Flotation Behavior and Mechanism of Andalusite and Quartz Under the Sodium Dodecyl Sulfonate System
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Test Methods
2.2.1. Zeta Potential Measurements
2.2.2. Infrared Spectroscopic Analysis
2.2.3. MS Calculation Method
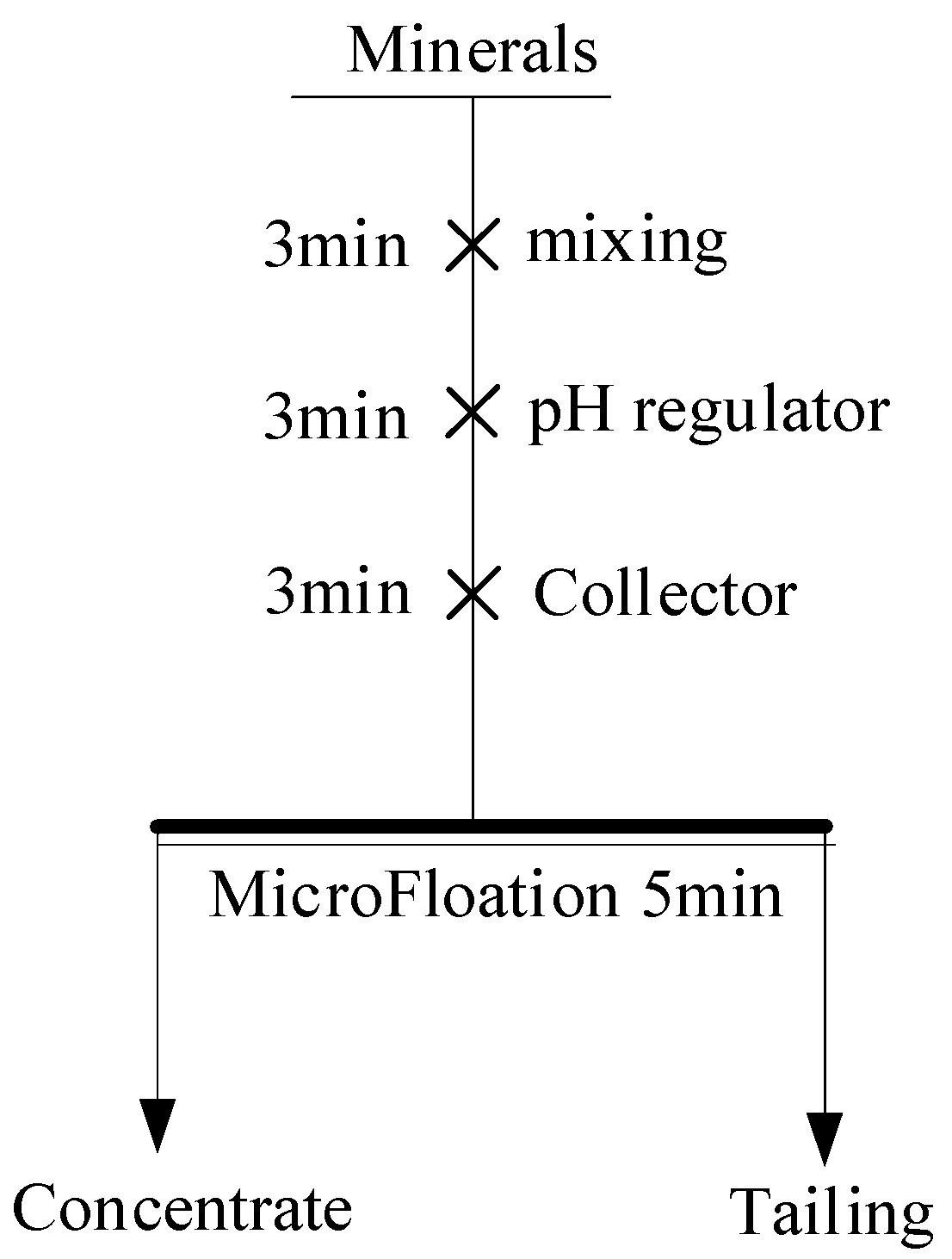
2.2.4. Single-Mineral Flotation Tests
3. Result and Discussion
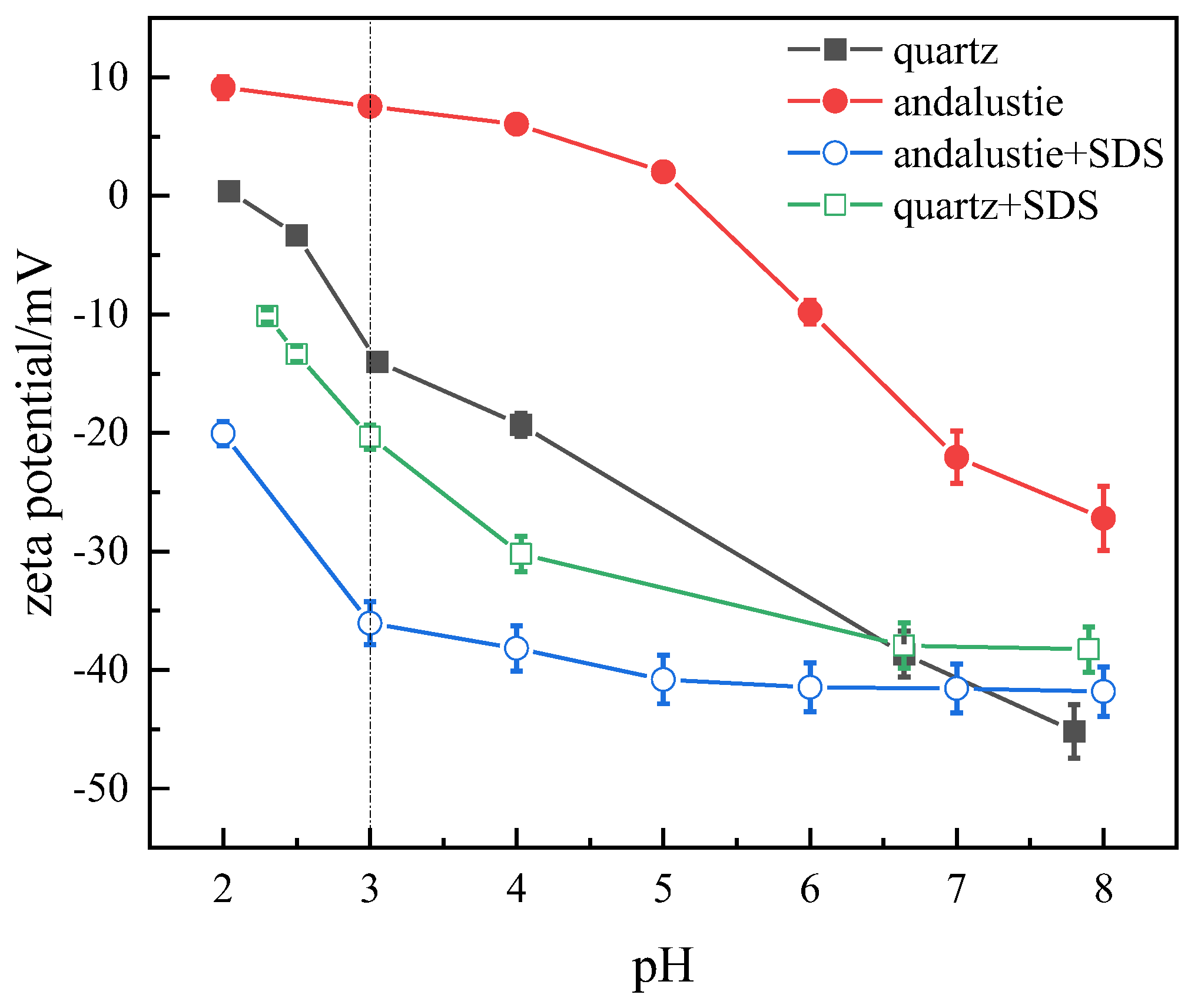
3.1. Variations in Surface Zeta Potential of Andalusite and Quartz Under SDS Treatment
3.2. Infrared Spectroscopy Analysis Under the Action of SDS
3.2.1. Infrared Spectroscopic Analysis of Interaction Between Andalusite and SDS
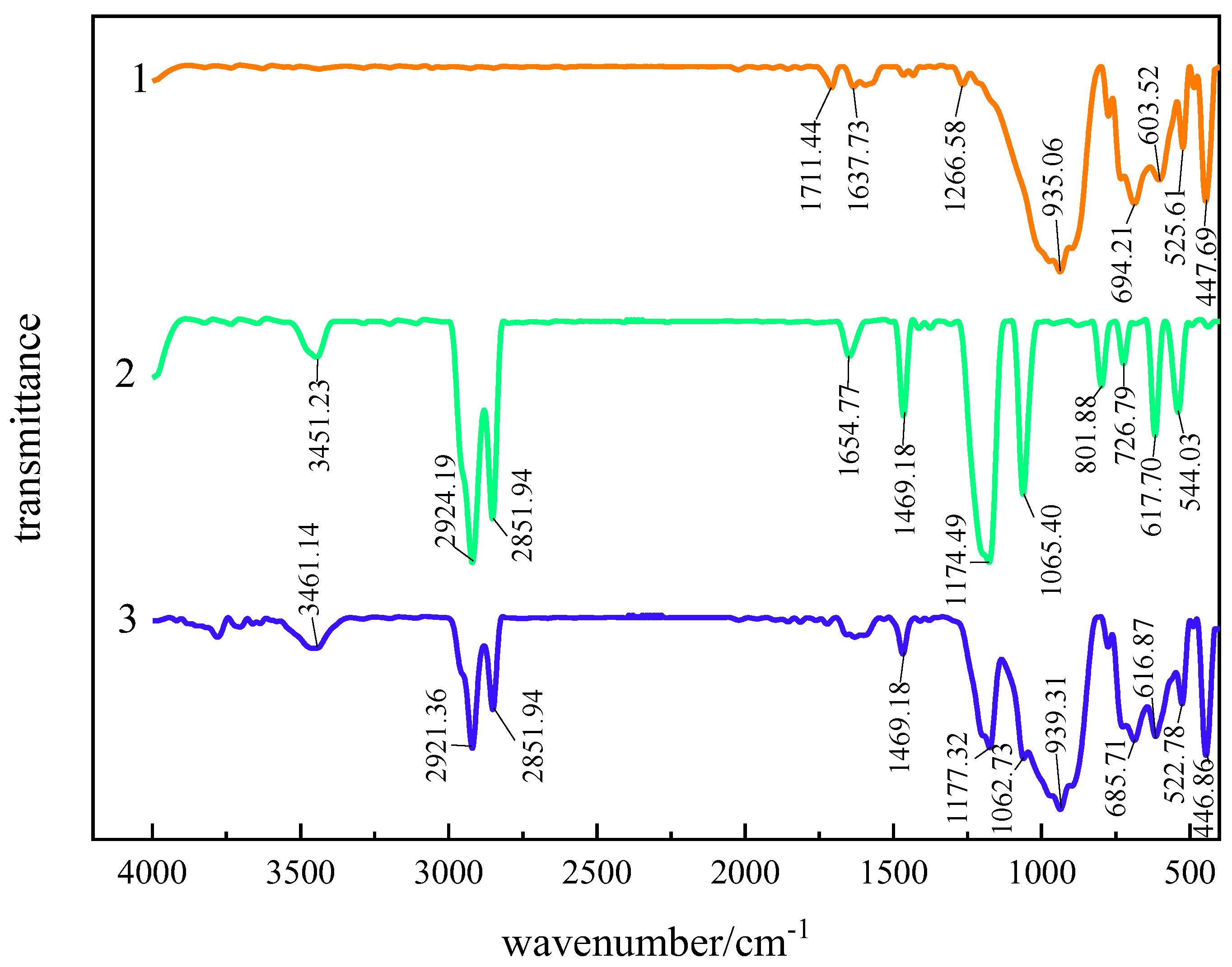
3.2.2. Infrared Spectroscopic Analysis of Interaction Between Quartz and SDS

3.3. Quantum Chemistry of SDS Adsorption on Andalusite Surface
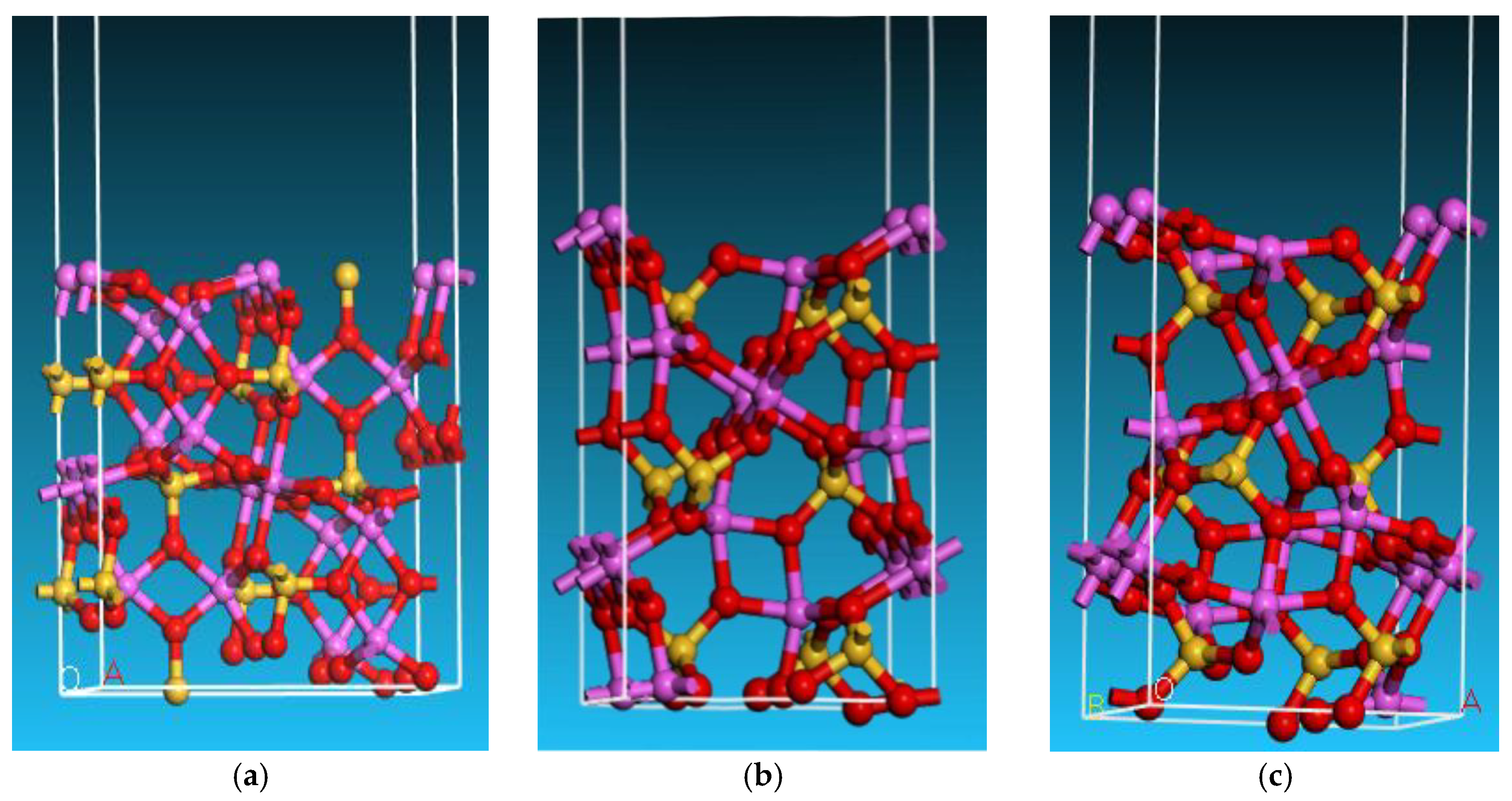
3.3.1. Selection of Andalusite Surfaces
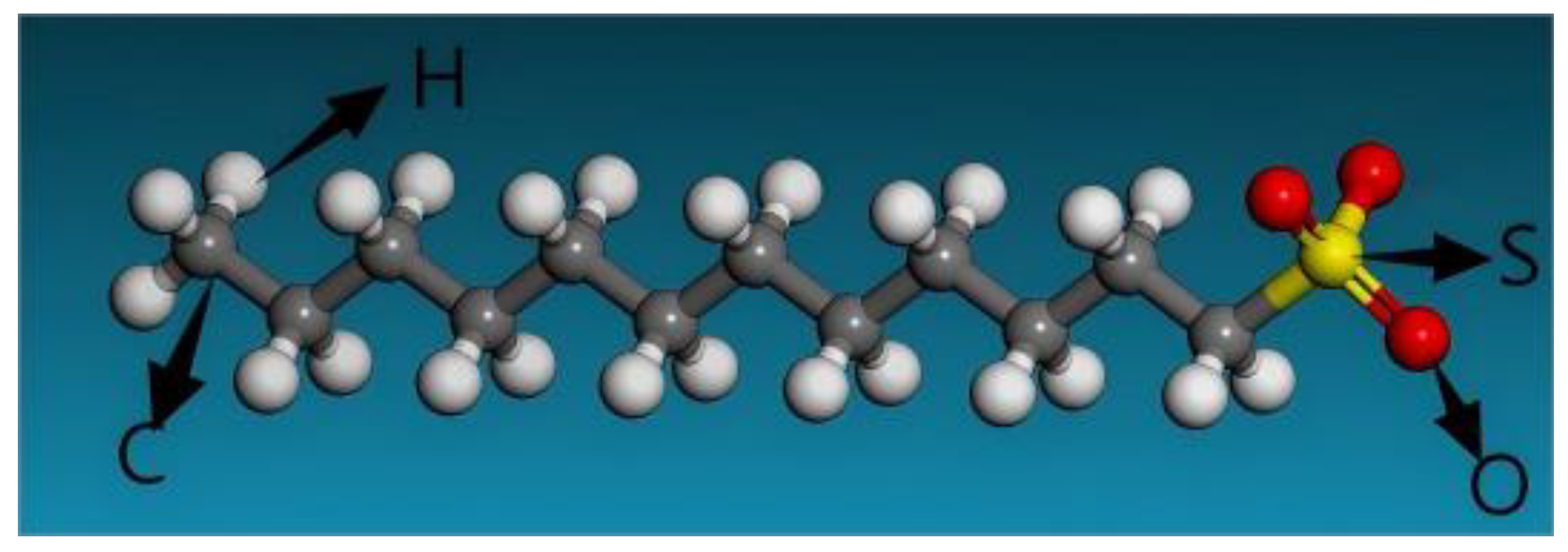
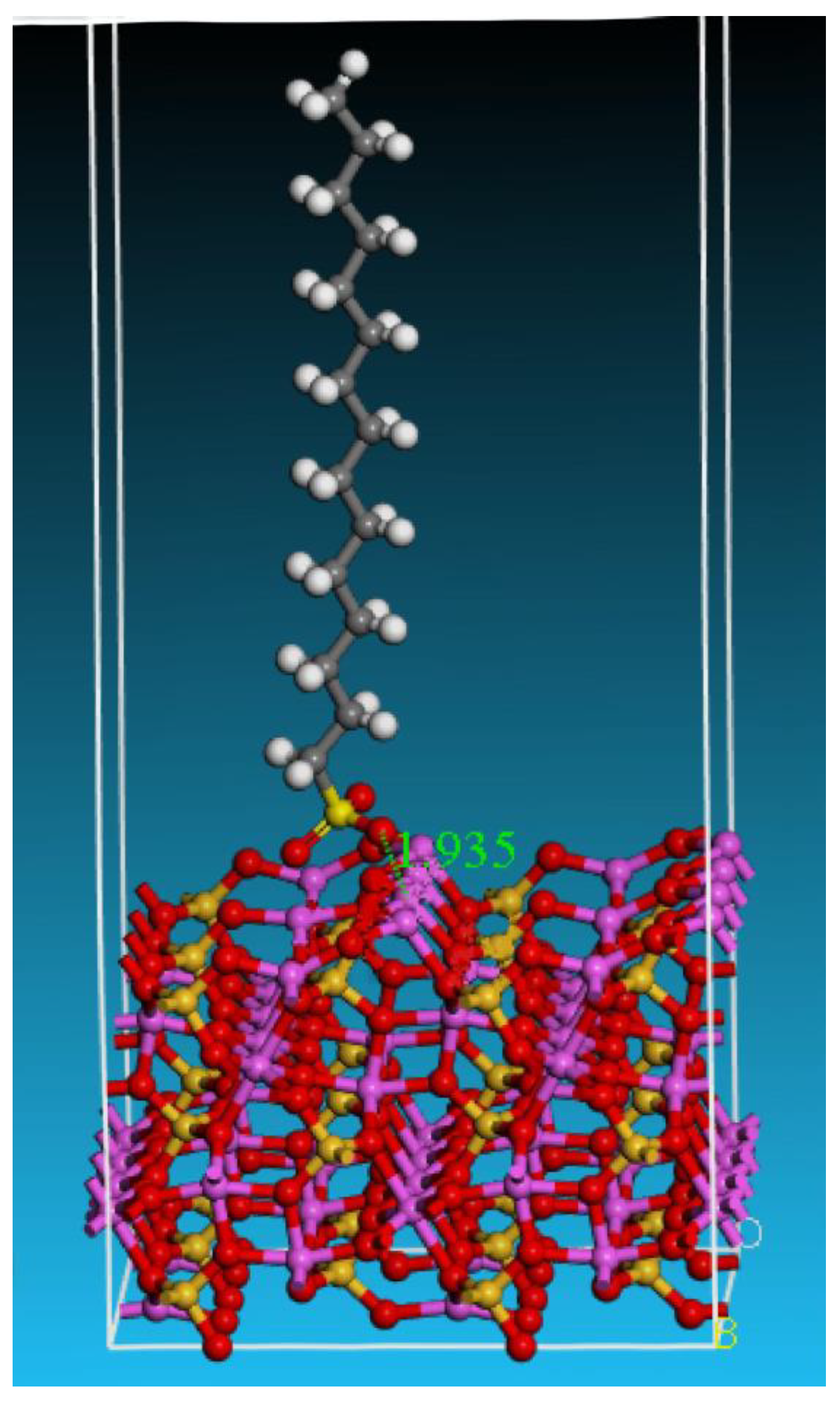
3.3.2. Adsorption of SDS on Andalusite {1 0 0} Surface
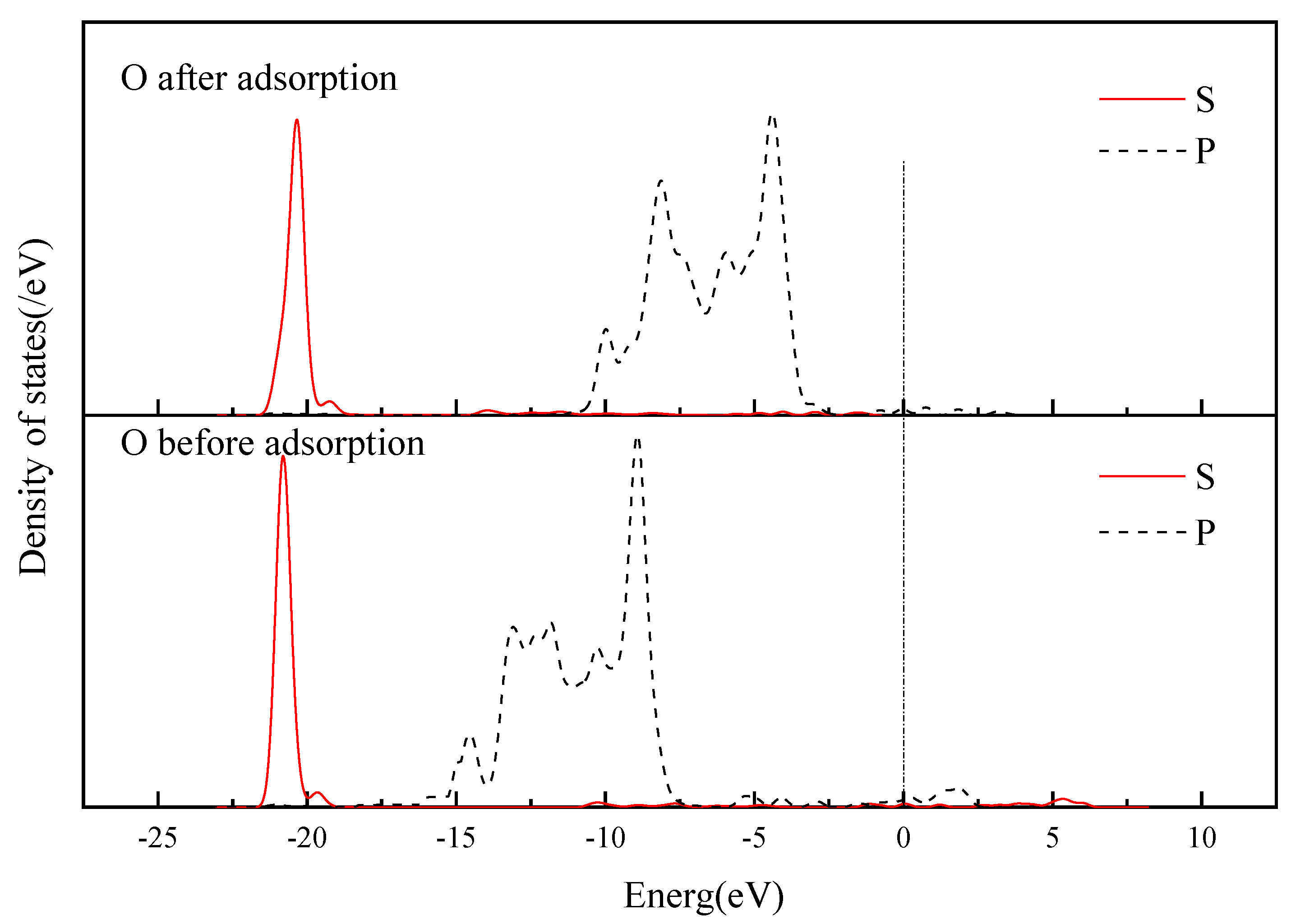
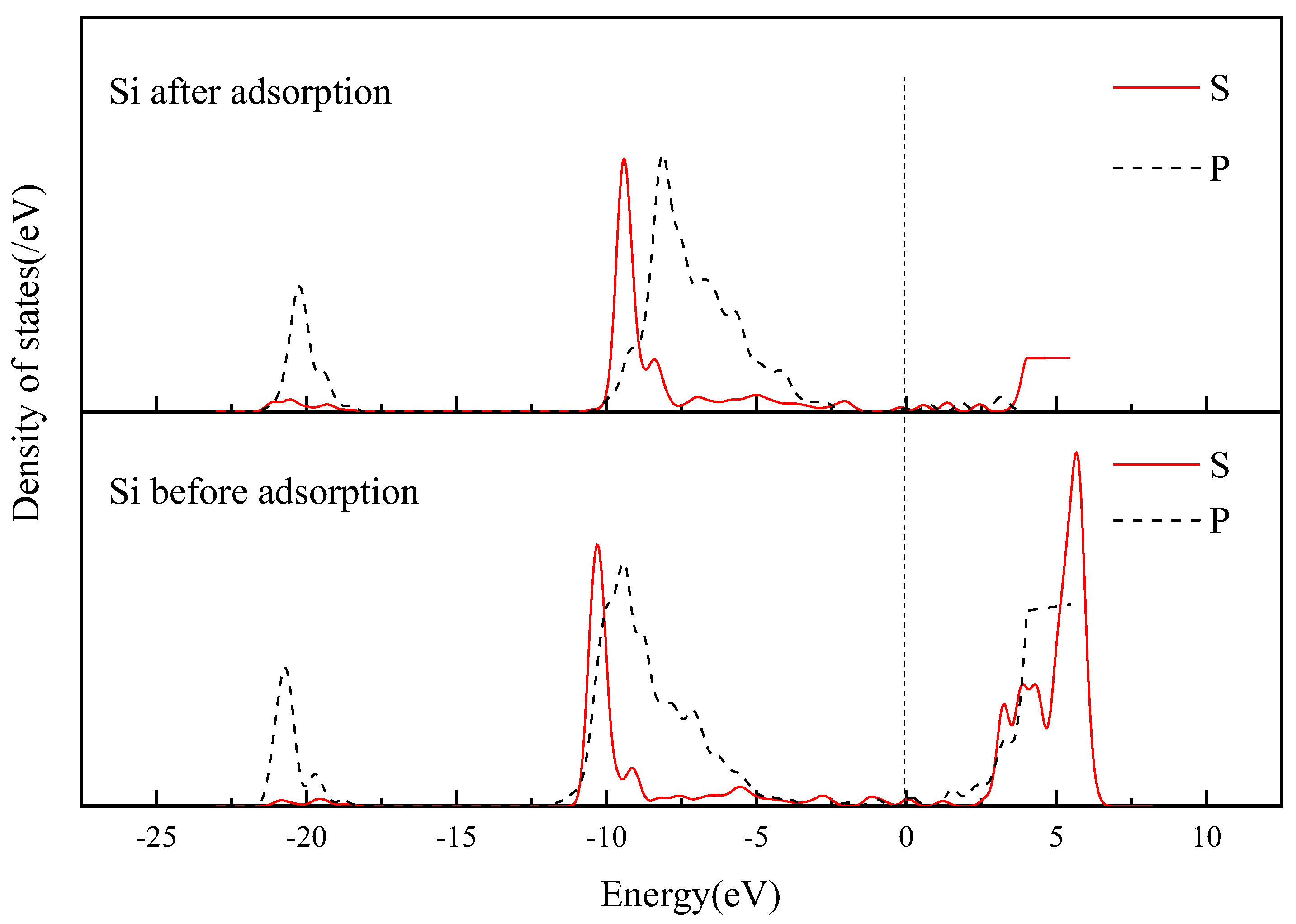
3.3.3. Mulliken Charge Analysis of Andalusite {1 0 0} Surface Adsorbed with SDS
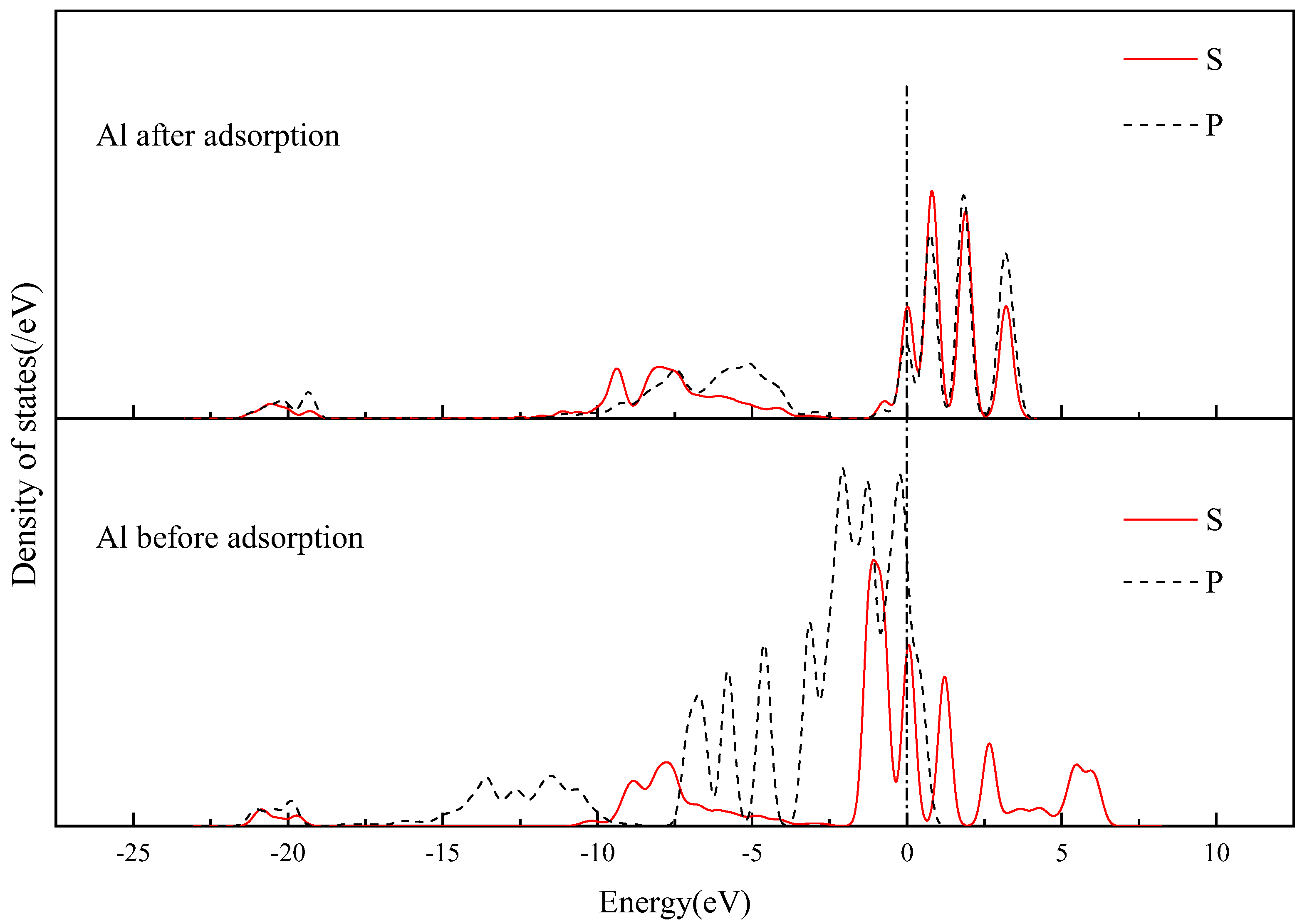
3.3.4. Electronic Structure of Andalusite {1 0 0} Surface Adsorbed by SDS
3.4. Effect of SDS on the Flotation Behavior of Andalusite and Quartz
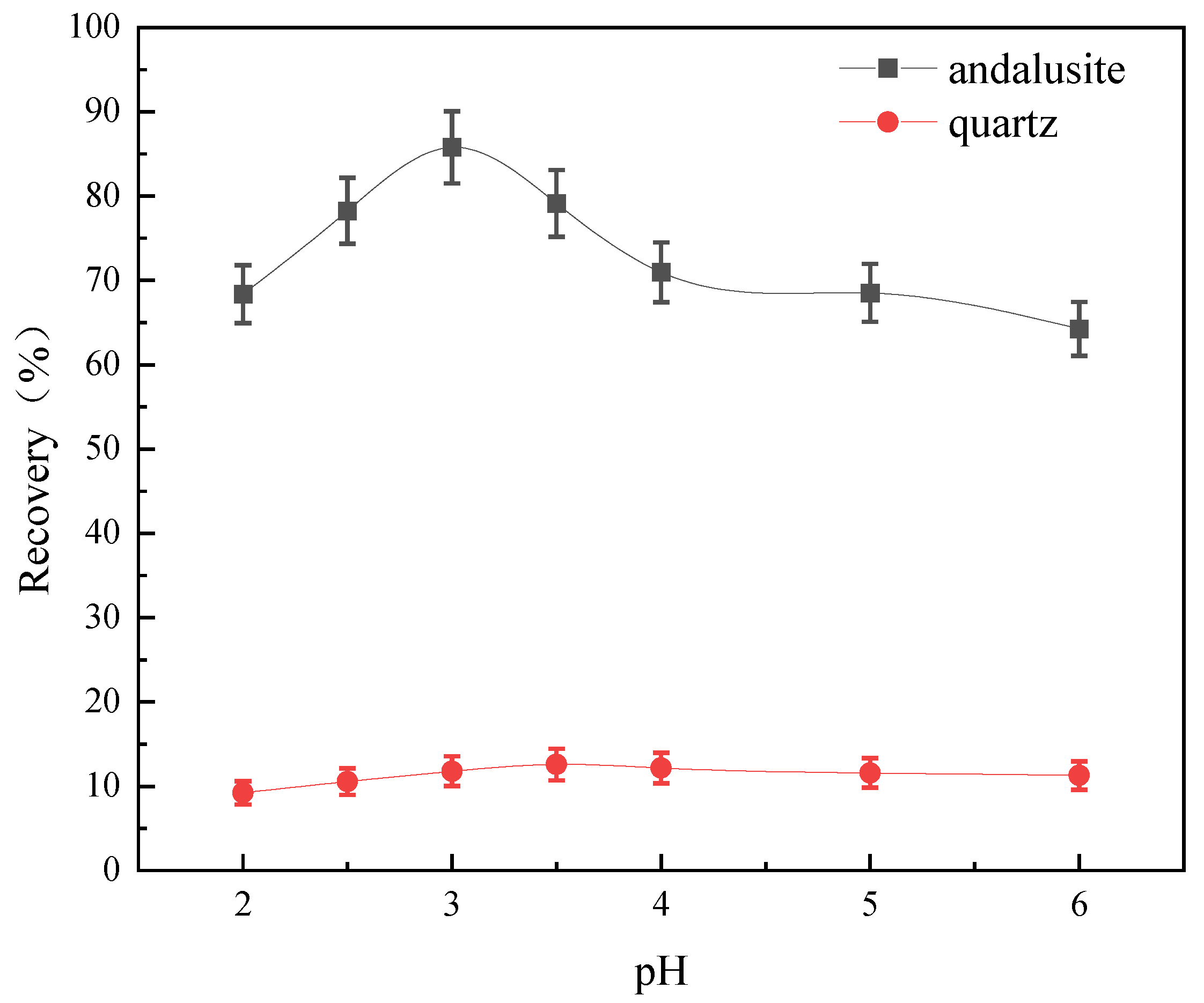
3.4.1. The Influence of Medium pH Value on the Floatability of Minerals
3.4.2. The Influence of Collector Types on the Floatability of Minerals
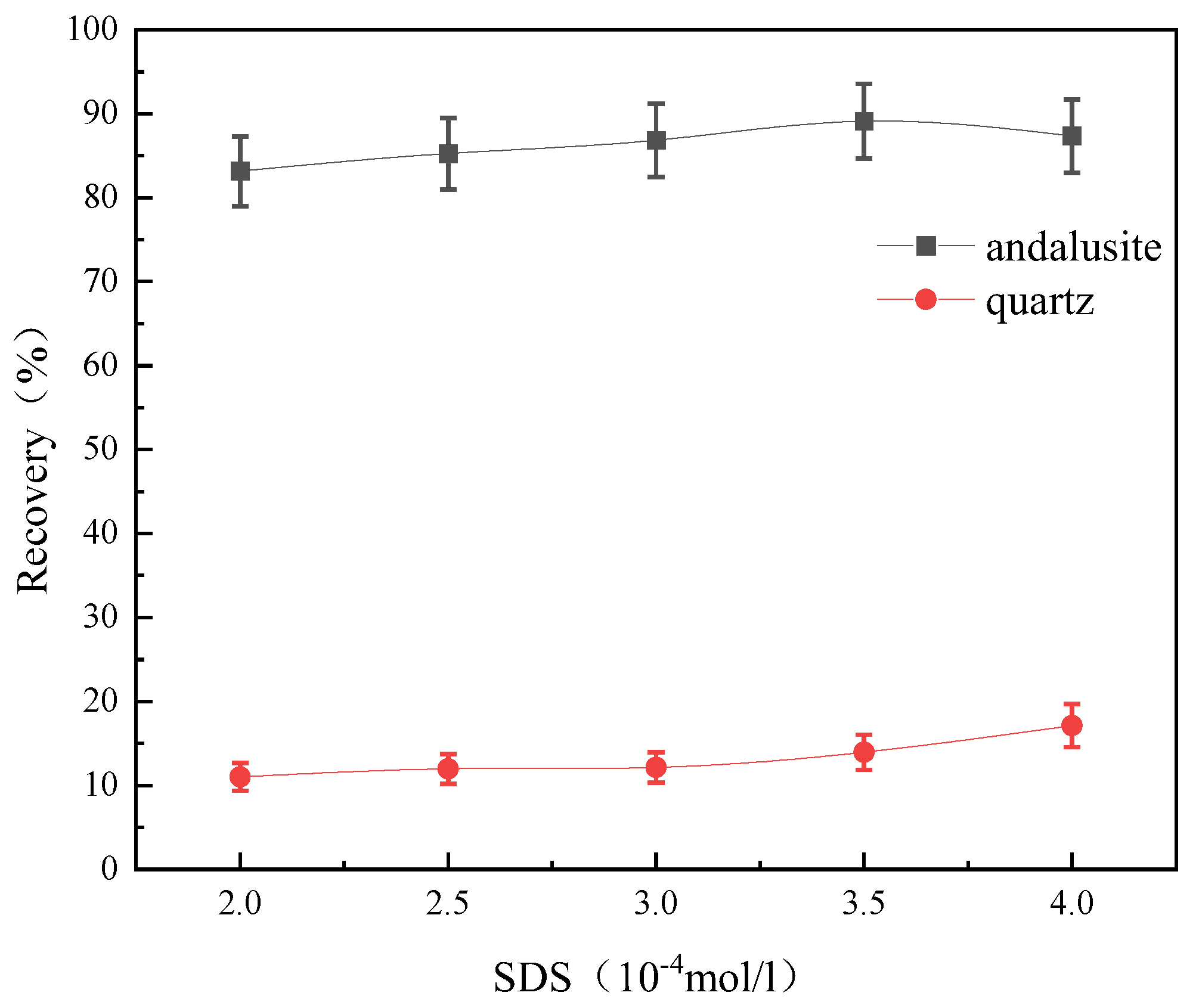
3.4.3. Effect of SDS Dosage on Mineral Floatability
4. Conclusions
- (1)
- The zeta potential test indicates that the surface potential of andalusite decreases significantly under the SDS system. Under the test pH value of 3, the absolute value of the zeta potential on the surface of andalusite increases significantly, while the absolute value of the zeta potential on the quartz surface does not change significantly. This indicates that SDS has undergone strong adsorption on the surface of andalusite. However, the adsorption effect on quartz is not obvious. The FTIR analysis results further demonstrate that SDS undergoes chemisorption on the surface of andalusite, rather than physisorption.
- (2)
- Quantum chemical research was conducted on the adsorption of SDS on the surface of andalusite. It was found that the {1 0 0} surface of andalusite had the lowest surface energy and was the most important dissociation surface. After sodium dodecyl sulfate adsorbs on the {1 0 0} surface of andalusite, the surface atoms of andalusite show a slight downward relaxation. Mulliken charge analysis and density of states analysis indicate that the aluminum atoms on the surface of andalusite lose electrons and the positive charge they carry increases significantly, while the charge numbers of oxygen and silicon atoms change little. This indicates that sodium dodecyl sulfate adsorbs on the active sites of Al atoms on the surface of andalusite.
- (3)
- Flotation tests show that when SDS is used as the collector, andalusite has better floatability in a wide pH range, while quartz has poorer floatability. When the pH of the pulp was 3 and the dosage of SDS was 3.5 × 10−4 mol/L, the recovery rate of andalusite was 89.11% and that of quartz was 13.96%, indicating that andalusite and quartz had a good flotation separation effect under the SDS system.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, G.C.; Resende, W.S.; Rodrigues, J.A.; Ribeiro, S. Thermal shock resistance of a refractory castable containing andalusite aggregate. Ceram. Int. 2016, 42, 19167–19171. [Google Scholar] [CrossRef]
- Ding, D.F.; Zhao, Z.H.; Huang, D.W.; Wu, S.H. Effect of the calcined andalusite aggregates on the micro-crack formation and thermal shock resistance of mullite castables. Ceram. Int. 2022, 48, 21556–21560. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kanagawa, K.; Hiroi, Y.; Hirajima, T.; Svojtka, M.; Hokada, T.; Wallis, S.R.; Nagaya, T.; Miyake, A. Deformation-induced, retrograde transformation of kyanite to andalusite: An example of felsic granulite in the southern Bohemian Massif. Tectonophysics 2024, 877, 230293. [Google Scholar] [CrossRef]
- Dominik, H.; Jong-Won, S.; David, T.; Almuth, S.; Peter, Q. Thermal shock behavior of andalusite-based refractories: Microstructural investigation of crack bridging and grain-matrix interactions. J. Am. Ceram. Soc. 2025, 108, e20117. [Google Scholar]
- Shirin, K.; Masoomeh, M. High temperature corrosion resistance of various aluminosilicate refractory bricks. Ceram. Int. 2024, 50, 49125–49133. [Google Scholar] [CrossRef]
- Wang, P.; Yang, B.; Fan, X.X.; Ye, H.G.; Luo, G.W. Discovery of large-scale andalusite deposit in Maluli, Zhangxian county, Gansu Province. China Geol. 2024, 51, 701–702. [Google Scholar]
- Teresa, W.; Bronislaw, P.; Jerzy, K.; Katarzyna, S.; Jacek, P. Mullitization process of andalusite concentrates—Role of natural inclusions. Ceram. Int. 2014, 40, 5129–5136. [Google Scholar]
- Niu, Y.P.; Li, Y.; Xu, H.F. Study on the Interaction of Flotation between Andalusite and Quartz. Nonferrous Met. Eng. 2020, 10, 69–74. [Google Scholar]
- Likhanov, I.I.; Santosh, M. The ‘triple point’ paradigm of aluminosilicates revisited. Geol. J. 2020, 55, 4772–4789. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xu, H.J. Polymorphism and deformation of andalusite, kyanite and sillimanite. Bull. Mineral. Petrol. Geochem. 2023, 42, 402–419. [Google Scholar]
- Ding, D.F.; Ye, G.T.; Li, N.; Liao, G.H.; Tian, X.K.; Chen, L.G. Andalusite transformation and properties of andalusite-bearing refractories fired in different atmospheres. Ceram. Int. 2019, 45, 3186–3191. [Google Scholar] [CrossRef]
- Evans, H.V.; Rossman, G.R. Intervalence Charge Transfer in Aluminum Oxide and Aluminosilicate Minerals at Elevated Temperatures. Am. Mineral. 2024, 109, 2152–2161. [Google Scholar] [CrossRef]
- Abdi, M.S.; Ebadzadeh, T. Mullitization, microstructure and physical properties of mechanically activated andalusite sintered by microwave. Ceram. Int. 2013, 39, 1451–1454. [Google Scholar] [CrossRef]
- An, Y.L.; Liu, G.; Zhao, X.Q.; Chen, J.M.; Zhou, H.D.; Hou, G.L. Preparation and Microstructure Characterization of Mullite Coatings Made of Mullitized Natural Andalusite Powders. J. Therm. Spray Technol. 2011, 20, 479–485. [Google Scholar] [CrossRef]
- An, Y.L.; Chen, J.M.; Zhou, H.D.; Liu, G.A. Microstructure and thermal cycle resistance of plasma sprayed mullite coatings made from secondary mullitized natural andalusite powder. Surf. Coat. Technol. 2010, 205, 1897–1903. [Google Scholar] [CrossRef]
- Bouchetou, M.L.; Poirier, J.; Arbelaez Morales, L.; Chotard, T.; Joubert, O.; Weissenbacher, M. Synthesis of an innovative zirconia-mullite raw material sintered from andalusite and zircon precursors and an evaluation of its corrosion and thermal shock performance. Ceram. Int. 2019, 45, 12832–12844. [Google Scholar] [CrossRef]
- Razavi, A.; Hopp, V.; Hahn, D.; Sax, A.; Quirmbach, P. Microstructural X-Ray Computed Tomography Investigation of the Defect Evolution in Refractory Castings Based on Andalusite. Ceramics 2024, 7, 1867–1879. [Google Scholar] [CrossRef]
- Qi, B.; Tessier-Doyen, N.; Absi, J. Young’s modulus evolution with temperature of glass/andalusite model materials: Experimental and numerical approach. Comput. Mater. Sci. 2012, 55, 44–53. [Google Scholar] [CrossRef]
- Poirier, J.; Bouchetou, M.L.; Prigent, P. Andalusite: An amazing refractory raw material with excellent corrosion resistance to sodium vapours. Ceram. Int. 2011, 37, 2287–2296. [Google Scholar] [CrossRef]
- Wang, Z.F.; Ma, S.L.; Ren, B.; Li, Y.J.; Xu, Y.B.; Sang, S.B.; Li, Y.W. Microstructural Evolution of Andalusite at Elevated Temperature and Its Potassium Vapor Attack Resistance Behavior. Bull. Chin. Ceram. Soc. 2023, 42, 1106–1114. [Google Scholar]
- Kucera, M. Industrial Minerals and Rocks; Elsevier Science: Amsterdam, The Netherlands, 2013; p. 455. [Google Scholar]
- Hawkin, D.W. Kyanite, andalusite and sillimanite. Min. Eng. 2024, 76, 49–51. [Google Scholar]
- Jin, J.X.; Gao, H.M.; Chen, X.M.; Peng, Y.J.; Min, F.F. The flotation of aluminosilicate polymorphic minerals with anionic and cationic collectors. Miner. Eng. 2016, 99, 123–132. [Google Scholar] [CrossRef]
- He, Y.H.; Ren, Z.J.; Zhu, H.M.; Zheng, R.J.; Li, M.Y.; Song, Y.H.; Chen, Z.J. Application of dodecyl amine/dodium petroleum sulfonate mixed collector in quartz-feldspar flotation separation. Physicochem. Probl. Miner. Process. 2024, 60, 185553. [Google Scholar] [CrossRef]
- Zhou, H.L.; Liu, Y.S.; Liang, S. Study on the Effect of the Depressants on Flotation Separation of Andalusite and Quartz. J. Shanxi Inst. Energy 2020, 33, 100–102. [Google Scholar]
- Wang, Y.L.; Li, J.; Zhang, W.H.; Li, P.W.; Guo, J.; Yao, K. Citric acid inhibits the floatability of quartz in the Mg2+ system. Physicochem. Probl. Miner. Process. 2021, 57, 1–11. [Google Scholar]
- Zhou, L.C.; Zhang, Y.M. Flotation separation of Xixia andalusite ore. Trans. Nonferrous Met. Soc. China 2011, 21, 1388–1392. [Google Scholar] [CrossRef]
- Gao, G.; Sun, H.; Wang, Y.L.; Sun, Y.S.; Han, H.L.; Yuan, Z.G. Study on the separation effects of the novel collector ODD on magnesite and quartz. Physicochem. Probl. Miner. Process. 2024, 60, 1–12. [Google Scholar] [CrossRef]
- Yin, W.Z.; Sun, C.Y. Review on Research Status on Flotation Principles of Silicate Minerals. Conserv. Util. Miner. Resour. 2001, 109–130. [Google Scholar] [CrossRef]
- Zhou, H.L.; Liu, Y.S. Adsorption Mechanism of Sodium Oleate on Andalusite. Multipurp. Util. Miner. Resources 2020, 41, 198–202. [Google Scholar]
- Hong, X.; Luo, X.M.; Wen, S.; Jia, L.F.; Jiang, W.Q.; Song, Z.G.; Wang, Y.F. Flotation separation of hematite from quartz with dodecyl trimethyl ammonium chloride and sodium dodecyl sulfonate collector. J. Environ. Chem. Eng. 2024, 12, 113481. [Google Scholar] [CrossRef]
- Luo, X.M.; Jia, L.F.; Yang, S.L.; Guo, X.; Liu, X.Y.; Wang, Y.F. Enhanced efficacy of sodium dodecyl sulfonate in the reverse flotation of hematite with dodecylamine as the collector. Powder Technol. 2025, 450, 120459. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Xu, H.; Tang, X.K.; Zhou, H.P.; Xie, T.; Shen, L.; Guo, L.; Luo, X. Application of a novel mixed anionic/cationic collector in the selective flotation separation of lepidolite and quartz. Colloids Surf. Physicochem. Eng. Aspects 2024, 701, 134919. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, Y. A first-principle study of the effect of vacancy defects and impurity on adsorption of O2 on sphalerite surface. Colloids Surf. Physicochem. Eng. Aspects 2011, 363, 56–63. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, Y.; Li, Y.Q. Effect of vacancy defects on electronic properties and activation of sphalertite(110) surface by first-principles. Trans. Nonferrous Met. Soc. China 2010, 20, 502–506. [Google Scholar] [CrossRef]
- Chen, J.H.; Long, X.H.; Zhao, C.H.; Kang, D.; Guo, J. DFT calculation on relaxation and electronic structure of sulfide minerals surface in presence of H2O molecule. J. Cent. South Univ. 2014, 21, 3945–3954. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.H. The first-principle study of the effect of lattice impurity on adsorption of CN on sphalerite surface. Miner. Eng. 2010, 23, 676. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhong, J.L.; Li, Y.Q.; Chen, Y.; Guo, J. Electronic structures and floatability of pyrite, marcasite and pyrrhotite. Chin. J. Nonferrous Met. 2011, 21, 1719–1726. [Google Scholar]
- Xv, H.F.; Nin, Y.P. Flotation Separation Mechanism of Kyanite Group Minerals and Quartz: A Computational Chemistry Simulation. Min. Metall. Eng. 2024, 44, 72–76. [Google Scholar]
- Zhou, L.C.; Zhang, Y.M. Quantum chemical study on the Interaction mechanism of several commonly used collectors with andalusite. J. Wuhan Univ. (Nat. Sci. Ed.) 2010, 33, 632–636. [Google Scholar] [CrossRef]
- He, X.M.; Deng, H.B.; Zhu, H.L.; Wu, C.H. Progress on Research of Mineral Processing Technology and Separation Theory of Andalusite in China. Morden Min. 2010, 26, 4–7. [Google Scholar] [CrossRef]
- Chen, Z.J.; Gao, H.M.; Li, Y.B.; Jin, J.X.; Ren, Z.J.; Wang, W. Evaluation of sodium petroleum sulfonates with different molecular weights for flotation of kyanite ore. Physicochem. Probl. Miner. Process. 2017, 53, 956–968. [Google Scholar]
- Molaei, N.; Razavi, H.; Chelgani, S.C. Designing different beneficiation techniques by Taguchi method for upgrading Mehdi-Abad white barite ore. Mineral Processing and Extractive Metallurgy Review. Miner. Process. Extr. Metall. Rev. 2018, 39, 198–201. [Google Scholar] [CrossRef]
- Hu, Y.W.; Wang, J.L.; Qin, Q.Z.; Cao, Z.; Lu, W.D.; Shi, J.Y.; Wu, X.; Wang, P.; Sun, Y.S. Enhancing the flotation of columbite via the synergistic effect of mixed collectors. Miner. Eng. 2025, 225, 109235. [Google Scholar] [CrossRef]
- Ao, Y.X.; Han, C.; Kong, L.H.; Shen, Y.B.; Zhao, S.; Liu, W.G.; Zhou, S.J. Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine. Molecules 2024, 29, 2256. [Google Scholar] [CrossRef]
- Lu, J.; Ren, Z.J.; Gao, H.M.; Yi, Z.C.; Chen, Z.J.; Huang, Y.; Wen, J.Y. Experimental Research on Preconcentration of an Andalusite Ore in Xinjiang. Non-Met. Mines 2016, 39, 69–72. [Google Scholar]
- Fan, X.Y.; Xiao, T.T.; Zhou, C.Y.; Wang, H.R.; Pan, Z.Q.; Wu, H.J.; Zhou, H. Mixed surfactants with solubilization behaviors: Separation of feldspar and quartz by self-assembly flotation. Miner. Eng. 2025, 221, 109130. [Google Scholar] [CrossRef]
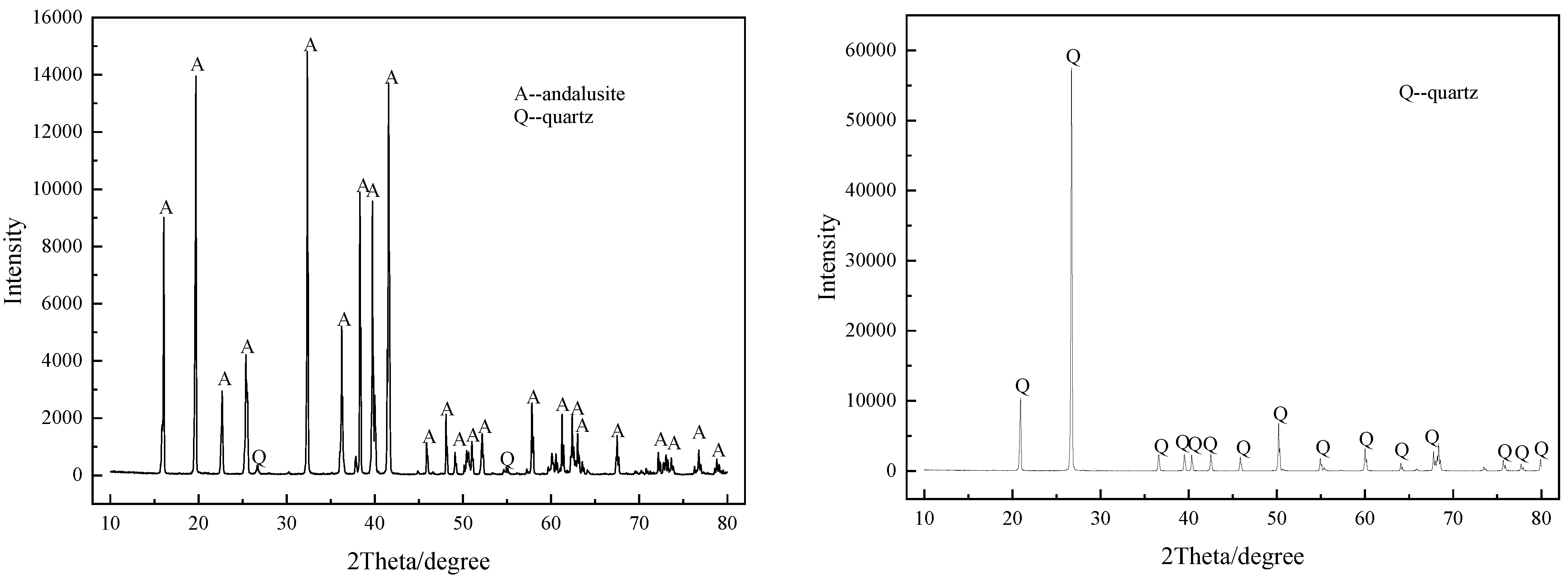

| Mineral | SiO2 | Al2O3 | TFe | MgO | TiO2 | K2O | Ignition Loss |
|---|---|---|---|---|---|---|---|
| Andalusite | 37.68 | 61.25 | 0.25 | 0.083 | 0.59 | 0.17 | 1.03 |
| Quartz | 97.69 | 0.17 | 0.06 | 0.50 | - | 0.26 | - |
| Andalusite surface | {1 1 0} surface | {1 0 0} surface | {0 0 1} surface |
| Surface energy | −3962.74 | −4234.90 | −4172.40 |
| Atomic Number | Atomic Displacement/nm | ||
|---|---|---|---|
| ΔX | ΔY | ΔZ | |
| 1(Al) | −0.0019 | 0.0039 | −0.0042 |
| 2(Al) | 0.0042 | −0.0018 | −0.0059 |
| 3(Al) | −0.0043 | −0.0071 | −0.0063 |
| 4(O) | −0.0010 | 0.0011 | −0.0008 |
| 5(O) | −0.0060 | 0.0011 | −0.0023 |
| 6(O) | −0.0231 | −0.0058 | −0.0331 |
| 7(Al) | −0.0019 | 0.0013 | −0.0023 |
| 8(Al) | 0.0025 | 0.0031 | 0.0221 |
| 9(O) | 0.0011 | 0.0011 | −0.0042 |
| 10(O) | −0.0060 | 0.0019 | 0.0064 |
| 11(Si) | 0.0004 | −0.0029 | 0.0009 |
| 12(Si) | −0.0036 | −0.0004 | 0.0026 |
| 13(Si) | 0.0006 | 0.0006 | −0.0022 |
| Atomic Number | 1(Al) | 2(Al) | 3(Al) | 4(O) | 5(O) | 6(O) | 7(Al) |
|---|---|---|---|---|---|---|---|
| Net charge before adsorption (e) | 0.688 | 0.688 | 0.688 | −0.876 | −0.936 | −0.876 | 1.371 |
| Net charge after adsorption (e) | 0.65 | 1.024 | 0.769 | −0.876 | −0.956 | −0.886 | 1.371 |
| Atomic number | 8(Al) | 9(O) | 10(O) | 11(Si) | 12(Si) | 13(Si) | |
| Net charge before adsorption (e) | 1.371 | −0.95 | −0.95 | 1.915 | 1.857 | 1.857 | |
| Net charge after adsorption (e) | 1.432 | −0.949 | −0.95 | 1.918 | 1.855 | 1.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Zhao, G.; Qiu, T.; Deng, C.; Yang, W.; Zhou, X. Flotation Behavior and Mechanism of Andalusite and Quartz Under the Sodium Dodecyl Sulfonate System. Minerals 2025, 15, 959. https://doi.org/10.3390/min15090959
Lin L, Zhao G, Qiu T, Deng C, Yang W, Zhou X. Flotation Behavior and Mechanism of Andalusite and Quartz Under the Sodium Dodecyl Sulfonate System. Minerals. 2025; 15(9):959. https://doi.org/10.3390/min15090959
Chicago/Turabian StyleLin, Liqiang, Guanfei Zhao, Tingsheng Qiu, Chong Deng, Wenhui Yang, and Xiaowen Zhou. 2025. "Flotation Behavior and Mechanism of Andalusite and Quartz Under the Sodium Dodecyl Sulfonate System" Minerals 15, no. 9: 959. https://doi.org/10.3390/min15090959
APA StyleLin, L., Zhao, G., Qiu, T., Deng, C., Yang, W., & Zhou, X. (2025). Flotation Behavior and Mechanism of Andalusite and Quartz Under the Sodium Dodecyl Sulfonate System. Minerals, 15(9), 959. https://doi.org/10.3390/min15090959






