Reflectance Spectral Characteristics of Minerals in the Mboukoumassi Sylvite Deposit, Kouilou Province, Congo
Abstract
:1. Introduction
2. Geological Backgrounds
3. Samples and Analytical Methods
4. Results
5. Discussion
6. Conclusions
- (1)
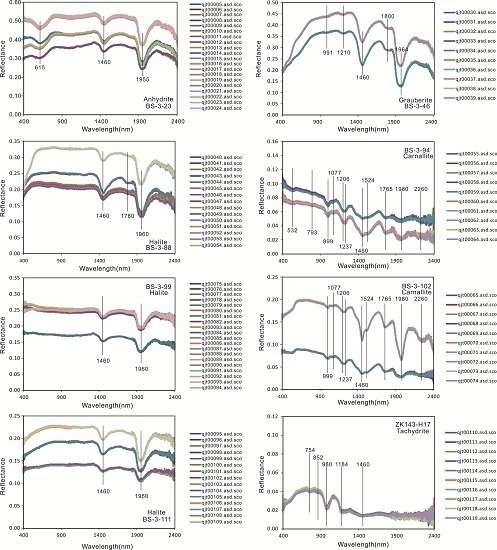
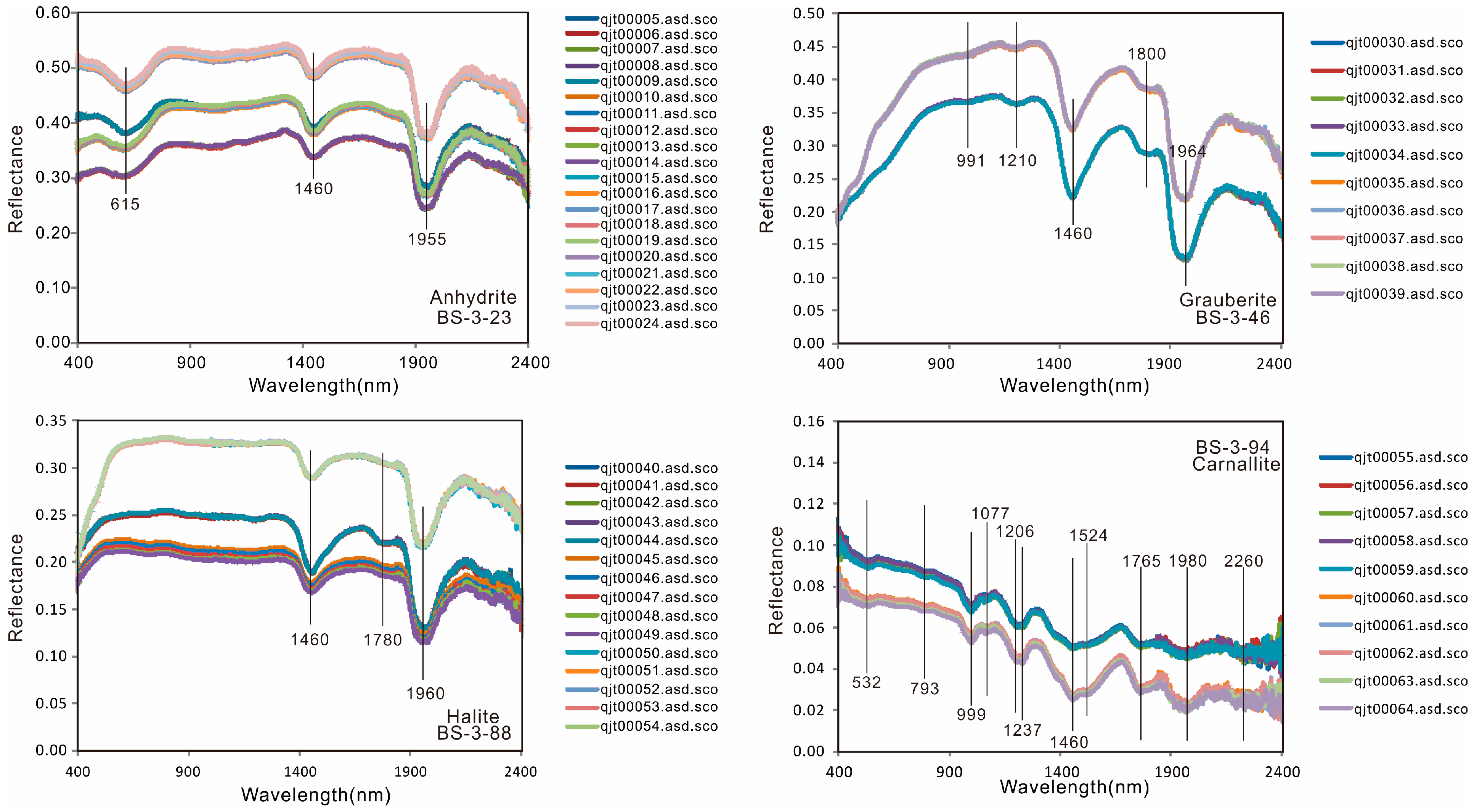
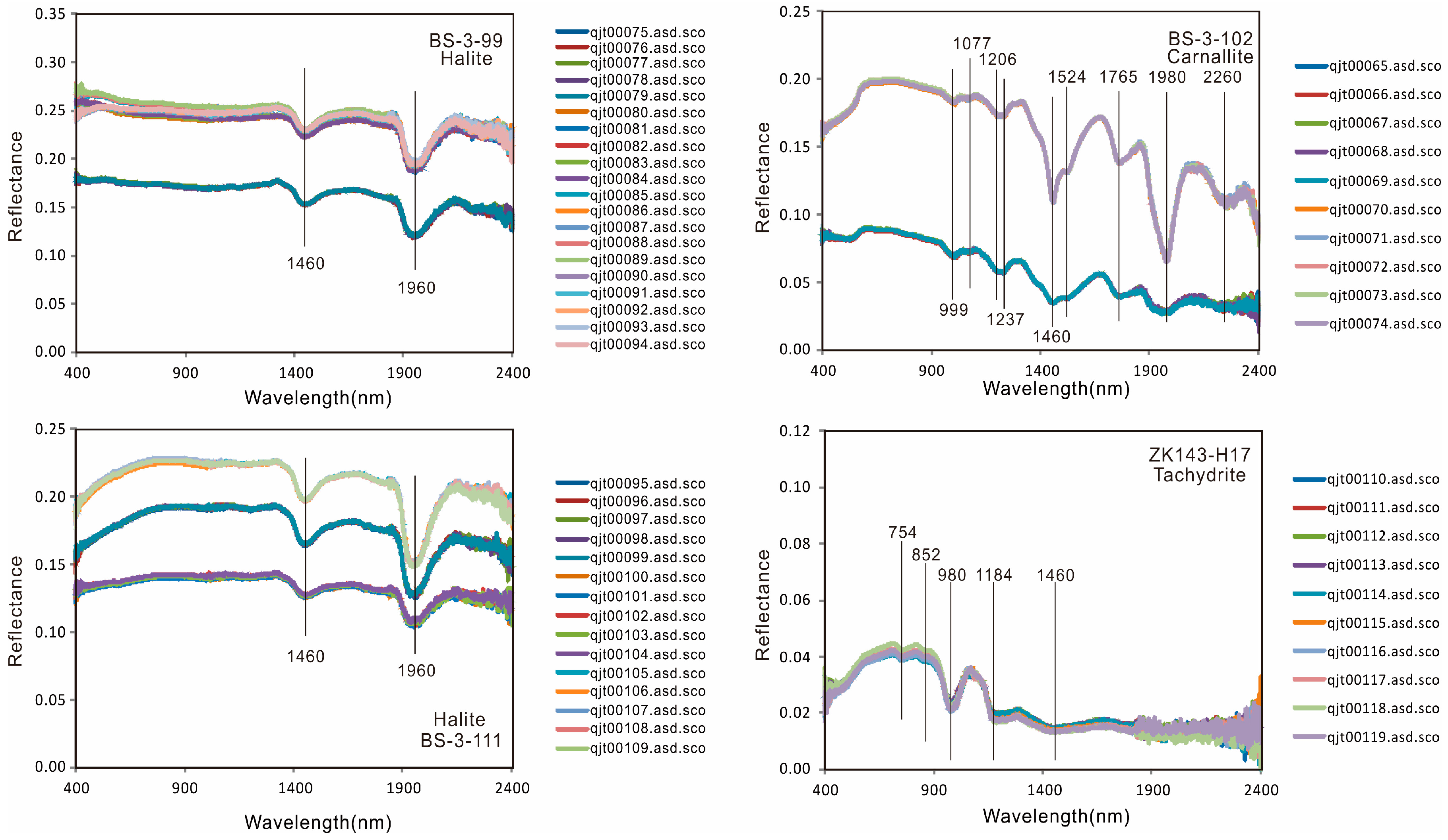
- The spectra of five main types of minerals from the Mboukoumassi deposit, Democratic Republic of the Congo, were determined: anhydrite, glauberite, halite, carnallite, and tachydrite. Anhydrite shows absorption peaks at 615, 1460, and 1955 nm. Glauberite has absorption peaks at 991, 1210, 1460, 1800, and 1964 nm. Halite displays absorption features at 1460 and 1960 nm. Carnallite has absorption peaks at 999, 1077, 1206, 1237, 1460, 1524, 1765, 1980, and 2260 nm. Tachydrite displays absorption features at 754, 852, 980, 1184, and 1460 nm.
- (2)
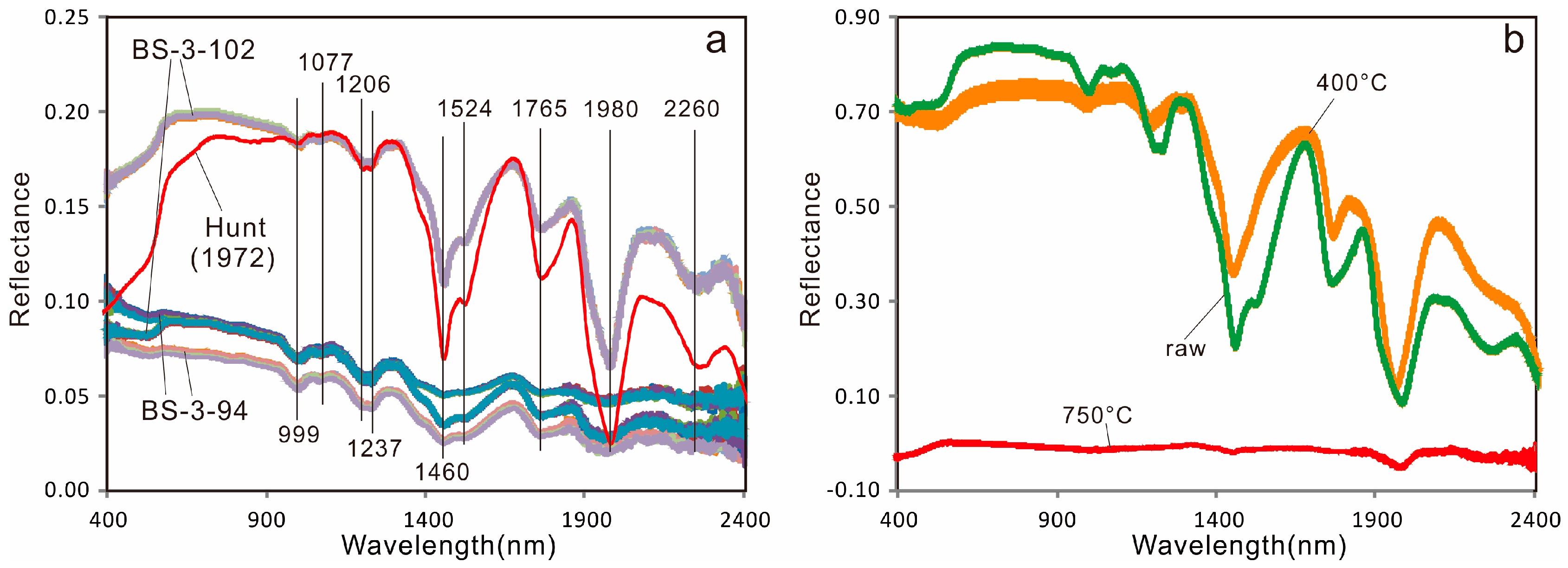
- The absorption features at 999, 1077, 1206, 1237, 1524 and 1765 nm can be used to distinguish carnallite from halite in samples and thereby assist in metallurgical extraction.
- (3)
- The high spectral absorption reproducibility of carnallite suggests that mineral identification based on absorption features is more reliable than that based on general spectral shape.
- (4)
- The absorption features of carnallite (KCl·MgCl2·6H2O) are clearly related to hydration or hydroxyl addition in its crystallographic structure, as demonstrated by the heating experiment.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chabrillat, S.; Pinet, P.C.; Ceuleneer, G.; Johnson, P.E.; Mustard, J.F. Ronda peridotite massif: Methodology for its geological mapping and lithological discrimination from airborne hyperspectral data. Int. J. Remote Sens. 2000, 21, 2363–2388. [Google Scholar] [CrossRef]
- Chen, X.; Warner, T.A.; Campagna, D.J. Integrating visible, near-infrared and short-wave infrared hyperspectral and multispectral thermal imagery for geological mapping at Cuprite, Nevada: A rule-based system. Int. J. Remote Sens. 2010, 31, 1733–1752. [Google Scholar] [CrossRef]
- Harris, J.R.; McGregor, R.; Budkewitsch, P. Geological analysis of hyperspectral data over southwest Baffin Island: Methods for producing spectral maps that relate to variations in surface lithologies. Can. J. Remote Sens. 2010, 36, 412–435. [Google Scholar] [CrossRef]
- Slavinski, H.; Morris, B.; Ugalde, H.; Spicer, B.; Skulski, T.; Rogers, N. Integration of lithological, geophysical, and remote sensing information: A basis for remote predictive geological mapping of the Baie Verte Peninsula, Newfoundland. Can. J. Remote Sens. 2010, 36, 99–118. [Google Scholar] [CrossRef]
- Qiu, J.-T.; Li, P.-J.; Yu, Z.-F.; Li, P. Petrology and spectroscopy studies on Danxia Geoheritagein Southeast Sichuan Area, China: Implications for Danxia surveying and monitoring. Geoheritage 2015, 7, 307–318. [Google Scholar] [CrossRef]
- Babu, P.S.; Majumdar, T.J.; Bhattacharya, A.K. Study of spectral signatures for exploration of Bauxite ore deposits in Panchpatmali, India. Geocarto Int. 2015, 30, 545–559. [Google Scholar] [CrossRef]
- Boesche, N.K.; Rogass, C.; Lubitz, C.; Brell, M.; Herrmann, S.; Mielke, C.; Tonn, S.; Appelt, O.; Altenberger, U.; Kaufmann, H. Hyperspectral REE (Rare Earth Element) mapping of outcrops—Applications for neodymium detection. Remote Sens. 2015, 7, 5160–5186. [Google Scholar] [CrossRef]
- Qiu, J.-T.; Zhang, C.; Hu, X. Integration of concentration-area fractal modeling and spectral angle mapper for ferric iron alteration mapping and uranium exploration in the Xiemisitan Area, NW China. Remote Sens. 2015, 7, 13878–13894. [Google Scholar] [CrossRef]
- Nair, A.M.; Mathew, G. Effect of bulk chemistry in the spectral variability of igneous rocks in VIS-NIR region: Implications to remote compositional mapping. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 227–237. [Google Scholar] [CrossRef]
- Martini, J.E.J.; Bowles, M. Metallogenic Map of the Republic of Congo, Scale 1:1,000,000; Republic of Congo Ministry of Mines and Energy: Brazzaville, Congo, 1994.
- Clark, R.N.; Swayze, G.A.; Gallagher, A.J.; King, T.V.V.; Calvin, W.M. The U.S. Geological Survey, Digital Spectral Library: Version 1: 0.2 to 3.0 Microns; U.S. Geological Survey Open File Report (No.93-592); U.S. Geological Survey: Reston, VA, USA, 1993; p. 1340.
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks: V. Halides, phosphates, arsenates, vanadates and borates. Mod. Geol. 1972, 3, 121–132. [Google Scholar]
- Clark, R.N. Spectroscopy of rocks and minerals, and principles of spectroscopy. In Manual of Remote Sensing; Rencz, A.N., Ed.; John Wiley and Sons: New York, NY, USA, 1999; Volume 3, pp. 3–58. [Google Scholar]
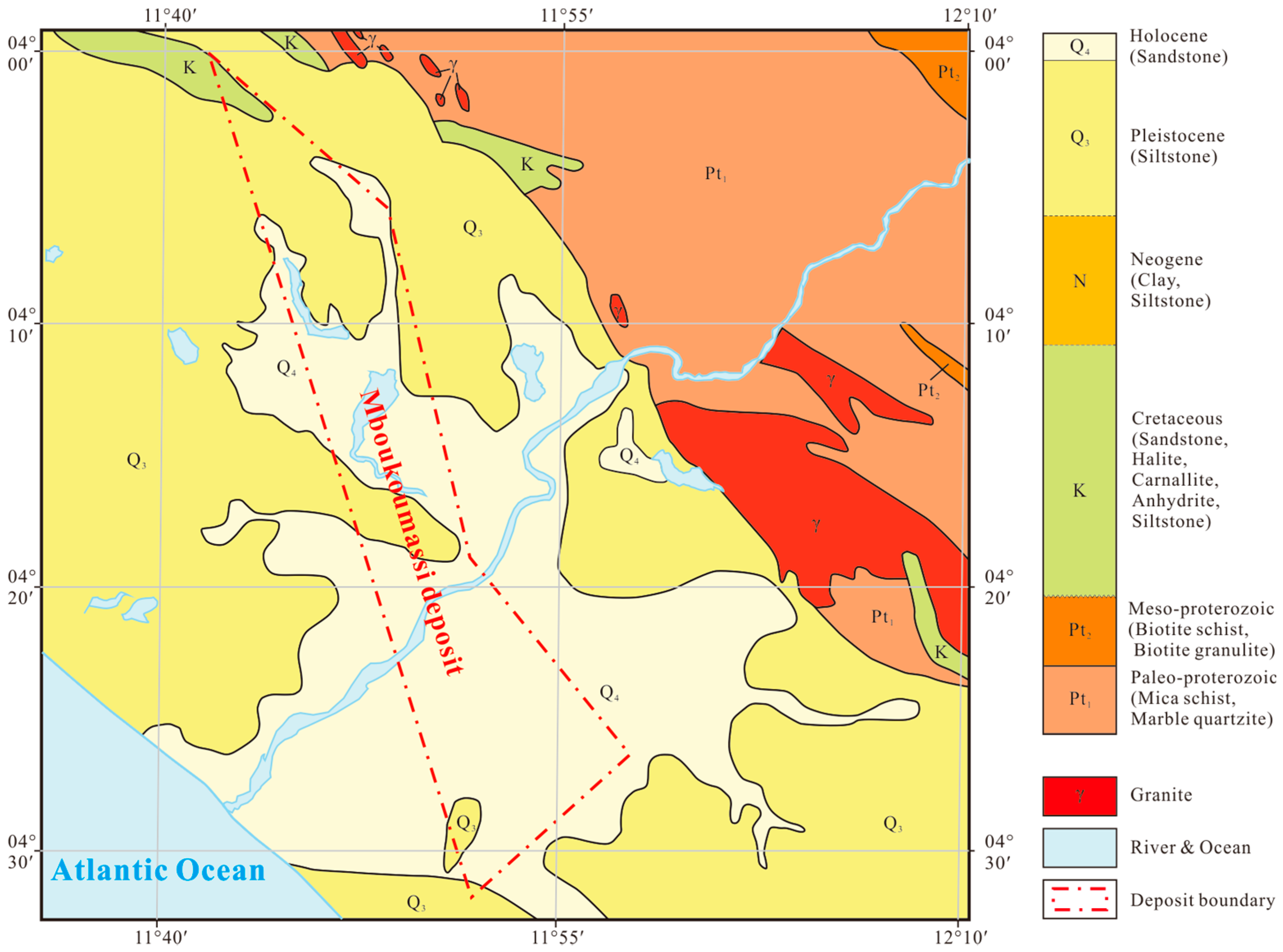

| Sample No. | Lithology | Location1 | Description |
|---|---|---|---|
| BS-3-23 | Anhydrite | D.C. BS-3 −240.80 m | White or gray in color, opaque, blocky structure, earthy luster |
| BS-3-46 | Grauberite | D.C. BS-3 −308.10 m | Gray in color, opaque, blocky structure, earthly luster |
| BS-3-88 | Halite | D.C. BS-3 −447.31 m | Gray in color, translucent, fine to medium grained, blocky structure, vitreous to earthly luster |
| BS-3-94 | Carnallite | D.C. BS-3 −453.71 m | Lightpink in color, translucent, blocky structure, oily luster |
| BS-3-99 | Halite | D.C. BS-3 −464.73 m | Gray to white in color, translucent, blocky structure, vitreous to earthly luster |
| BS-3-102 | Carnallite | D.C. BS-3 −474.55 m | Salmon to darksalmon in color, translucent, blocky structure, oily luster |
| BS-3-111 | Halite | D.C. BS-3 −494.19 m | Gray in color, translucent, blocky structure, vitreous to earthly luster |
| ZK143-H17 | Tachydrite | D.C. ZK143 −435.00 m | Goldenrod in color, translucent, oily luster, very soluble |
| Sample | Mg | K | Sr | Na | Ca | Li | B | S | Cl | Br |
|---|---|---|---|---|---|---|---|---|---|---|
| BS-3-23 | 0.1646 | 0.0072 | 0.0864 | 0.0666 | 24.9031 | 0.0023 | 0.0195 | 64.5483 | 0.1919 | 1.7114 |
| BS-3-46 | 0.1637 | 0.0187 | 0.0665 | 9.3709 | 20.5751 | 0.0066 | 0.1195 | 53.2418 | 17.4755 | 2.1987 |
| BS-3-88 | 0.0775 | 0.0928 | 0.0022 | 39.0701 | 0.2392 | 0.0002 | 0.0004 | 0.1292 | 60.2938 | 0.0456 |
| BS-3-94 | 7.4666 | 11.3368 | 0.0004 | 6.2572 | 0.0557 | 0.0001 | 0.0000 | 0.0307 | 43.1870 | 0.4341 |
| BS-3-99 | 0.3203 | 0.6943 | 0.0025 | 36.3044 | 0.4268 | 0.0001 | 0.0029 | 0.2901 | 59.4677 | 0.0590 |
| BS-3-102 | 6.6710 | 10.9012 | 0.0005 | 8.3753 | 0.0657 | 0.0000 | 0.0000 | 0.0385 | 38.7926 | 0.2900 |
| BS-3-111 | 0.0478 | 0.0363 | 0.0021 | 38.2802 | 0.3930 | 0.0001 | 0.0010 | 0.2672 | 60.5064 | 0.0337 |
| ZK143-H17 | 11.5903 | 0.3040 | 0.1885 | 0.2841 | 5.1510 | 0.0000 | 0.0107 | 0.1136 | 44.4799 | 5.7306 |
| Item | Parameter |
|---|---|
| Spectral Range | 350 to 2500 nm |
| Spectral Resolutions | 3 nm @ 700 nm |
| 6 nm @ 1400 nm | |
| 6 nm @ 2100 nm | |
| Sampling Intervals | 1.4 nm between 350 and 1000 nm |
| 2 nm between 1000 and 2500 nm | |
| Signal to Noise Values | 9500 DN @ 700 nm |
| 5000 DN @ 1400 nm | |
| 800 DN @ 2100 nm |
| File Name List | Sample No. | Comment |
|---|---|---|
| qjt00000.asd.sco to qjt00004.asd.sco | White Reference | Standard (1 measurement) |
| qjt00005.asd.sco to qjt00024.asd.sco | BS-3-23 | Anhydrite (4 measurements) |
| qjt00030.asd.sco to qjt00039.asd.sco | BS-3-46 | Grauberite (2 measurements) |
| qjt00040.asd.sco to qjt00054.asd.sco | BS-3-88 | Halite (3 measurements) |
| qjt00055.asd.sco to qjt00064.asd.sco | BS-3-94 | Carnallite (2 measurements) |
| qjt00075.asd.sco to qjt00094.asd.sco | BS-3-99 | Halite (4 measurements) |
| qjt00065.asd.sco to qjt00074.asd.sco | BS-3-102 | Carnallite (2 measurements) |
| qjt00095.asd.sco to qjt00109.asd.sco | BS-3-111 | Halite (3 measurements) |
| qjt00110.asd.sco to qjt00119.asd.sco | ZK143-H17 | Tachydrite (2 measurements) |
| qjt00155.asd.sco to qjt00159.asd.sco | White Reference | Repeat Standard (1 measurement) |
| Mineral | Absorption(s) @ nm | Reference |
|---|---|---|
| BS-3-23 (Anhydrite) | 615, 1460, 1955 | this study |
| BS-3-46 (Grauberite) | 991, 1210, 1460, 1800, 1964 | this study |
| BS-3-88 (Halite) | 1460, 1780, 1960, | this study |
| BS-3-94 (Carnallite) | 532, 793, 999, 1077, 1206, 1237, 1460, 1524, 1765, 1980, 2260 | this study |
| BS-3-99 (Halite) | 1460, 1960 | this study |
| BS-3-102 (Carnallite) | 999, 1077, 1206, 1237, 1460, 1524, 1765, 1980, 2260 | this study |
| BS-3-111 (Halite) | 1460, 1960 | this study |
| ZK143-H17 (Tachydrite) | 754, 852, 980, 1184, 1460 | this study |
| GDS42 (Anhydrite) | 1464, 1955 | USGS |
| HS433.3B (Halite) | 1464, 1965 | USGS |
| NMNH98011 (Carnallite) | 1006, 1074, 1209, 1234, 1464, 1529, 1764, 1985, 2255 | USGS |
| HS430.3B(Carnallite) | 994, 1074, 1199, 1224, 1454, 1520, 1764, 1975, 2265 | USGS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.-F.; Wang, Z.-Q.; Qiu, J.-T.; Song, Y. Reflectance Spectral Characteristics of Minerals in the Mboukoumassi Sylvite Deposit, Kouilou Province, Congo. Minerals 2016, 6, 55. https://doi.org/10.3390/min6020055
Zhao X-F, Wang Z-Q, Qiu J-T, Song Y. Reflectance Spectral Characteristics of Minerals in the Mboukoumassi Sylvite Deposit, Kouilou Province, Congo. Minerals. 2016; 6(2):55. https://doi.org/10.3390/min6020055
Chicago/Turabian StyleZhao, Xian-Fu, Zong-Qi Wang, Jun-Ting Qiu, and Yang Song. 2016. "Reflectance Spectral Characteristics of Minerals in the Mboukoumassi Sylvite Deposit, Kouilou Province, Congo" Minerals 6, no. 2: 55. https://doi.org/10.3390/min6020055
APA StyleZhao, X.-F., Wang, Z.-Q., Qiu, J.-T., & Song, Y. (2016). Reflectance Spectral Characteristics of Minerals in the Mboukoumassi Sylvite Deposit, Kouilou Province, Congo. Minerals, 6(2), 55. https://doi.org/10.3390/min6020055