Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China
Abstract
:1. Introduction
2. Geologic Setting
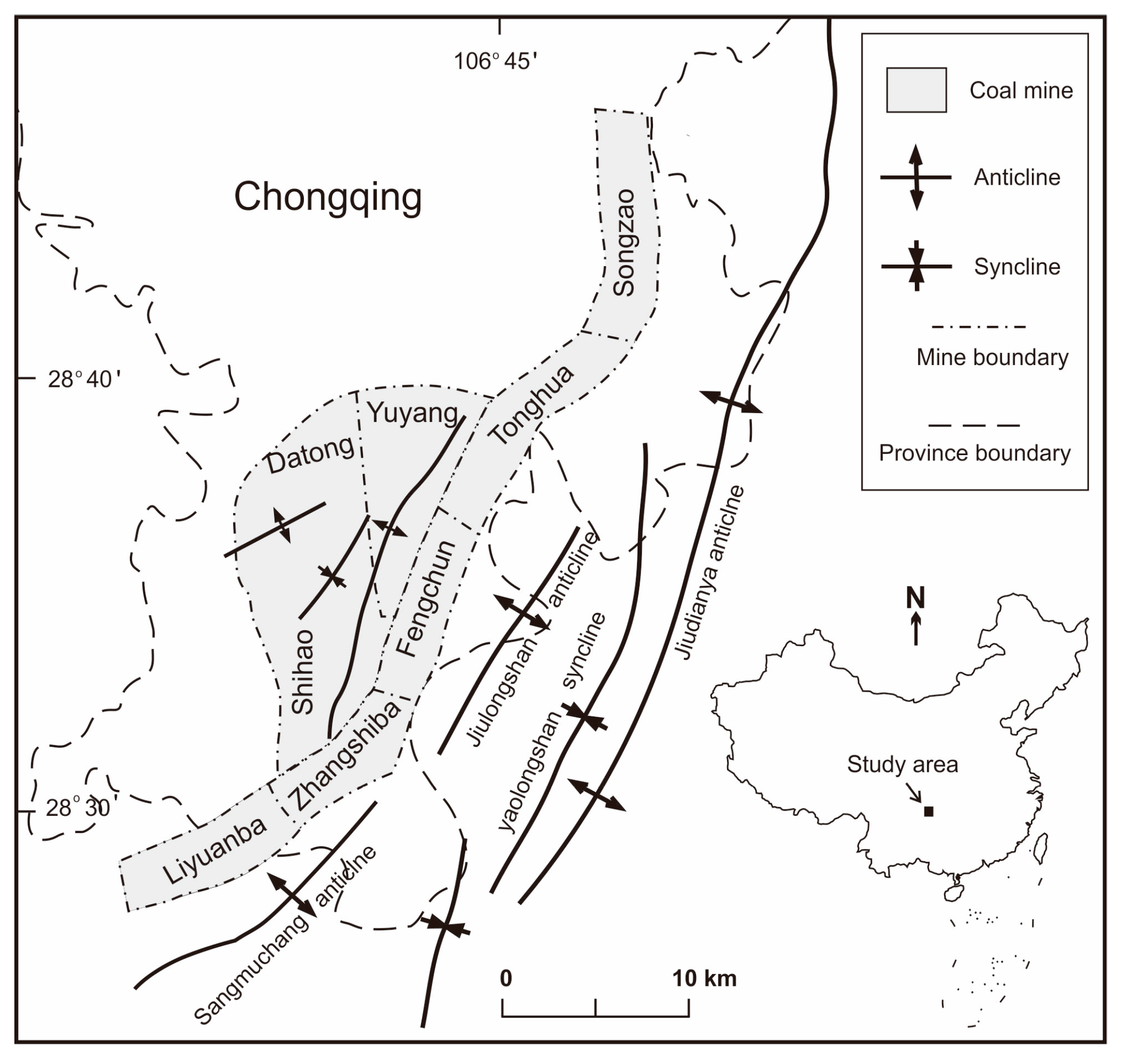

3. Sampling and Methods
4. Results and Discussion
4.1. Coal Characteristics
| Sample | Thickness (cm) | Ash Yield | VMdaf | FCdaf | Total Sulfur | Pyritic Sulfur | Rv,max | Clay Minerals | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Kao (+ Chl) | I | E | ||||||||
| dt-7-0 | - | - | - | - | - | - | - | 71 | 7 | 23 |
| dt-7-1 | 15 | 20.6 | 10.0 | 90.0 | 6.73 | 5.00 | 2.22 | 86 | 0 | 14 |
| dt-7-2 | 22 | 18.2 | 10.3 | 89.7 | 4.89 | - | 2.17 | 100 | 0 | 0 |
| dt-7-3 | 12 | 19.4 | 9.4 | 90.6 | 4.62 | 4.10 | 2.36 | 86 | 0 | 14 |
| dt-7-4 | 24 | 23.2 | 8.0 | 92.0 | 3.73 | - | 2.33 | 78 | 4 | 18 |
| dt-7-5 | 20 | 35.4 | 9.5 | 90.5 | 3.21 | - | 2.29 | 80 | 7 | 12 |
| dt-7-6 | - | - | - | - | - | - | - | 69 | 26 | 5 |
| th-k2b-0 | - | - | - | - | - | - | - | 11 | 25 | 64 |
| th-k2b-1 | 30 | 32.3 | 8.1 | 91.9 | 2.97 | - | 2.40 | 76 | 1 | 22 |
| th-k2b-2 | 7 | 43.7 | 6.9 | 93.1 | 0.78 | - | 2.42 | 89 | 0 | 10 |
| th-k2b-3 | - | - | - | - | - | - | - | 22 | 28 | 50 |
| th-k2b-4 | 11 | 36.6 | 11.1 | 88.1 | 10.10 | - | 2.30 | 78 | 0 | 22 |
| th-k2b-5 | - | - | - | - | - | - | - | 66 | 10 | 25 |
| th-k2b-6 | 6 | 36.6 | 10.7 | 89.3 | 1.22 | - | 2.42 | 90 | 0 | 10 |
| th-k2b-7 | - | - | - | - | - | - | - | 40 | 35 | 26 |
| yy-11-0 | - | - | - | - | - | - | - | 27 | 45 | 28 |
| yy-11-1 | 10 | 35.9 | 7.0 | 93.0 | 8.11 | - | 2.09 | 100 | 0 | 0 |
| yy-11-2 | 7 | 24.1 | 8.9 | 91.2 | 8.13 | - | 2.25 | 100 | 0 | 0 |
| yy-11-3 | 7 | 24.2 | 10.0 | 90.0 | 13.39 | 11.0 | 2.31 | 100 | 0 | 0 |
| yy-11-4 | 14 | 23.6 | 9.5 | 90.5 | 10.08 | 8.50 | 2.40 | 100 | 0 | 0 |
| yy-11-5 | 8 | 41.3 | 7.7 | 92.3 | 3.03 | 2.40 | 2.28 | 93 | 0 | 7 |
| yy-11-6 | - | - | - | - | - | - | - | 88 | 0 | 12 |
| yy-11-7 | 11 | 31.5 | 8.4 | 91.6 | 1.64 | - | 2.25 | 93 | 0 | 7 |
| yy-11-8 | - | - | - | - | - | - | - | 41 | 26 | 33 |
4.2. Geochemical Associations in Coal Samples
| Sample | Major Element Oxide (wt %) and Trace Element (ppm) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | TiO2 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | MnO | Li | Be | F | Sc | V | Cr | Co | Ni | Cu | Zn | Ga | Ge | As | |
| dt-7-0 | 36.9 | 24.9 | 3.82 | 9.04 | 0.66 | 0.50 | 0.870 | 1.042 | 0.226 | 0.148 | 197 | 4.9 | 354 | 62.0 | 360 | 168 | 65.4 | 101 | 192 | 213 | 44.0 | 2.2 | 9.3 |
| dt-7-1 | 6.9 | 4.8 | 0.30 | 7.35 | 0.06 | 0.44 | 0.079 | 0.073 | 0.048 | 0.006 | 57 | 2.1 | 71 | 10.0 | 47 | 22 | 11.6 | 19 | 35 | 12 | 8.0 | 1.7 | 3.9 |
| dt-7-2 | 5.3 | 4.0 | 0.20 | 5.63 | 0.08 | 1.77 | 0.040 | 0.056 | 0.029 | 0.011 | 57 | 1.6 | 64 | 11.4 | 108 | 23 | 15.8 | 15 | 60 | 16 | 6.1 | 2.0 | 3.0 |
| dt-7-3 | 7.1 | 5.0 | 0.26 | 5.11 | 0.11 | 1.11 | 0.053 | 0.118 | 0.011 | 0.008 | 69 | 1.4 | 41 | 11.5 | 97 | 24 | 20.4 | 23 | 56 | 49 | 8.6 | 1.7 | 2.9 |
| dt-7-4 | 10.2 | 6.8 | 0.68 | 3.99 | 0.13 | 0.52 | 0.114 | 0.255 | 0.015 | 0.004 | 70 | 6.0 | 76 | 18.9 | 157 | 56 | 23.1 | 41 | 98 | 251 | 10.1 | 1.1 | 4.4 |
| dt-7-5 | 17.0 | 7.6 | 0.59 | 4.36 | 0.32 | 3.37 | 0.108 | 0.351 | 0.027 | 0.015 | 107 | 1.4 | 96 | 25.2 | 154 | 50 | 35.5 | 213 | 71 | 34 | 13.0 | 1.1 | 2.5 |
| dt-7-6 | 42.1 | 31.5 | 5.99 | 1.41 | 0.36 | 0.21 | 1.697 | 1.203 | 0.067 | 0.010 | 235 | 4.6 | 309 | 65.5 | 433 | 297 | 22.9 | 55 | 152 | 85 | 51.4 | 2.7 | <1 |
| th-k2b-0 | 41.3 | 16.1 | 1.79 | 11.19 | 0.72 | 0.64 | 0.907 | 1.965 | 0.114 | 0.050 | 53 | 5.6 | 1697 | 47.6 | 279 | 137 | 51.5 | 125 | 88 | 81 | 31.2 | 1.8 | 36.7 |
| th-k2b-1 | 15.6 | 7.7 | 0.51 | 4.20 | 0.72 | 1.81 | 0.181 | 0.343 | 0.037 | 0.020 | 80 | 3.5 | 533 | 33.7 | 335 | 65 | 43.3 | 91 | 334 | 24 | 14.6 | 1.7 | 2.9 |
| th-k2b-2 | 23.1 | 15.2 | 1.79 | 1.00 | 0.25 | 0.75 | 0.290 | 0.395 | 0.056 | 0.006 | 185 | 4.2 | 536 | 48.4 | 457 | 103 | 9.6 | 39 | 305 | 43 | 24.5 | 2.5 | 0.8 |
| th-k2b-3 | 39.6 | 28.6 | 4.09 | 4.17 | 0.53 | 0.25 | 2.016 | 1.300 | 0.051 | 0.025 | 186 | 12.8 | 1014 | 65.8 | 385 | 201 | 32.8 | 140 | 260 | 110 | 53.3 | 4.5 | 1.0 |
| th-k2b-4 | 8.3 | 4.9 | 0.32 | 13.80 | 1.46 | 3.36 | 0.145 | 0.177 | 0.040 | 0.033 | 50 | 2.2 | 326 | 19.5 | 170 | 32 | 18.7 | 76 | 81 | 42 | 9.0 | 1.1 | 5.4 |
| th-k2b-5 | 42.5 | 31.0 | 1.44 | 0.88 | 0.55 | 0.22 | 1.205 | 1.375 | 0.031 | 0.007 | 211 | 9.4 | 1288 | 33.6 | 27 | 8 | 3.5 | 34 | <2 | 45 | 75.3 | 3.1 | <1 |
| th-k2b-6 | 13.7 | 7.2 | 0.44 | 7.24 | 1.71 | 3.70 | 0.080 | 0.174 | 0.067 | 0.040 | 97 | 1.6 | 365 | 26.0 | 160 | 41 | 25.7 | 70 | 69 | 48 | 12.7 | 1.5 | 5.2 |
| th-k2b-7 | 42.2 | 31.4 | 5.28 | 2.39 | 0.65 | 0.24 | 2.188 | 1.359 | 0.050 | 0.014 | 233 | 6.8 | 1184 | 60.9 | 502 | 234 | 21.7 | 111 | 124 | 86 | 59.1 | 4.4 | <1 |
| yy-11-0 | 50.4 | 20.3 | 1.84 | 5.26 | 0.89 | 0.28 | 1.260 | 2.459 | 0.112 | 0.029 | 39 | 5.8 | 1635 | 45.1 | 182 | 39 | 25.6 | 42 | 144 | 119 | 39.8 | 2.0 | 2.3 |
| yy-11-1 | 21.6 | 2.3 | 0.37 | 10.56 | 0.02 | 0.12 | 0.014 | 0.040 | 0.012 | 0.011 | 18 | 17.4 | 58 | 16.6 | 33 | 15 | 15.4 | 28 | 18 | 13 | 5.6 | 3.8 | 4.8 |
| yy-11-2 | 11.0 | 2.1 | 0.28 | 9.17 | 0.06 | 0.61 | 0.022 | 0.041 | 0.007 | 0.005 | 15 | 6.8 | 70 | 10.2 | 24 | 15 | 17.2 | 22 | 17 | 10 | 6.2 | 3.6 | 5.3 |
| yy-11-3 | 5.1 | 2.1 | 0.17 | 13.49 | 0.09 | 0.92 | 0.019 | 0.024 | 0.009 | 0.006 | 14 | 7.8 | 69 | 6.1 | 18 | 11 | 13.1 | 6 | 23 | 11 | 6.7 | 3.4 | 6.9 |
| yy-11-4 | 7.7 | 3.9 | 0.27 | 13.91 | 0.15 | 0.96 | 0.015 | 0.059 | 0.019 | 0.009 | 30 | 5.9 | 98 | 6.7 | 22 | 15 | 107 | 68 | 92 | 11 | 8.5 | 3.7 | 6.9 |
| yy-11-5 | 21.0 | 13.6 | 0.59 | 3.53 | 0.30 | 0.95 | 0.186 | 0.334 | 0.027 | 0.010 | 110 | 4.9 | 530 | 16.5 | 26 | 14 | 4.2 | 10 | 7 | 42 | 26.5 | 3.8 | 2.3 |
| yy-11-6 | 41.6 | 31.8 | 1.42 | 0.37 | 0.36 | 0.20 | 0.575 | 0.809 | 0.018 | 0.008 | 251 | 14.0 | 1225 | 27.7 | 11 | 5 | 9.8 | 6 | <2 | 20 | 62.4 | 7.9 | <1 |
| yy-11-7 | 16.8 | 8.0 | 0.24 | 2.26 | 0.42 | 2.17 | 0.093 | 0.159 | 0.050 | 0.021 | 59 | 7.3 | 343 | 15.1 | 23 | 13 | 3.7 | 16 | 3 | 9 | 12.2 | 2.0 | 1.6 |
| yy-11-8 | 43.0 | 25.6 | 2.94 | 4.81 | 0.96 | 0.31 | 0.739 | 2.949 | 0.073 | 0.024 | 75 | 9.3 | 2587 | 52.8 | 300 | 89 | 30.6 | 65 | 123 | 182 | 55.0 | 3.4 | 16.6 |
| Sample | Trace Element (ppm) | ||||||||||||||||||||||
| Se | Rb | Cs | Sr | Y | Zr | Nb | Mo | Ag | Cd | Sn | Sb | Te | Ba | Hf | Ta | W | Hg (ppb) | Tl | Pb | Bi | Th | U | |
| dt-7-0 | 3.43 | 24.05 | 1.94 | 613 | 56 | 536 | 69.0 | 1.3 | 1.34 | 0.22 | 4.65 | 0.70 | 1.14 | 216.9 | 26.7 | 6.48 | 1.76 | 108 | <0.02 | 16.6 | 0.18 | 20.6 | 6.1 |
| dt-7-1 | 8.63 | 1.67 | 0.18 | 206 | 13 | 56 | 5.6 | 1.7 | 0.12 | 0.09 | 1.06 | 0.47 | 0.16 | 18.2 | 3.1 | 0.50 | 0.37 | 459 | 0.06 | 16.9 | 0.35 | 6.3 | 2.5 |
| dt-7-2 | 9.17 | 1.51 | 0.18 | 213 | 18 | 49 | 3.8 | 1.0 | 0.10 | 0.10 | 1.00 | 0.53 | 0.23 | 14.6 | 3.2 | 0.48 | 0.21 | 450 | 0.03 | 13.8 | 0.33 | 6.7 | 1.9 |
| dt-7-3 | 12.32 | 3.16 | 0.44 | 133 | 17 | 67 | 10.0 | 2.4 | 0.16 | 0.46 | 1.79 | 1.05 | 0.29 | 17.6 | 4.3 | 0.64 | 1.09 | 852 | 0.02 | 16.5 | 0.48 | 10.3 | 2.3 |
| dt-7-4 | 15.30 | 6.06 | 0.73 | 120 | 23 | 99 | 10.4 | 1.7 | 0.22 | 0.58 | 1.65 | 0.86 | 0.11 | 36.6 | 6.2 | 0.96 | 0.54 | 1102 | 0.04 | 46.6 | 0.32 | 10.8 | 2.6 |
| dt-7-5 | 13.05 | 8.48 | 1.07 | 292 | 32 | 221 | 12.4 | 1.5 | 0.35 | 0.22 | 3.31 | 1.14 | 0.20 | 45.1 | 9.1 | 1.34 | 0.49 | 679 | 0.06 | 10.1 | 0.48 | 11.8 | 3.7 |
| dt-7-6 | 6.46 | 21.32 | 1.17 | 841 | 49 | 611 | 107.5 | 1.5 | 1.69 | 0.22 | 5.85 | 0.78 | 1.06 | 266.2 | 30.9 | 8.25 | 2.24 | 855 | <0.02 | 6.8 | 0.13 | 25.1 | 5.6 |
| th-k2b-0 | 8.21 | 45.79 | 2.94 | 719 | 38 | 521 | 73.0 | 20.4 | 1.47 | 0.34 | 5.96 | 1.27 | 0.80 | 173.3 | 28.5 | 7.64 | 0.97 | 414 | 0.03 | 21.7 | 0.30 | 25.2 | 10.2 |
| th-k2b-1 | 10.81 | 7.22 | 1.00 | 285 | 77 | 162 | 20.2 | 2.0 | 0.81 | 0.25 | 4.77 | 0.50 | 0.36 | 35.0 | 9.9 | 1.83 | 0.58 | 906 | 0.07 | 22.8 | 0.38 | 17.6 | 4.2 |
| th-k2b-2 | 5.28 | 8.54 | 1.09 | 301 | 42 | 283 | 51.0 | 1.2 | 0.92 | 0.15 | 4.12 | 0.39 | 0.18 | 57.3 | 17.2 | 4.01 | 1.56 | 196 | 0.03 | 10.0 | 0.42 | 23.0 | 4.6 |
| th-k2b-3 | 10.64 | 22.92 | 1.44 | 1134 | 37 | 581 | 82.8 | 2.2 | 1.60 | 0.31 | 6.18 | 1.15 | 0.58 | 214.5 | 30.4 | 7.35 | 3.17 | 369 | 0.05 | 30.2 | 0.17 | 21.5 | 4.7 |
| th-k2b-4 | 15.62 | 3.68 | 0.53 | 311 | 70 | 334 | 41.6 | 3.5 | 0.76 | 0.39 | 4.94 | 0.86 | 0.29 | 20.9 | 15.1 | 1.42 | 0.80 | 781 | 0.03 | 23.3 | 0.41 | 8.3 | 4.3 |
| th-k2b-5 | 3.29 | 23.10 | 1.65 | 825 | 37 | 857 | 355.6 | 1.1 | 5.16 | 0.34 | 13.16 | 0.75 | 0.70 | 183.8 | 66.8 | 31.71 | 1.87 | 457 | <0.02 | 9.2 | 0.63 | 76.8 | 9.3 |
| th-k2b-6 | 11.93 | 3.97 | 0.59 | 432 | 101 | 665 | 38.1 | 6.0 | 0.76 | 0.34 | 5.69 | 0.78 | 0.41 | 23.7 | 26.6 | 2.04 | 1.16 | 361 | 0.03 | 11.7 | 0.39 | 13.7 | 5.0 |
| th-k2b-7 | 3.07 | 23.97 | 1.60 | 971 | 53 | 739 | 106.6 | 2.7 | 1.67 | 0.29 | 6.93 | 0.72 | 0.27 | 204.1 | 36.1 | 7.79 | 3.07 | 148 | 0.03 | 10.6 | 0.19 | 24.7 | 5.0 |
| yy-11-0 | 9.70 | 43.50 | 2.55 | 729 | 41 | 531 | 88.7 | 1.5 | 1.47 | 0.22 | 5.71 | 0.92 | 0.28 | 167.5 | 30.5 | 7.00 | 2.89 | 196 | 0.03 | 22.8 | 0.07 | 25.8 | 4.4 |
| yy-11-1 | 7.88 | 0.73 | 0.14 | 72 | 13 | 106 | 7.3 | 3.2 | 0.17 | 0.09 | 1.44 | 0.43 | 0.15 | 9.1 | 5.6 | 0.91 | 0.70 | 917 | 0.50 | 8.9 | 0.24 | 4.9 | 2.9 |
| yy-11-2 | 12.05 | 0.76 | 0.14 | 99 | 14 | 85 | 6.4 | 3.5 | 0.14 | 0.06 | 1.25 | 0.39 | <0.1 | 9.0 | 4.6 | 0.77 | 0.20 | 764 | 0.46 | 7.8 | 0.18 | 5.8 | 1.8 |
| yy-11-3 | 14.01 | 0.51 | 0.10 | 112 | 16 | 68 | 4.9 | 5.8 | 0.12 | 0.12 | 0.87 | 0.43 | <0.1 | 5.2 | 3.7 | 0.64 | 0.38 | 599 | 0.37 | 20.0 | 0.09 | 4.0 | 1.3 |
| yy-11-4 | 12.57 | 1.11 | 0.20 | 95 | 22 | 123 | 11.9 | 4.1 | 0.21 | 0.11 | 1.26 | 0.41 | <0.1 | 7.4 | 6.6 | 1.29 | 0.13 | 861 | 0.57 | 16.6 | 0.13 | 7.1 | 2.6 |
| yy-11-5 | 7.06 | 6.04 | 0.99 | 239 | 56 | 537 | 134.2 | 2.5 | 2.58 | 0.28 | 8.55 | 0.55 | 0.18 | 37.5 | 32.2 | 12.69 | 1.55 | 443 | 0.11 | 21.0 | 0.30 | 27.2 | 6.5 |
| yy-11-6 | 0.24 | 12.20 | 1.98 | 418 | 40 | 529 | 403.8 | 2.5 | 6.28 | 0.13 | 14.48 | 0.78 | 0.43 | 84.4 | 31.7 | 33.27 | 4.22 | 93 | <0.02 | 1.2 | 0.17 | 29.2 | 4.8 |
| yy-11-7 | 6.28 | 3.72 | 0.65 | 204 | 75 | 773 | 57.4 | 1.8 | 1.12 | 0.16 | 6.12 | 0.36 | 0.23 | 19.6 | 37.8 | 3.82 | 0.24 | 203 | 0.02 | 8.2 | 0.11 | 23.4 | 7.8 |
| yy-11-8 | 10.85 | 57.98 | 4.01 | 794 | 69 | 1068 | 155.7 | 3.4 | 2.46 | 0.40 | 8.60 | 1.01 | 0.47 | 174.5 | 50.3 | 12.22 | 7.57 | 175 | 0.09 | 30.0 | 0.24 | 37.7 | 10.3 |
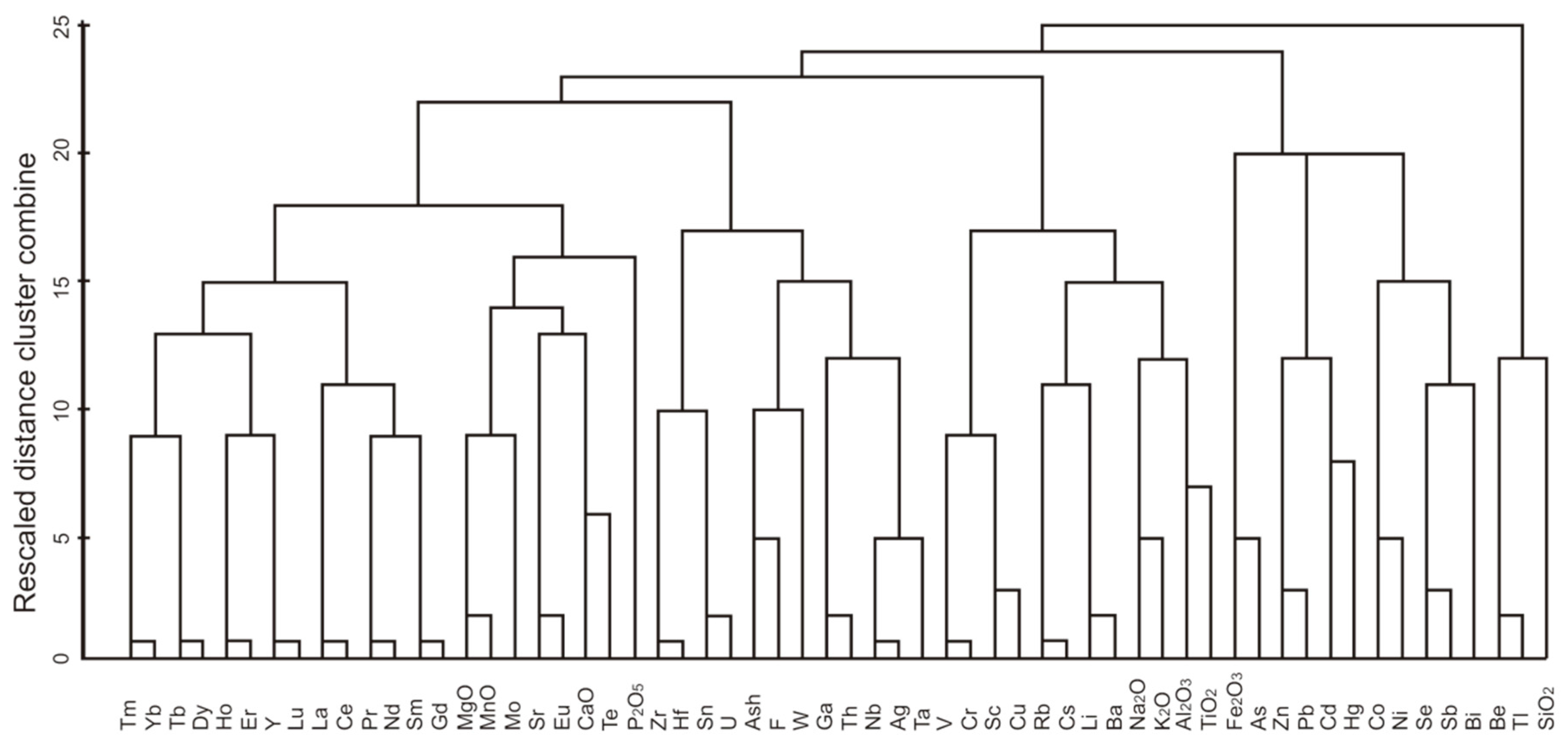
| Group | Element (Correlation Coefficient) | Element or Mineral to which the Elements in the Group Correlate |
|---|---|---|
| Group A | Al2O3 (1), TiO2 (0.79), Rb (0.82), Cs (0.86), Li (0.92), Ba (0.87), Na2O (0.9), K2O (0.86), Sc (0.73), Cr (0.63), V(0.59), Cu (0.47) | R(E-Al2O3) |
| Group B | Hf (0.63), Sn (0.72), U (0.69), F (0.8), W (0.74), Ga (0.97), Th (0.91), Nb (0.75), Ag (0.76), Ta (0.73), Zr (0.5) | R(E-Al2O3) |
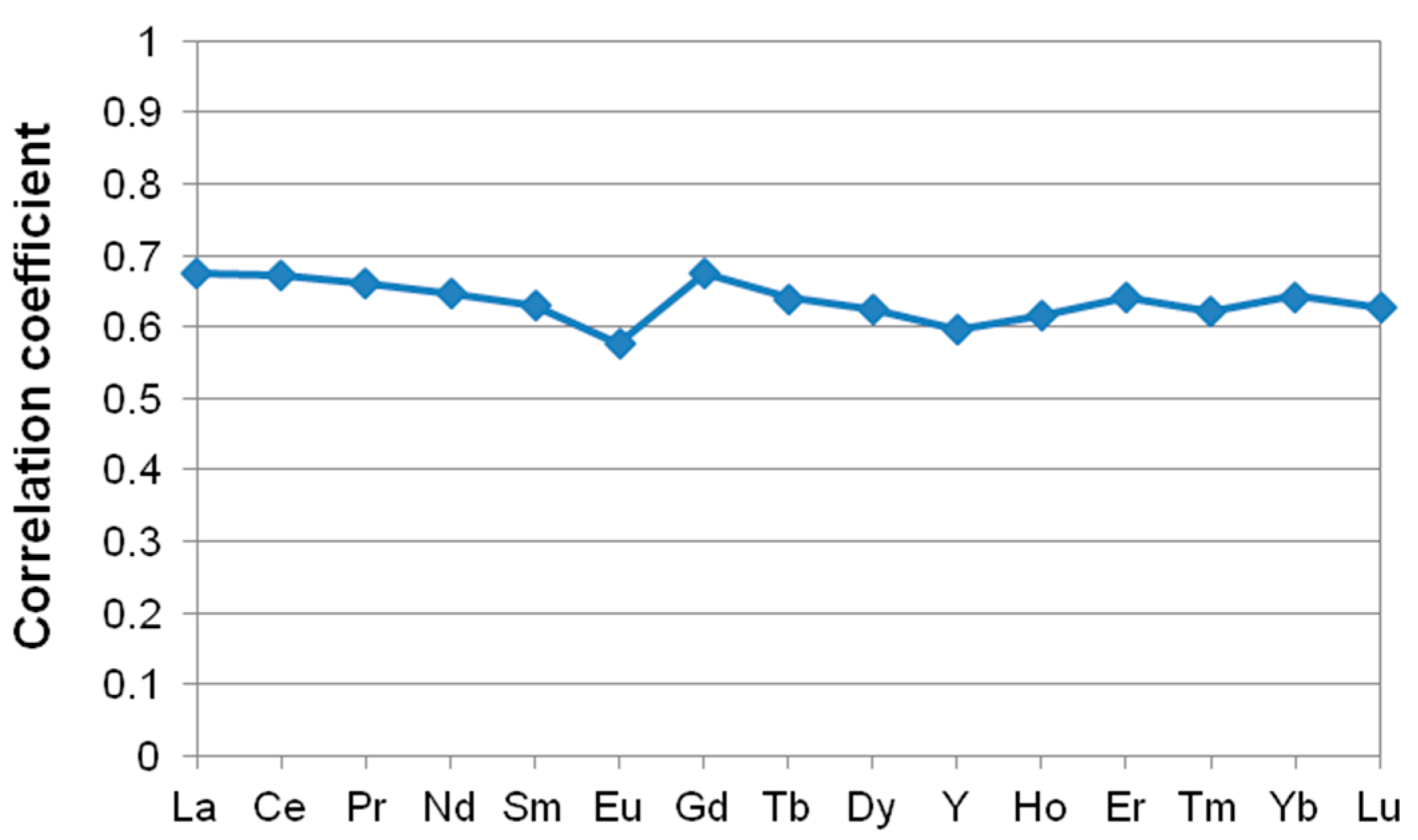
| Group C | Tm (0.44), Yb (0.47), Tb (0.52), Dy (0.5), Ho (0.47), Er (0.49), Y (0.46), Lu (0.43), La (0.59), Ce (0.57), Pr (0.55), Nd (0.53), Sm (0.51), Gd (0.59) | R(E-Al2O3) |
| Group D | CaO (1), MgO (0.8), MnO (0.86), Sr (0.77), Eu (0.42), Te (0.66), P2O5 (0.52), Mo (0.19) | R(E-CaO) |
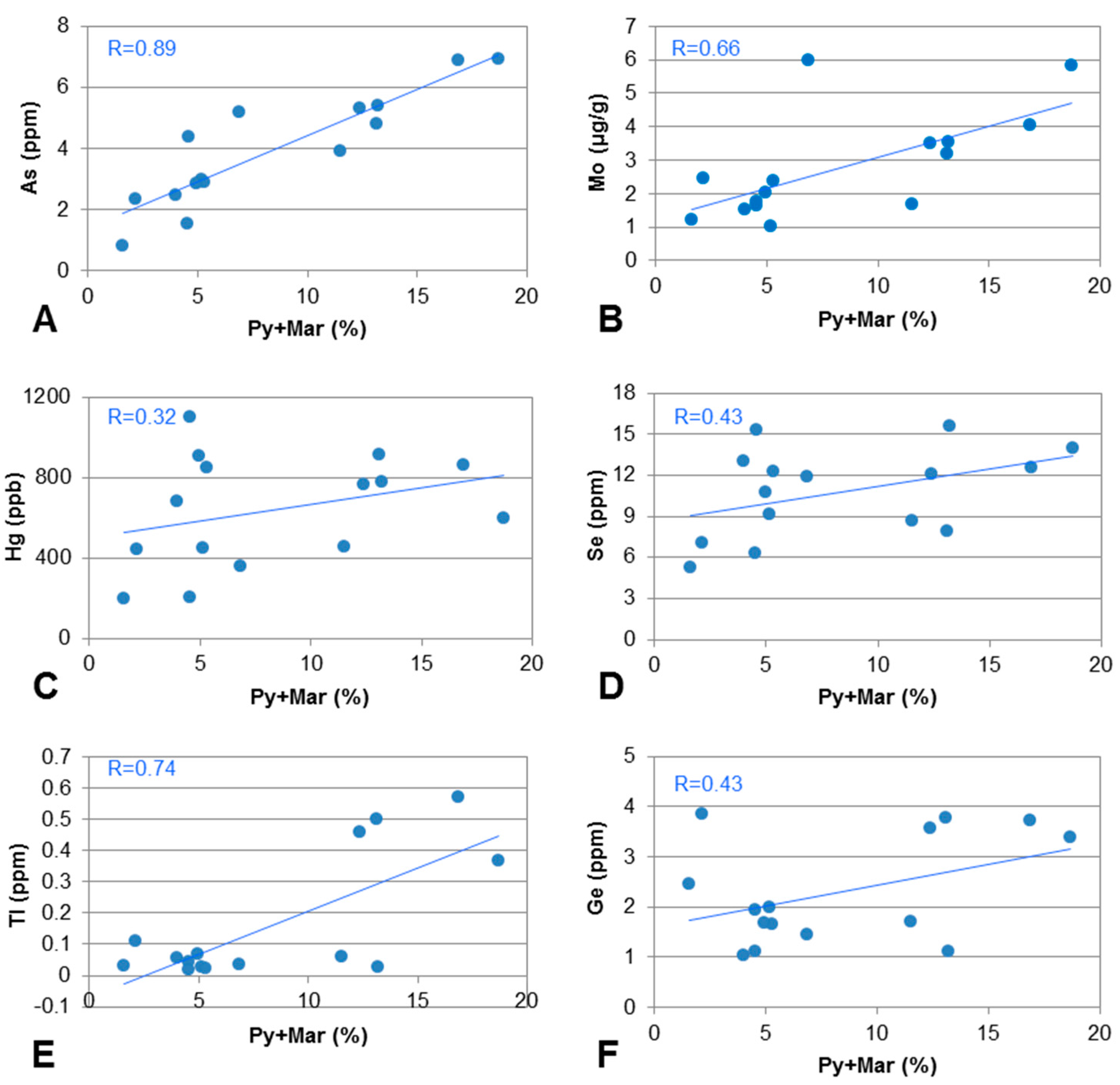
| Group E | Fe2O3 (0.95), As (0.89), Hg (0.32), Zn (−0.32), Pb (−0.06), Cd (−0.37), Co (0.35), Ni (−0.17), Se (0.43), Sb (−0.3), Bi (−0.58) | R(E-(Py + Mar)) |
| Group F | Be (−0.28), Tl (−0.16), SiO2 (0.84) | R(E-Al2O3) |
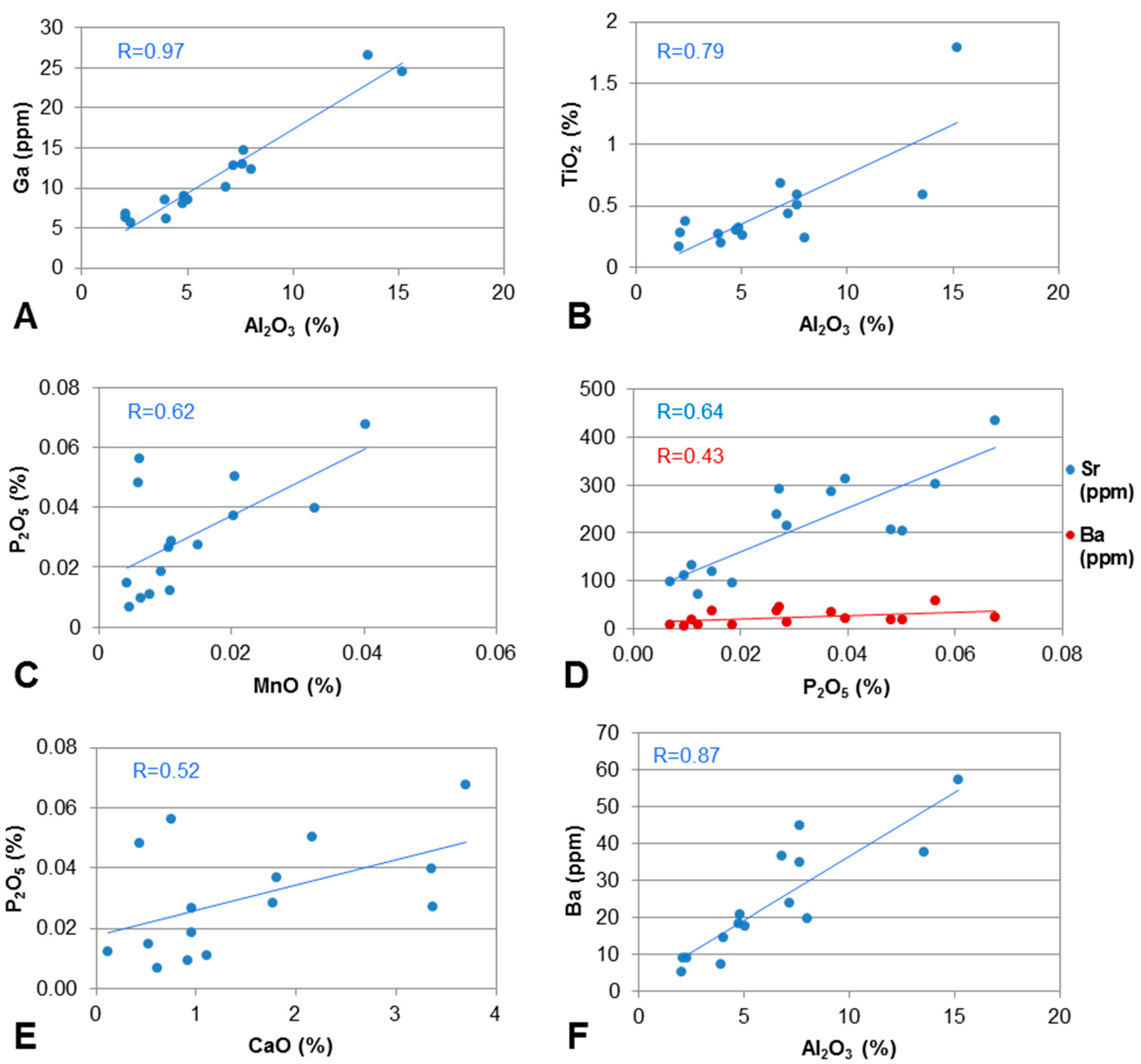
4.3. Associations of Major Elements in the Coals
4.4. Selected Elements in the Roof, Floor and Claystone Samples
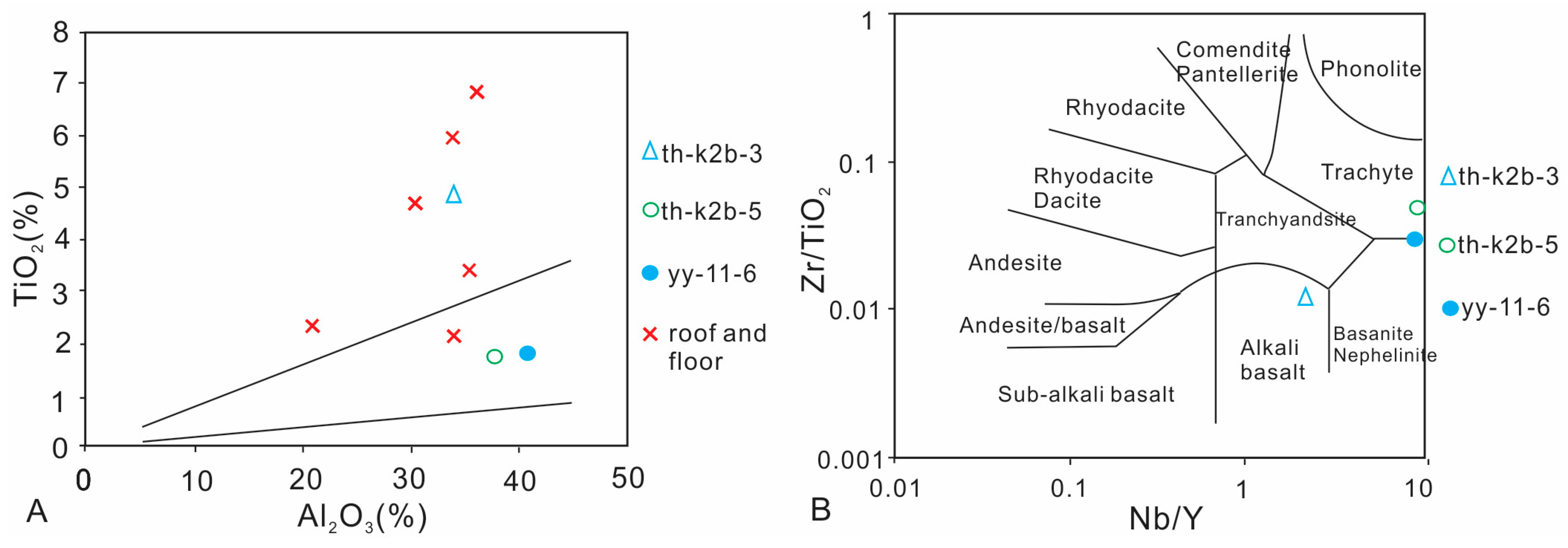
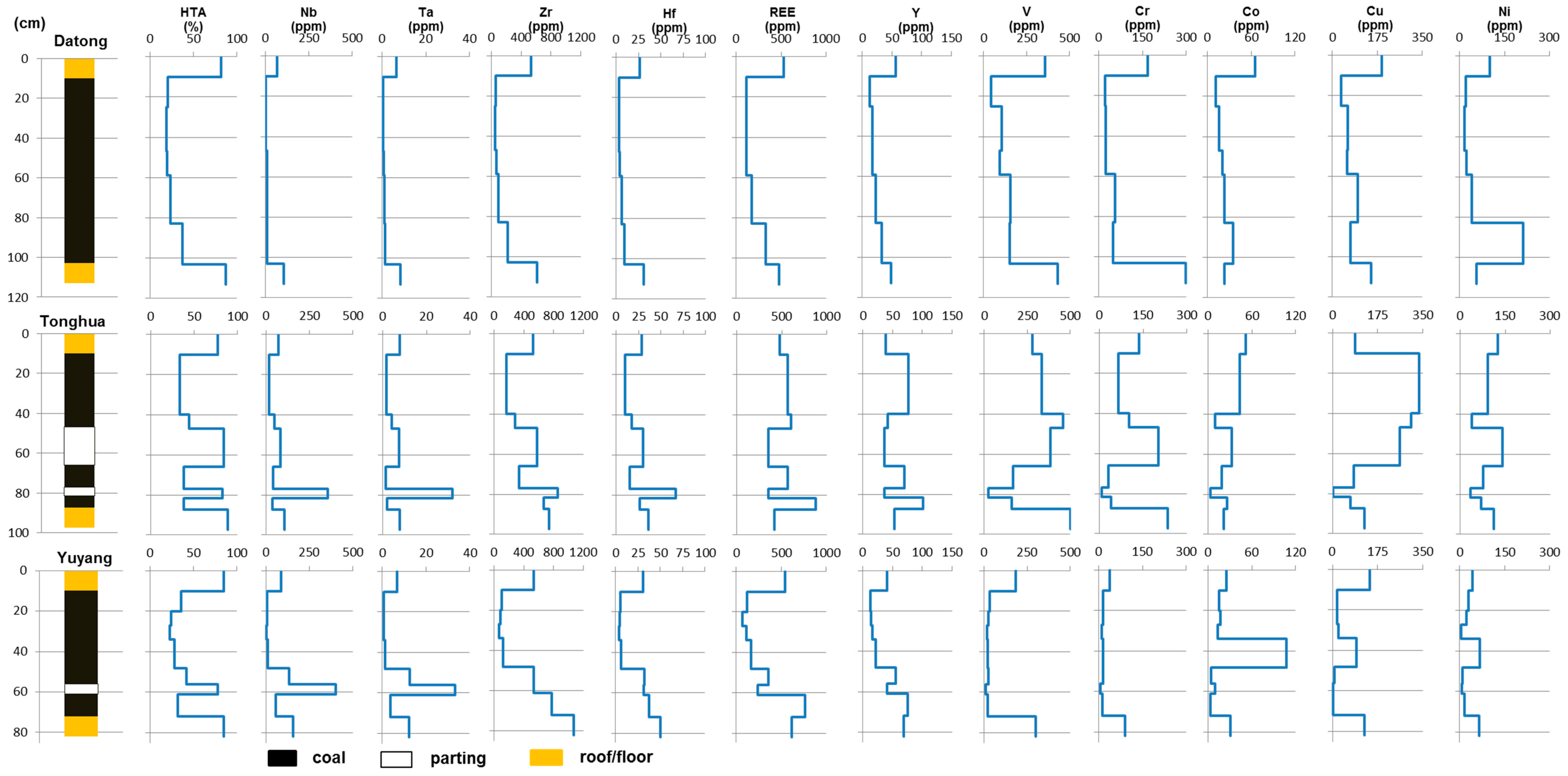
4.5. Selected Trace Elements in Coal Samples
4.5.1. Sr and Ba
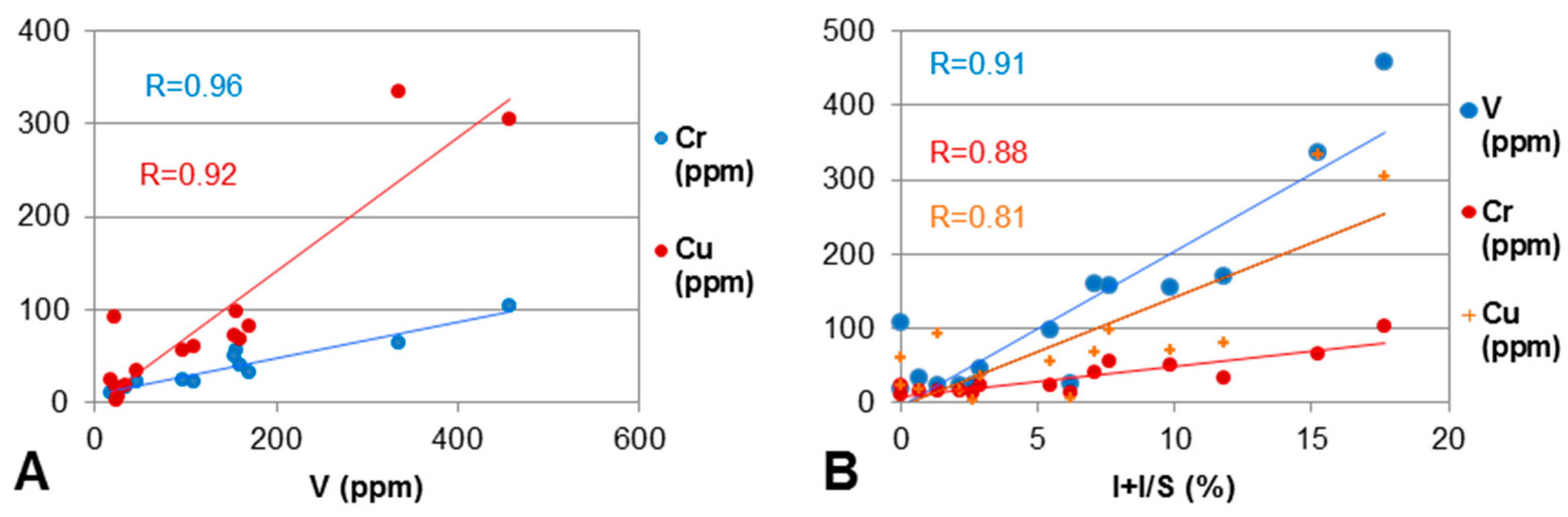
4.5.2. V, Cr, Cu, Co and Ni
4.5.3. Chalcophile Elements
4.5.4. Nb, Ta, Zr, Hf, Th, U and REE
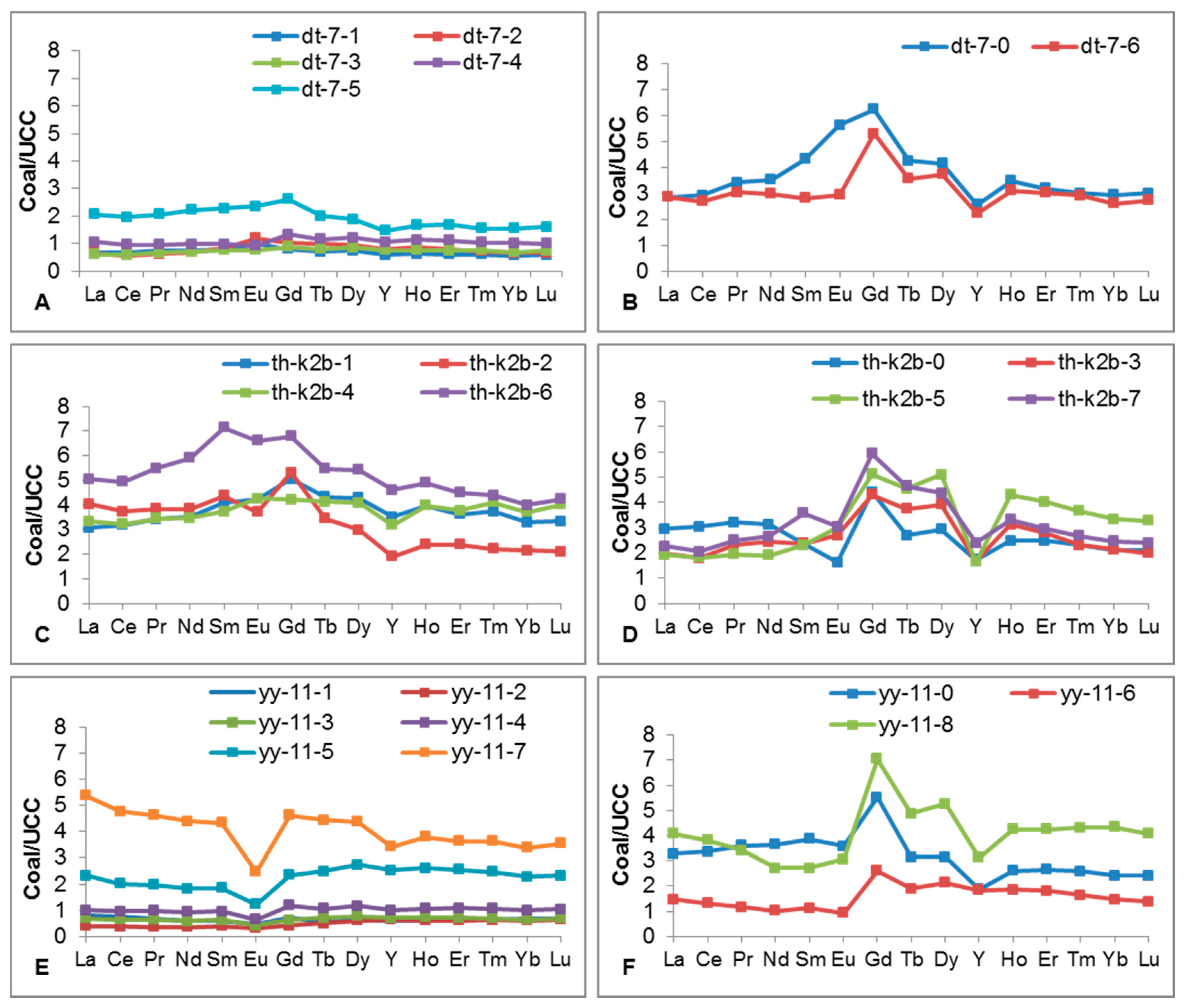
4.6. Distribution and Affinity of REE and Y
| Element | Sample | ||||||||||||||
| dt-7-0 | dt-7-1 | dt-7-2 | dt-7-3 | dt-7-4 | dt-7-5 | dt-7-6 | th-k2b-0 | th-k2b-1 | th-k2b-2 | th-k2b-3 | th-k2b-4 | th-k2b-5 | th-k2b-6 | th-k2b-7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | 85.56 | 20.33 | 19.49 | 18.38 | 32.03 | 61.68 | 85.92 | 88.63 | 92.56 | 121.1 | 59.46 | 100 | 58.17 | 151.5 | 68.12 |
| Ce | 187.1 | 43.26 | 36.89 | 38.05 | 61.1 | 125.4 | 172.2 | 194.2 | 203.3 | 239.2 | 115.1 | 206.6 | 117.2 | 316.3 | 131.9 |
| Pr | 24.32 | 5.35 | 4.53 | 4.68 | 6.82 | 14.65 | 21.57 | 22.78 | 24.27 | 27.36 | 16.45 | 24.54 | 13.88 | 39 | 17.82 |
| Nd | 91.54 | 19.59 | 17.81 | 18.4 | 25.51 | 57.94 | 77.68 | 81.41 | 91.4 | 99.71 | 63.58 | 90.02 | 49.82 | 153.4 | 69.01 |
| Sm | 19.43 | 3.59 | 3.73 | 3.43 | 4.4 | 10.24 | 12.66 | 10.67 | 18.37 | 19.69 | 10.7 | 16.85 | 10.38 | 32.14 | 16.1 |
| Eu | 4.96 | 0.88 | 1.05 | 0.68 | 0.82 | 2.08 | 2.59 | 1.44 | 3.73 | 3.26 | 2.38 | 3.75 | 2.65 | 5.81 | 2.68 |
| Gd | 23.72 | 3.05 | 4 | 3.37 | 5.08 | 9.95 | 20.11 | 16.8 | 19.17 | 20.19 | 16.37 | 15.99 | 19.48 | 25.83 | 22.52 |
| Tb | 2.72 | 0.46 | 0.63 | 0.52 | 0.74 | 1.29 | 2.29 | 1.73 | 2.78 | 2.2 | 2.4 | 2.64 | 2.9 | 3.5 | 2.98 |
| Dy | 14.52 | 2.58 | 3.37 | 3.02 | 4.25 | 6.6 | 13.08 | 10.29 | 14.99 | 10.45 | 13.74 | 14.32 | 17.82 | 18.98 | 15.28 |
| Y | 56.21 | 12.85 | 17.64 | 16.87 | 23.29 | 32.44 | 48.98 | 38.27 | 77.11 | 42.29 | 36.78 | 70.26 | 36.54 | 101.4 | 52.67 |
| Ho | 2.79 | 0.51 | 0.7 | 0.61 | 0.92 | 1.33 | 2.48 | 1.98 | 3.17 | 1.92 | 2.51 | 3.19 | 3.44 | 3.92 | 2.66 |
| Er | 7.36 | 1.41 | 1.8 | 1.7 | 2.59 | 3.87 | 6.96 | 5.72 | 8.36 | 5.49 | 6.48 | 8.71 | 9.26 | 10.4 | 6.78 |
| Tm | 0.99 | 0.2 | 0.24 | 0.25 | 0.35 | 0.51 | 0.96 | 0.77 | 1.23 | 0.73 | 0.76 | 1.36 | 1.21 | 1.45 | 0.88 |
| Yb | 6.45 | 1.27 | 1.48 | 1.53 | 2.26 | 3.42 | 5.75 | 4.69 | 7.27 | 4.76 | 4.71 | 8.19 | 7.34 | 8.79 | 5.43 |
| Lu | 0.96 | 0.19 | 0.21 | 0.24 | 0.32 | 0.52 | 0.88 | 0.67 | 1.07 | 0.67 | 0.64 | 1.29 | 1.05 | 1.36 | 0.77 |
| REY | 529 | 116 | 114 | 112 | 170 | 332 | 474 | 480 | 569 | 599 | 352 | 568 | 351 | 874 | 416 |
| REO | 634 | 139 | 136 | 134 | 205 | 398 | 569 | 576 | 683 | 719 | 422 | 681 | 421 | 1049 | 499 |
| REOa | 777 | 675 | 747 | 691 | 884 | 1124 | 652 | 742 | 2115 | 1645 | 501 | 1861 | 512 | 2866 | 562 |
| Eu/Eu * | 1.07 | 1.24 | 1.27 | 0.93 | 0.8 | 0.96 | 0.73 | 0.48 | 0.93 | 0.76 | 0.81 | 1.07 | 0.81 | 0.95 | 0.64 |
| Ce/Ce * | 0.93 | 0.94 | 0.9 | 0.93 | 0.94 | 0.95 | 0.91 | 0.98 | 0.98 | 0.95 | 0.84 | 0.95 | 0.94 | 0.94 | 0.86 |
| Y/Y * | 0.67 | 0.85 | 0.87 | 0.94 | 0.9 | 0.83 | 0.65 | 0.64 | 0.85 | 0.71 | 0.47 | 0.79 | 0.35 | 0.89 | 0.62 |
| (La/Lu)N | 0.95 | 1.13 | 0.98 | 0.81 | 1.07 | 1.27 | 1.05 | 1.41 | 0.93 | 1.92 | 0.997 | 0.83 | 0.59 | 1.19 | 0.95 |
| (La/Sm)N | 0.66 | 0.85 | 0.78 | 0.8 | 1.09 | 0.9 | 1.02 | 1.25 | 0.76 | 0.92 | 0.83 | 0.89 | 0.84 | 0.71 | 0.63 |
| (Gd/Lu)N | 2.08 | 1.34 | 1.58 | 1.17 | 1.34 | 1.62 | 1.93 | 2.11 | 1.51 | 2.52 | 2.17 | 1.04 | 1.56 | 1.6 | 2.47 |
| Type | H-M | L-M | H-M | H-M | L | L-M | L | L | H-M | L-M | M | M | M | L-M | H-M |
| Element | Sample | ||||||||||||||
| yy-11-0 | yy-11-1 | yy-11-2 | yy-11-3 | yy-11-4 | yy-11-5 | yy-11-6 | yy-11-7 | yy-11-8 | |||||||
| La | 98.11 | 24.25 | 11.69 | 20.84 | 30.15 | 69.42 | 44.3 | 160.9 | 122.1 | ||||||
| Ce | 215.3 | 47.44 | 23.26 | 41 | 60.41 | 128.6 | 83.98 | 304.5 | 244.6 | ||||||
| Pr | 25.64 | 4.75 | 2.56 | 4.5 | 6.91 | 13.99 | 8.33 | 32.81 | 24.22 | ||||||
| Nd | 94.73 | 15.57 | 9.08 | 15 | 24.36 | 47.5 | 26.09 | 114 | 70.8 | ||||||
| Sm | 17.4 | 2.69 | 1.81 | 2.75 | 4.28 | 8.35 | 5.03 | 19.46 | 12.26 | ||||||
| Eu | 3.15 | 0.4 | 0.28 | 0.37 | 0.56 | 1.09 | 0.81 | 2.18 | 2.69 | ||||||
| Gd | 20.98 | 2.78 | 1.54 | 2.38 | 4.53 | 8.92 | 9.93 | 17.58 | 26.77 | ||||||
| Tb | 2.02 | 0.36 | 0.32 | 0.46 | 0.67 | 1.59 | 1.22 | 2.84 | 3.11 | ||||||
| Dy | 11.04 | 2.16 | 2.13 | 2.72 | 4.05 | 9.54 | 7.45 | 15.31 | 18.37 | ||||||
| Y | 41.25 | 12.77 | 14.24 | 15.65 | 21.85 | 55.61 | 40.32 | 75.1 | 68.58 | ||||||
| Ho | 2.09 | 0.47 | 0.48 | 0.58 | 0.85 | 2.09 | 1.48 | 3.03 | 3.39 | ||||||
| Er | 6.11 | 1.43 | 1.37 | 1.64 | 2.48 | 5.88 | 4.19 | 8.32 | 9.75 | ||||||
| Tm | 0.85 | 0.21 | 0.21 | 0.23 | 0.35 | 0.82 | 0.54 | 1.2 | 1.42 | ||||||
| Yb | 5.33 | 1.47 | 1.3 | 1.4 | 2.2 | 5 | 3.25 | 7.42 | 9.52 | ||||||
| Lu | 0.78 | 0.22 | 0.21 | 0.22 | 0.33 | 0.74 | 0.44 | 1.13 | 1.3 | ||||||
| REY | 545 | 117 | 70 | 110 | 164 | 359 | 237 | 766 | 619 | ||||||
| REO | 654 | 140 | 85 | 132 | 197 | 431 | 285 | 919 | 743 | ||||||
| REOa | 768 | 390 | 353 | 545 | 835 | 1044 | 365 | 2917 | 871 | ||||||
| Eu/Eu * | 0.76 | 0.68 | 0.78 | 0.67 | 0.6 | 0.59 | 0.49 | 0.55 | 0.63 | ||||||
| Ce/Ce * | 0.98 | 1 | 0.97 | 0.96 | 0.95 | 0.94 | 0.99 | 0.95 | 1.02 | ||||||
| Y/Y * | 0.65 | 0.96 | 1.07 | 0.95 | 0.9 | 0.95 | 0.92 | 0.84 | 0.66 | ||||||
| (La/Lu)N | 1.35 | 1.19 | 0.61 | 1.01 | 0.98 | 0.998 | 1.07 | 1.52 | 0.999 | ||||||
| (La/Sm)N | 0.85 | 1.35 | 0.97 | 1.13 | 1.06 | 1.25 | 1.32 | 1.24 | 1.49 | ||||||
| (Gd/Lu)N | 2.28 | 1.08 | 0.63 | 0.91 | 1.16 | 1.01 | 1.89 | 1.31 | 1.73 | ||||||
| Type | L-M | L | H | L | H | H | L | L | H | ||||||
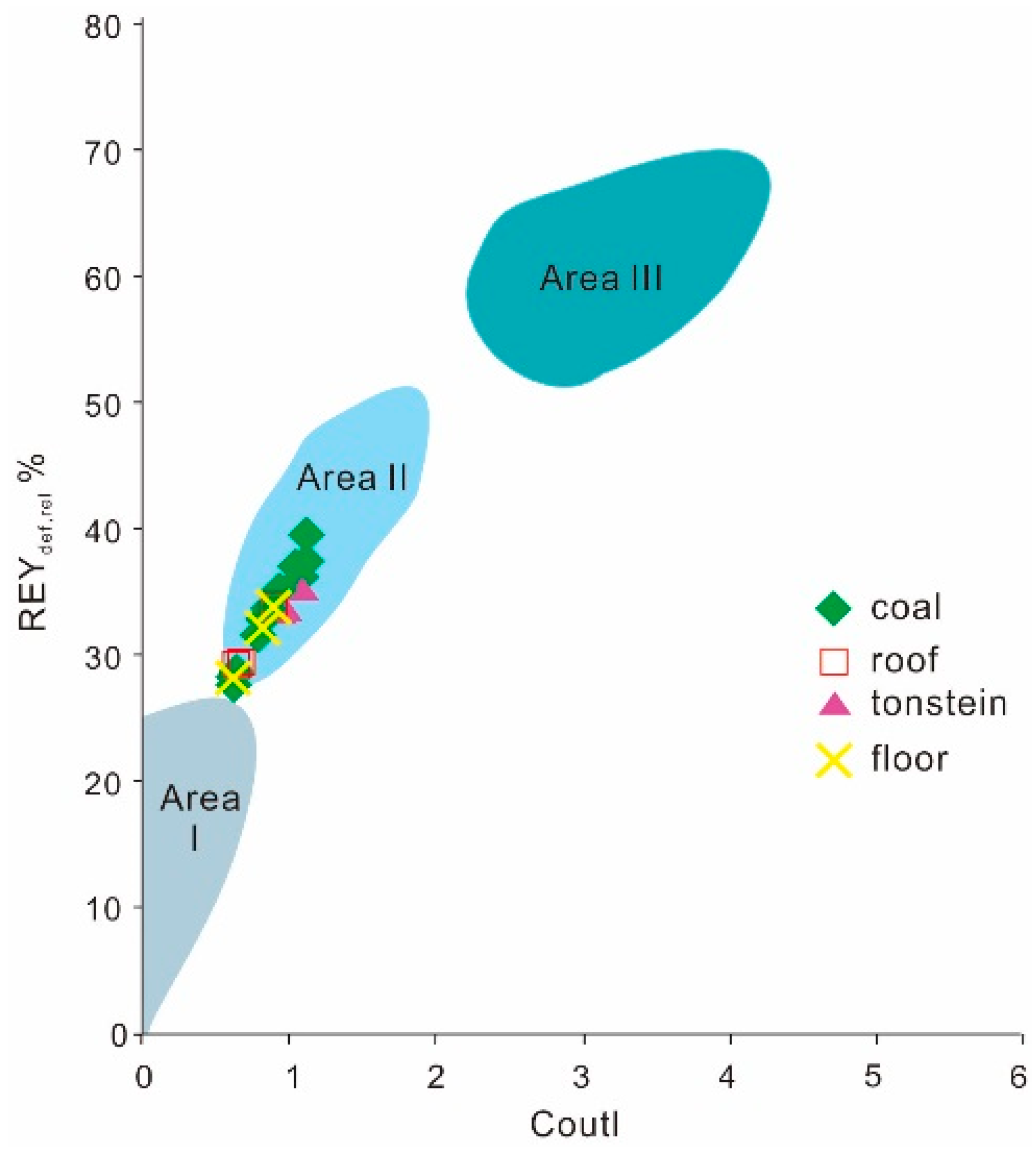
4.7. Potential Industrial Value of REY in Coal Ashes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swaine, D.J. Trace Elements in Coal; Butterworths: London, UK, 1990; p. 278. [Google Scholar]
- Kolker, A.; Finkelman, R.B. Potentially hazardous elements in coal: Modes of occurrence and summary of concentration data for coal components. Coal Prep. 1998, 19, 133–157. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Bouška, V. Geochemistry of Coal; Elsevier: Amsterdam, The Nederland, 1981; p. 284. [Google Scholar]
- Hower, J.C.; Rimmer, S.M.; Bland, A.E. Geochemistry of the Blue Gem coal bed, Knox County, Kentucky. Int. J. Coal Geol. 1991, 18, 211–231. [Google Scholar] [CrossRef]
- Crowley, S.S.; Stanton, R.W.; Ryer, T.A. The effects of volcanic ash on the maceral and chemical composition of the C coal bed, Emery Coal Field, Utah. Org. Geochem. 1989, 14, 315–331. [Google Scholar] [CrossRef]
- Hower, J.C.; Ruppert, L.F.; Eble, C.F. Lanthanide, yttrium, and zirconium anomalies in the Fire Clay coal bed, eastern Kentucky. Int. J. Coal Geol. 1999, 39, 141–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, D.; Ren, Y. Characteristics of zircons from volcanic ash-derivd tonsteins in Late Permian coalfields of eastern Yunnan, China. Acta Sedimentol. Sin. 1992, 10, 28–38. (In Chinese) [Google Scholar]
- Zhou, Y.; Ren, Y.; Tang, D.; Bohor, B. Characteristics of zircons from volcanic ash-derived tonsteins in Late Permian coal fields of eastern Yunnan, China. Int. J. Coal Geol. 1994, 25, 243–264. [Google Scholar] [CrossRef]
- Zhou, Y.; Bohor, B.F.; Ren, Y. Trace element geochemistry of altered volcanic ash layers (tonsteins) in Late Permian coal-bearing formations of eastern Yunnan and western Guizhou provinces, China. Int. J. Coal Geol. 2000, 44, 305–324. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, Y.; Bohor, B.F. Origin and distribution of tonsteins in Late Permian coal seams of southwestern China. Int. J. Coal Geol. 1982, 2, 49–77. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, Y. Element geochemistry of volcanic ash derived tonsteins in Late Permian coal-bearing formation of eastern Yunnan and western Guizhou, China. Acta Sedimentol. Sin. 1994, 12, 123–132. (In Chinese) [Google Scholar]
- Zhou, Y.P. The alkali pyroclastic tonsteins of early stage of Late Permian age in southwestern China. Coal Geol. Explor. 1999, 27, 5–9. (In Chinese) [Google Scholar]
- Dai, S.; Zhou, Y.; Zhang, M.; Wang, X.; Wang, J.; Song, X.; Jiang, Y.; Luo, Y.; Song, Z.; Yang, Z.; et al. A new type of Nb(Ta)-Zr(Hf)-REE-Ga polymetallic deposit in the Late Permian coal-bearing strata, eastern Yunnan, southwestern China: Possible economic significance and genetic implications. Int. J. Coal Geol. 2010, 83, 55–63. [Google Scholar] [CrossRef]
- Dai, S.; Chekryzhov, I.Y.; Seredin, V.V.; Nechaev, V.P.; Graham, I.T.; Hower, J.C.; Ward, C.R.; Ren, D.; Wang, X. Metalliferous coal deposits in east Asia (Primorye of Russia and south China): A review of geodynamic controls and styles of mineralization. Gondwana Res. 2015, in press. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Ren, D.; Wang, X.; Li, D.; Zhao, L. Geochemistry and mineralogy of the Late Permian coals from the Songzao Coalfield, Chongqing, southwestern China. Sci. China Ser. D Earth Sci. 2007, 50, 678–688. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Zhou, Y.; Hower, J.C.; Li, D.; Chen, W.; Zhu, X.; Zou, J. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the Late Permian coals from the Songzao Coalfield, Chongqing, southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I.T. Mineralogical composition of Late Permian coal seams in the Songzao Coalfield, southwestern China. Int. J. Coal Geol. 2013, 116–117, 208–226. [Google Scholar] [CrossRef]
- Li, W. A preliminary evaluation of coal resources in the Songzao Coalfield. Inf. Sci. Technol. Econ. 2007, 17, 148–150. (In Chinese) [Google Scholar]
- Dai, S.; Wang, X.; Chen, W.; Li, D.; Chou, C.-L.; Zhou, Y.; Zhu, C.; Li, H.; Zhu, X.; Xing, Y.; et al. A high-pyrite semianthracite of Late Permian age in the Songzao Coalfield, southwestern China: Mineralogical and geochemical relations with underlying mafic tuffs. Int. J. Coal Geol. 2010, 83, 430–445. [Google Scholar] [CrossRef]
- China Coal Geology Bureau. Sedimentary Environments and Coal Accumulation of Late Permian Coal Formation in Western Guizhou, Southern Sichuan, and Eastern Yunnan, China; Chongqing University Press: Chongqing, China, 1996; p. 216. (In Chinese) [Google Scholar]
- Standards Australia. Coal and Coke—Analysis and Testing Part 3: Proximate Analysis of Higher Rank Coal. Australian Standard 1038.22; Standards Australia: Strathfield, Australia, 2000. [Google Scholar]
- Li, X.; Dai, S.; Zhang, W.; Li, T.; Zheng, X.; Chen, W. Determination of As and Se in coal and coal combustion products using closed vessel microwave digestion and collision/reaction cell technology (CCT) of inductively coupled plasma mass spectrometry (ICP-MS). Int. J. Coal Geol. 2014, 124, 1–4. [Google Scholar] [CrossRef]
- Beijing Research Institute of Coal Chemistry. Chinese National Standard GB/T 4633-1997, Determination of Fluorine in Coal; Standand Press of China: Beijing, China, 1997. [Google Scholar]
- American Society for Testing and Materials (ASTM). ASTM Standard D388. Classification of Coals By Rank; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Lyons, P.C.; Spears, D.A.; Outerbridge, W.F.; Congdon, R.D.; Evans, H.T. Euramerican tonsteins: Overview, magmatic origin, and depositional-tectonic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 106, 113–134. [Google Scholar] [CrossRef]
- Spears, D.A. The origin of tonsteins, an overview, and links with seatearths, fireclays and fragmental clay rocks. Int. J. Coal Geol. 2012, 94, 22–31. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of occurrence of environmentally-sensitive trace elements in coal. In Environmental Aspect of Trace Elements in Coal; Swaine, D.J., Goodarzi, F., Eds.; Kluwer Academic Publishers: Amsterdam, The Nederland, 1995. [Google Scholar]
- Ren, D.; Zhao, F.; Dai, S.; Zhang, J.; Luo, K. Geochemistry of Trace Elements in Coal; Science Press: Beijing, China, 2006; p. 556. (In Chinese) [Google Scholar]
- Ward, C.R.; Spears, D.A.; Booth, C.A.; Staton, I.; Gurba, L.W. Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int. J. Coal Geol. 1999, 40, 281–308. [Google Scholar] [CrossRef]
- Price, N.B.; Duff, P.M.D. Mineralogy and chemistry of tonsteins from Carboniferous sequences in Great Britain. Sedimentology 1969, 13, 45–69. [Google Scholar] [CrossRef]
- Spears, D.A.; Rice, C.M. An Upper Carboniferous tonstein of volcanic origin. Sedimentology 1973, 20, 281–294. [Google Scholar] [CrossRef]
- Spears, D.A.; Kanaris-Sotiriou, R. Titanium in some Carboniferous sediments from Great Britain. Geochim. Cosmochim. Acta. 1976, 40, 345–351. [Google Scholar] [CrossRef]
- Addison, R.; Harrison, R.K.; Land, D.H.; Young, B.R.; Davis, A.E.; Smith, T.K. Volcanogenic tonsteins from Tertiary coal measures, east Kalimantan, Indonesia. Int. J. Coal Geol. 1983, 3, 1–30. [Google Scholar] [CrossRef]
- Burger, K.; Zhou, Y.; Ren, Y. Petrography and geochemistry of tonsteins from the 4th Member of the Upper Triassic Xujiahe Formation in southern Sichuan province, China. Int. J. Coal Geol. 2002, 49, 1–17. [Google Scholar] [CrossRef]
- Spears, D.A.; Kanaris-Sotiriou, R. A geochemical and mineralogical investigation of some British and other European tonsteins. Sedimentology 1979, 26, 407–425. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Zielinski, R.A. Element mobility during alteration of silicic ash to kaolinite-a study of tonstein. Sedimentology 1985, 32, 567–579. [Google Scholar] [CrossRef]
- Brownfield, M.E.; Affolter, R.H.; Cathcart, J.D.; Johnson, S.Y.; Brownfield, I.K.; Rice, C.A. Geologic setting and characterization of coals and the modes of occurrence of selected elements from the Franklin coal zone, Puget Group, John Henry No. 1 mine, King County, Washington, USA. Int. J. Coal Geol. 2005, 63, 247–275. [Google Scholar] [CrossRef]
- Rao, P.D.; Walsh, D.E. Nature and distribution of phosphorus minerals in Cook Inlet coals, Alaska. Int. J. Coal Geol. 1997, 33, 19–42. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I.T. Mineralogy of the volcanic-influenced Great Northern coal seam in the Sydney Basin, Australia. Int. J. Coal Geol. 2012, 94, 94–110. [Google Scholar] [CrossRef]
- Glick, D.C.; Davis, A. Variability in the inorganic element content of U.S. coals including results of cluster analysis. Org. Geochem. 1987, 11, 331–342. [Google Scholar] [CrossRef]
- Spears, D.A.; Martinez-Tarazona, M.R. Geochemical and mineralogical characteristics of a power station feed-coal, Eggborough, England. Int. J. Coal Geol. 1993, 22, 1–20. [Google Scholar] [CrossRef]
- Querol, X.; Cabrera, L.; Pickel, W.; López-Soler, A.; Hagemann, H.W.; Fernández-Turiel, J.L. Geological controls on the coal quality of the Mequinenza subbituminous coal deposit, northeast Spain. Int. J. Coal Geol. 1996, 29, 67–91. [Google Scholar] [CrossRef]
- Kolker, A. Minor element distribution in iron disulfides in coal: A geochemical review. Int. J. Coal Geol. 2012, 94, 32–43. [Google Scholar] [CrossRef]
- Dai, S.; Chou, C.-L.; Yue, M.; Luo, K.; Ren, D. Mineralogy and geochemistry of a Late Permian coal in the Dafang Coalfield, Guizhou, China: Influence from siliceous and iron-rich calcic hydrothermal fluids. Int. J. Coal Geol. 2005, 61, 241–258. [Google Scholar] [CrossRef]
- Finkelman, R.B. Mode of Occurrence of Trace Elements in Coal; University of Maryland: College Park, MD, USA, 1980. [Google Scholar]
- Miller, R.N.; Given, P.H. The association of major, minor and trace inorganic elements with lignites. I. Experimental approach and study of a North Dakota lignite. Geochim. Cosmochim. Acta 1986, 50, 2033–2043. [Google Scholar] [CrossRef]
- Querol, X.; Klika, Z.; Weiss, Z.; Finkelman, R.B.; Alastuey, A.; Juan, R.; López-Soler, A.; Plana, F.; Kolker, A.; Chenery, S.R.N. Determination of element affinities by density fractionation of bulk coal samples. Fuel 2001, 80, 83–96. [Google Scholar] [CrossRef]
- Huggins, F.E.; Shah, N.; Zhao, J.; Lu, F.; Huffman, G.P. Nondestructive determination of trace element speciation in coal and coal ash by XAFS spectroscopy. Energy Fuels 1993, 7, 482–489. [Google Scholar] [CrossRef]
- Kolker, A.; Huggins, F.E.; Palmer, C.A.; Shah, N.; Crowley, S.S.; Huffman, G.P.; Finkelman, R.B. Mode of occurrence of arsenic in four US coals. Fuel Proc. Technol. 2000, 63, 167–178. [Google Scholar] [CrossRef]
- Lindahl, P.C.; Finkelman, R.B. Factors influencing major, minor, and trace element variations in U.S. Coals. In Mineral Matter and Ash in Coal; Vorres, K.S., Ed.; American Chemical Society: Washington, DC, USA, 1986; Volume 301, pp. 61–69. [Google Scholar]
- Spears, D.A.; Zheng, Y. Geochemistry and origin of elements in some UK coals. Int. J. Coal Geol. 1999, 38, 161–179. [Google Scholar] [CrossRef]
- Diehl, S.F.; Goldhaber, M.B.; Hatch, J.R. Modes of occurrence of mercury and other trace elements in coals from the Warrior field, Black Warrior Basin, northwestern Alabama. Int. J. Coal Geol. 2004, 59, 193–208. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Wang, X. Geochemistry of Late Triassic coals in the Changhe mine, Sichuan Basin, southwestern China: Evidence for authigenic lanthanide enrichment. Int. J. Coal Geol. 2009, 80, 167–174. [Google Scholar] [CrossRef]
- Eskenazy, G.M. Aspects of the geochemistry of rare earth elements in coal: An experimental approach. Int. J. Coal Geol. 1999, 38, 285–295. [Google Scholar] [CrossRef]
- Dai, S.; Zou, J.; Jiang, Y.; Ward, C.R.; Wang, X.; Li, T.; Xue, W.; Liu, S.; Tian, H.; Sun, X.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Adaohai mine, Daqingshan coalfield, Inner Mongolia, China: Modes of occurrence and origin of diaspore, gorceixite, and ammonian illite. Int. J. Coal Geol. 2012, 94, 250–270. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.-L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the haerwusu surface mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Li, S.; Jiang, Y. Mineralogy and geochemistry of the No. 6 coal (Pennsylvanian) in the Junger coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; p. 312. [Google Scholar]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Zhao, L. Mineralogy and geochemistry of Permian coal seams of the Sydney Basin, Australia, and the Songzao Coalfield, SW China. Ph.D. Thesis, University of New South Wales, Australia, 2012. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Ward, C.R.; French, D.; Graham, I.T. Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China. Minerals 2015, 5, 870-893. https://doi.org/10.3390/min5040531
Zhao L, Ward CR, French D, Graham IT. Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China. Minerals. 2015; 5(4):870-893. https://doi.org/10.3390/min5040531
Chicago/Turabian StyleZhao, Lei, Colin R. Ward, David French, and Ian T. Graham. 2015. "Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China" Minerals 5, no. 4: 870-893. https://doi.org/10.3390/min5040531
APA StyleZhao, L., Ward, C. R., French, D., & Graham, I. T. (2015). Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China. Minerals, 5(4), 870-893. https://doi.org/10.3390/min5040531







