Study of the Extraction Kinetics of Lithium by Leaching β-Spodumene with Hydrofluoric Acid
Abstract
:1. Introduction
2. Materials and Methods
Experimental Equipment and Procedure
3. Results
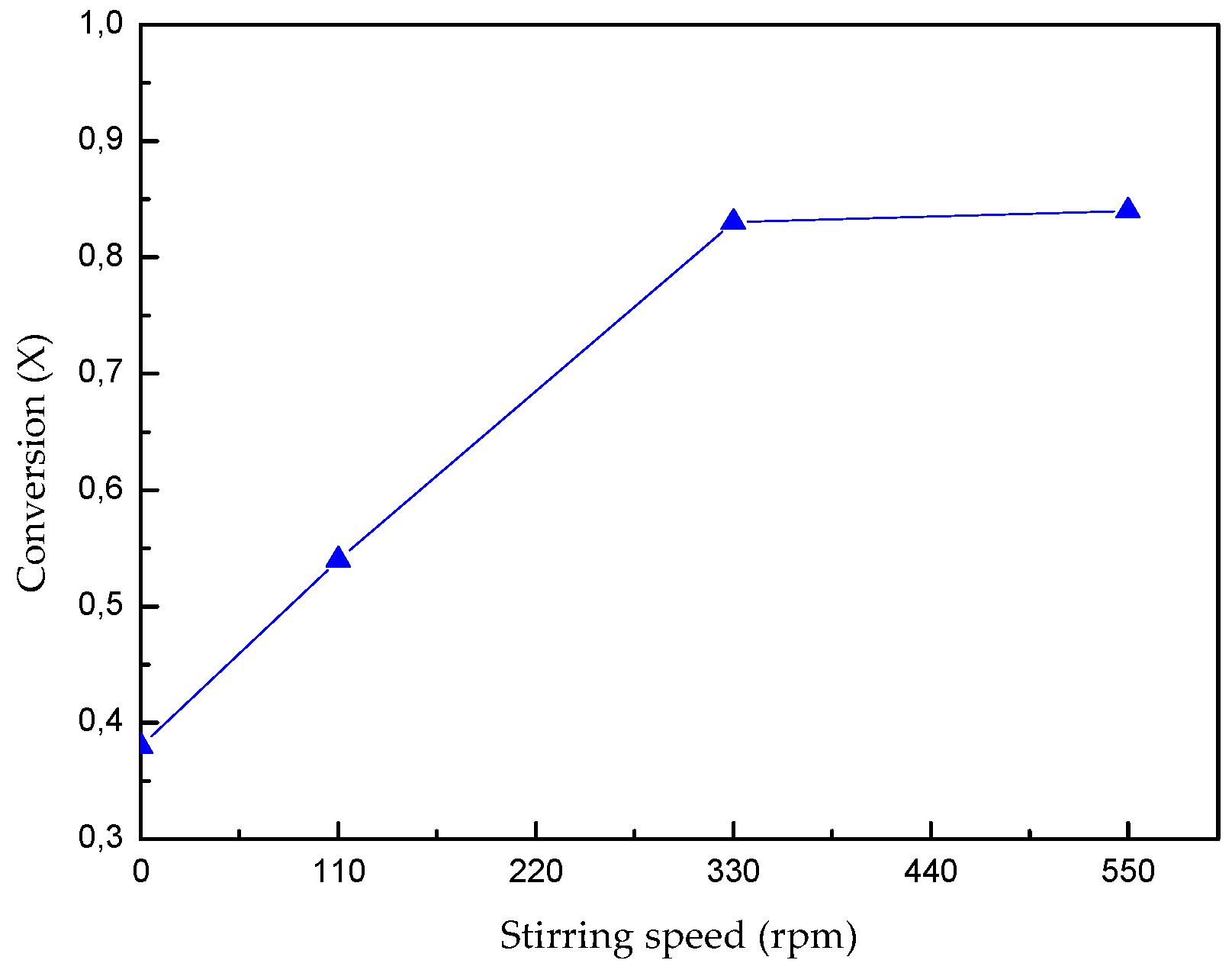
3.1. Effect of Stirring Speed
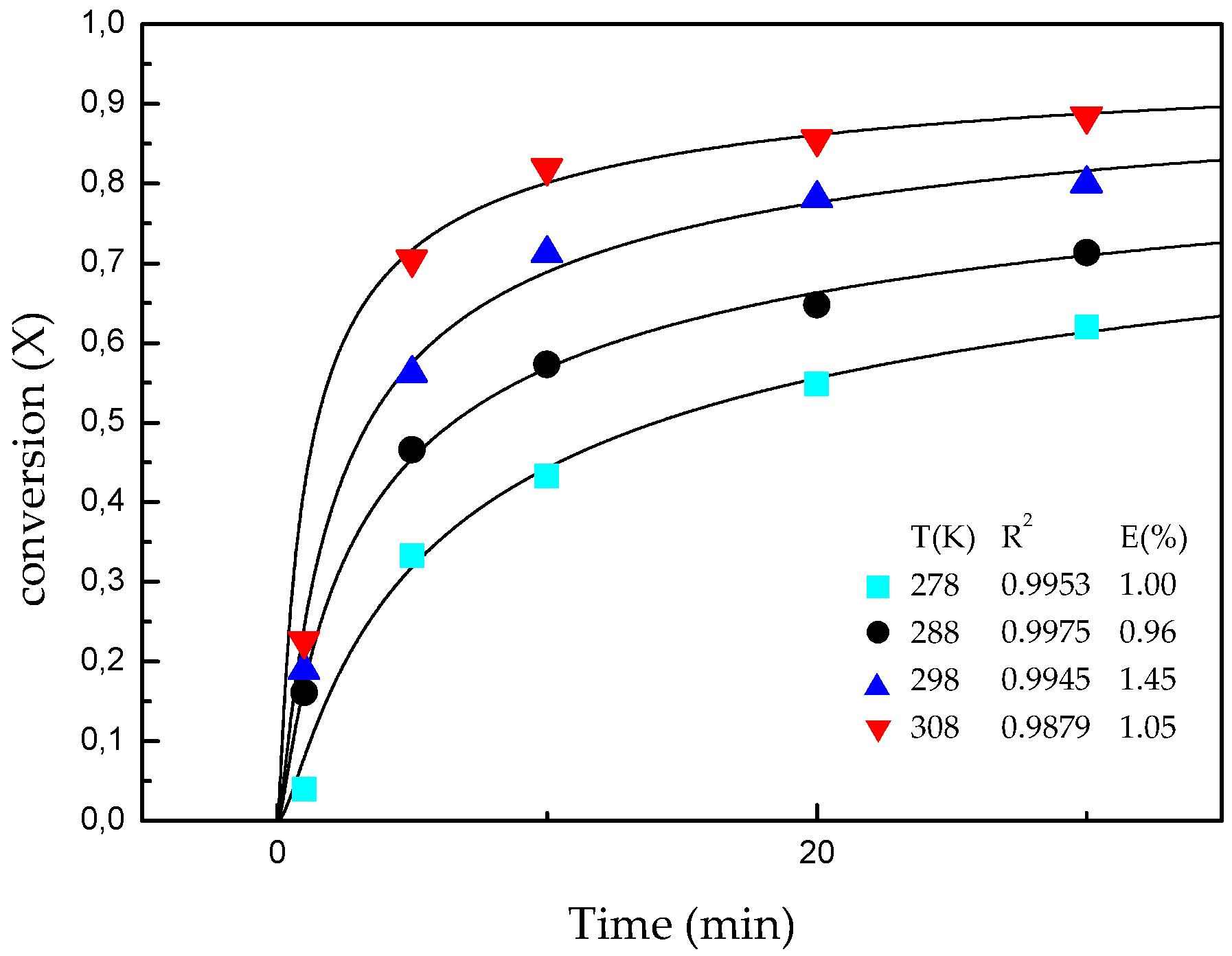
3.2. Effect of Temperature and Reaction Time
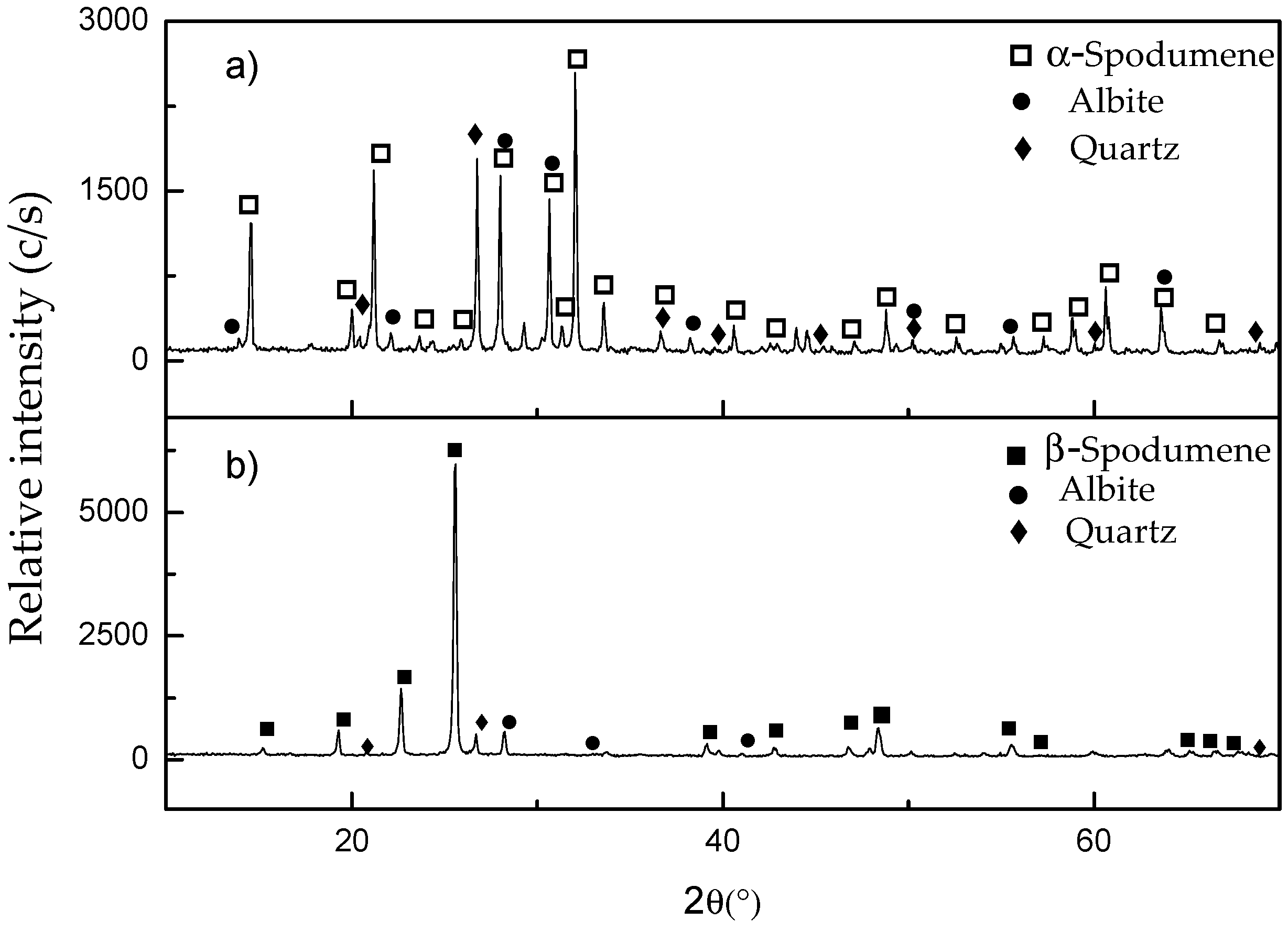
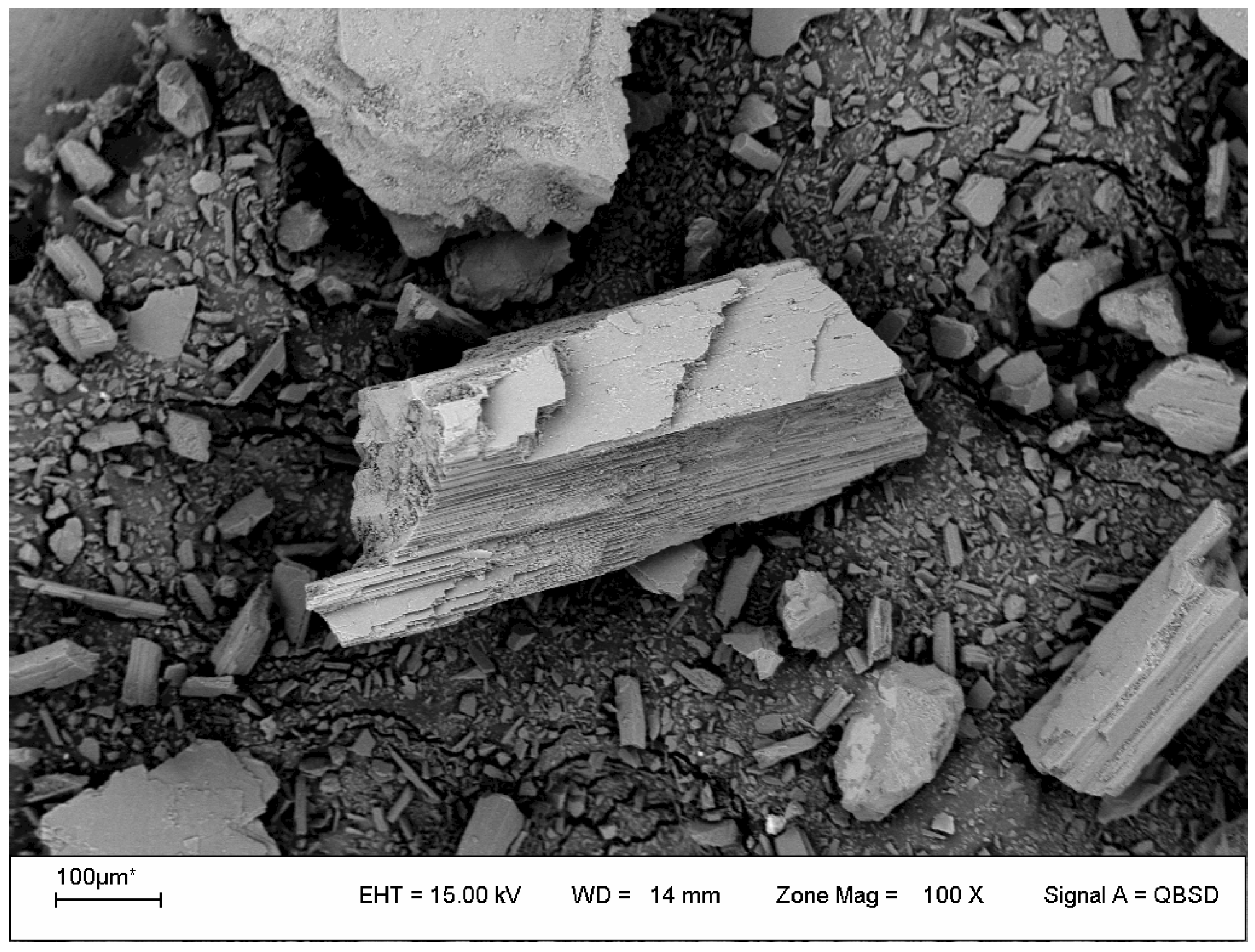
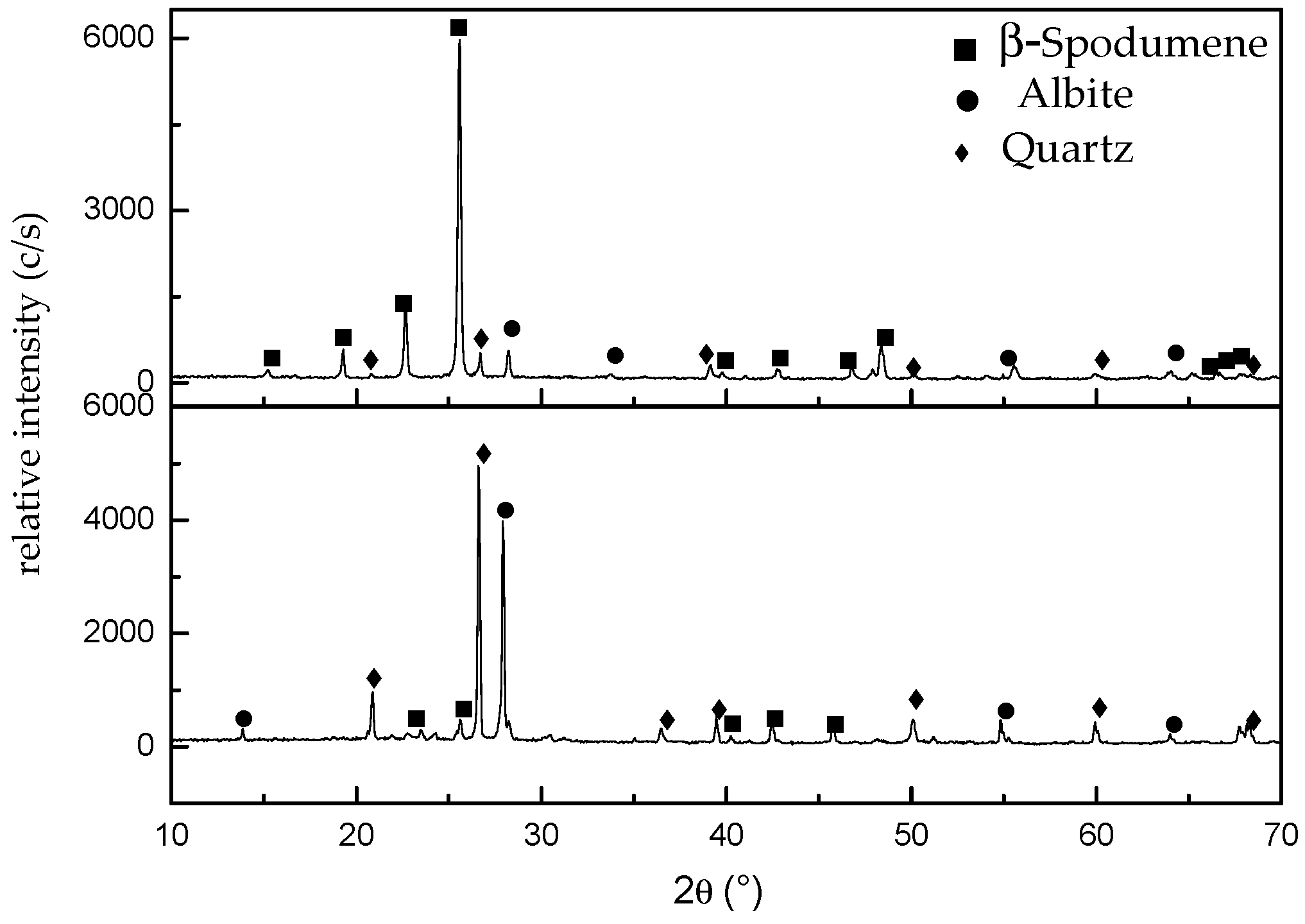
3.3. Characterization of the Leaching Residues
3.4. Kinetic Model
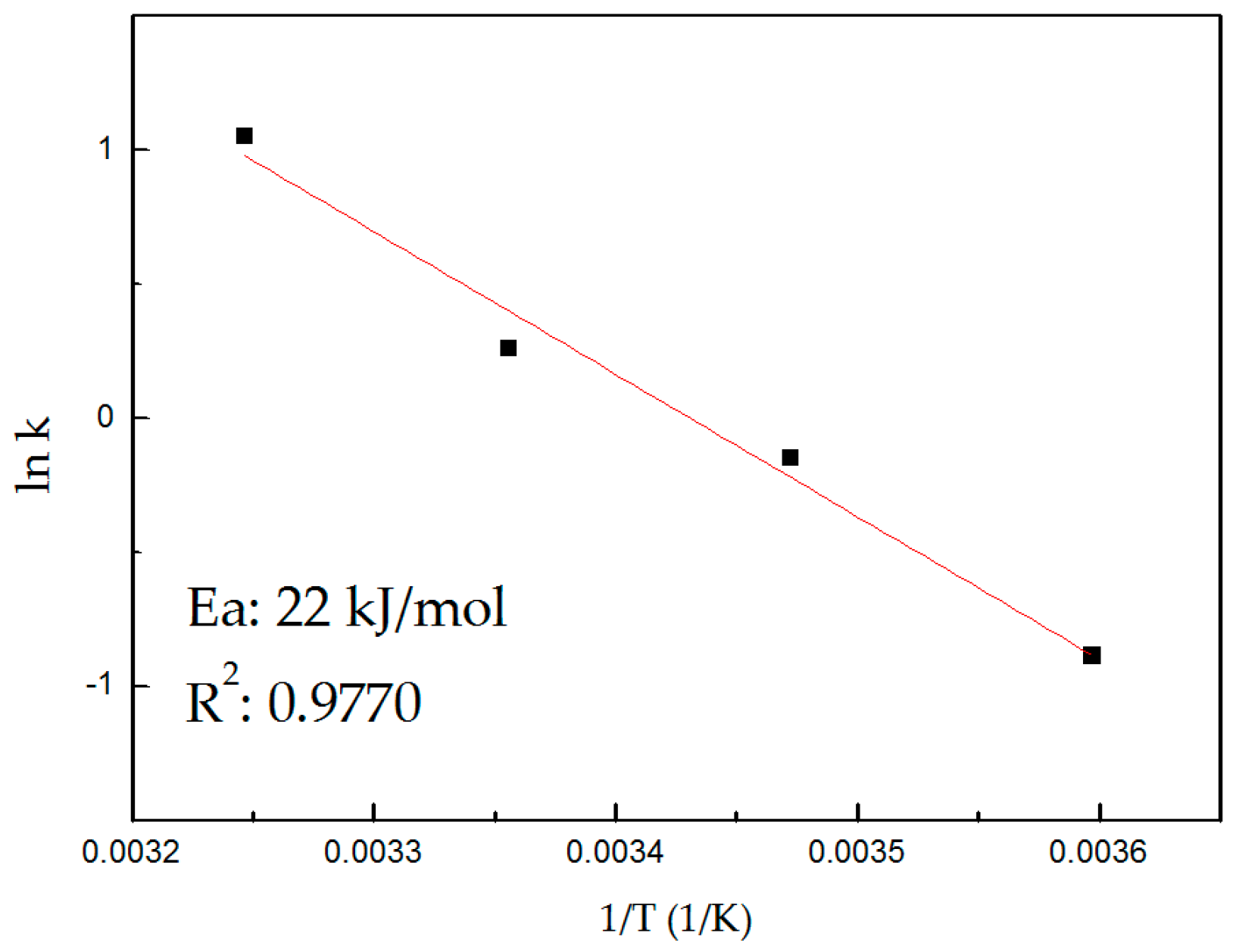
3.4.1. Process Controlling Stage
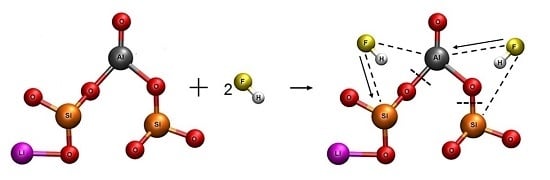
3.4.2. Reaction Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Symbols
| b | Stoichiometric coefficient |
| b1 and b2 | Coefficients defined by Equations (4) and (5), respectively |
| CA | Concentration of HF evaluated on the interface of reaction, mol/L |
| Initial particle diameter, mm | |
| Ea | Activation energy, kJ/mol |
| k | Kinetic coefficient of the reaction rate, m/s |
| Kinetic coefficient of the formation of the sites, m2/s | |
| MB | Molecular weight of the solid reactant, g/mol |
| Number of moles per surface unit of a chemical specie E | |
| Initial number of the sites than can be activated per unit of initial surface of the particle | |
| NS | Number of the active sites per surface unit |
| p | Growth factor |
| R | Gas constant, kJ/mol K |
| Reaction solid–fluid rate, mol/cm2 min | |
| T | Temperature, K |
| t | Time, min |
| X | Solid conversion |
Greek Symbols
| ρ | Solid density, kg/m3. |
| Ω0 | Initial particle surface, m2. |
| Coefficient of shape of the hole. |
References
- Garret, D.E. Part 1—Lithium. In Handbook of Lithium and Natural Calcium Chloride; Elsevier Ltd.: London, UK, 2004. [Google Scholar]
- Siame, E.; Pascoe, R.D. Extraction of lithium from micaceous waste from China clay production. Min. Eng. 2011, 24, 1595–1602. [Google Scholar] [CrossRef]
- Habashi, F. Alkali Metals-Lithium. In Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997; Volume IV. [Google Scholar]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from β-spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Barbosa, L.; Valente, G.; Orosco, R.P.; Gonzalez, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Min. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Barbosa, L.; Ruiz, M.C.; Gonzalez, J.A. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Rosales, G.; Ruiz, M.C.; Rodriguez, M. Alkaline metal fluorides synthesis as subproduct of β-spodumene leaching. Hydrometallurgy 2013, 139, 73–78. [Google Scholar] [CrossRef]
- Rosales, G.; Ruiz, M.C.; Rodriguez, M. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Kline, E.; Fogler, S. Dissolution kinetics: The nature of the particle attack of layered silicates in HF. Chem. Eng. Sci. 1980, 36, 871–884. [Google Scholar] [CrossRef]
- Kline, E.; Fogler, S. Dissolution kinetics: Catalysis by salts. J. Colloid Interface Sci. 1981, 82, 103–115. [Google Scholar] [CrossRef]
- Kumar, M.; Mankhand, T.R.; Murthy, D.S.R.; Mukhopadhyay, R.; Prasad, P.M. Refining of a low-grade molybdenite concentrate. Hydrometallurgy 2006, 86, 56–62. [Google Scholar] [CrossRef]
- Kumar, M.; Nani, M.; Mankhand, T.; Pandey, B. Precipitation of sodium silicofluoride (Na2SiF6) and cryolite (Na3AlF6) from HF/HCl leach liquors of alumino-silicates. Hydrometallurgy 2010, 104, 304–307. [Google Scholar] [CrossRef]
- Brumbaugh, R.; Fanus, W. Determination of lithium in spodumene by flame photometry. Anal. Chem. 1954, 26, 463–465. [Google Scholar] [CrossRef]
- Salakjani, N.; Singh, P.; Nikoloski, A.N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventional heating. Min. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Quiroga, O.D.; Avanza, J.R.; Fusco, A.J. Modelado Cinético de las Transformaciones Fluido-Sólido Reactivo; Editorial Universitaria de la Universidad del Nordeste (EUDENE): Buenos Aires, Argentina, 1996. (In Spanish) [Google Scholar]
- Barbosa, L.; Valente, G.; Gonzalez, J.A. Kinetic study on the chlorination of β-spodumene for lithium extraction with Cl2 gas. Thermochim. Acta 2014, 557, 61–67. [Google Scholar] [CrossRef]
- Tunez, F.; Orosco, P.; Gonzalez, J.A.; Ruiz, M.C. Kinetic study on the chlorination of indium oxide. Thermochim. Acta 2011, 524, 151–156. [Google Scholar] [CrossRef]
- Rodriguez, M.; Quiroga, O.; Ruiz, M.C. Kinetic study of ferrocolumbite dissolution in hydrofluoric acid medium. Hydrometallurgy 2007, 85, 87–94. [Google Scholar] [CrossRef]
- Quiroga, O.D. Modelado. In Software para el Tratamiento Cinético de Transformaciones Fluido Sólido-Reactivo; INIQUI (UNSa-CONICET): Salta, Argentina, 2002. (In Spanish) [Google Scholar]
- Delmon, B. Introduction a la Cinétique Hétérogène; Editions Technip: Paris, France, 1969. (In French) [Google Scholar]
- Avrami, M. Kinetic of phase change. I General theory. J. Chem. Phys. 1963, 7, 1103. [Google Scholar] [CrossRef]
- Habashi, F. Principles of Extractive Metallurgy; Gordon and Breach: New York, NY, USA, 1980; Volume I. [Google Scholar]
- Fernández Lisbona, D.; Steel, M.K. Recovery of fluoride values from spent pot-lining: Precipitation of an aluminum hydroxyfluoride hydrate product. Sep. Purif. Technol. 2008, 61, 182–192. [Google Scholar] [CrossRef]




| Component | % w/w |
|---|---|
| SiO2 | 68.3 |
| Al2O3 | 18.6 |
| Fe2O3 | 3.21 |
| CaO | 0.52 |
| MgO | 0.29 |
| K2O | 0.14 |
| Na2O | 0.78 |
| Li2O | 7.03 |
| TiO | 0.11 |
| Others | 0.12 |
| Temperature (K) | % O | % Al | % Si | % F |
|---|---|---|---|---|
| 278 | 32 | 17 | 51 | - |
| 288 | 40 | 16 | 43 | - |
| 298 | 37 | 16 | 46 | - |
| 308 | 48 | 12 | 39 | - |
| Temperature (K) | b1 | b2 | k |
|---|---|---|---|
| 278 | 0.3698 | 1.116 | 0.4126 |
| 288 | 0.3812 | 2.257 | 0.8603 |
| 298 | 0.5020 | 2.580 | 1.295 |
| 308 | 0.5346 | 5.355 | 2.862 |
| Ea (kJ/mol) | Control |
|---|---|
| <12.5 | Diffusional |
| 21–33.5 | Intermediate |
| >41.8 | Chemical |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, G.D.; Ruiz, M.C.; Rodriguez, M.H. Study of the Extraction Kinetics of Lithium by Leaching β-Spodumene with Hydrofluoric Acid. Minerals 2016, 6, 98. https://doi.org/10.3390/min6040098
Rosales GD, Ruiz MC, Rodriguez MH. Study of the Extraction Kinetics of Lithium by Leaching β-Spodumene with Hydrofluoric Acid. Minerals. 2016; 6(4):98. https://doi.org/10.3390/min6040098
Chicago/Turabian StyleRosales, Gustavo D., María C. Ruiz, and Mario H. Rodriguez. 2016. "Study of the Extraction Kinetics of Lithium by Leaching β-Spodumene with Hydrofluoric Acid" Minerals 6, no. 4: 98. https://doi.org/10.3390/min6040098
APA StyleRosales, G. D., Ruiz, M. C., & Rodriguez, M. H. (2016). Study of the Extraction Kinetics of Lithium by Leaching β-Spodumene with Hydrofluoric Acid. Minerals, 6(4), 98. https://doi.org/10.3390/min6040098