Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Separation Products of Reductive Products of Ilmenite Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Conditions
2.2. Measuring Principles of Microwave Absorbing Characteristics
2.3. XRD Characterization
3. Results and Discussion
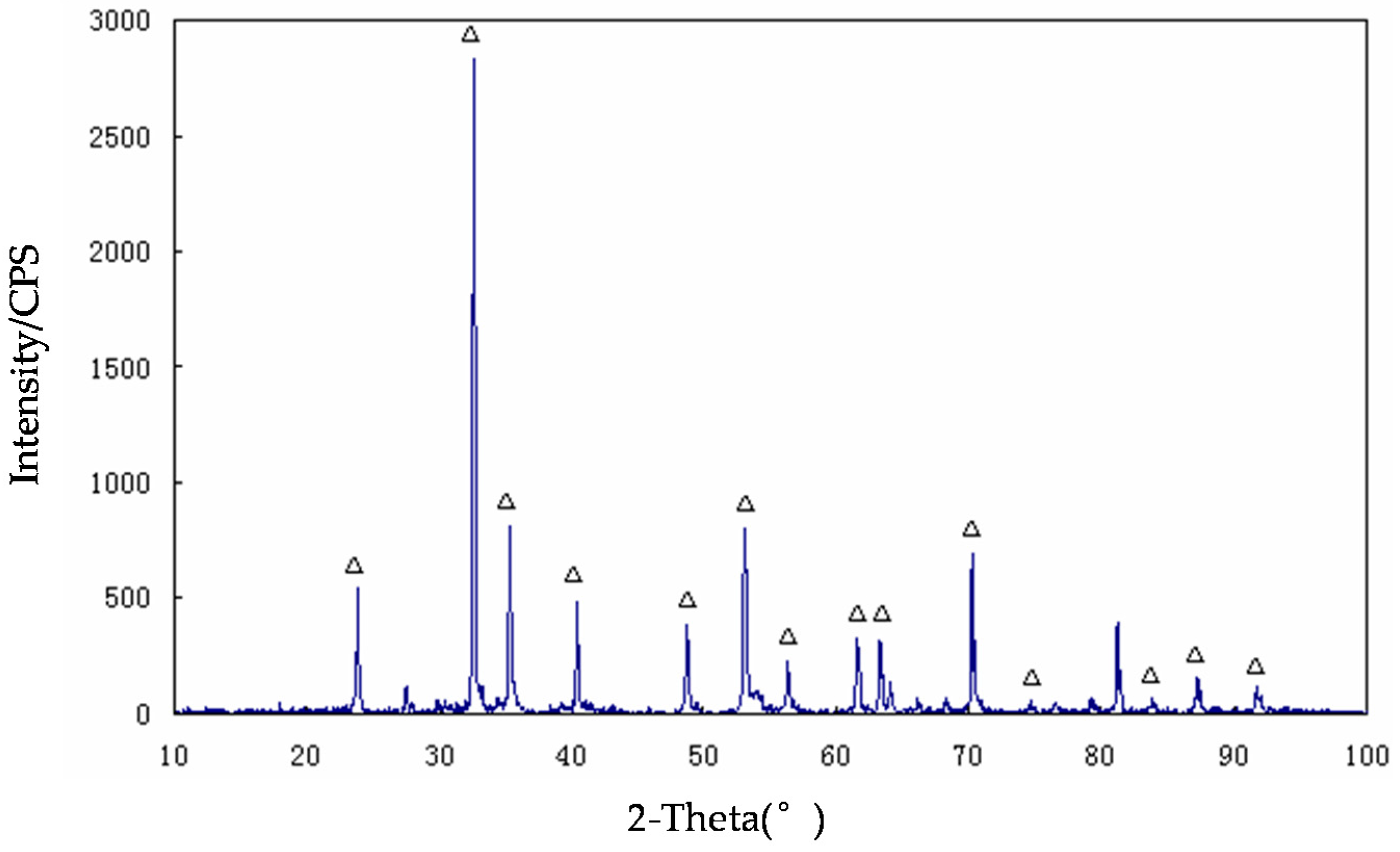
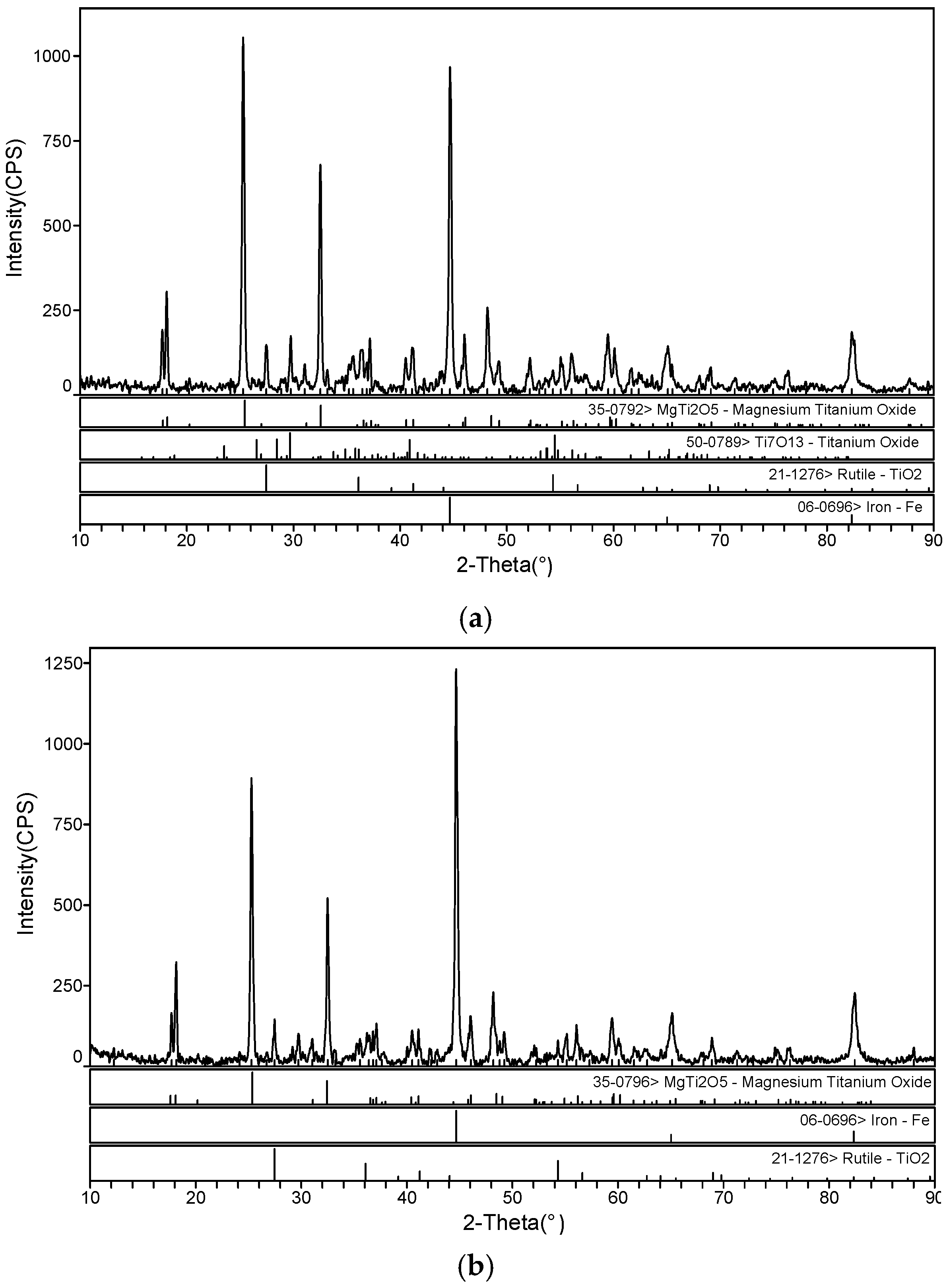
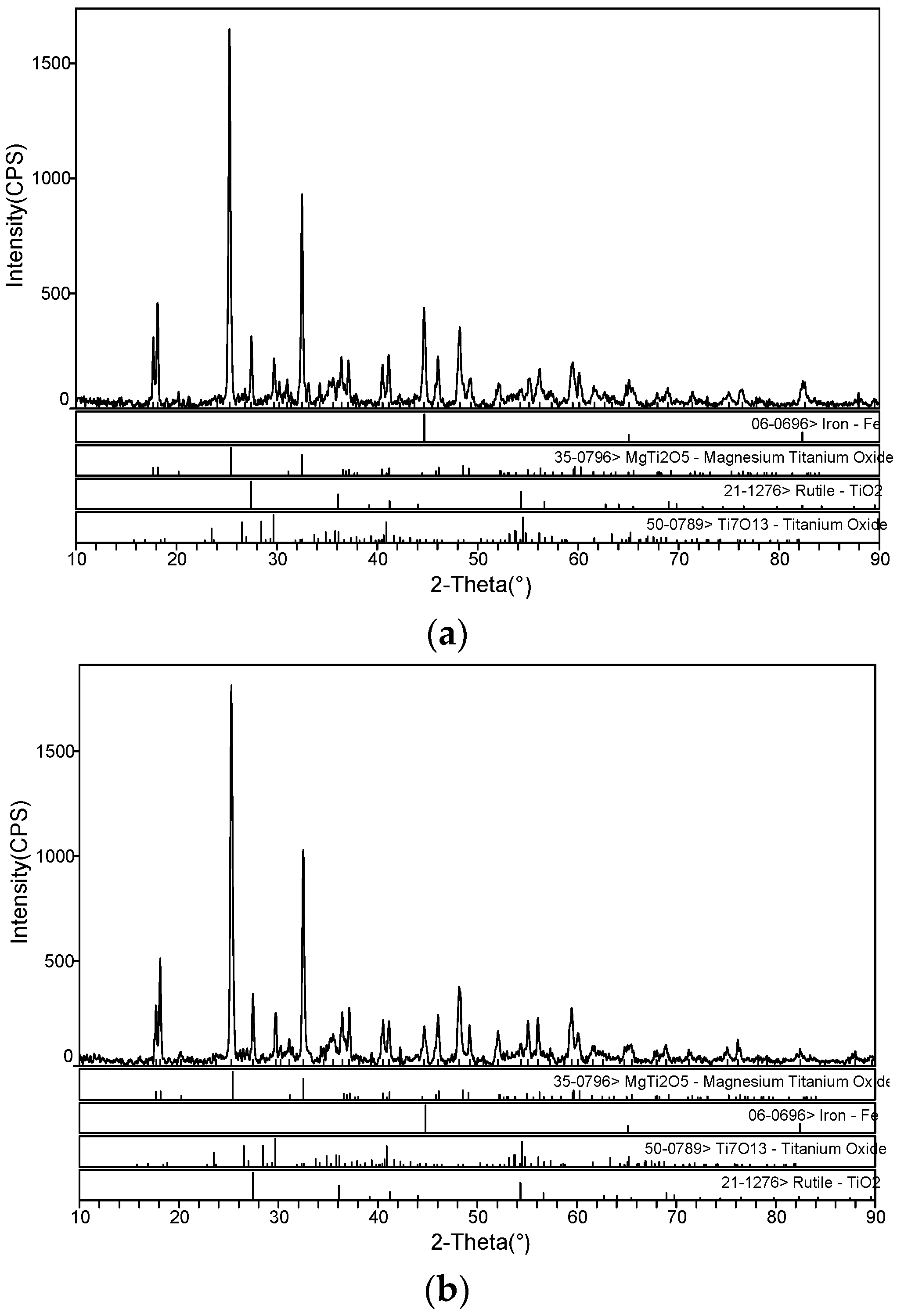
3.1. The Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Products
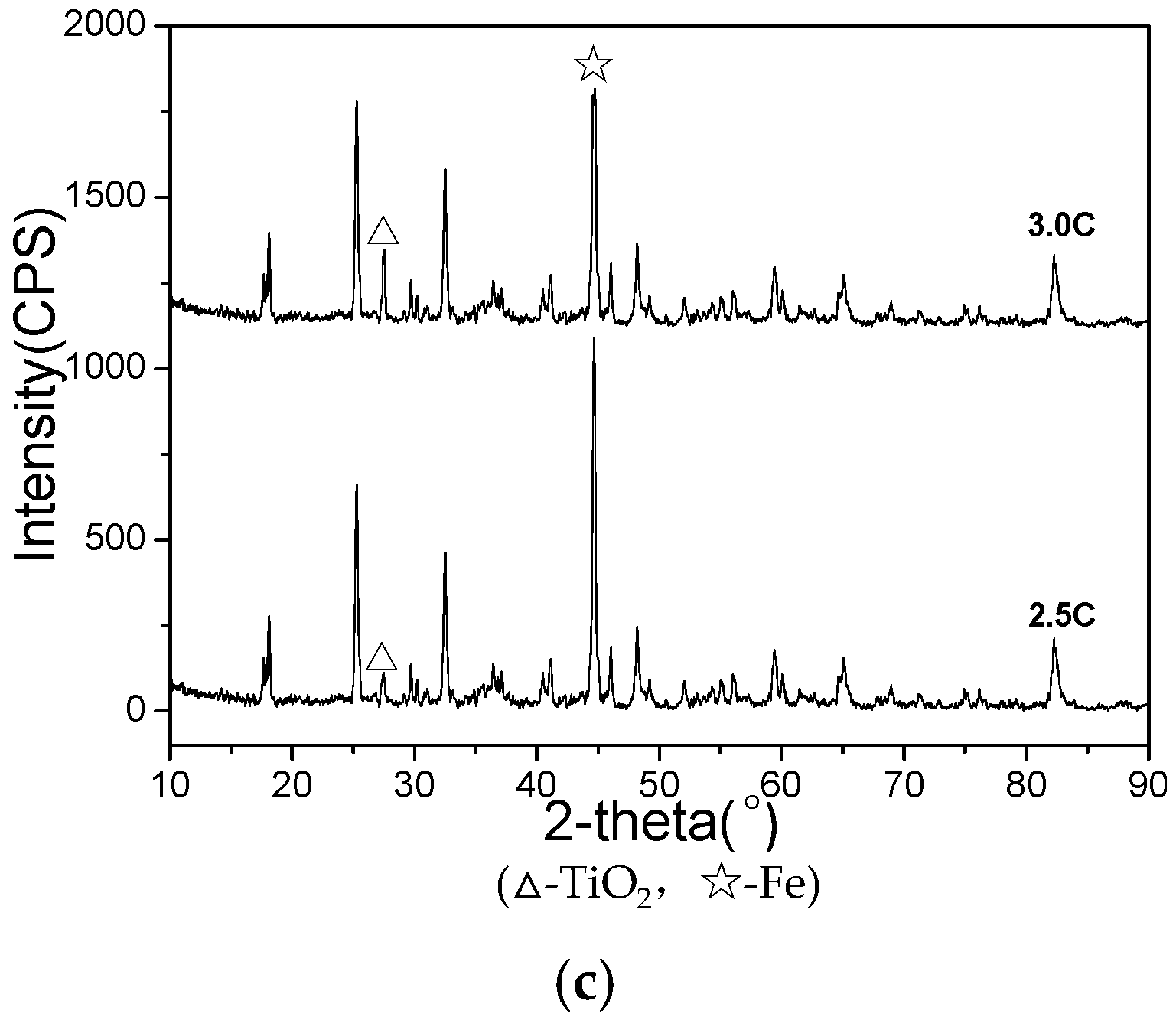
3.2. The Microwave-Absorbing Characteristics and XRD Characterization of Non-Magnetic Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kucukkaragoz, C.S.; Eric, R.H. Solid state reduction of a natural ilmenite. Miner. Eng. 2006, 19, 334–337. [Google Scholar]
- El-Tawil, S.Z.; Morsi, I.M.; Yehia, A.; Francis, A.A. Alkali reductive roasting of ilmenite ore. Can. Metall. Q. 1996, 35, 31–37. [Google Scholar] [CrossRef]
- Sarker, M.K.; Rashid, A.K.M.B.; Kurny, A.S.W. Kinetics of leaching of oxidized and reduced ilmenite in dilute hydrochloric acid solutions. Int. J. Miner. Process. 2006, 80, 223–228. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Hwang, J.Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave processing of materials and applications in manufacturing industries: A review. Mater. Manuf. Process. 2015, 30, 1–29. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Rattanadecho, P.; Makul, N. Microwave-assisted drying: A review of the State-of-the-Art. Dry. Technol. 2016, 34, 1–38. [Google Scholar] [CrossRef]
- Wang, N.; Wang, P. Study and application status of microwave in organic wastewater treatment: A review. Chem. Eng. J. 2016, 283, 193–214. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave-material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Composites: Part A 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Kelly, R.M.; Rowson, N.A. Microwave reduction of oxidised ilimenite concentrate. Miner. Eng. 1995, 8, 1427–1438. [Google Scholar] [CrossRef]
- Kingman, S.W.; Corfield, G.M.; Rowson, N.A. Effects of microwave radiation upon the mineralogy and magnetic processing of a massive Norwegian ilmenite ore. Magn. Electr. Sep. 1999, 9, 131–148. [Google Scholar] [CrossRef]
- Cutmore, N.; Evans, T.; Crnokark, D.; Middleton, A.; Stoddard, S. Microwave technique for analysis of mineral sands. Miner. Eng. 2000, 13, 729–736. [Google Scholar] [CrossRef]
- Tong, Z.F.; Bi, S.W.; Yang, Y.H. Present situation of study on microwave heating application in metallurgy. J. Mater. Metall. 2004, 3, 117–120. [Google Scholar]
- Itoh, S.; Suga, T.; Takizawa, H.; Nagasaka, T. Application of 28 GHz Microwave irradiation to oxidation of ilmenite ore for new rutile extraction process. ISIJ Int. 2007, 47, 1416–1421. [Google Scholar] [CrossRef]
- Fang, X.F.; Waters, K.E.; Rowson, N.A.; Parker, D.J. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J. Colloid Interface Sci. 2009, 329, 167–172. [Google Scholar]
- Guo, S.H.; Chen, G.; Peng, J.H.; Chen, J.; Li, D.B.; Liu, L.J. Microwave assisted grinding of ilmenite ore. Trans. Nonferrous Met. Soc. China 2011, 21, 2122–2126. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Li, J.; Guo, S.H.; Srinivasakannan, C.; Peng, J.H. Optimization of combined microwave pretreatment-magnetic separation parameters of ilmenite using response surface methodology. Powder Technol. 2012, 232, 58–63. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, J.; Chang, X.D.; Guo, S.H.; Srinivasakannan, C.; Chen, G.; Peng, J.H. Effect of microwave irradiation on selective heating behavior and magnetic separation characteristics of Panzhihua ilmenite. Appl. Surf. Sci. 2014, 300, 171–177. [Google Scholar] [CrossRef]
- Nuri, O.S.; Mehdilo, A.; Irannajad, M. Influence of microwave irradiation on ilmenite surface properties. Appl. Surf. Sci. 2014, 311, 27–32. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M. Comparison of microwave irradiation and oxidation roasting as pretreatment methods for modification of ilmenite physicochemical properties. J. Ind. Eng. Chem. 2016, 33, 59–72. [Google Scholar] [CrossRef]
- Guo, S.H.; Li, W.; Peng, J.H.; Niu, H.; Huang, M.Y.; Zhang, L.B.; Zhang, S.M.; Huang, M. Microwave-absorbing characteristics of mixtures of different carbonaceous reducing agents and oxidized ilmenite. Int. J. Miner. Process. 2009, 93, 289–293. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.H.; Guo, S.H.; Zhang, L.B.; Chen, G.; Xia, H.Y. Carbothermic reduction kinetics of ilmenite concentrates catalyzed by sodium silicate and microwave-absorbing characteristics of reductive products. Chem. Ind. Chem. Eng. Q. 2013, 19, 423–433. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.H.; Guo, S.H.; Zhang, L.B.; Chen, G.; Xia, H.Y.; Liu, B.G. Carbothermic reduction kinetics of ilmenite concentrates catalyzed by sodium chloride and microwave-absorbing characteristics of reductive products. Miner. Metall. Process. 2013, 30, 108–116. [Google Scholar]
- Li, W.; Peng, J.H.; Guo, S.H.; Zhang, L.B.; Chen, G.; Xia, H.Y.; Liu, B.G. Carbothermic reduction kinetics of ilmenite concentrates catalyzed by sodium chloride and sodium borate and microwave-absorbing characteristics of reductive products. Metal. Int. 2013, 18, 19–25. [Google Scholar]
- Wang, X.Y.; Li, W.; Yang, B.; Guo, S.H.; Zhang, L.B.; Chen, G.; Peng, J.H.; Luo, H.L. Microwave-absorbing of carbothermic reduced products of ilmenite and oxidized ilmenite. J. Microw. Power Electromag. Energ. 2014, 48, 192–202. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhang, L.B.; Peng, J.H.; Liu, B.G.; Xia, H.Y.; Gu, X.C.; Shi, Y.F. The effect of temperature on dielectric property and microwave heating behavior of low grade Panzhihua ilmenite ore. Trans. Nonferrous Met. Soc. China 2013, 23, 3462–3469. [Google Scholar] [CrossRef]
- Huang, M.; Peng, J.H.; Yang, J.J.; Wang, J.Q. Microwave cavity perturbation technique for measuring the moisture content of sulphide minerals concentrates. Miner. Eng. 2007, 20, 92–94. [Google Scholar] [CrossRef]
- Huang, M. Non-Destructive Detection Method for Egg Freshness by Using Electromagnetic Wave Resonant Cavity. China Patent 02125071.5, 21 June 2006. [Google Scholar]
- Carter, R.D. Accuracy of microwave cavity perturbation measurements. Microwave theory and techniques. IEEE Trans. Microw. Theory Tech. 2001, 49, 918–923. [Google Scholar] [CrossRef]




| Chemical Composition | TFe | TiO2 | CaO | MgO | SiO2 | Al2O3 | S |
|---|---|---|---|---|---|---|---|
| Content (wt %) | 32.18 | 47.85 | 1.56 | 6.56 | 5.6 | 3.16 | ≤0.1 |
| Water Content | Ash | Volatile | Fixed Carbon | Total Sulfur (Dry Basis) | Calorific Value (Air Dry Basis) |
|---|---|---|---|---|---|
| 1.93% | 27.80% | 1.41% | 70.80% | 2.68% | 23.64 MJ/Kg |
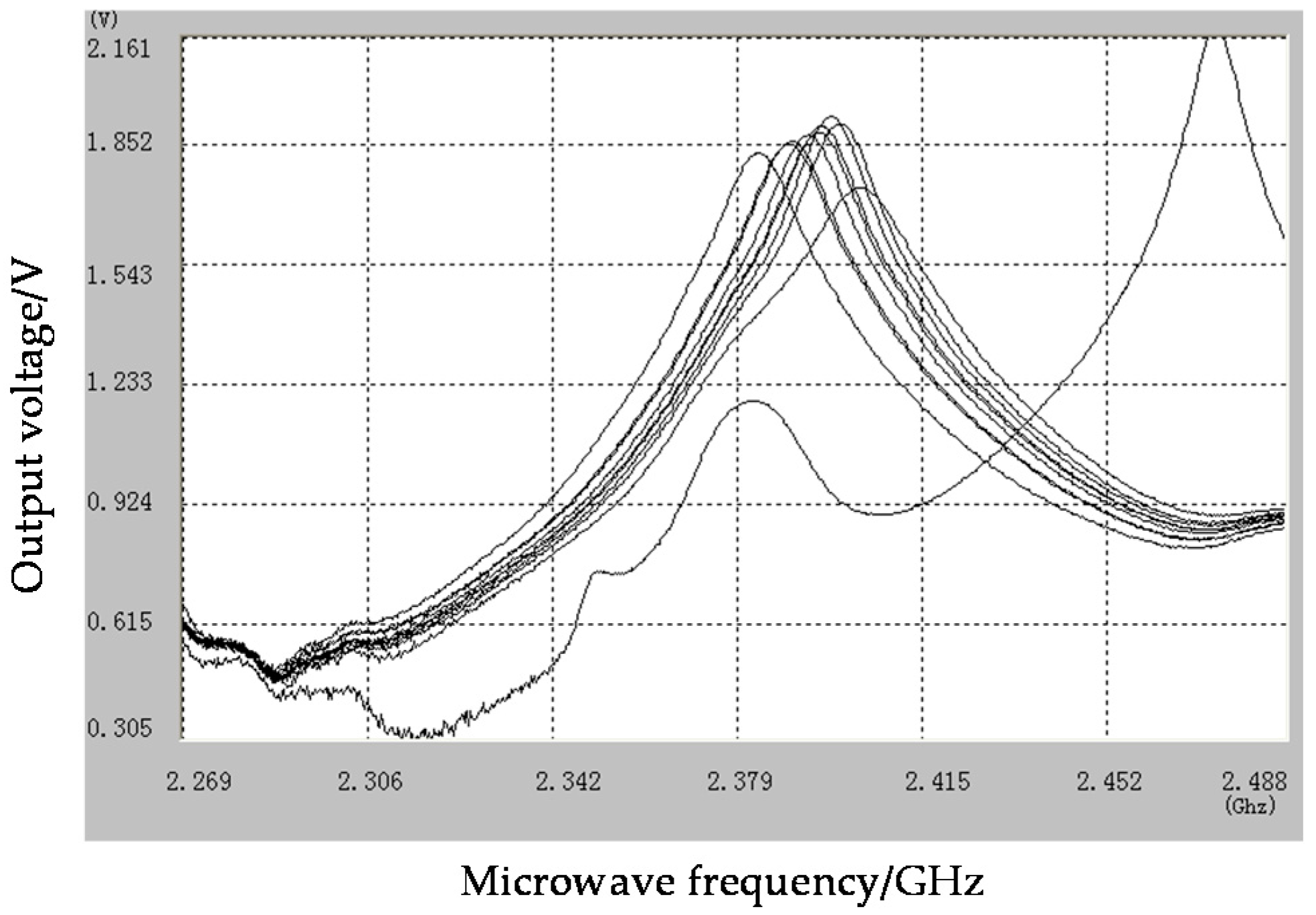
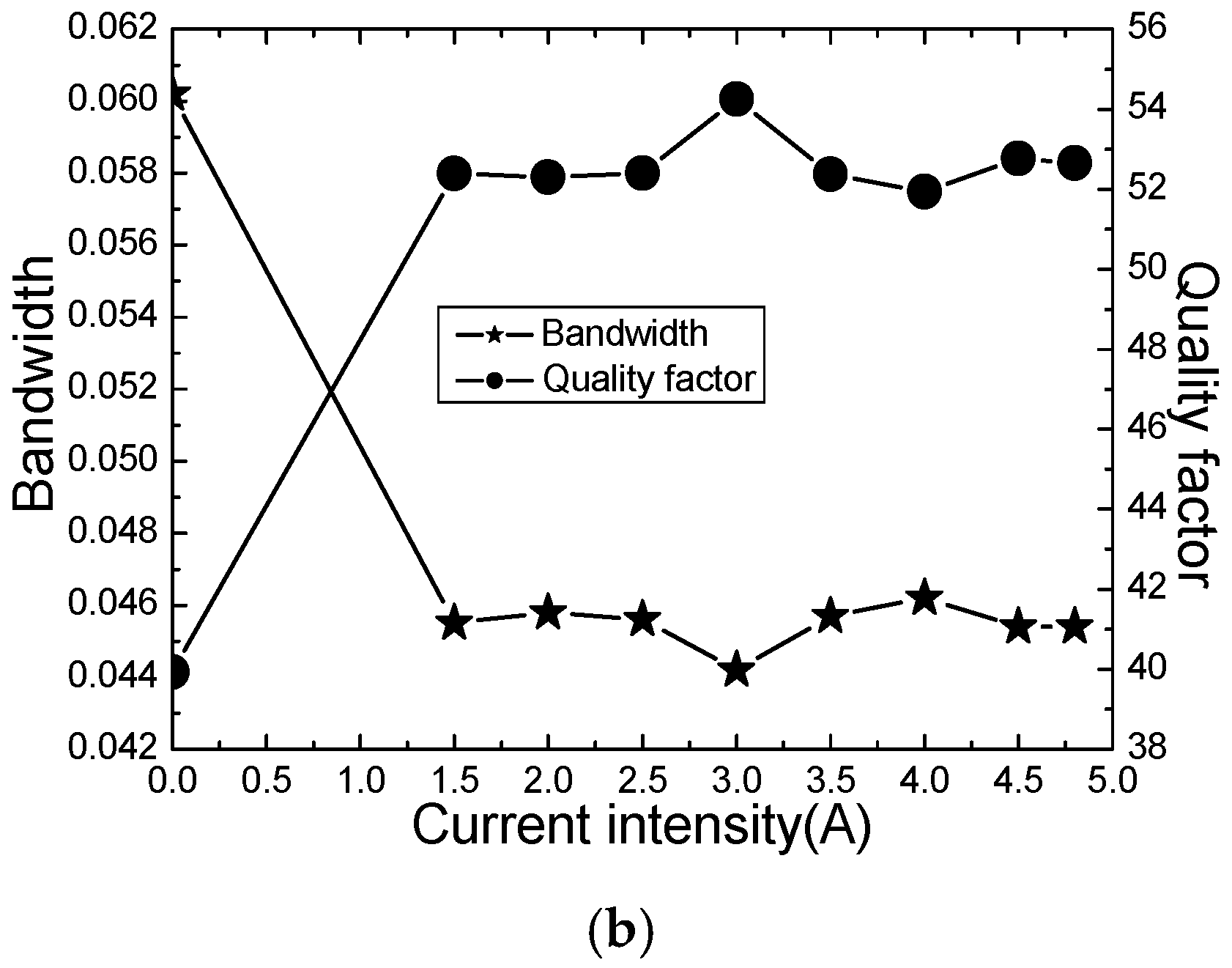
| NO | Intensity (A) | Attenuation Voltage (V) | Frequency (Ghz) | Bandwidth (Ghz) | Quality Factors (Q) |
|---|---|---|---|---|---|
| Empty cavity | 0.0 | 2.2030 | 2.4744 | 0.0325 | 76.14 |
| Raw material | 0.0 | 1.7659 | 2.4037 | 0.0602 | 39.93 |
| 1.5C | 1.5 | 1.8577 | 2.3836 | 0.0455 | 52.39 |
| 2.0C | 2.0 | 1.9130 | 2.3955 | 0.0458 | 52.30 |
| 2.5C | 2.5 | 1.8819 | 2.3897 | 0.0456 | 52.40 |
| 3.0C | 3.0 | 1.9543 | 2.3979 | 0.0442 | 54.25 |
| 3.5C | 3.5 | 1.9033 | 2.3935 | 0.0457 | 52.37 |
| 4.0C | 4.0 | 1.9355 | 2.3999 | 0.0462 | 51.94 |
| 4.5C | 4.5 | 1.9283 | 2.3960 | 0.0454 | 52.77 |
| 4.8C | 4.8 | 1.8895 | 2.3904 | 0.0454 | 52.65 |
| NO | Intensity (A) | Attenuation Voltage (V) | Frequency (Ghz) | Bandwidth (Ghz) | Quality Factors (Q) |
|---|---|---|---|---|---|
| Empty cavity | 0.0 | 2.2029 | 2.4745 | 0.0325 | 76.14 |
| Raw material | 0.0 | 1.8204 | 2.4127 | 0.0614 | 39.29 |
| 1.5F | 1.5 | 1.7885 | 2.4245 | 0.0714 | 33.95 |
| 2.0F | 2.0 | 1.7657 | 2.4194 | 0.0708 | 34.17 |
| 2.5F | 2.5 | 1.7888 | 2.4208 | 0.0690 | 35.08 |
| 3.0F | 3.0 | 1.7628 | 2.3990 | 0.0574 | 41.79 |
| 3.5F | 3.5 | 1.7400 | 2.4274 | 0.0790 | 30.72 |
| 4.0F | 4.0 | 1.7234 | 2.4250 | 0.0797 | 30.42 |
| 4.5F | 4.5 | 1.7463 | 2.4257 | 0.0781 | 31.06 |
| 4.8F | 4.8 | 1.7495 | 2.4305 | 0.0605 | 40.17 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, W.; Zhang, L.; Peng, J. Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Separation Products of Reductive Products of Ilmenite Concentrate. Minerals 2016, 6, 99. https://doi.org/10.3390/min6040099
Wang X, Li W, Zhang L, Peng J. Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Separation Products of Reductive Products of Ilmenite Concentrate. Minerals. 2016; 6(4):99. https://doi.org/10.3390/min6040099
Chicago/Turabian StyleWang, Xinying, Wei Li, Libo Zhang, and Jinhui Peng. 2016. "Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Separation Products of Reductive Products of Ilmenite Concentrate" Minerals 6, no. 4: 99. https://doi.org/10.3390/min6040099
APA StyleWang, X., Li, W., Zhang, L., & Peng, J. (2016). Microwave-Absorbing Characteristics and XRD Characterization of Magnetic Separation Products of Reductive Products of Ilmenite Concentrate. Minerals, 6(4), 99. https://doi.org/10.3390/min6040099





