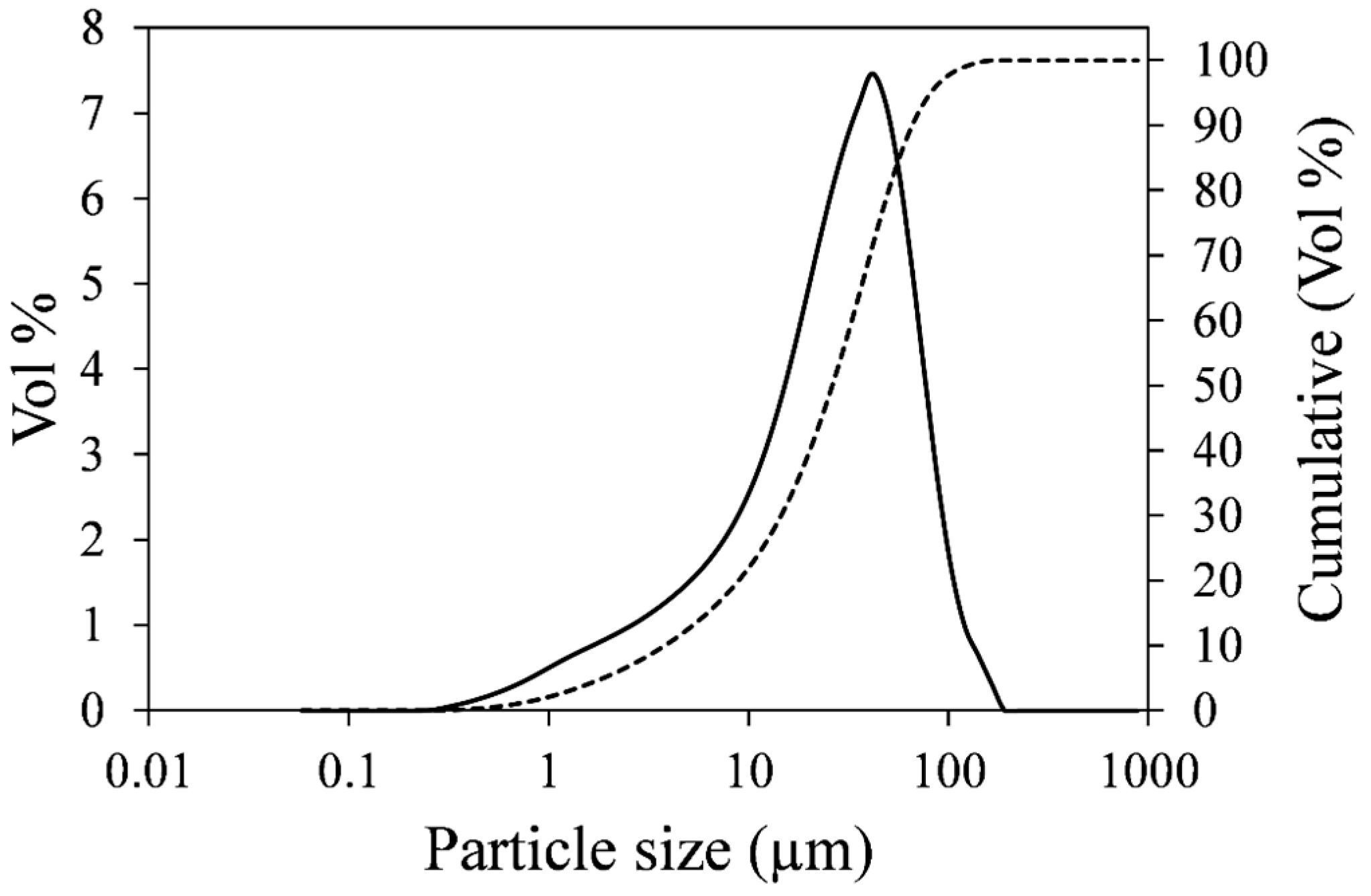
3.1. Effect of Temperature on Nickel Extraction
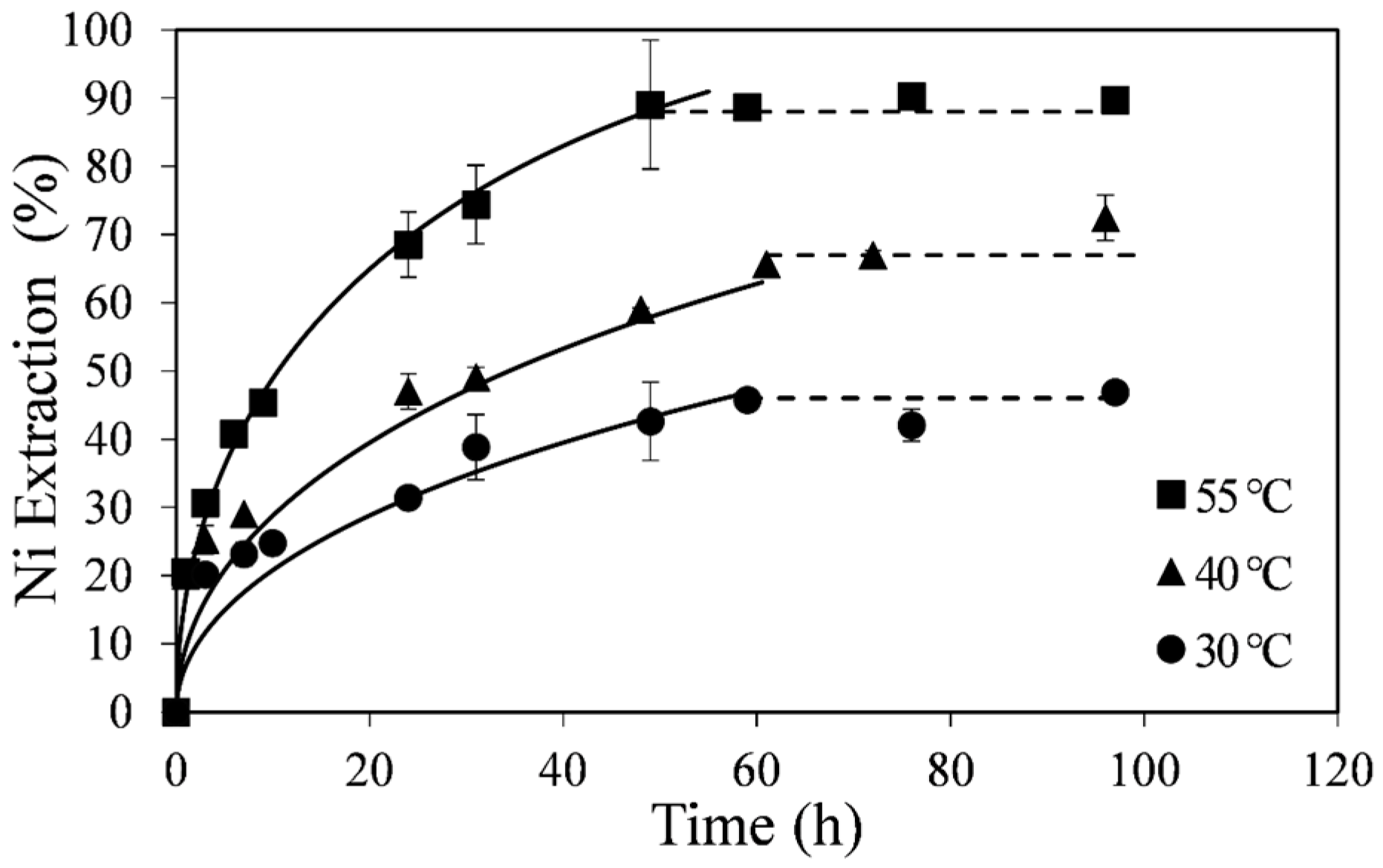
The effect of temperature on the kinetics of nickel extraction from the upgraded pyrrhotite tailings is shown in
Figure 2. These results were obtained by leaching about 0.14 wt % solids in acidic ferric sulfate solution at 30, 40, and 55 °C, 0.12 M Fe
2(SO
4)
3, and 0.2 M H
2SO
4 (Experiments A1-A3). A low % solids value was used to ensure near constant ferric concentration in order to apply the Shrinking Core Model. About 90% of the nickel in the tailings sample was extracted within 50 h at 55 °C, whereas at 30 and 40 °C the observed nickel extraction rate at any given time was lower. In all cases the total nickel in solution appeared to level off before complete dissolution (
Figure 2, dotted lines), with higher temperatures corresponding to higher total nickel extraction. Curve fittings in
Figure 2 (solid lines) were made with the diffusion-control shrinking core model (SCM) as discussed below.
Figure 2.
Effect of temperature on nickel extraction kinetics. Conditions: 0.14 wt % solids, 0.12 M Fe2(SO4)3 and 0.2 M H2SO4. The error bars represent standard deviation of triplicate tests.
Figure 2.
Effect of temperature on nickel extraction kinetics. Conditions: 0.14 wt % solids, 0.12 M Fe2(SO4)3 and 0.2 M H2SO4. The error bars represent standard deviation of triplicate tests.
The extraction of nickel from the pyrrhotite tailings involves a reaction between Fe
2(SO
4)
3 and the nickel-containing sulfide minerals pyrrhotite and pentlandite, which exist as a mixture. Although tailings are non-uniform particulate material in terms of composition and size, the SCM was tested as a semi-empirical kinetics model for the overall nickel dissolution kinetics [
17]. The nickel extraction
vs. time data at different temperatures were plotted for three rate-controlling forms of the SCM: liquid film diffusion (Equation (2)); diffusion through the product layer (Equation (3)); and chemical reaction (Equation (4)), where k
m, k
d and k
r are the apparent reaction rate constants, and x is the fraction of the solid converted.
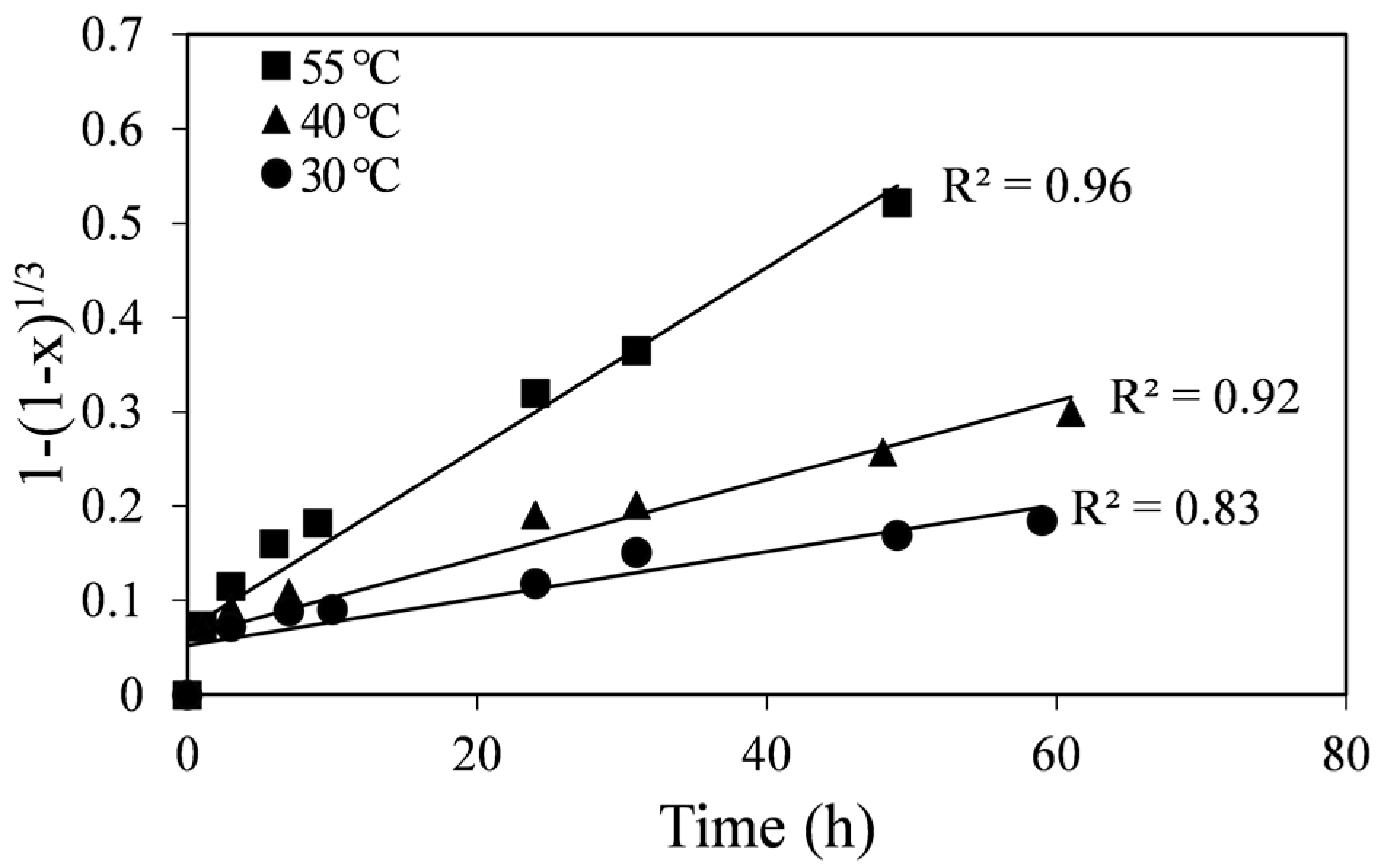
Since the curves in
Figure 2 are not linear, liquid film diffusion (Equation (2)) was ruled out as a controlling process. The straight-line plots in
Figure 3 show higher correlation coefficients for all the temperatures in comparison to the chemical reaction-control SCM (
Figure 4). Moreover, in
Figure 3, the fitted lines at different temperatures extrapolate through the origin or are very close to the origin, whereas in
Figure 4 they do not. Apparent diffusion-control rate constants (k
d), obtained from the slopes of the linear fits in
Figure 3, are shown in
Table 4.
Table 4.
Apparent rate constant values for diffusion control (kd) at various temperatures.
Table 4.
Apparent rate constant values for diffusion control (kd) at various temperatures.
| Temperature (°C) | kd (h−1) |
|---|
| 55 | 1.05 × 10−2 |
| 40 | 0.32 × 10−2 |
| 30 | 0.16 × 10−2 |
Figure 3.
Plot of the diffusion-control SCM vs. time at different temperatures.
Figure 3.
Plot of the diffusion-control SCM vs. time at different temperatures.
Figure 4.
Plot of the chemical reaction-control SCM vs. time at different temperatures.
Figure 4.
Plot of the chemical reaction-control SCM vs. time at different temperatures.
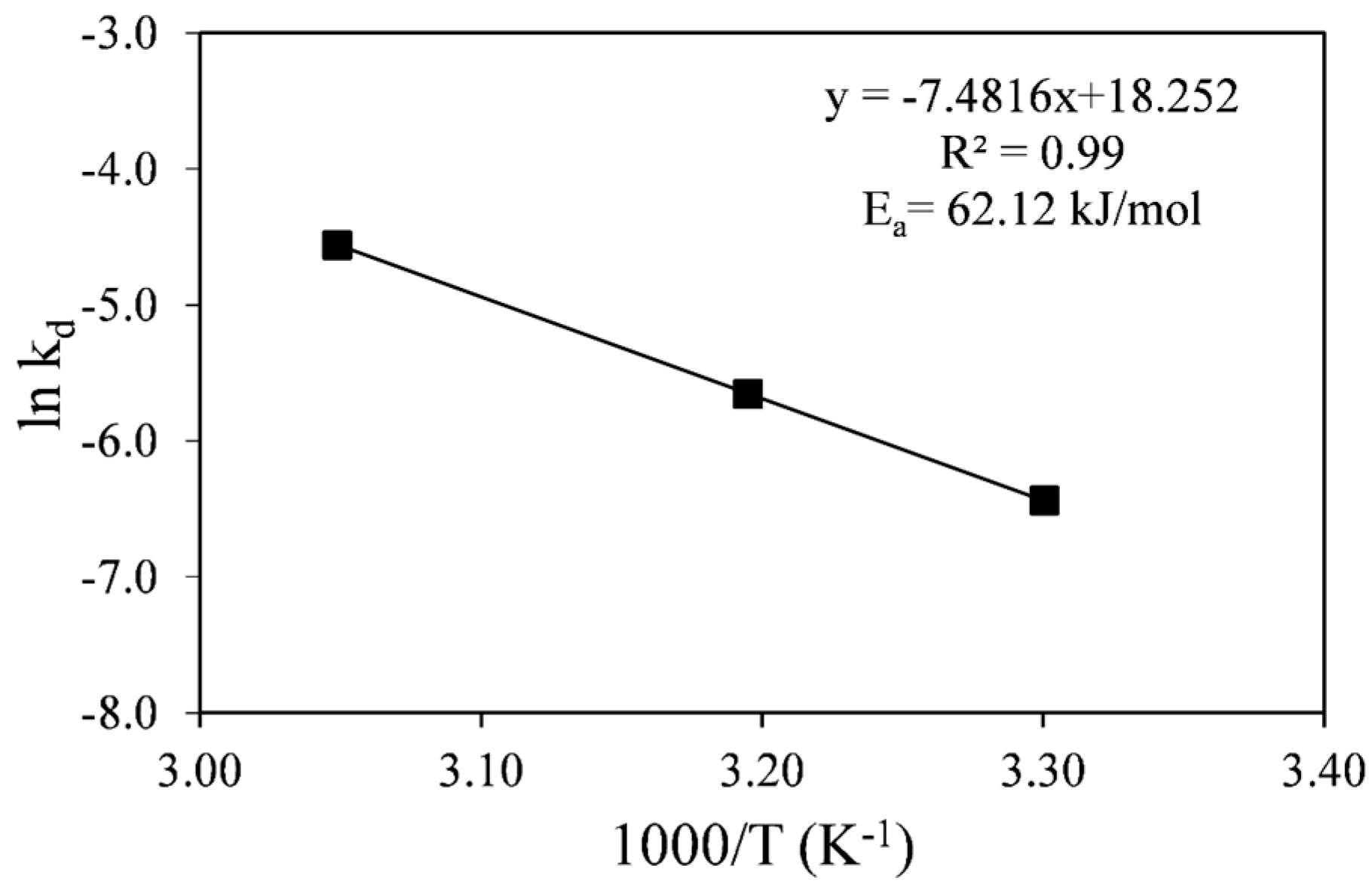
The k
d values obtained were used in an Arrhenius plot shown in
Figure 5. The linear fit to the data in
Figure 5 gave an activation energy value of E
a = 62.12 kJ/mol with a correlation coefficient close to unity (
R2 = 0.99). This is a high activation energy value commonly attributed to chemical reaction-control processes rather than diffusion-control processes, since the former are more sensitive to temperature than the latter [
17]. However, a number of kinetic studies on different systems have reported high values of activation energy for diffusion-control dissolution of sulfide minerals [
18,
19]. Particle size variability within a sample may affect the interpretation of leaching data when the SCM is applied; for example, Gbor and Jia [
20] showed mathematically that when the coefficient of variation (CV) of the PSD is large (0.7 < CV < 1.5), a chemical reaction control process can be mistakenly interpreted as an inert layer diffusion control process. The CV of the PSD of the pyrrhotite tailings was found to be within this range (0.82), however, accounting for the PSD according to Gbor and Jia [
20] resulted in a better fit for the diffusion control process (data not shown). The temperature dependent levelling off of nickel concentrations is also more consistent with the formation of a product layer that may limit and ultimately stop the reaction.
Figure 5.
Arrhenius plot for the diffusion-control process.
Figure 5.
Arrhenius plot for the diffusion-control process.
The fact that the tailings are composed of two individual nickel-bearing minerals, each containing almost half of the total nickel, may complicate things. To obtain an accurate kinetic model for nickel extraction, the oxidation kinetics of pyrrhotite and pentlandite need to be known separately. This information needs to become available by tracking the conversion of each individual mineral in the mixture. A method proposed by Ingraham
et al. [
21], based on the idea that pyrrhotite is an acid soluble mineral, while pentlandite is essentially not acid soluble could be applied to the pyrrhotite tailings. Given the very small amount of pentlandite (1.2 wt %) in the tailings and the experimentation under very low percent solids (~1.4 wt %), it was not possible to achieve this in the present study, and therefore we decided to treat the tailings as a uniform mixture until we develop a mineral separation (physical or chemical) protocol.
3.2. Effect of Ferric Sulfate Concentration on Nickel Extraction
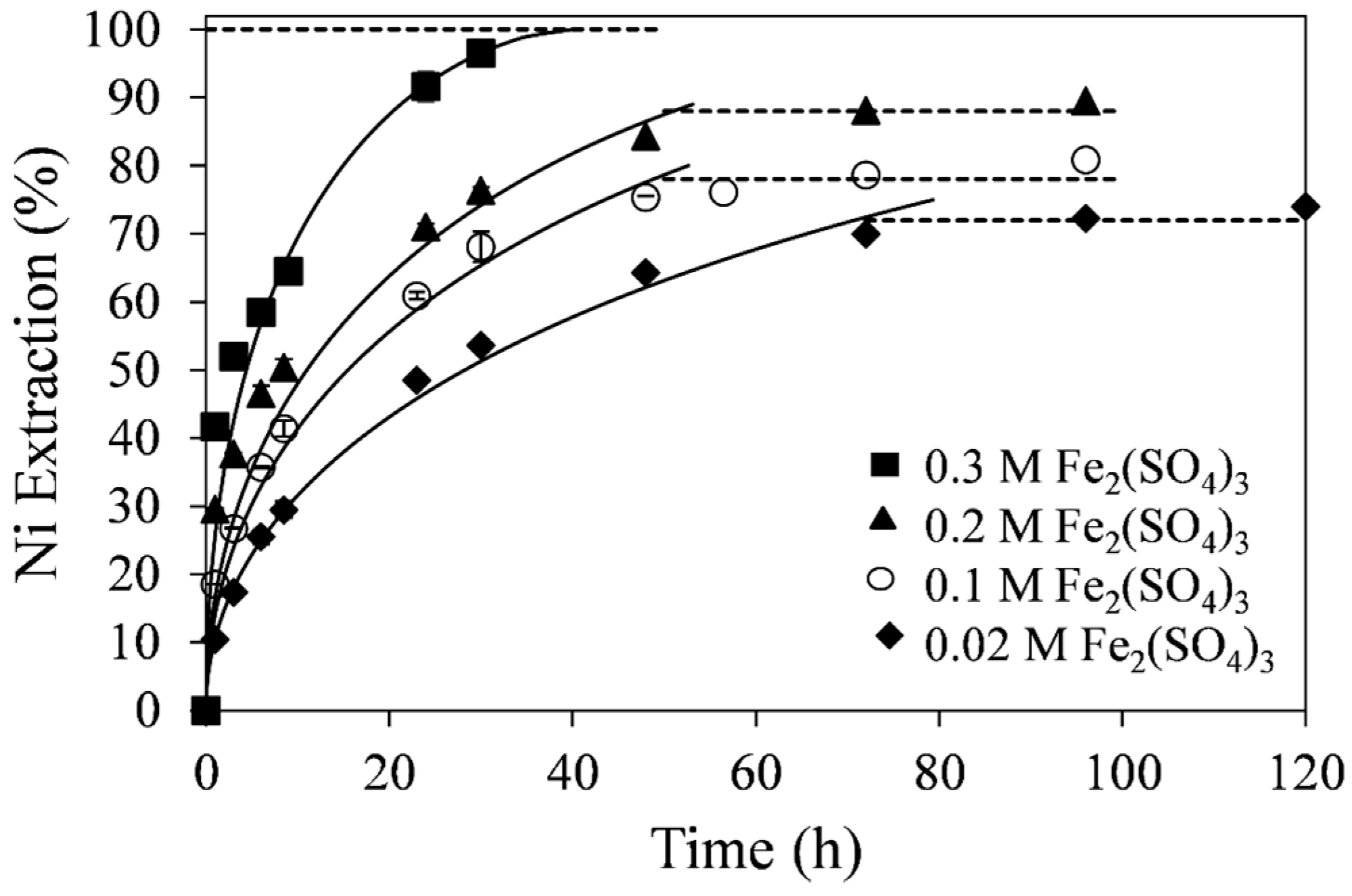
The effect of ferric sulfate concentration on the percent extraction of nickel at 55 °C is shown in
Figure 6. As expected, the rate of nickel extraction increased with increasing ferric sulfate concentration from 0.02 to 0.3 M. At 0.3 M ferric sulfate, 96% nickel extraction was achieved by about 30 h of retention time. At lower concentrations of ferric sulfate, the kinetics were slower and the extraction curves tended to reach a plateau before complete dissolution of the tailings was achieved. The curve fitting in
Figure 6 was made with the diffusion-control SCM. The diffusion-control SCM produced linear fits with higher correlation coefficients for all ferric sulfate concentrations tested than the chemical reaction-control SCM. Apparent diffusion-control rate constants (k
d), shown in
Table 5, were obtained from the slopes of the linear fits.
Figure 6.
Effect of Fe2(SO4)3 concentration on nickel extraction kinetics. Conditions: 55 °C, 0.14 wt % solids, and 0.2 M H2SO4. The error bars represent standard deviation from triplicate tests.
Figure 6.
Effect of Fe2(SO4)3 concentration on nickel extraction kinetics. Conditions: 55 °C, 0.14 wt % solids, and 0.2 M H2SO4. The error bars represent standard deviation from triplicate tests.
Table 5.
Diffusion-control apparent rate constants at various concentrations of ferric sulfate.
Table 5.
Diffusion-control apparent rate constants at various concentrations of ferric sulfate.
| Fe2(SO4)3 (M) | kd (h−1) | R2 |
|---|
| 0.3 | 2.50 × 10−2 | 0.99 |
| 0.2 | 1.00 × 10−2 | 0.97 |
| 0.1 | 0.71 × 10−2 | 0.98 |
| 0.02 | 0.39 × 10−2 | 0.99 |
Using the mixed solvent-electrolyte (MSE) chemical model of the OLI software (OLI Analyzer Studio 3.2; Cedar Knolls, NJ, USA), the concentrations of different Fe species were calculated at 55 °C, 0.2 M H
2SO
4, and different ferric sulfate concentrations (0.02–0.3 M). It can be seen in
Table 6 that ferric ion was found to be the dominant species, while Fe(OH)
2+ concentration was low and did not vary significantly over the range of ferric sulfate concentrations tested. Therefore, the order of reaction was determined with respect to ferric ion concentration.
Table 6.
Concentrations of Fe species obtained using OLI-MSE model.
Table 6.
Concentrations of Fe species obtained using OLI-MSE model.
| Fe2(SO4)3 (M) | Fe3+ (M) | Fe(OH)2+ (M) |
|---|
| 0.3 | 0.597 | 0.0031 |
| 0.2 | 0.396 | 0.0038 |
| 0.1 | 0.196 | 0.0037 |
| 0.02 | 0.039 | 0.0011 |
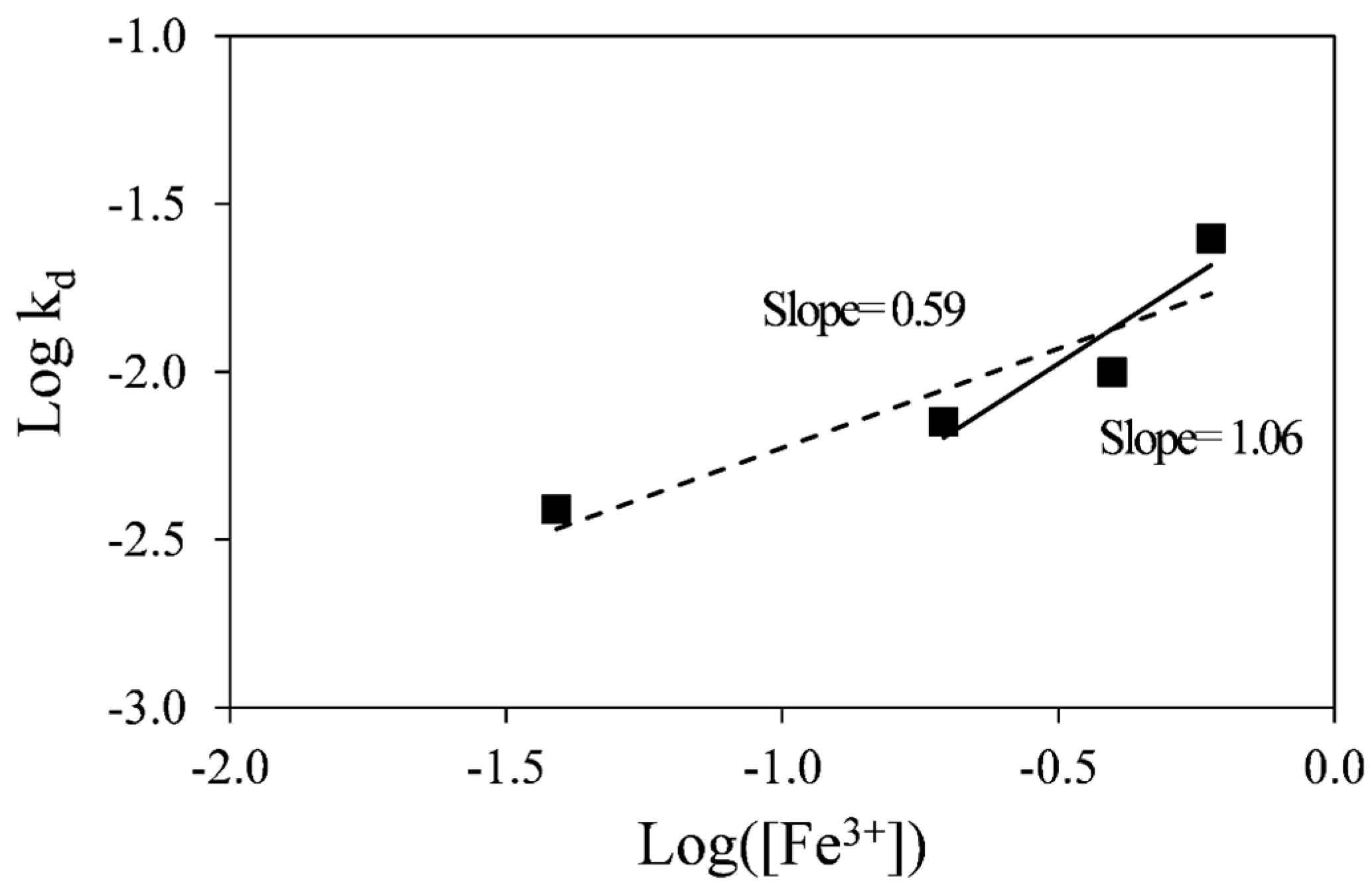
A plot of the log
10 of k
d against the log
10 of ferric concentration is shown in
Figure 7. The linear fit to the data indicates that the order of reaction with respect to ferric ion is about 0.6. However, the order of reaction with respect to ferric ion within the range of 0.1 to 0.3 M ferric sulfate gives a value of 1.0, which is consistent with the expected value for a diffusion-control process, since Fick’s law is first order with respect to diffusing species. This discrepancy is likely due to the fact that at 0.02 M ferric sulfate the stoichiometric ratio of ferric sulfate: pyrrhotite is only 1.2:1 and would therefore not meet the requirements for the use of the SCM, where the concentration of the reactant is required to remain constant during the reaction. Therefore, the lowest ferric sulfate concentration (0.02 M) was ruled out for determination of the reaction order with respect to ferric ion.
Figure 7.
Determination of reaction order with respect to ferric ion.
Figure 7.
Determination of reaction order with respect to ferric ion.
3.3. Elemental Sulfur Formation
The extraction of nickel and elemental sulfur formation with time at 55 °C, 0.2 M Fe
2(SO
4)
3, and 0.2 M H
2SO
4 is shown in
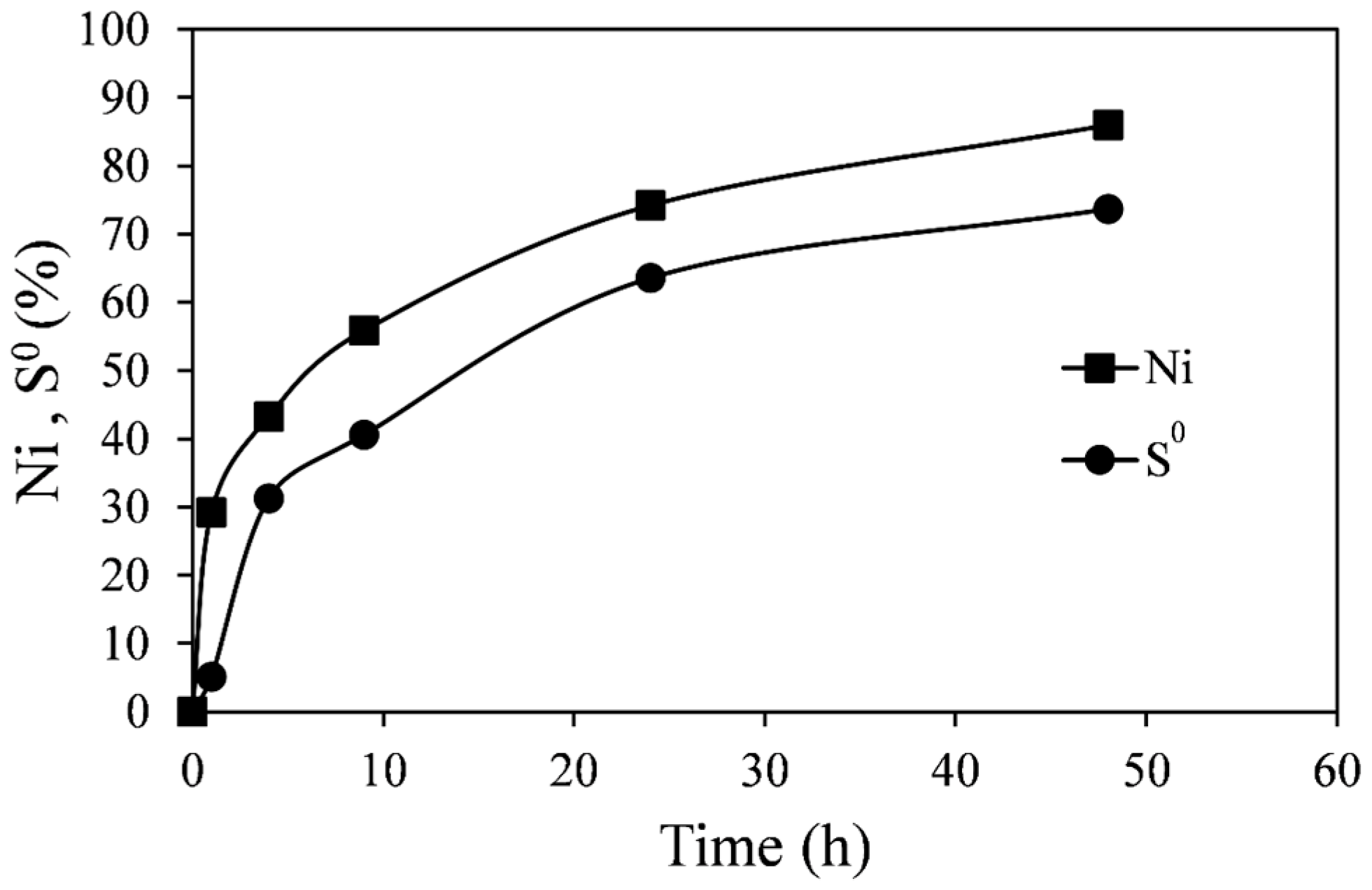
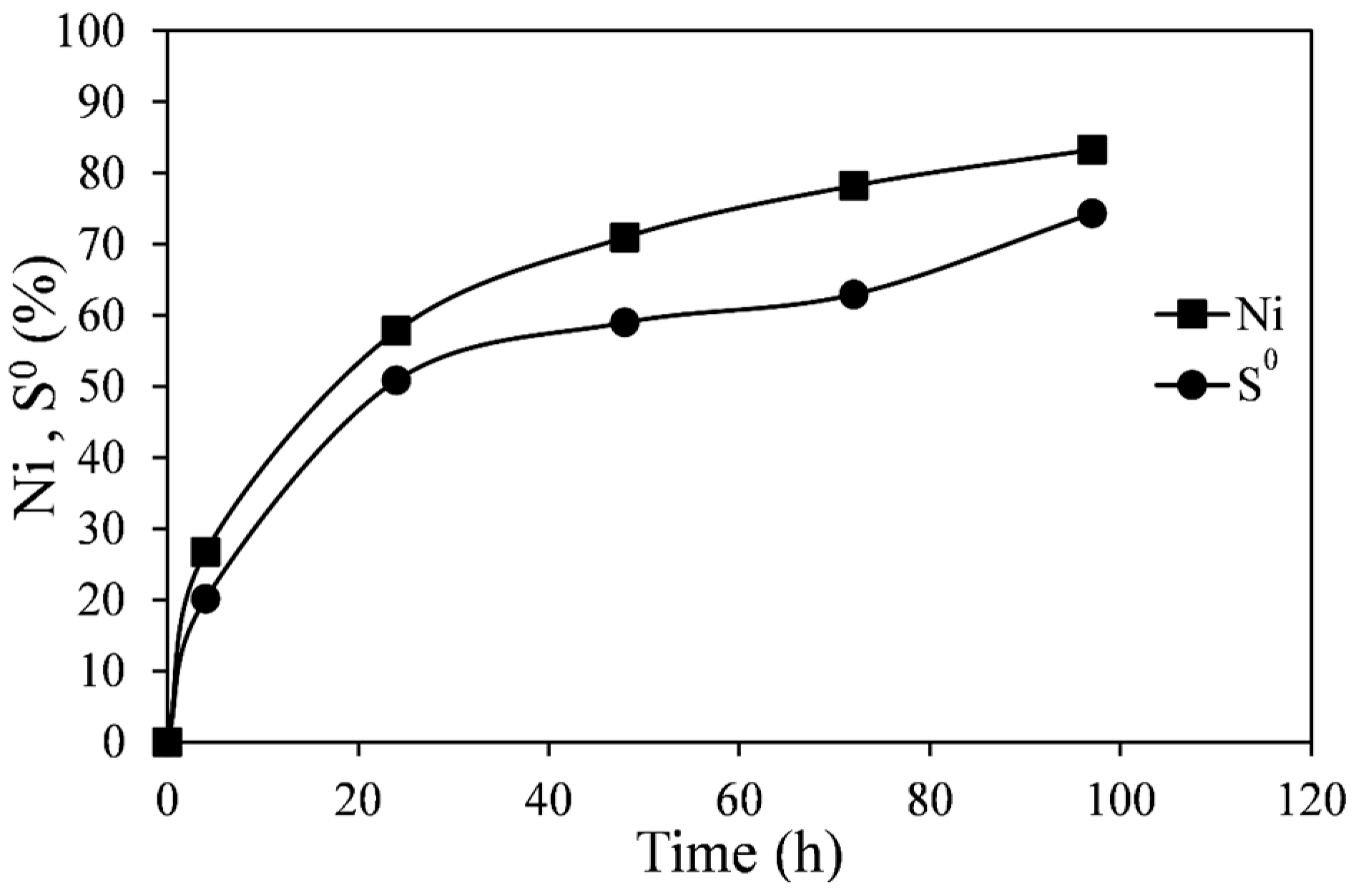
Figure 8 (Experiment C1). Each pair of points represents a separate flask, since the low percent solids employed did not permit sufficient slurry sampling for sulfur analysis. As in previous experiments, the pyrrhotite tailings dissolved relatively quickly, and after about 50 h 85% extraction of nickel was achieved. After the same period of time about 75% of the sulfide in the pyrrhotite tailings had been converted into elemental sulfur. Although the nickel extraction and sulfur production curves follow a similar trend, the elemental sulfur extraction is consistently lower. The difference should be the amount of sulfide oxidized to sulfate ions, although the amount of sulfate could not be determined due to the high sulfate background in the ferric sulfate solution.
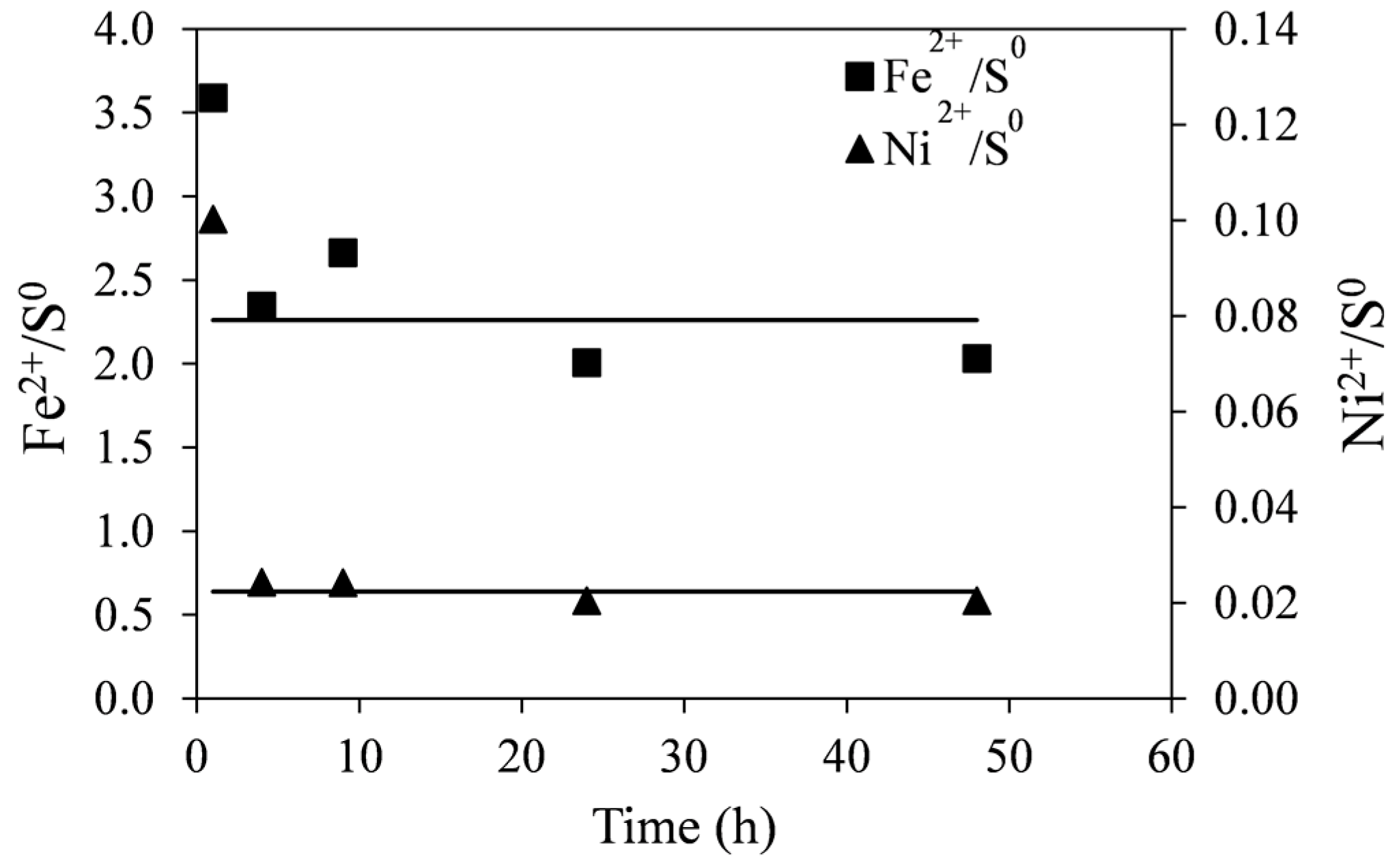
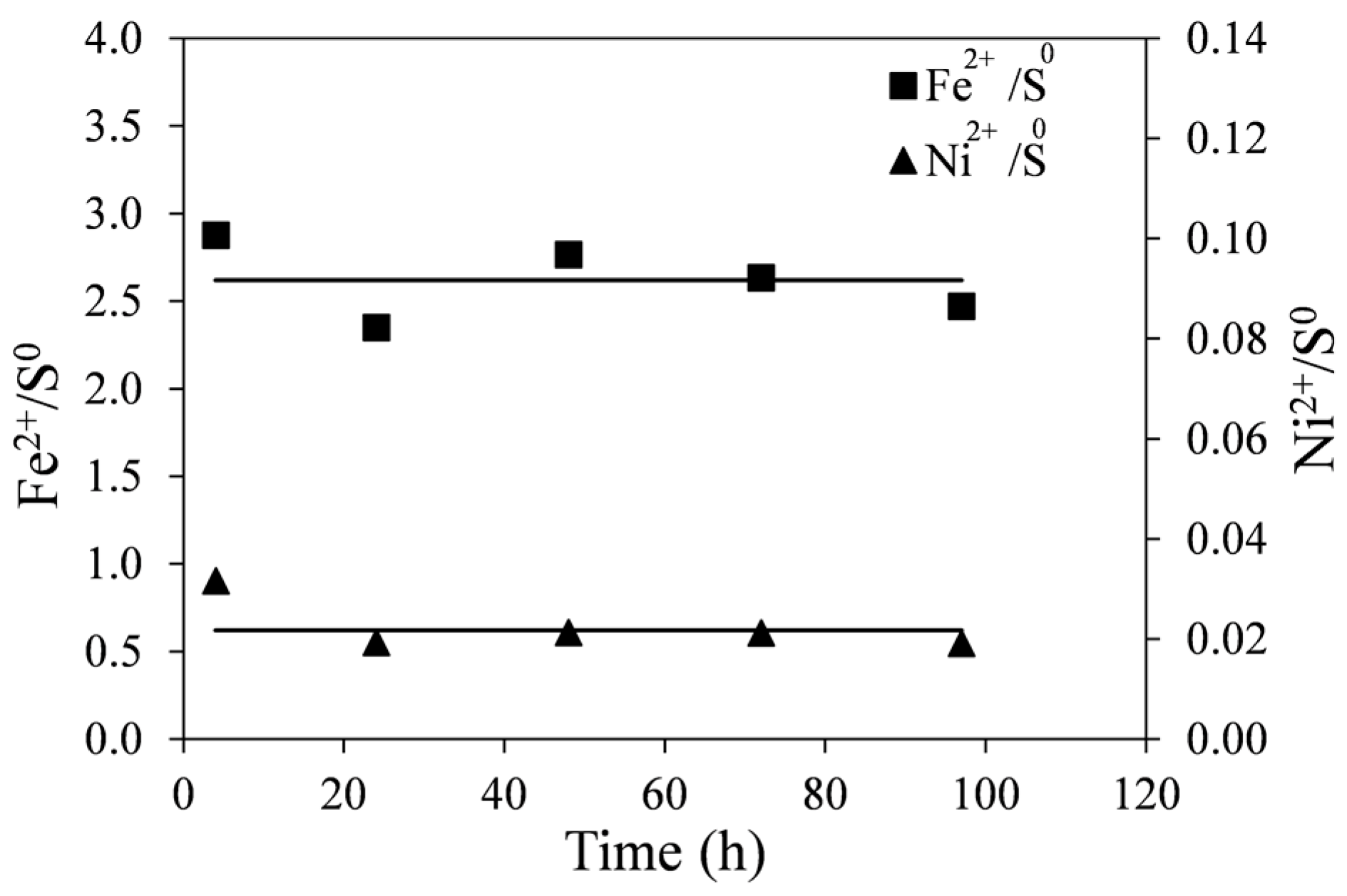
The molar ratios of Fe
2+/S
0 and Ni
2+/S
0 with time are shown in
Figure 9. These were calculated from data shown in
Figure 8. Initially (at ~1 h), the molar ratios of Fe
2+/S
0 and Ni
2+/S
0 are high suggesting that the dissolution of the tailings at the beginning of leaching does not produce as much elemental sulfur as at later stages of reaction. This is likely due to the direct acid attack of pyrrhotite with the formation of dissolved hydrogen sulfide, shown in Equation (5). However, as no noticeable gaseous hydrogen sulfide was detected, it is possible that subsequent oxidation of hydrogen sulfide by ferric ions occurs at a slower rate according to Equation (6). In any case, initial surface oxidation of sulfides in the tailings could not account for the initially seen high ratios. These values were determined to be about 1%–2% of total nickel in the tailings samples. The lines in
Figure 9 are least-square linear fits to the data points (excluding
t = 1 h), producing a Fe
2+/S
0 ratio of 2.26 and the Ni
2+/S
0 ratio of 0.022.
It can be seen in Reaction (7) that if monoclinic pyrrhotite were completely dissolved, the molar ratio of Fe2+/S0 would be 2.6. The fact that the pyrrhotite tailings are not pure and contain small quantities of other sulfide minerals (pentlandite, pyrite and chalcopyrite) may explain the difference between the expected and observed values.
Figure 8.
Extraction of Ni and S0 formation from leaching of pyrrhotite tailings. Conditions: 55 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 8.
Extraction of Ni and S0 formation from leaching of pyrrhotite tailings. Conditions: 55 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 9.
Molar ratios of Fe2+/S0 and Ni2+/S0 generated during leaching of pyrrhotite tailings. Conditions: 55 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4. The lines are least square linear fits to the data excluding the first time point (1 h).
Figure 9.
Molar ratios of Fe2+/S0 and Ni2+/S0 generated during leaching of pyrrhotite tailings. Conditions: 55 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4. The lines are least square linear fits to the data excluding the first time point (1 h).
Pyrrhotite is the dominant mineral of the tailings (86.2 wt %), and therefore most of the elemental sulfur produced is the by-product of pyrrhotite dissolution. However, nickel in the tailings is attributed to both pentlandite and pyrrhotite, accounting for 40% and 60% of the total nickel, respectively. Accordingly, if pyrrhotite and pentlandite were to react at different rates, the Ni
2+/S
0 ratio would be expected to change during the reaction. However, as seen in
Figure 9, the Ni
2+/S
0 ratio remains relatively constant over time. This suggests that the dissolution rates of pyrrhotite and pentlandite are similar, and that no significant galvanic interaction has occurred between the two minerals, most likely due to their high degree of liberation. This supports the use of the SCM for the analysis of the dissolution kinetics. The theoretical value of Ni
2+/S
0 obtained based on the assumption that pyrrhotite and pentlandite react at a similar rate is 0.02 which is comparable with the molar ratios obtained for both 55 and 40 °C (Ni
2+/S
0 = 0.022).
To examine whether ferrous ion is re-oxidized to ferric ion due to oxygen entrainment, a control experiment was conducted in which 0.02 M ferrous ion was added to 0.2 M ferric sulfate solution at 55 °C and 250 rpm. After one week, the concentration of ferrous ion remained reasonably constant over time (1% overall reduction). This suggests that there was no oxygen entrainment in these tests, and all oxidative reactions are due to ferric attack.
Figure 10 shows the extraction of nickel and elemental sulfur formation from the leaching of the pyrrhotite tailings at a lower temperature of 40 °C, 0.2 M Fe
2(SO
4)
3 and 0.2 M H
2SO
4, where each pair of points represents a separate experiment.
Figure 10.
Extraction of Ni and S0 formation from leaching of pyrrhotite tailings. Conditions: 40 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 10.
Extraction of Ni and S0 formation from leaching of pyrrhotite tailings. Conditions: 40 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
It can be seen in
Figure 10 that after about 100 h, 80% of nickel is extracted, while only about 70% of sulfide is converted to elemental sulfur. Although the curve for elemental sulfur follows a similar trend to that of nickel, the elemental sulfur conversion is consistently lower due to a small fraction (about 20%) converting to sulfate, as explained above.
Figure 11 shows the molar ratios of Fe
2+/S
0 and Ni
2+/S
0 with time from data in
Figure 10. The lines are least-square linear fits to the data points, where the ratios of Ni
2+/S
0 and Fe
2+/S
0 are 2.62 and 0.022, respectively. The relatively constant Ni
2+/S
0 ratio shows that the rates of nickel extraction from both pyrrhotite (60% of total Ni) and pentlandite (40% of total Ni) must be very similar. The constant molar ratios of Ni
2+/S
0 and Fe
2+/S
0 are similar at both temperatures of 40 and 55 °C indicating that the measured elemental sulfur yield was essentially independent of the temperature of the leach solution.
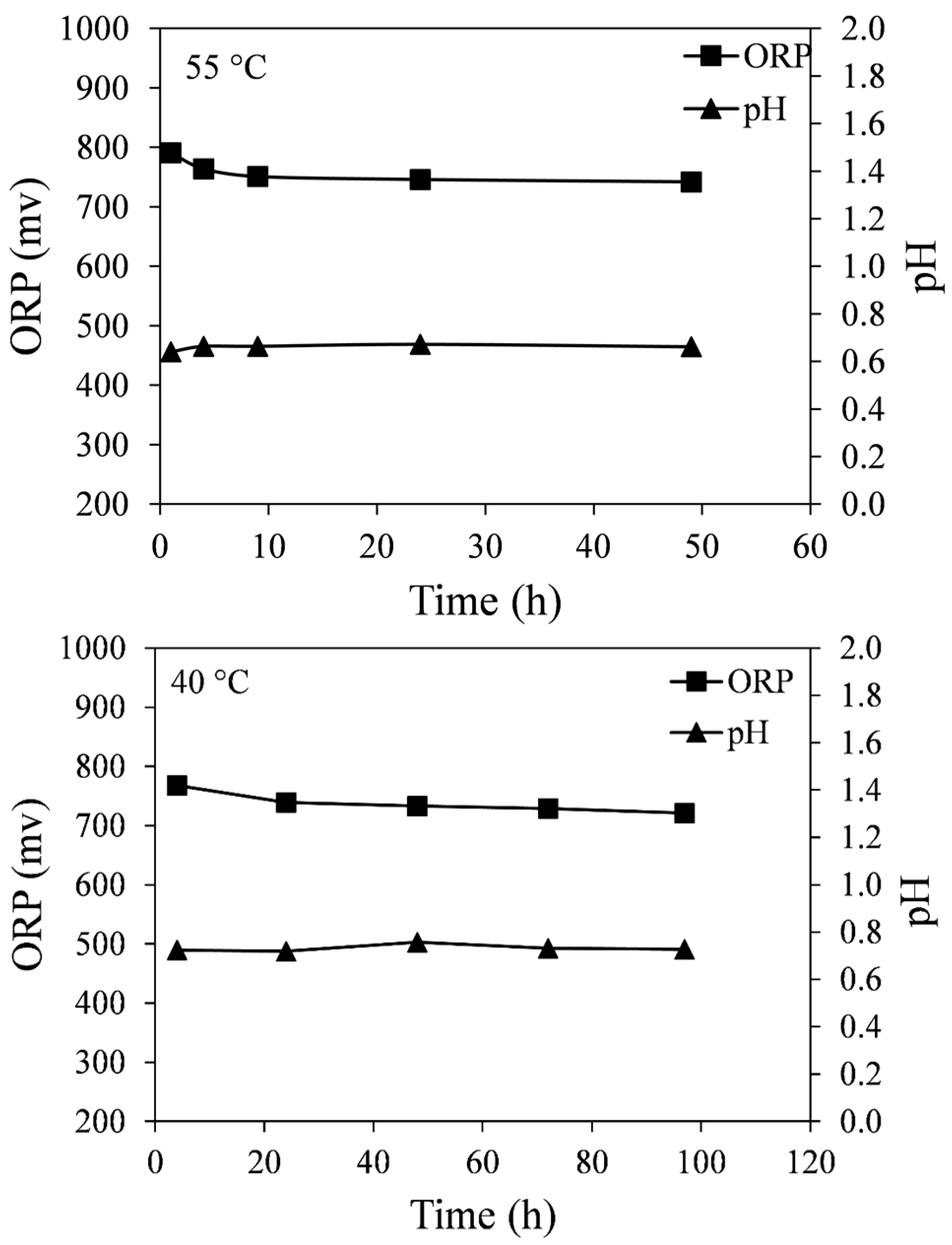
Figure 12 shows that the ORP and pH values of the leach solution remain relatively constant at both temperatures.
Figure 11.
Molar ratios of Fe2+/S0 and Ni2+/S0 generated during leaching of pyrrhotite Tailings. Conditions: 40 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4. The lines are least-square linear fits to the data.
Figure 11.
Molar ratios of Fe2+/S0 and Ni2+/S0 generated during leaching of pyrrhotite Tailings. Conditions: 40 °C, 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4. The lines are least-square linear fits to the data.
Figure 12.
ORP and pH values of the leach solution measured at different times for 55 and 40 °C. Conditions: 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 12.
ORP and pH values of the leach solution measured at different times for 55 and 40 °C. Conditions: 0.14 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
3.4. Morphology of Elemental Sulfur
To observe elemental sulfur and its morphology on partially leached pyrrhotite particles at different percent extractions of nickel, Experiment D1 was performed by adding about 2.8 g of the pyrrhotite tailings into 500 mL leach solution containing 0.2 M ferric sulfate and 0.2 M sulfuric acid at 55 °C. After 19, 72, and 144 h of leaching, the contents of the flasks were vacuum filtered, and the residues were dried, epoxy mounted, polished, and subjected to SEM examination. The ICP analysis of the leach solutions showed that the 19, 72, and 144 h of leaching correspond to 50%, 80% and 90% nickel extractions, respectively. These values are lower than those obtained with 1.4 g/L of initial solids loading due to different stoichiometric excess of ferric ion.
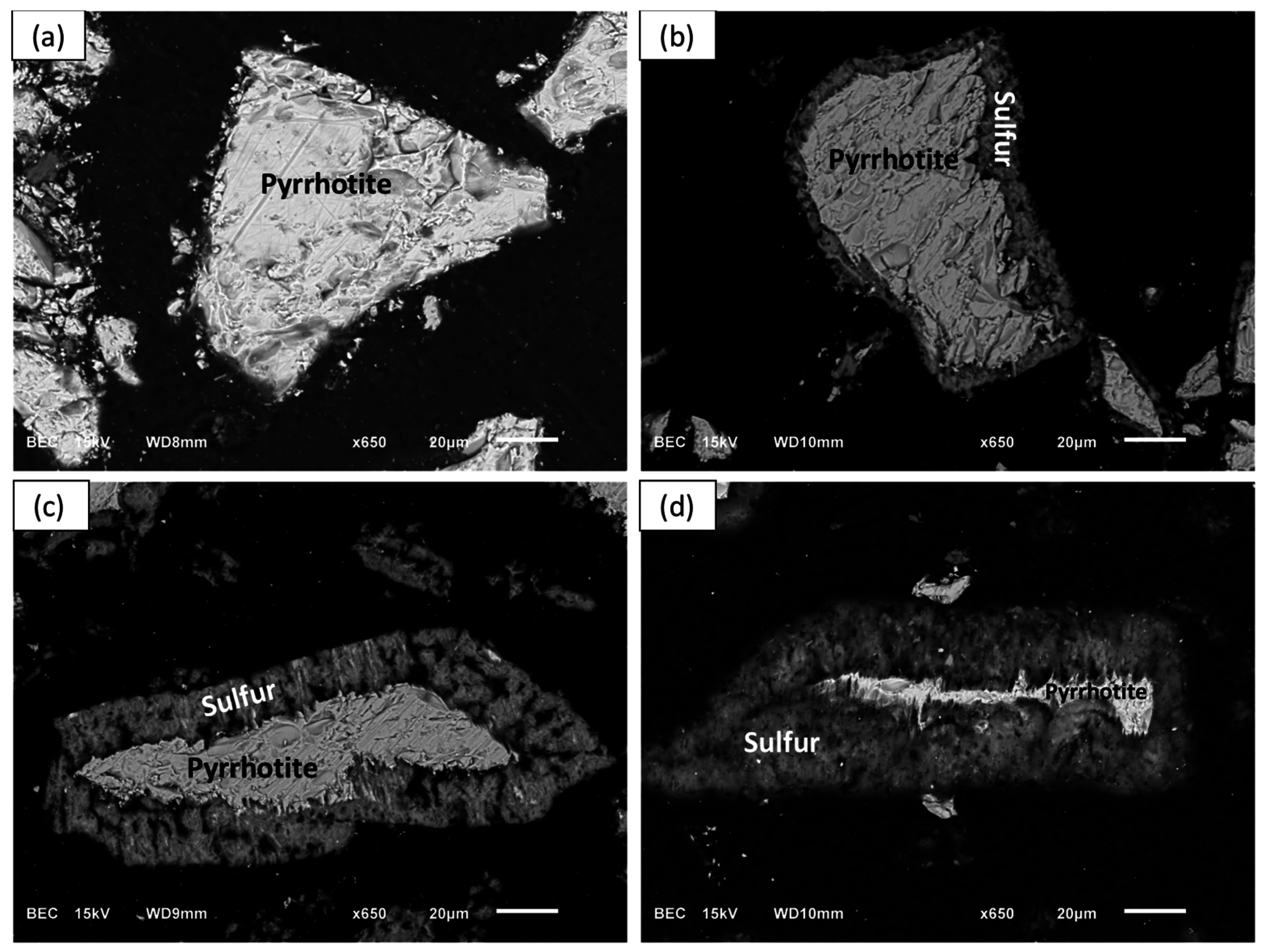
The SEM-BSE images of the leached pyrrhotite particles at 0% (a), 50% (b), 80% (c), and 90% (d) nickel extractions are shown in
Figure 13. Clearly, a distinct and growing layer of elemental sulfur develops on the pyrrhotite particles.
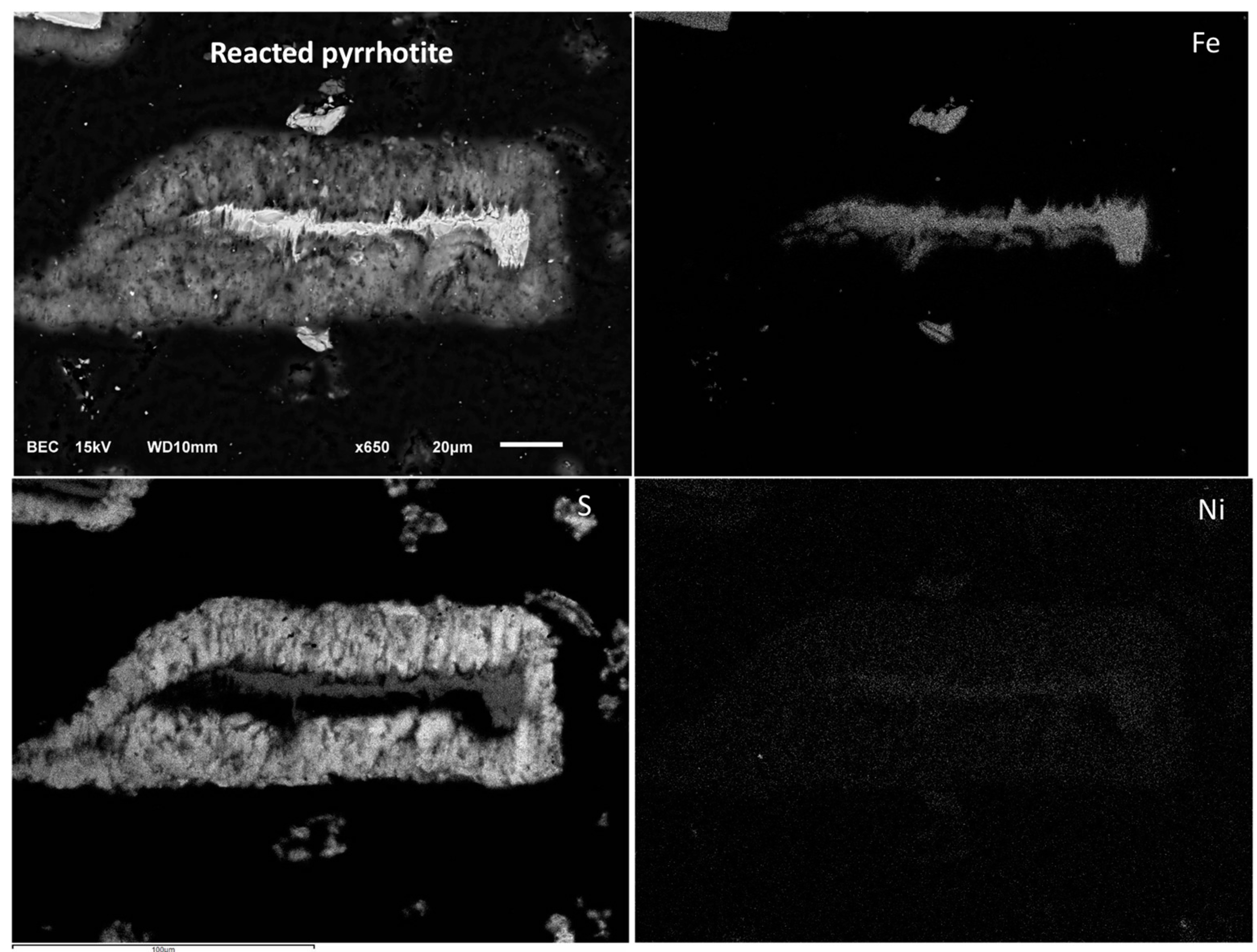
Figure 13b,d shows that a relatively porous sulfur layer forms, which allows the diffusion of the reactants to the reacting interface. This is consistent with the postulated SCM for diffusion through the product layer-control processes. It seems, however, that as the layer grows it reaches a point that halts the reaction. This phenomenon is less apparent as the temperature increases and accelerates the rate of sulfur formation, perhaps allowing sulfur to remain amorphous and porous for a longer time allowing the completion of iron and nickel dissolution. Elemental mapping of a pyrrhotite particle after 90% nickel extraction was obtained with the help of EDX and is shown in
Figure 14.
Figure 13.
SEM-BSE images of partially leached pyrrhotite particles after (a) time 0 (0%); (b) 19 h (50%); (c) 72 h (80%) and (d) 144 h (90%) of leaching. Conditions: 55 °C, 0.56 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 13.
SEM-BSE images of partially leached pyrrhotite particles after (a) time 0 (0%); (b) 19 h (50%); (c) 72 h (80%) and (d) 144 h (90%) of leaching. Conditions: 55 °C, 0.56 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 14.
Elemental maps of a pyrrhotite particle after 90% nickel extraction. Conditions: 55 °C, 0.56 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.
Figure 14.
Elemental maps of a pyrrhotite particle after 90% nickel extraction. Conditions: 55 °C, 0.56 wt % solids, 0.2 M Fe2(SO4)3, and 0.2 M H2SO4.