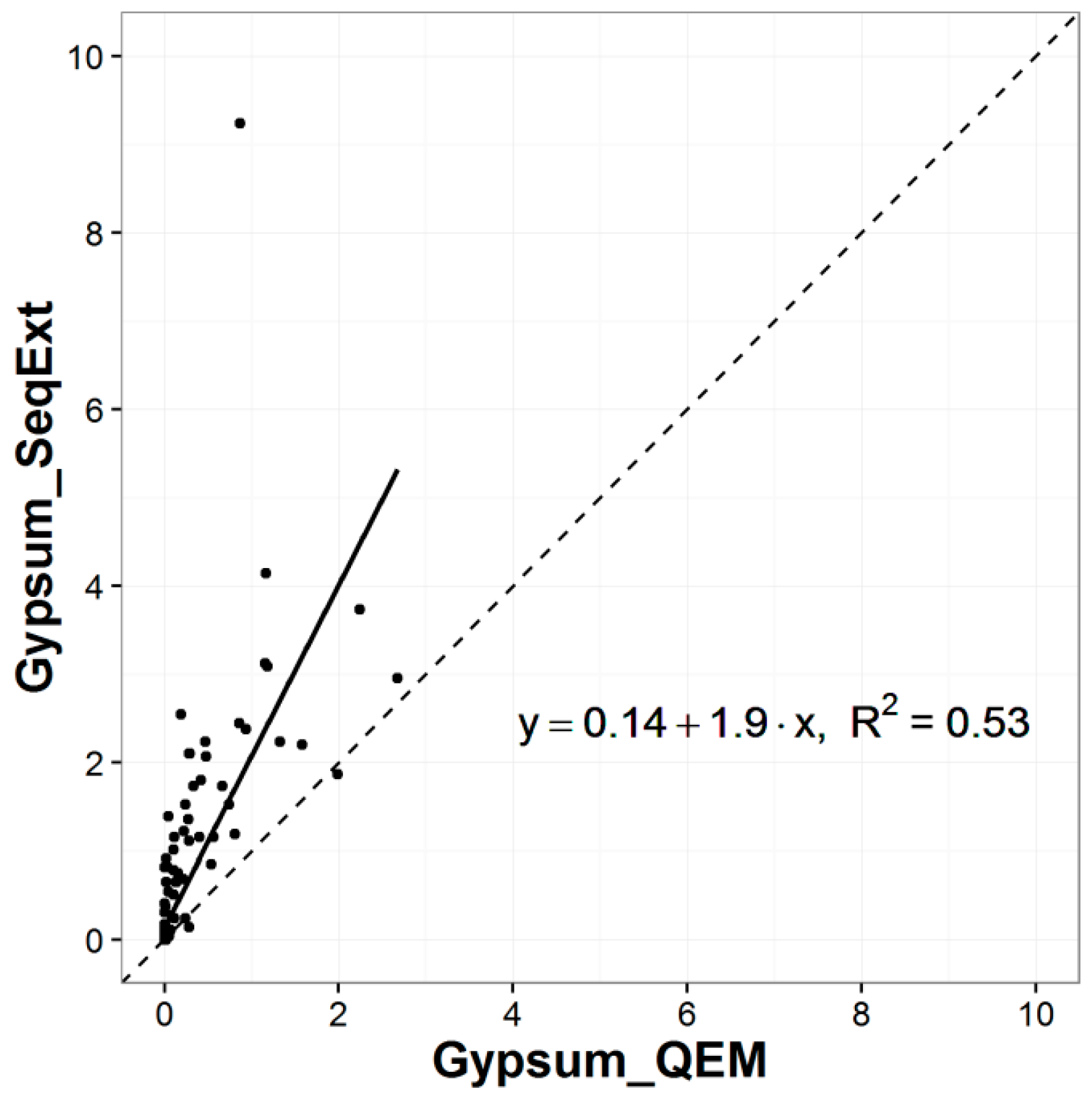

| Sequential Extraction Analysis Sequence | Preferentially Dissolved Minerals |
|---|---|
| secondary sulfates, e.g., bonattite, chalcanthite, pickeringite, magnesioauberite, gypsum |
| calcite, vermiculite-type mixed-layer, absorbed and exchangeable ions |
| schwertmannite, two-line ferrihydrite, secondary jarosite, MnO2 |
| goethite, jarosite, Na-jarosite, hematite, magnetite, higher ordered ferrihydrites |
| organic matter, covellite, chalcocite-digenite |
| pyrite, chalcopyrite, bornite, sphalerite, galena, molybdenite, Cu-As-Sb sulfosalts, cinnabar, orpiment, stibnite |
| silicates, other residual phases |
| Ore Deposit | Codelco, Andina Division |
|---|---|
| Deposit type | porphyry copper |
| Gangue mineralogy | quartz, albite, K-feldspar, biotite, ankerite, siderite, calcite, gypsum, sericite, chlorite, epidote, tourmaline |
| Hypogene ore mineralogy | pyrite, chalcopyrite, bornite, molybdenite, sphalerite, galena, tennantite-tetrahedrite, magnetite, hematite, ilmenite |
| Supergene mineralogy | Chalcocite-digenite, covellite |
| Test No. | Test 1 | Test 2 | Test 3 |
| Size ranges used (µm) | ≥850, <850 ≥150, <150 | ≥150, <150 | ≥150, <150 |
| No. polished sections/size fraction | 3, 2, 1 | 3, 1 | 2, 1 |
| Test No. | Test 4 | Test 5 | Test 6 (A–B) * |
| Size Ranges used (µm) | (as received) | (as received) | Reduced to <212 µm |
| No. polished sections/size fraction | 2 | 2 | 1 |
| Mineral/Element | Pyrite_QEM | Fe_Chem | S_Chem | |
|---|---|---|---|---|
| Pyrite_QEM | Pearson Correlation | 1.00 | 0.49 ** | 0.86 ** |
| Sig. (2-tailed) | - | 0.00 | 0.00 | |
| N | 253 | 253 | 253 | |
| Fe_Chem | Pearson Correlation | 0.49 ** | 1.00 | 0.51 ** |
| Sig. (2-tailed) | 0.00 | - | 0.00 | |
| N | 253 | 253 | 253 | |
| S_Chem | Pearson Correlation | 0.86 ** | 0.51 ** | 1.00 |
| Sig. (2-tailed) | 0.00 | 0.00 | - | |
| N | 253 | 253 | 253 | |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pooler, R.; Dold, B. Optimization and Quality Control of Automated Quantitative Mineralogy Analysis for Acid Rock Drainage Prediction. Minerals 2017, 7, 12. https://doi.org/10.3390/min7010012
Pooler R, Dold B. Optimization and Quality Control of Automated Quantitative Mineralogy Analysis for Acid Rock Drainage Prediction. Minerals. 2017; 7(1):12. https://doi.org/10.3390/min7010012
Chicago/Turabian StylePooler, Robert, and Bernhard Dold. 2017. "Optimization and Quality Control of Automated Quantitative Mineralogy Analysis for Acid Rock Drainage Prediction" Minerals 7, no. 1: 12. https://doi.org/10.3390/min7010012
APA StylePooler, R., & Dold, B. (2017). Optimization and Quality Control of Automated Quantitative Mineralogy Analysis for Acid Rock Drainage Prediction. Minerals, 7(1), 12. https://doi.org/10.3390/min7010012





