Investigating the Effects of Se Solid Phase Substitution in Jarosite Minerals Influenced by Bacterial Reductive Dissolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aqueous Phase Analysis
2.1.1. No-Bacteria Controls (Ln and Hn)
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard errors (n = 3).
; error bars are standard errors (n = 3).
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard errors (n = 3).
; error bars are standard errors (n = 3).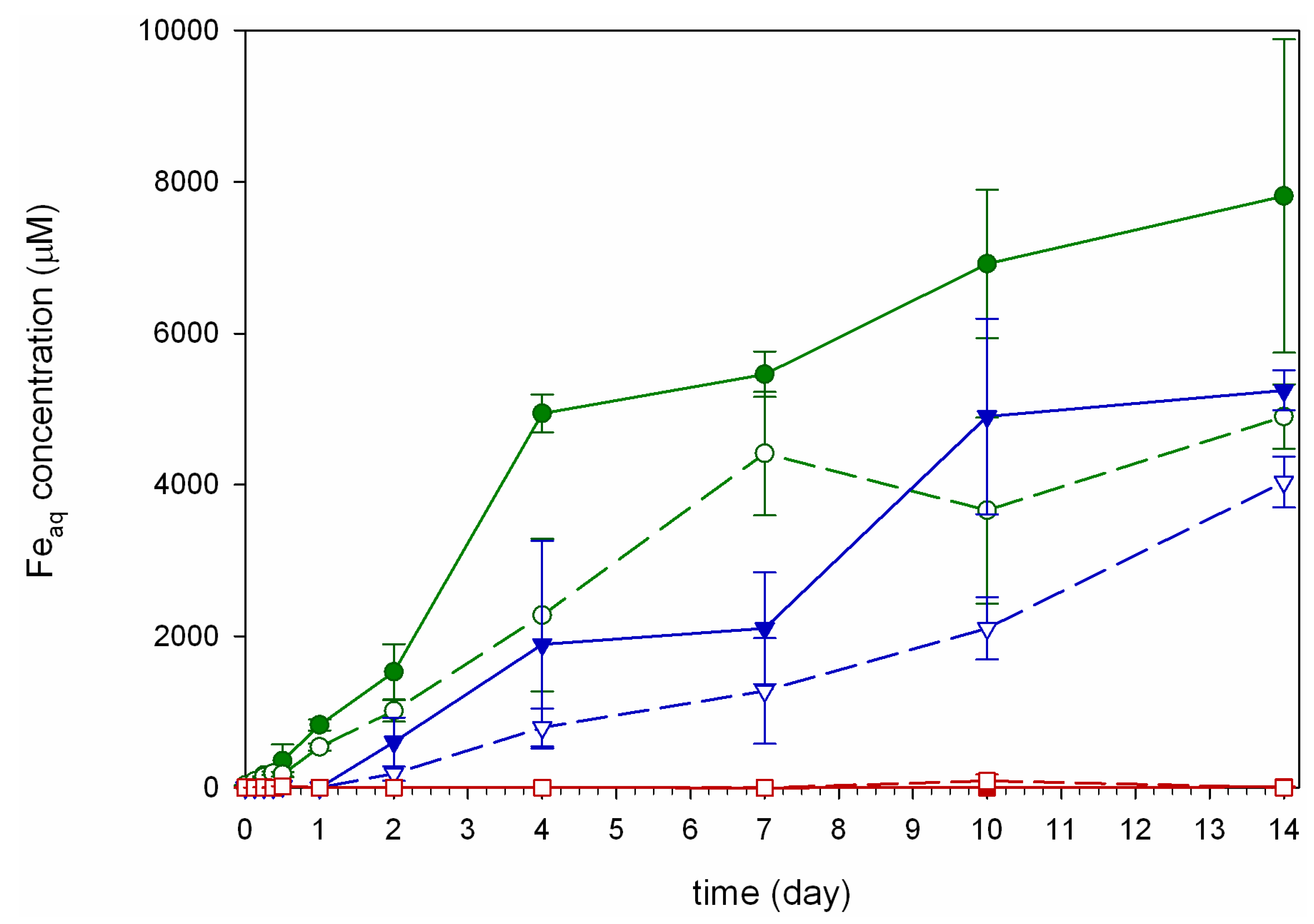
| Time-frame treatment | 0–24 h | 24 h–7 days | 7–14 days | 0–14 days |
|---|---|---|---|---|
| La | 31.87 ± 4.52 | 39.14 ± 2.06 | −8.16 ± 2.27 | 20.06 ± 2.04 |
| (0.7566) | (0.9756) | (0.6497) | (0.7627) | |
| 18.63 ± 1.59 | 21.22 ± 1.88 | 4.31 ± 2.86 | 13.83 ± 0.85 | |
| (0.8953) | (0.9409) | (0.3129) | (0.9035) | |
| Time-frame treatment | 0–12 h | 12 h–7 days | 7–14 days | 0–14 days |
| Ld | 1.29 ± 0.31 | 12.82 ± 2.92 | 17.58 ± 7.75 | 17.66 ± 1.32 |
| (0.5676) | (0.6364) | (0.4618) | (0.8643) | |
| 1.28 ± 0.31 | 5.05 ± 1.49 | 20.50 ± 2.82 | 19.80 ± 0.72 | |
| (0.5619) | (0.5344) | (0.8979) | (0.8893) | |
| 1.17 ± 0.26 | −0.07 ± 0.03 | 0.06 ± 0.02 | 0.01 ± 0.01 | |
| (0.6057) | (0.2957) | (0.4493) | (0.0369) | |
| 1.19 ± 0.36 | −0.08 ± 0.03 | −0.04 ± 0.45 | 0.09 ± 0.07 | |
| (0.4597) | (0.2779) | (0.0013) | (0.0477) |
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard error (n = 3).
; error bars are standard error (n = 3).
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard error (n = 3).
; error bars are standard error (n = 3).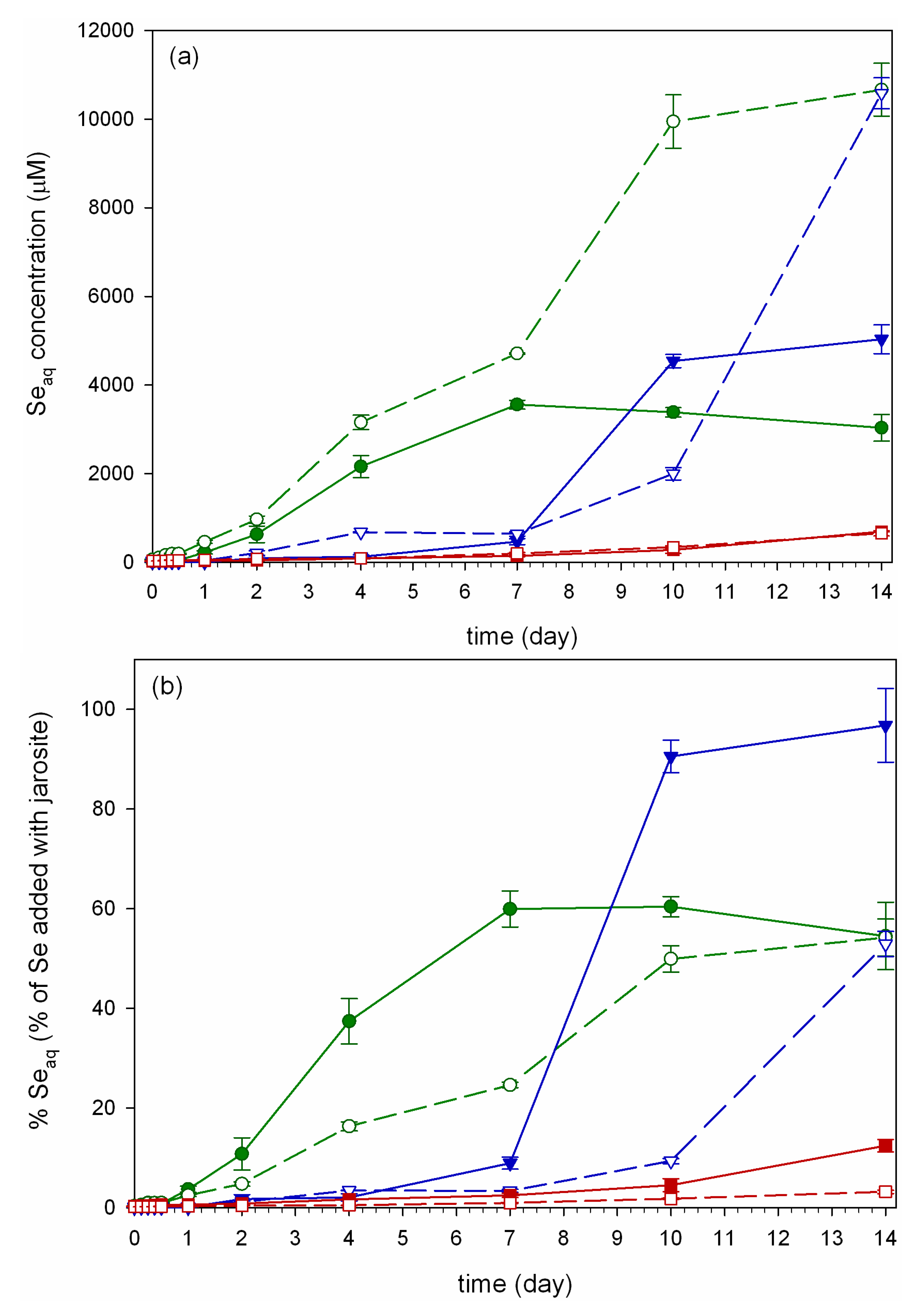
| Time-frame treatment | Se release rates (μM h−1) | Se release rates (% h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–24 h | 24 h–7 days | 7–14 days | 0–14 days | 0–24 h | 24 h–7 days | 7–14 days | 0–14 days | |
| La | 8.37 ± 1.14 | 23.87 ± 1.59 | −3.15 ± 1.5 | 11.85 ± 1.09 | 0.140 ± 0.030 | 0.400 ± 0.040 | −0.034 ± 0.019 | 0.210± 0.030 |
| (0.7718) | (0.9576) | (0.3884) | (0.7934) | (0.8457) | (0.9841) | (07587) | (0.8305) | |
| 16.07 ± 1.10 | 44.60 ± 9.71 | 23.16 ±13.72 | 33.37 ± 2.37 | 0.001 ± 0.000 | 0.058 ± 0.014 | 0.170± 0.086 | 0.300 ± 0.045 | |
| (0.9305) | (0.6783) | (0.2894) | (0.8648) | (0.5575) | (0.9006) | (0.7931) | (0.8274) | |
| 0.05 ± 0.02 | 11.98 ± 5.54 | 16.22 ± 7.97 | 13.65 ± 1.37 | 0.014 ± 0.003 | 0.013 ± 0.001 | 0.500 ± 0.300 | 0.029 ± 0.004 | |
| (0.3682) | (0.3189) | (0.372) | (0.7621) | (0.8452) | (0.9898) | (0.7337) | (0.8457) | |
| 0.42 ± 0.13 | 7.61 ± 1.99 | 57.83 ± 8.15 | 24.60 ± 2.63 | 0.087 ± 0.010 | 0.160 ± 0.019 | 0.300 ± 0.100 | 0.018 ± 0.010 | |
| (0.4104) | (0.6183) | (0.8936) | (0.7503) | (0.9452) | (0.9733) | (0.8948) | (0.9714) | |
| 1.07 ± 0.09 | 0.77 ± 0.07 | 2.74 ± 0.71 | 1.48 ± 0.13 | 0.002 ± 0.001 | 0.023 ± 0.009 | 0.061 ± 0.015 | 0.110 ± 0.025 | |
| (0.8986) | (0.9294) | (0.7143) | (0.8227) | (0.6252) | (0.7703) | (0.9410) | (0.6937) | |
| 1.40 ± 0.18 | 0.95 ± 0.16 | 2.74 ± 0.35 | 1.63 ± 0.10 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.013 ± 0.001 | 0.008 ± 0.001 | |
| (0.7965) | (0.8045) | (0.8989) | (0.8996) | (0.9493) | (0.9573) | (0.9964) | (0.9363) | |
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard error (n = 3).
; error bars are standard error (n = 3).
 , Ha =
, Ha =  , Ld =
, Ld =  , Hd =
, Hd =  , Ln =
, Ln =  , and Hn =
, and Hn =  ; error bars are standard error (n = 3).
; error bars are standard error (n = 3).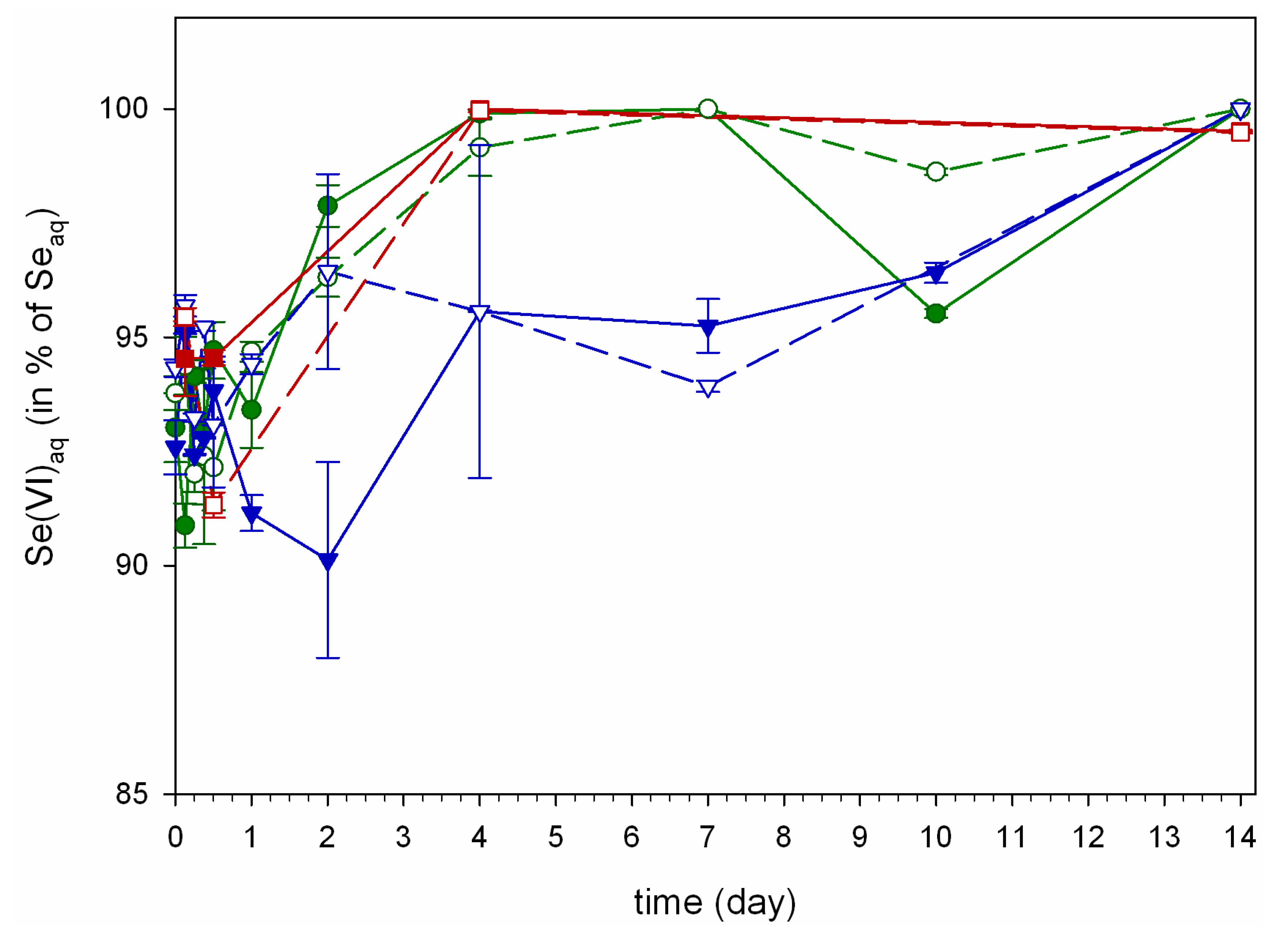
2.1.2. Dead-Bacteria Treatment (Ld and Hd)
2.1.3. Live Bacteria (La and Ha)
2.2. Solid Phase Analysis
2.2.1. Scanning Electron Microscopy
2.2.2. Transmission Electron Microscopy
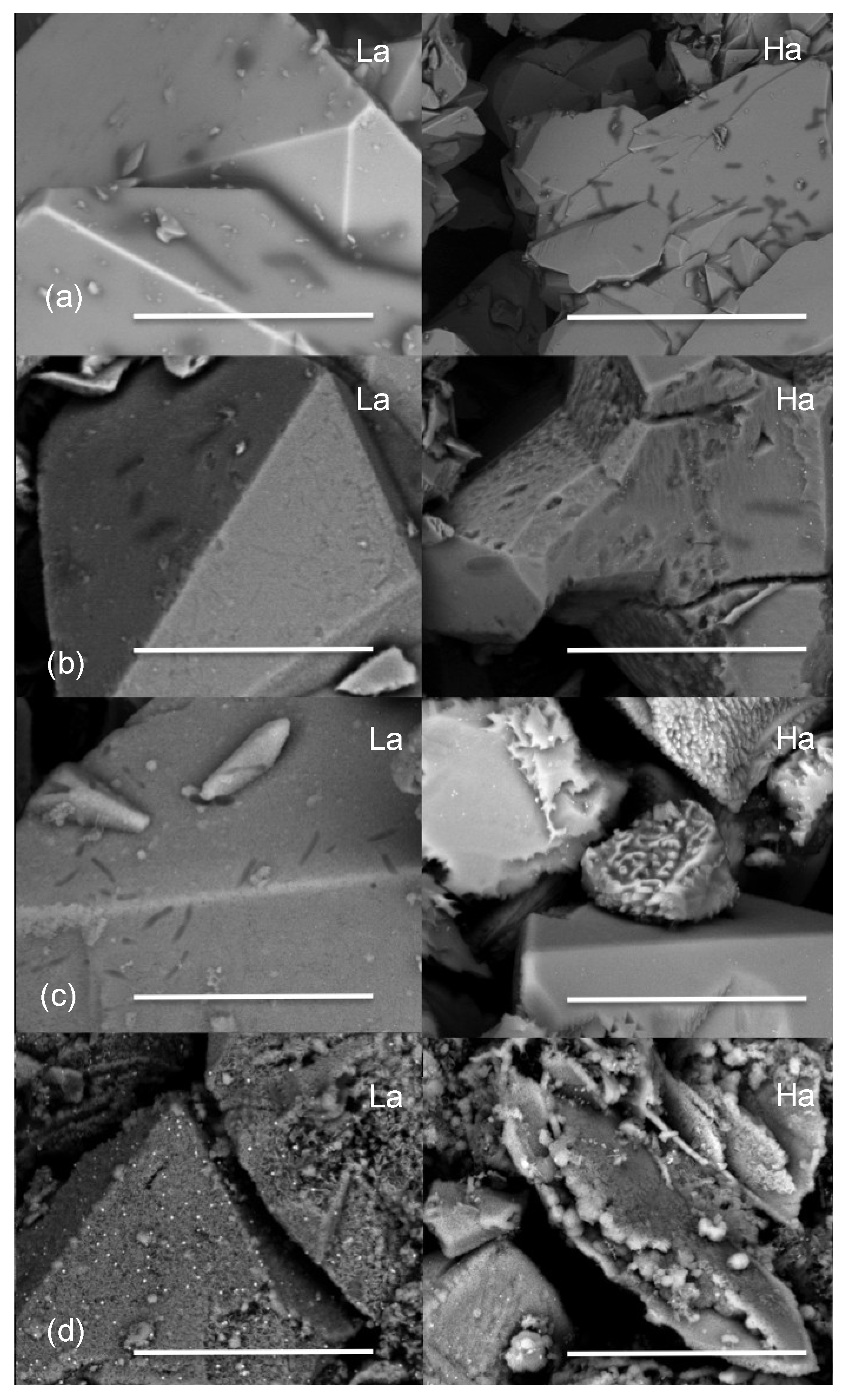
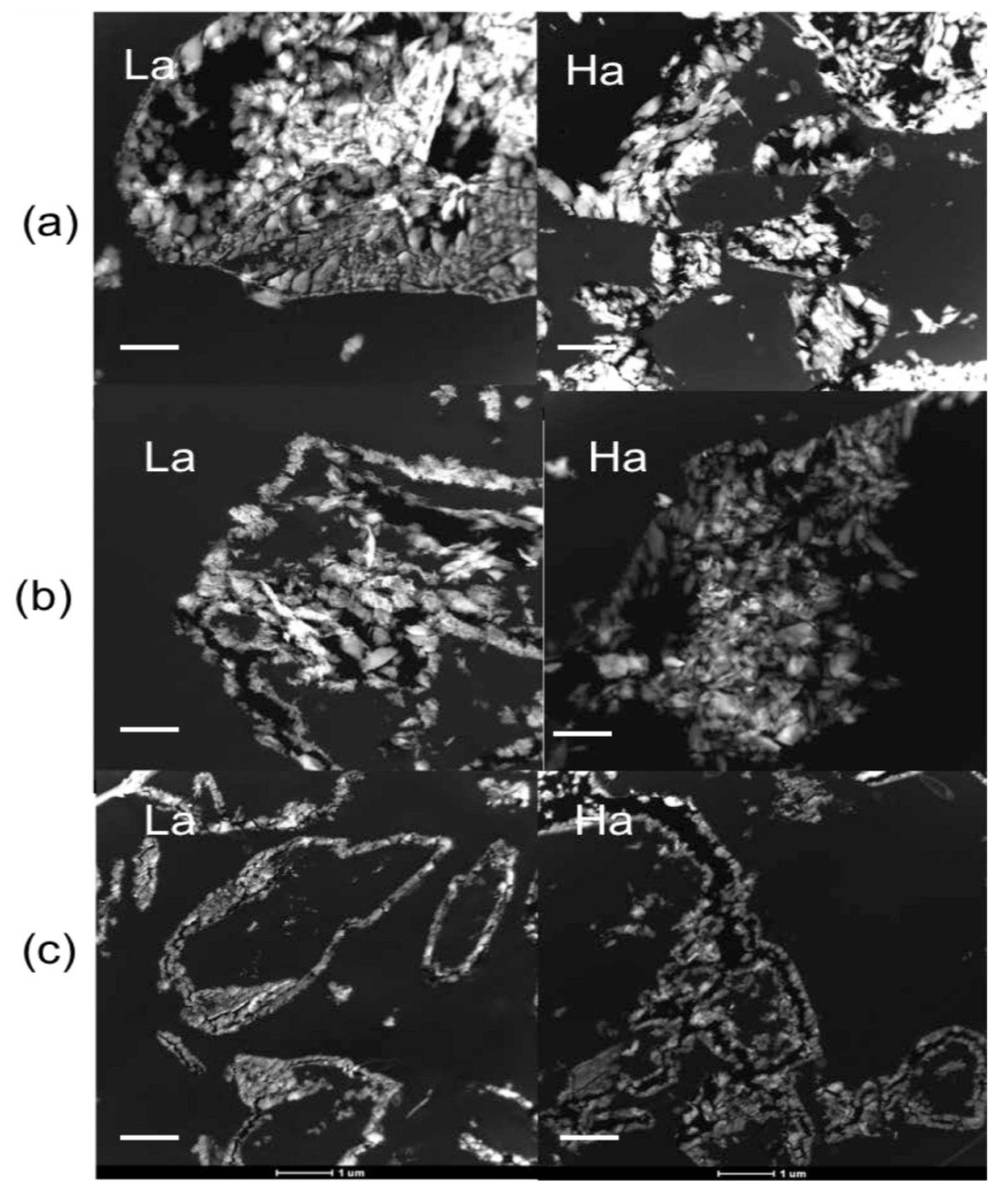
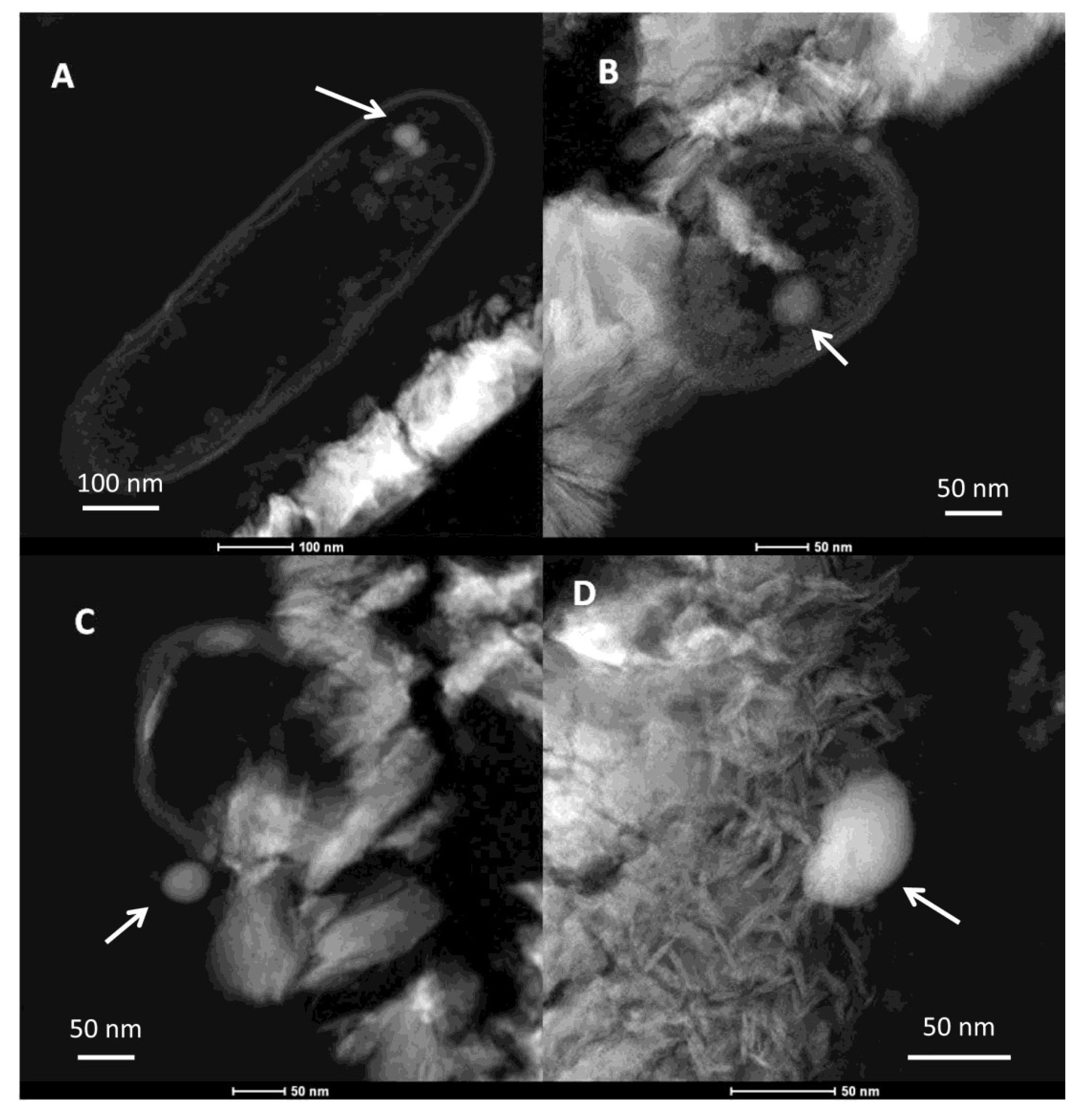

2.2.3. Selenium Speciation in the Solid Phase
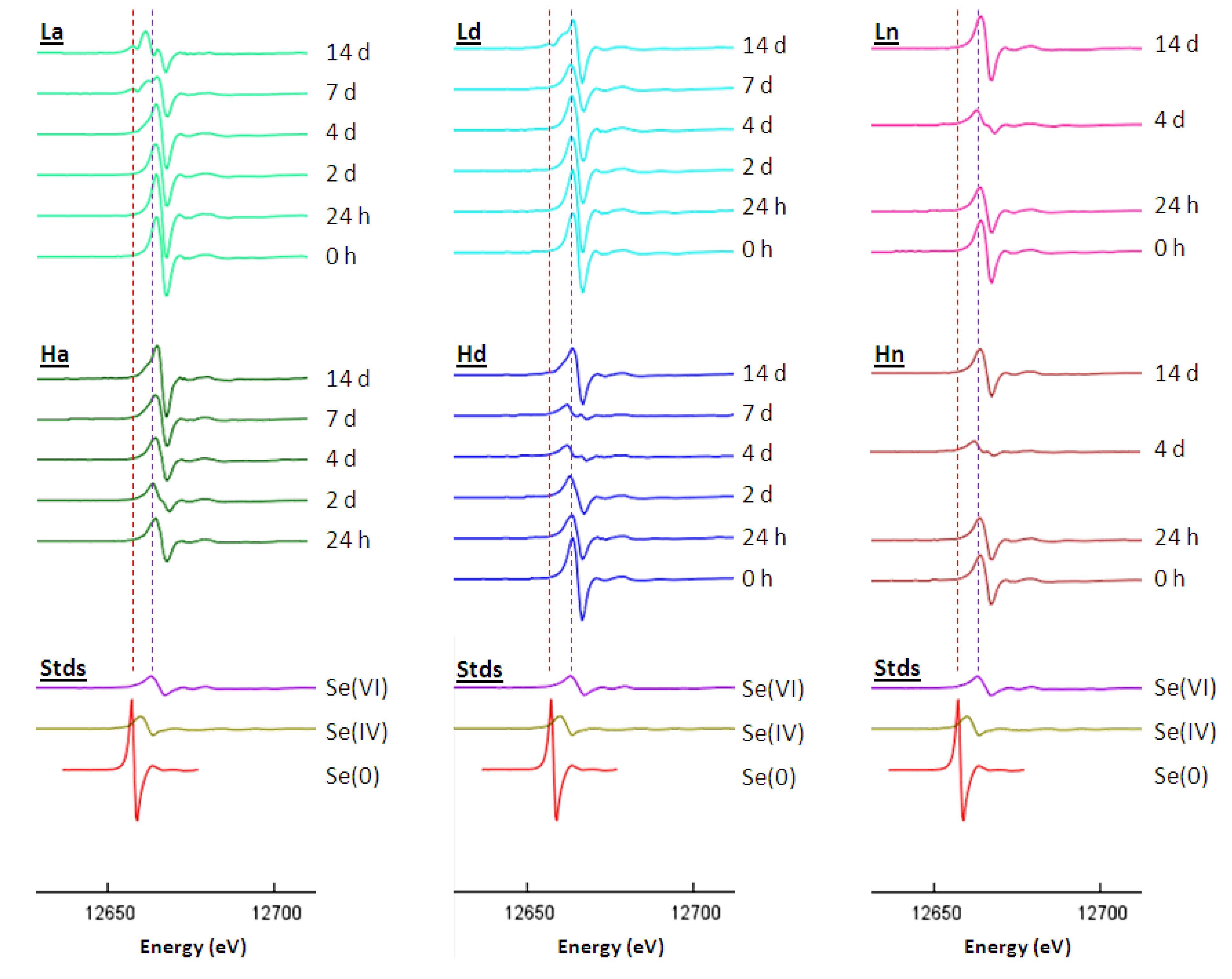
3. Experimental Section
3.1. Jarosite Synthesis
3.2. Bacterial Culture
3.3. Setup and Sampling Procedure
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Manceau, A.; Tamura, N.; Marcus, M.A.; Alastair, A.M.; Celestre, R.S.; Sublett, R.E.; Sposito, G.; Padmore, H.A. Deciphering Ni sequestration in soil ferromanganese nodules by combining X-ray fluorescence, absorption, and diffraction at micrometer scales of resolution. Am. Mineral. 2002, 87, 1494–1499. [Google Scholar]
- Strawn, D.; Doner, H.; Zavarin, M.; McHugo, S. Microscale investigation into the geochemistry of arsenic, selenium, and iron in soil developed in pyritic shale materials. Geoderma 2002, 108, 237–257. [Google Scholar] [CrossRef]
- Tamaki, S.; Frankenberger, W.T., Jr. Environmental biochemistry of arsenic. Rev. Environ. Contam. Toxicol. 1992, 124, 79–110. [Google Scholar]
- Fanning, D.S.; Fanning, M.C.B. Soil: Morphology, Genesis and Classification; John Wiley & Sons: Hoboken, NJ, USA, 1989; p. 395. [Google Scholar]
- Evangelou, V.P.; Zhang, Y.L. A review: Poxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Jambor, J.L. Nomenclature of the alunite supergroup. Can. Mineral. 1999, 37, 1323–1341. [Google Scholar]
- Smeaton, C.M.; Fryer, B.J.; Weisener, C.G. Intracellular precipitation of Pb by Shewanella putrefaciens CN32 during the reductive dissolution of Pb-jarosite. Environ. Sci. Technol. Lett. 2009, 43, 8086–8091. [Google Scholar]
- Smeaton, C.M.; Walshe, G.E.; Fryer, B.J.; Weisener, C.G. Reductive dissolution of Tl(I)-jarosite by Shewanella putrefaciens: Providing new insights into Tl biogeochemistry. Environ. Sci. Technol. Lett. 2012, 46, 11086–11094. [Google Scholar]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Walshe, G.E.; Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K.; Beale, A.M.; Fryer, B.J.; Weisener, C.G. Simultaneous release of Fe and As during the reductive dissolution of Pb-As jarosite by Shewanella putrefaciens CN32. Environ. Sci. Technol. Lett. 2012, 46, 12823–12831. [Google Scholar]
- Dutrizac, J.E.; Dinardo, O.; Kaiman, S. Selenate analogs of jarosite-type compounds. Hydrometallurgy 1981, 6, 327–337. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Pratt, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmochim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Foster, A.L.; Brown, G.E., Jr.; Tingle, T.N.; Parks, G.A. Quantitative arsenic speciation in mine tailings using X-ray absorption spectroscopy. Am. Mineral. 1998, 83, 553–568. [Google Scholar]
- Deverel, S.J.; Millard, S.P. Distribution and mobility of selenium and other trace elements in shallow groundwater of the western San Joaquin Valley, California. Environ. Sci. Technol. Lett. 1988, 22, 697–702. [Google Scholar]
- Zhang, Y.Q.; Moore, J.N.; Frankenberger, W.T., Jr. Speciation of soluble selenium in agricultural drainage waters and aqueous soil-sediment extracts using hydride generation atomic absorption spectrometry. Environ. Sci. Technol. Lett. 1999, 33, 1652–1656. [Google Scholar]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Arsenic and selenium chemistry as affected by sediment redox potential and pH. J. Environ. Qual. 1991, 20, 522–527. [Google Scholar] [CrossRef]
- Chizhikov, D.M.; Ščastlivij, V.P. Selenium and Selenides; Collet’s: Wellingborough, UK, 1968. [Google Scholar]
- Frost, D.V.; Lish, P.M. Selenium in biology. Annu. Rev. Pharmacol. Toxicol. 1975, 15, 259–284. [Google Scholar] [CrossRef]
- Flohe, L.; Günzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta 2005, 1726, 1–13. [Google Scholar] [CrossRef]
- Brown, T.A.; Shrift, A. Selenium: Toxicity and tolerance in higher plants. Biol. Rev. 1982, 57, 59–84. [Google Scholar] [CrossRef]
- Letavayová, L.; Vlcková, V.; Brozmanová, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. Lett. 2012, 46, 571–579. [Google Scholar]
- Barceloux, D.G. Selenium. J. Toxicol. Clin. Toxicol. 1999, 37, 145–172. [Google Scholar] [CrossRef]
- Hockin, S.L.; Gadd, G.M. Linked redox precipitation of sulfur and selenium under anaerobic conditions by sulfate-reducing bacterial biofilms. Appl. Environ. Microbiol. 2003, 69, 7063–7072. [Google Scholar] [CrossRef]
- Stolz, J.F.; Oremland, R.S. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 1999, 23, 615–627. [Google Scholar] [CrossRef]
- Tomei, F.A.; Barton, L.L.; Lemanski, C.L.; Zocco, T.G.; Fink, N.H.; Sillerud, L.O. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J. Ind. Microbiol. 1995, 14, 329–336. [Google Scholar] [CrossRef]
- Frankenberger, W.T., Jr.; Arshad, M. Bioremediation of selenium-contaminated sediments and water. Biofactors 2001, 14, 241–254. [Google Scholar] [CrossRef]
- Kashiwa, M.; Nishimoto, S.; Takahashi, K.; Ike, M.; Fujita, M. Factors affecting soluble selenium removal by a selenate-reducing bacterium Bacillus sp. SF-1. J. Biosci. Bioeng. 2000, 89, 528–533. [Google Scholar] [CrossRef]
- Kessi, J.; Ramuz, M.; Wehrli, E.; Spycher, M.; Bachofen, R. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 1999, 65, 4734–4740. [Google Scholar]
- Klonowska, A.; Heulin, T.; Vermeglio, A. Selenite and tellurite reduction by Shewanella oneidensis. Appl. Environ. Microbiol. 2005, 71, 5607–5609. [Google Scholar] [CrossRef]
- Macy, J.M.; Lawson, S.; Demoll-Decker, H. Bioremediation of selenium oxyanions in San Jaoquin drainage water using Thauera selenatis in a biological reactor system. Appl. Microbiol. Biotechnol. 1993, 40, 588–594. [Google Scholar]
- Oremland, R.S.; Blum, J.S.; Bindi, A.B.; Dowdle, P.R.; Herbel, M.; Stolz, J.F. Simultaneous reduction of nitrate and selenate by cell suspensions of selenium-respiring bacteria. Appl. Environ. Microbiol. 1999, 65, 4385–4392. [Google Scholar]
- Schmidt, R.; Tantoyotai, P.; Fakra, S.C.; Marcus, M.A.; Yang, S.I.; Pickering, I.J.; Bañuelos, G.S.; Hristova, K.R.; Freeman, J.L. Selenium biotransformations in an engineered aquatic ecosystem for bioremediation of agricultural wastewater via brine shrimp production. Environ. Sci. Technol. Lett. 2013, 47, 5057–5065. [Google Scholar]
- Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K. Dissolution of jarosite [KFe3(SO4)2(OH)6] at pH 2 and 8: Insights from batch experiments and computational modelling. Geochim. Cosmochim. Acta 2006, 70, 608–621. [Google Scholar] [CrossRef]
- Smith, A.M.L.; Dubbin, W.E.; Wright, K.; Hudson-Edwards, K.A. Dissolution of lead- and lead–arsenic-jarosites at pH 2 and 8 and 20 °C: Insights from batch experiments. Chem. Geol. 2006, 229, 344–361. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Kenward, P.A.; Fowle, D.A.; Yee, N. Microbial selenate sorption and reduction in nutrient limited systems. Environ. Sci. Technol. Lett. 2006, 40, 3782–3786. [Google Scholar]
- Paktunc, D.; Dutrizac, J.E. Characterization of arsenate-for-sulfate substitution in synthetic jarosite using X-ray diffraction and X-ray absorption spectroscopy. Can. Mineral. 2003, 41, 905–919. [Google Scholar] [CrossRef]
- Stolz, J.F.; Basu, P.; Santini, J.M.; Oremland, R.S. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 2006, 60, 107–130. [Google Scholar] [CrossRef]
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60. [Google Scholar] [CrossRef]
- Zehr, J.P.; Oremland, R.S. Reduction of selenate to selenide by sulfate-respiring bacteria: Experiments with cell suspensions and estuarine sediments. Appl. Environ. Microbiol. 1987, 53, 1365–1369. [Google Scholar]
- Tomei, F.A.; Barton, L.L.; Lemanski, C.L.; Zocco, T.G. Reduction of selenate and selenite to elemental selenium by Wolinella succinogenes. Can. J. Microbiol. 1992, 38, 1328–1333. [Google Scholar] [CrossRef]
- Silverberg, B.A.; Wong, P.T.S.; Chau, Y.K. Localization of selenium in bacterial cells using TEM and energy dispersive X-ray analysis. Arch. Microbiol. 1976, 107, 1–6. [Google Scholar] [CrossRef]
- Losi, M.E.; Frankenberger, W.T. Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: Isolation and growth of the bacterium and its expulsion of selenium particles. Appl. Environ. Microbiol. 1997, 63, 3079–3084. [Google Scholar]
- Dungan, R.S.; Yates, S.R.; Frankenberger, W.T., Jr. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ. Microbiol. 2003, 5, 287–295. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Bridge, T.A.M.; Johnson, D.B. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 2000, 17, 193–206. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Franzblau, R.E.; Loick, N.; Weisener, C.G. Investigating the Effects of Se Solid Phase Substitution in Jarosite Minerals Influenced by Bacterial Reductive Dissolution. Minerals 2014, 4, 17-36. https://doi.org/10.3390/min4010017
Franzblau RE, Loick N, Weisener CG. Investigating the Effects of Se Solid Phase Substitution in Jarosite Minerals Influenced by Bacterial Reductive Dissolution. Minerals. 2014; 4(1):17-36. https://doi.org/10.3390/min4010017
Chicago/Turabian StyleFranzblau, Rachel E., Nadine Loick, and Christopher G. Weisener. 2014. "Investigating the Effects of Se Solid Phase Substitution in Jarosite Minerals Influenced by Bacterial Reductive Dissolution" Minerals 4, no. 1: 17-36. https://doi.org/10.3390/min4010017
APA StyleFranzblau, R. E., Loick, N., & Weisener, C. G. (2014). Investigating the Effects of Se Solid Phase Substitution in Jarosite Minerals Influenced by Bacterial Reductive Dissolution. Minerals, 4(1), 17-36. https://doi.org/10.3390/min4010017




