Abstract
The sulfur dioxide removal in thermoelectric plants occurs through flue gas desulfurization (FGD), which produces waste that needs to be correctly disposed of. This exploratory research aims to characterize waste obtained from an FGD plant in Candiota, Rio Grande do Sul, Brazil, and evaluate its potential as alternative mineral source in obtaining alkali-activated materials (AAM). The dried and processed waste was called FGD-D, and AAM was produced by mixing FGD-D with sodium-based alkaline activating solutions. The amounts of FGD at formulations ranged from 31.6 (F1) to 38.9 wt.% (F4), and the use of metakaolin was not necessary. The results show that the chemical composition of FGD-D is composed mainly of calcium oxides (38 wt.%), sulfur (22 wt.%), and silica (19 wt.%). Crystalline phases and a high amorphous fraction were identified in the residual samples. The use of FGD-D in AAM proved to be an alternative mineral source, showing an exothermic reaction with subsequent rapid hardening and increased compressive strength values ranged from 7.7 ± 1.3 Mpa for F1 to 14.4 ± 1.8 Mpa for F4 at seven days. The results demonstrate the potential of using FGD-D in AAM formulations, opening positive perspectives for a more sustainable destination for these residual materials.
1. Introduction
Fossil fuels such as coal, oil, and natural gas account for about 86% of primary energy generation worldwide [1]. However, coal is the principal source, accounting for 38% of global electricity consumption in 2018 [2]. China, a significant consumer, accounted for 57.64% of total global coal consumption in 2019, primarily to fuel its industrial production [3].
The global economy’s reliance on coal burning is well-known. Still, concerns about boiler emissions have spurred the frequent application of techniques to contain particulate matter (PM), sulfur dioxide (SO2), and nitrogen oxides (NOx). In recent years, extensive research has been dedicated to removing these conventional pollutants from coal combustion, leading to significant advancements in control technologies.
Regarding sulfur dioxide, its removal occurs through the flue gas desulfurization (FGD) process, which reduces 99% of SO2 emissions into the atmosphere [4]. Two FGD processes are most commonly used worldwide: wet and semi-dry [3]. In both FGD processes, the flue gas SO2 (and partially CO2) reacts with aqueous calcium and magnesium inputs, producing the solid byproduct of this stage and evaporating water [5]. The difference between wet and dry flue gas desulfurization (FGD) methods lies in how flue gas is treated. In wet FGD, limestone, air, and water are utilized. In contrast, semi-dry FGD uses lime and water, but in smaller quantities [6].
Thus, the by-products of the two techniques are different: in the wet FGD process, gypsum and CaSO3 are obtained, while in the semi-dry FGD process, solid waste is generated with varying levels of sulfites, sulfates, carbonates, calcium and magnesium hydroxides, and fly ash.
The wet FGD byproduct has been successfully explored in concrete and construction materials [7], including lightweight and porous concrete [8]. More recently, scientific research has explored commercial applications of the semi-dry FGD byproduct, driven by its distinct compositional characteristics.
Desulfurization through the semi-dry FGD process occurs at the expense of the production of daily tons of solid by-products, generally disposed of in landfills, which implies operating costs, territorial areas, and environmental concerns. In this context, new technological routes and investments have been proposed to make the energy generation chain through coal-fired thermoelectric plants increasingly sustainable, seeking to value the by-products generated by the desulfurization process of gases from coal combustion.
Aiming to add value combined with sustainable development and technological innovation, there is potential for using FGD byproducts to obtain geopolymers and alkali-activated materials. These materials could be used to manufacture products for civil construction and infrastructure. Using byproducts in AAM reduces the costs of the thermoelectric plant related to the transportation and storage of the FGD byproduct in landfills.
Geopolymers and alkali-activated materials, known as third-generation cement, significantly impact the construction field. Due to their lower CO2 emissions and adequate or superior mechanical properties, they have been proposed as potential substitutes for Portland cement [9]. Recently, their properties as catalyst supports and adsorption agents have been explored in environmental applications such as energy generation and pollutant removal [10,11]. Their excellent durability contributes to developing geopolymer structures and expanding their application in areas such as executing rapid repairs. One of the most impressive features of geopolymers is their rapid curing time, reaching 70% of their final strength within the first 3–4 h of curing, as reported by Khale and Chaudhary [9].
The potential to use various wastes in the composition of these materials is a notable factor and has been studied by several authors. Radioactive Cs and Sr waste generated by uranium fission were successfully encapsulated, respecting adequate molar concentrations in a geopolymeric matrix [12,13].
Environmental progress in the development of geopolymers and AAM is related to the valorization of the FGD byproduct and the principles of sustainable development. Davidovits [13], one of the most renowned researchers in these materials, states that for the manufacture of Portland cement, CO2 emissions are 1000 kg CO2/ton of cement. In the manufacture of geopolymers, this value is reduced by approximately 80%, with emissions of around 200 kg CO2/ton of geopolymer [13].
Waste is valorized when it can be integrated into a production cycle, when it has the potential for use and for improving the functionality of a product, and when there is an innovative design. According to the properties of the FGD byproduct, it has the potential to be integrated into the composition of geopolymers and AAM due to the chemical similarities between them. Using FGD byproducts in the composition of these materials will reduce the areas of landfills required for waste disposal.
Oliveira et al. (2022) discuss in their review article that the durability of AAM or geopolymers made with industrial by-products, such as fly ash, blast furnace slag, and FGD waste, yielded promising results [14]. These materials demonstrate significant potential in reducing CO2 emissions, facilitating the recycling and reuse of waste, and promoting sustainable development in civil construction [14].
Oliveira et al. (2023) analyzed the production potential of a geopolymer paste made from metakaolin (MK) and Flue Gas Desulfurization (FGD) waste [15]. The compressive strength results indicated that the optimal compositions were 100% MK (0% FGD) and 95% MK (5% FGD), both cured thermally at 65 °C. The compressive strength values recorded were 47.65 Mpa and 28.44 Mpa, surpassing the requirements of the main structural standards [15]. Hanjitsuwan et al. (2020) investigated the drying shrinkage, strength, and microstructure of an alkali-activated high-calcium fly ash (AAHF) paste that utilized FGD and dolomite (DLM) as expansive additives [16]. They replaced fly ash (HF) with FGD and DLM at varying dosages of 0%, 2.5%, 5.0%, 7.5%, and 10% by weight of the binder. The results showed that the setting time of the AAHF paste decreased with the addition of FGD and DLM, while the strength increased. However, the strength development of AAHF paste incorporating FGD tended to decline after 120 days of curing [16]. In the study conducted by Zhang et al. (2023), the efflorescence problem became more severe with the addition of FGD, leading to the formation of a new efflorescence product, Na2SO4 [17]. In contrast to these studies, which employed FGD as a partial replacement with other aluminosilicate precursors, the present work explores an alkali-activated binder formulated exclusively with FGD-D (100%) as the primary precursor. This approach provides novel insights into the feasibility and performance of fully FGD-based AAMs.
Thus, studying sustainable alternatives that reduce the byproducts generated by the coal desulfurization process is a relevant topic, considering the possible increase in energy generation and its contribution to environmental preservation. Technological innovation in the search for alternatives to landfills is a viable and feasible solution that involves numerous economic, social, and environmental advantages and benefits. From an environmental point of view, this method economically and efficiently reduces large volumes of waste discharged into the environment, which can cause soil contamination.
Therefore, this study’s primary objective is to conduct an exploratory investigation into the applicability of FGD waste as a precursor in alkali-activated materials (AAMs). Rather than confirming a definitive substitution or presenting an optimized formulation, the focus is assessing this waste’s preliminary reactivity, chemical behavior, and physical characteristics within an AAM system. In this context, the work serves as an initial step toward evaluating the potential of FGD waste to partially or fully replace conventional precursors, such as metakaolin, in future studies and formulations.
2. Materials and Methods
2.1. Materials
The flue gas desulfurization raw waste product (FGD-R) used in this study was obtained from a thermoelectric power plant in Candiota City, State Rio Grande do Sul, Brazil. It is pale grey, and the moisture is about 20%. The moisture was obtained by heating at 100 °C until it reached a constant weight. The FGD-R was dried at 100 °C to obtain powders (0 wt.% moisture) and was prepared by dry-milling for five minutes to disintegrate. This dried and processed material was called FGD-D. In addition, to study the decomposition behavior of FGD-R, the sample was calcined at 950 °C in an air atmosphere, with 10 °C/min heat rate, and called FGD-C. This variation (FGD-C) was produced to compare its reactivity with the other samples (FGD-R and FGD-D) and identify the most suitable precursor for AAM production.
Alkaline solutions were used to evaluate the potential of FGD-D to obtain AAM. A commercial product from DAV-QUÍMICA (Içara, Brazil), sodium silicate, was used as an alkaline activator, with a content of 14.72 wt.% Na2O, 32.40 wt.% SiO2 (SiO2/Na2O ratio of 2.20), and 52.88 wt.% water.
2.2. Alkali-Activated Material Preparation
Table 1 summarizes the formulations containing FGD-D, sodium silicate, and water. Across the formulations, the proportions of FGD-D change from 31.6%–38.9%. The water content in each formulation was adjusted based on the workability observed during preliminary tests. We used four fixed proportions of FGD-D waste as a baseline. During the mixing process, we incrementally added water and sodium silicate to achieve molding consistency. It is important to note that this study is exploratory; it aims to demonstrate the potential use of FGD waste in AAM, rather than to establish an optimized or standardized formulation at this stage.

Table 1.
AAM formulations from FGD-D.
First, the liquid fractions were mixed at 60 rpm for 1 min using a planetary mixer, and then the FGD-D was combined with the alkaline activator solution at 60 rpm. Thorough mixing was performed until a uniform consistency of the paste was obtained. This methodology was based on previous works [18].
After mixing, the AAM paste was molded using plastic molds with a height of 10 cm and a diameter of 5 cm. All the samples were cured at room temperature (23 ± 1 °C) for 24 h. To test the compressive strength, the demolded specimens were cured at ambient temperature (~25 °C) and humidity (~80%) for 7 and 28 days.
2.3. Methods Applied for the Characterization of FGD and AAM
The characterization was divided into two stages: Characterization of the wastes (FGD-R, FGD-D, and FGD-C) and characterization of the obtained products.
The waste was characterized using different techniques, each selected for its specific contribution to understanding the material. Differential thermal analysis (DTA, TA Instruments SDTQ 600, New Castle, DE, USA; 10 K min−1 in air) was employed to evaluate thermal stability and detect phase transformations upon heating. X-ray diffractometry (XRD, Bruker–D8, Billerica, MA, USA; CuKα radiation, 2θ range 10–70°, 0.02° steps) was used to identify crystalline phases present in the samples. Chemical composition was determined by X-ray fluorescence spectrometry (Panalytical Axios Max, Worcestershire, UK) and atomic absorption spectrometry (UNICAM SOLAR 969, London, UK). Fourier transform infrared spectroscopy (FT-IR, Perkin–Elmer UATR Two, Waltham, MA, USA, 4000–400 cm−1, KBr pellets) was performed to detect functional groups and assess the presence of specific bonds related to mineral phases. Before all analyses, samples were ground in an agate mortar and pestle to a particle size below 75 μm.
For the FT-IR test, an equal amount of potassium bromide (KBr) powder and the sample were mixed using an agate mortar and pestle. The mixture was homogenized using an agate pestle to ensure a uniform particle size. It was then pressed with a force of 1.5 tons in a hydraulic press before being sent for FT-IR analysis.
The preparation procedure to quantify the crystalline phases found in the XRD follows De La Torre et al. [19] work by adding a high-purity alpha-alumina internal standard. Phase quantification used the Rietveld method and GSAS (General Structure Analysis System) software in conjunction with the EXPGUI graphical interface. Crystallographic information was collected from COD (Crystallography Open Database). The internal standard (Alumina, Almatis, 99.99%) was refined as a crystalline phase and used to determine the amorphous fraction of the samples. The refinement quality was evaluated using the least squares indexes, Rwp, and the graph. The results presented consider an overall experimental error of 2%.
The real density was measured using a helium pycnometer (Quantachrome Ultrapyc 1200e, Boynton Beach, FL, USA). The microstructures were analyzed with a scanning electron microscope (SEM Zeiss EVO MA10, Oberkochen, Germany), on powdered FGD-D and the products (formulation F2), which utilized gold deposition and did not involve polishing. Particle size was determined with a particle size analyzer (Mastersizer 2000, Malvern Instruments Ltd., Gloucester, UK), and the specific surface area was evaluated using a BET surface area analyzer (QuantaChrome NOVA 1200e).
The FGD’s hazardousness was characterized per Brazilian Standard NBR 10004 [20], with the procedures detailed in previous works [21]. This Brazilian standard (NBR) is based on the American USEPA SW 846 [22]. The standard generally seeks to classify waste as hazardous (Class I) or non-hazardous (Class II) through leaching, corrosivity, and reactivity tests. The toxicity (leaching) tests were performed by NBR 10005 [23] with extractant solution number 2 and a leaching time of 18 h. To determine whether the class II waste is inert (II-B) or non-inert (II-A), water solubilization tests were performed following NBR 10006 [24].
AAM (F2 sample at 28 days) was characterized using the same parameters for the waste by XRD, He pycnometer, and SEM using cut specimens, FT-IR, and compressive strength. The XRD analysis of the AAM was conducted immediately after the 28-day curing period, without applying any procedure to stop the reaction.
3. Results and Discussion
3.1. Waste Characterization
The chemical composition of the FGD-D studied (Table 2) is mainly composed of calcium oxides (CaO, ~38 wt.%), sulfur (SO3, ~22 wt.%), and silica (SiO2, ~19 wt.%), with minor oxides being alumina (Al2O3, ~5 wt.%), iron (Fe2O3, ~3 wt.%) and potassium (K2O, ~1 wt.%). Likewise, the literature consulted contains mainly calcium, sulfur, and other minor oxides. Variations in the concentrations of these oxides may occur primarily due to the residual ash that the coal combustion gases can carry. When comparing the SiO2 content of FGD-D (~19 wt.%) with the samples in the literature (<5 wt.%), a higher concentration of SiO2 can be observed in FGD-D. Furthermore, a loss on ignition (8.8 wt.%) can be observed, attributed to chemically bound water in the hydrated crystalline phases and decarbonization of the materials, later confirmed in XRD and thermal analysis tests.
The XRD tests found in the literature have revealed compositional differences depending on the process used. The literature indicates that the main crystalline phases in FGD are hemihydrate CaSO4·0.5H2O [25], gypsum (CaSO4·2H2O), muscovite, and dolomite [26,27], with gypsum concentrations reaching 90% [27]. This aligns with the chemical composition of most of the FGDs we have consulted, which have CaO and SO3 values similar to commercial gypsum (Table 2). Our sample characterization work has identified crystalline phases such as quartz, calcite, hannebacite, tobermorite, ettringite, calcium silicate, Gehlenite, Portlandite, Anhydrite, in addition to a high amorphous fraction (Figure 1). The FGD-R sample (as received) contained the following phases: quartz (3 wt.%), calcite (6 wt.%), hannebacite (7 wt.%), tobermorite (4 wt.%), ettringite (3 wt.%) and amorphous (77 wt.%). Drying the material (FGD-D) resulted in a few changes in the amount of each phase, with a slight increase in the amorphous fraction. The thermal treatment of the waste eliminated most of the volatile compounds from the calcite and hannebacite phases and formed the crystalline phases of calcium silicate, gehlenite, and anhydrite (FGD-C, Figure 1). Therefore, the phases found in the FGD-C (Calcined) sample were quartz (3 wt.%), tobermorite (4 wt.%), calcium silicate (5 wt.%), gehlenite (5 wt.%), anhydrite (12 wt.%), and amorphous (77 wt.%).
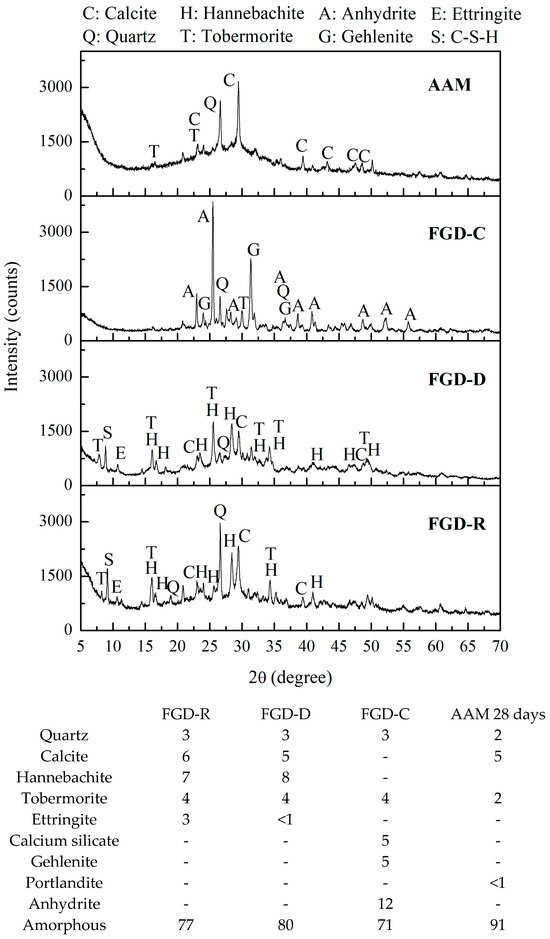
Figure 1.
XRD with phase quantification (wt.%).
The activated FGD-D paste’s XRD patterns show a high amorphous content, consistent with the formation of binding gels such as C-S-H, N-A-S-H, and C-A-S-H, predominantly amorphous and poorly crystalline. Future studies will include complementary techniques to more accurately quantify and characterize the formation of specific binding gels and relate them to the mechanical performance of FGD-D-based alkali-activated materials [28].
The gehlenite crystalline phase with a principal peak at ~31° in the FGD-C calcined sample is also commonly found in blast furnace slags, being a highly reactive material in alkaline activation reactions [29]. A significant study by Guo et al. [30] explored the inclusion of FGD waste (at different calcination temperatures) in producing geopolymers from ash. The authors reported that burning FGD waste at different temperatures changes the chemical properties of the material, making it more reactive for the geopolymerization process.

Table 2.
Chemical composition (XRF) of FGD-D waste.
Table 2.
Chemical composition (XRF) of FGD-D waste.
| Oxides | FGD-D | Lei et al. [31] | Wu et al. [25] | Wang et al. [27] | Zhang et al. [32] | Zhong et al. [33] | Gypsum Lei et al. [31] |
|---|---|---|---|---|---|---|---|
| CaO | 38.14 | 35.94 | 30.90 | 39.50 | 31.60 | 29.40 | 34.83 |
| SO3 | 22.18 | 41.54 | 41.50 | 47.80 | 42.40 | 39.60 | 38.97 |
| SiO2 | 19.78 | 1.05 | 1.27 | 1.05 | 2.70 | 4.37 | 3.12 |
| Al2O3 | 5.54 | 0.34 | 0.81 | 0.93 | 0.70 | 1.73 | 0.69 |
| Fe2O3 | 3.15 | 0.11 | 0.08 | 0.30 | 0.50 | 0.87 | 0.18 |
| K2O | 1.09 | 0.09 | - | 0.09 | - | 0.12 | 0.05 |
| Na2O | - | 0.82 | - | - | - | - | 0.49 |
| MgO | 0.61 | 0.17 | 2.14 | 0.17 | 1.00 | 0.64 | 1.73 |
| TiO2 | 0.43 | - | - | - | - | - | - |
| SrO | 0.17 | - | - | - | - | - | - |
| Cr2O3 | 0.02 | - | - | - | - | - | - |
| MnO | 0.01 | - | - | - | - | - | - |
| ZrO2 | 0.02 | - | - | - | - | - | - |
| V2O5 | 0.02 | - | - | - | - | - | - |
| CuO | 0.02 | - | - | - | - | - | - |
| Rb2O | 0.01 | - | - | - | - | - | - |
| ZnO | 0.01 | - | - | - | - | - | - |
| Y2O3 | 0.01 | - | - | - | - | - | - |
| Crystal water | - | 18.17 | - | 10.08 | - | 18.10 | 16.94 |
| LOI | 8.78 | - | - | - | 19.20 | - | - |
The thermogravimetry analyses (Figure 2) present thermal events characteristic of the calcium sulfate compounds found in the XRD tests (Figure 1). According to Engbrecht and Hirschfeld [34], a dihydrate calcium sulfate ore should exhibit four distinct thermal events, which are: (1) Endothermic reaction where the adsorbed water is removed from ~85 to 105 °C; (2) Endothermic reaction of hemihydrate formation (CaSO4.0.5H2O) from 150 to 175 °C; (3) Endothermic reaction of anhydrite formation from 225 to 250 °C; (4) Exothermic reaction of crystal reordering from monoclinic to orthorhombic from ~380 to 400 °C [34].
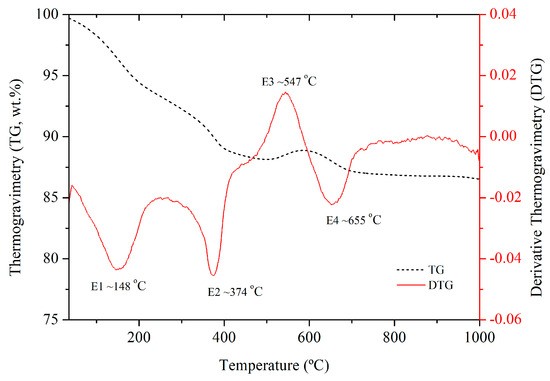
Figure 2.
Thermogravimetry (TG) and Derivative Thermogravimetry (DTG) of FGD-D.
The thermal analysis of the FGD-D sample revealed four distinct thermal events. The first event (E1), an endothermic reaction, indicates the evaporation of water adsorbed in the material and the formation of hemihydrate. The second event (E2), an exothermic reaction, signifies the crystalline reordering from monoclinic to orthorhombic of calcium sulfate [34]. The third event (E3), the combustion of residual coal, is a common observation between 500 and 600 °C [35,36]. The fourth event (E4) may be related to the decomposition of carbonates, which typically begins at approximately 700 °C and ends at approximately 970 °C [37]. A small weight gain observed between 500 and 600 °C is most likely due to undetected impurities in the residual coal. However, its magnitude was minimal and did not significantly affect the overall interpretation of the TG results.
FT-IR analyses show bands representing the composition of hydrated gypsum and aluminosilicates in the FGD-R and FGD-D samples. In this sense, region A (Figure 3) characterizes the hydrogen and oxygen bonds, representing the water adsorbed and chemically bound to calcium sulfate. The peaks at 3612, 3564 (A), and 1620 (B) cm−1 should be attributed to the O-H vibration of the crystalline water molecule [38,39,40]. Bands C and D can be associated with carbonate compounds, as they are related to symmetric stretching vibrations and bending vibrations of the CO32− of calcite, respectively [38,39,41]. The F, G, and J bands represent the S-O groups of calcium sulfates and sulfites present in the sample [39,40]. The J band also represents Si-O bonds [29]. The other FGD-C bands refer to the products formed after calcination, such as gelenite [42].
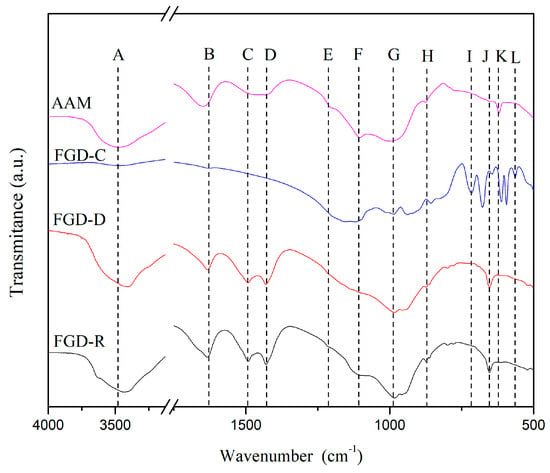
Figure 3.
FT-IR of FGD-R, FGD-D, FGD-C and, AAM (F2 sample) at 28 days.
These compositions containing high alkali content cause the pH values of the FGD sample to be high (~12.88), in comparison to the consulted literature, where reported values ranged between 7.7 and 9.9 [26,33,43]. It should be taken into account here that there is no specific methodology for controlling pH (solid/water ratio), and these variations may be significant for determining the difference between the values. In this work, the 1:1 ratio was used according to NBR 10004 [20] to classify the waste according to its hazardousness. Regarding geopolymerization, Davidovits [29] ratifies the importance of the samples’ pH to assess the alkalinity and rapid hardening of the materials in alkaline activations. Values between pH 8 and 11 are considered fast-setting, while pH values above 11 are considered flash-set. In this sense, the high alkalinity of FGD (12.88) makes this material a potential candidate for use in geopolymers and alkali-activated materials, increasing the reactivity of the formulations developed.
The estimated specific gravity of FGD-D of ~2.0 is similar to materials used in AAM, such as metakaolin and fly ash. Regarding the specific surface area, the result for the specific surface area of the FGD-D sample was 19.41 m2/g, which is well above the values reported in the literature [32], being even higher or similar to materials commonly used for AAM [44,45]. Combined with the high specific surface area, a low mean particle size of ~23 µm, similar to the materials used in AAM (Table 3 and Figure 4 and Figure 5), can be noted. The D10, D50, and D90 values were 2.90, 14.46, and 56.21 µm, respectively. These results demonstrate an interesting potential for use in AAM and cementitious materials, primarily due to the functionality of reactivity and filler effect.
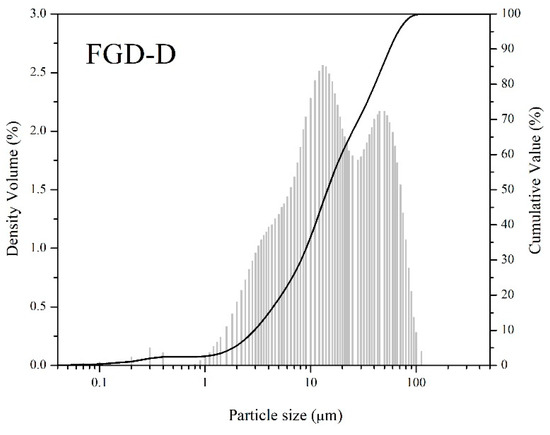
Figure 4.
Particle size distribution of FGD-D.
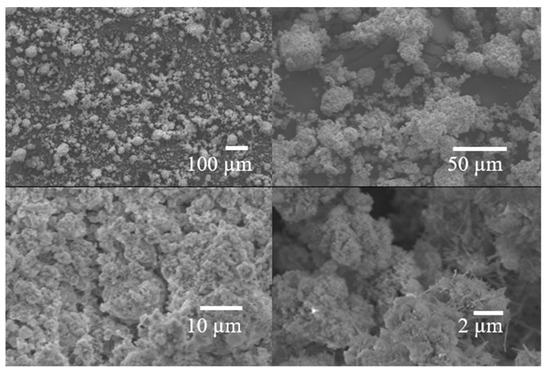
Figure 5.
Images by SEM of FGD-D sample.

Table 3.
Physical and chemical properties of FGD-D.
Table 3.
Physical and chemical properties of FGD-D.
| Properties | FGD-D | Lei et al. [31] | Gypsum Lei et al. [31] | Wu et al. [25] | Zhang et al. [32] | Li et al. [43] |
|---|---|---|---|---|---|---|
| Specific gravity (g/cm3) | 2.00 | - | - | - | - | 1.27 |
| BET surface area (m2/g) | 19.41 | - | - | - | 0.39 | - |
| Mean particle size (µm) | 22.82 | 32.67 | 26.55 | 37.13 | - | - |
The differences between FGD-R, FGD-D, and FGD-C in their amorphous fractions and the formation of more intense reactive phases were insignificant. Since FGD-C requires additional energy for the calcination process, FGD-D, which is obtained by drying the waste, was chosen to proceed with the study and to produce AAM.
3.2. Environmental Assessment of FGD-D (NBR 10004)
The results of NBR 10004 tests indicated that FGD-D has a light gray appearance (Figure 6), with a solids content of 87.6 wt.%, without a characteristic odor, and absence of free liquids (Table 4). The light gray color can be justified mainly by the high gypsum content and residual fly ash dragged from the combustion process. A difference between moisture at 42 (7.8 wt.%) and 105 °C (12.4 wt.%) can be observed due to dehydration reactions at different temperatures, as discussed in Section 3.1. In the same way, variations in moisture were also described in the literature, with humidity values between 3.8 and 23.0 wt.% [26,43].

Figure 6.
Picture of FGD-R (left) and FGD-C (right).

Table 4.
Environmental analysis of FGD-D.
Concerning FGD-D classification, the first step was to evaluate its corrosivity and reactivity characteristics. FGD-D was classified as non-reactive, as it does not present cyanide and sulfide ions in its constitution above the limits established by the NBR 10004 standard. FGD-D was shown to be a corrosive material in the corrosivity tests due to pH = 12.89, above the limits established at standard. These results classified FGD-D as a hazardous waste (Class I). In addition to the parameters mentioned above, toxicity is another important method for characterizing waste and detecting possible elements that can leach and cause environmental impacts when disposed of improperly. The results of the leachate extract (Table 5) show that all the elements evaluated are below the limit concentration established in the NBR 10004 standard. These values are consistent with those found in the literature [26,43,46,47]. Hao et al. [26] evaluated different flue gas desulfurization plaster (FGD) samples in 12 plants in Shanxi province, China, and the results revealed that the heavy metals contained in the leached solution of most samples were below the observed limits.

Table 5.
Leaching results (mg/L) of FGD-D.
As previously discussed, the hazardousness detected in FGD-D due to the high pH becomes a potential for use in geopolymers and alkali-activated materials. Also, the other parameters, such as reactivity and toxicity, were below the limit concentrations established by the NBR 10004 standard, confirming the possibility of FGD-D being used in alkali activation with reduced environmental impacts.
The FGD-D sample was also evaluated using the solubilization tests. In this case, two elements (total chromium and fluoride) were above the concentrations established by the NBR 10004 standard. These results show that FGD-D in this test is a non-inert waste (Table 6).

Table 6.
Solubilization results (mg/L) of FGD-D.
Both fluoride and total chromium can be found in several industrial wastes, such as energy generation waste in coal combustion plants [46,47]. In coal, fluorides can be in the form of fluorapatite or the rings of residual clay minerals (kaolinite, montmorillonite, and illite), replacing hydroxyl groups. This residual of solubilized fluoride can be from the desulfurization process that eliminates, in addition to sulfur, other toxic elements of the flue gases, such as F, As, Se, Sb, Hg, Cd, Cr, Cu, Ni, Pb, Zn, Ba, Mo. Fluorides have been of great concern due to their adverse effects on human health when ingested in excess, causing a disease called fluorosis [47]. Using the alkali activation process is essential to make these components chemically inert.
Although leaching tests were not performed in this study, numerous studies have demonstrated the capacity of geopolymers and alkali-activated materials to immobilize hazardous elements such as fluorides and heavy metals. Peng et al. (2023) showed that geopolymers effectively stabilized Cr6+ and Zn2+ within the matrix [48]. In another study, a geopolymer derived from sustainable FeCr slag achieved over 97% immobilization of Fe, Zn, Mn, Ni, and Cr [49]. Moreover, AAM specimens incorporating copper mine tailings and metakaolin achieved heavy-metal leaching levels (Fe, Cu, Pb, Cd) within EPA guidelines under both neutral and acidic conditions [50]. These findings suggest that, although FGD-D is classified as Class I hazardous waste due to its high alkalinity and potential solubilization of fluoride and chromium, the AAM process could still contribute to these species’ physical encapsulation and chemical binding. Consequently, despite the absence of direct leaching data in the current work, such literature supports the prospect of long-term stabilization of contaminants in AAM systems. Future studies should include targeted leaching assessments to confirm these immobilization mechanisms and evaluate regulatory compliance.
3.3. AAM from FGD-D
AAM material consisting of FGD-D was developed by adding sodium silicate for alkaline activation and water to improve rheology and workability. FGD-D use in AAM proved an alternative mineral source, showing an exothermic reaction with subsequent rapid hardening (in the first 10 min) and mechanical strength increase. This rapid hardening has compromised the molding and workability of samples. In the same way, studies show that adding blast furnace slag (reactive calcium) to geopolymers reduces the hardening time of the paste. In this sense, different approaches and additives could be studied to improve the properties of the fresh state and increase the workability time [51,52].
The compressive strength of the samples studied at seven days confirmed the reactivity of FGD-D in AAM, opening up new possibilities for its use in construction. The Uniaxial Compressive Strength values ranged from 7.7 ± 1.3 MPa for F1 to 14.4 ± 1.8 MPa for F4 (Table 7). F2, F3, and F4 variations were within the standard deviation, with no significant differences. The best results can be attributed to F4 since it had the highest compressive strength value, combined with a larger amount of FGD-D and a smaller amount of sodium silicate used in the other formulations. At 28 days, all samples showed a decrease in compressive strength values, but F4 maintained the highest values among all formulations.

Table 7.
FGD-D-based AAM compressive strength (MPA).
In the study by Hanjitsuwan et al. (2020), incorporating FGD and diatomaceous lime mortar into fresh Alkali-Activated Hybrid Fly Ash (AAHF) pastes reduced setting time and enhanced early strength development [16]. However, AAHF pastes with 5.0% FGD showed a slight strength reduction after 120 days of curing, indicating potential adverse effects on long-term strength [16]. Other researchers have reported similar findings [53,54], who identified CaO as a key factor in heat generation within the matrix, accelerating the geopolymerization process.
Although compressive strength tends to decrease with age, commercial precursor materials like metakaolin can enhance these properties and help mitigate the reduction. This exploratory study presents some inherent instability. Unlike most studies that use metakaolin to improve stability, this work did not offer unique insights into the material’s behavior without this additive. Future investigations may consider incorporating metakaolin to enhance system stability. The results indicated no differences between formulations, as means fell within the standard deviation. However, the findings confirm the potential use of FGD-D in AAM, and future works could improve the formulations. Previously, works using other types of waste [18,55] used metakaolin in the formulation, which had similar compressive strength.
FGD-D waste showed great potential for AAM and geopolymers, mainly because the formulations used only the waste (without metakaolin). Other studies employ formulations primarily based on metakaolin or other material, incorporating only minimal amounts of FGD waste (less than 15 wt.%) [15,16].
The chemical reaction involved in the study by Hua et al. [56] is described in Equation (1) and explains the hardening mechanism of FGD-D when mixed with sodium silicate, resulting in calcium silicate and sodium sulfate as a product [56].
Although this work does not employ metakaolin in its formulations, it is important to consider that the fly ashes used contain a high percentage of residual aluminosilicates. These components may participate in alkali activation reactions and contribute to the formation of binding phases commonly observed in geopolymeric systems, such as hydrated calcium silicate gel (CSH) [57,58], N-C-S-A-H [38] and C-A-H (calcium aluminate hydrate) [59,60]. Previous studies, such as Yip et al. [58], have shown that reactive calcium sources can accelerate the formation of C-S-H and other hydrated calcium aluminates, even when metakaolin is used as the precursor. Therefore, these aluminosilicate and calcium-rich phases in the fly ashes may partially explain the mechanical performance and phase composition observed in the materials produced in this study.
Other studies argue that geopolymers with Na2SO4 formation increase the pH value and accelerate the dissolution of chemical elements [61]. Zhang et al. [38] reported that FGD waste can improve compressive strength results due to SO4−2 ions accelerating the leaching rate of the [AlO4]− and [SiO4] tetrahedrons and consequently forming more hydration products. Ca2+ ions can participate in the process, forming other hydrated products [38], as previously discussed.
The alkaline activation reactions using FGD-D waste and calcium silicate previously discussed [56] were confirmed in the XRD and FT-IR analyses of the pastes obtained. The XRD shows a typical geopolymer amorphous halo between 25 and 35° with residual quartz and calcite phases from FGD-D (raw material). Other amorphous gel halos such as C-A-S-H (~30° in 2θ [29]) and C-S-H (~29° in 2θ [62,63]) were also found in the sample after alkaline activation. The quantified amorphous fraction was 91%, higher than that found in the FGD-D residue (80 wt.%) used as raw material. This increase in the amorphous fraction, along with the significant consumption of the hannebacite and tobermorite phases, further supports the reactivity of the FGD-D after alkaline activation. The FT-IR tests confirm the results indicated by the XRD, where the main bands of the source materials (FGD-D) were found, and specific bands to geopolymer materials are found: Si-O (1080–1100 cm−1) and Si (Al)-O (1008 cm−1) bonds [29].
SEM analyses of the F2 sample at 28 days (Figure 7) show a typical homogeneous and amorphous cementitious structure. However, numerous pore spaces were observed, including both rounded pores and microcracks. These cracks likely formed during the hardening process due to volumetric shrinkage associated with moisture loss. Such defects, which are commonly associated with rapid setting and water evaporation, may have negatively influenced the sample’s compressive strength (Table 7). Further studies are necessary to better understand and mitigate the formation of pores and cracks to enhance the material’s mechanical performance.
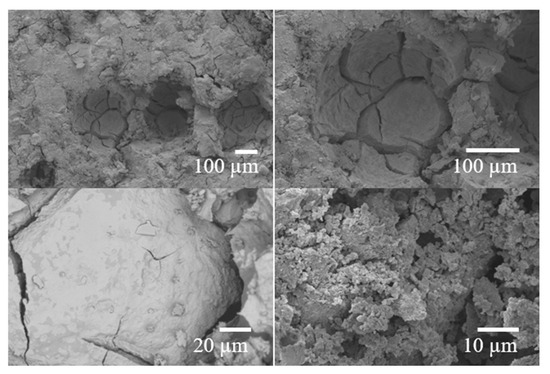
Figure 7.
Images by SEM of the F2 sample at 28 days.
4. Conclusions
This study provided an exploratory assessment of FGD-D waste from the Brazilian Candiota plant to evaluate its potential as a precursor in alkali-activated materials (AAMs). The chemical and mineralogical characterization revealed amounts of silica, calcium, and sulfur oxides, along with a substantial amorphous fraction—an essential feature for alkali activation. Preliminary compressive strength values ranging from 7.7 to 14.4 MPa support its potential in low-carbon binder systems for non-structural or moderately demanding applications.
An important environmental concern regarding FGD-D waste is its high pH, which classifies it as hazardous under specific regulations. However, in the context of alkali activation, this high alkalinity proves a beneficial feature, as precursors with elevated pH values tend to be more reactive and enhance the dissolution of aluminosilicate species during the geopolymerization process. Thus, what is generally considered an environmental liability becomes a functional advantage in AAM systems.
This work is a foundation for future studies, including blended systems investigations with traditional additives such as metakaolin, further mechanical and durability evaluations, and leaching tests on AAM specimens to assess the immobilization of potentially hazardous elements.
In conclusion, the findings demonstrate that FGD-D waste has great potential for alkali-activated materials and represents a sustainable and strategic route for valorizing industrial waste with otherwise problematic environmental characteristics.
Author Contributions
Conceptualization, P.M., L.S., A.D. and C.P.B.; methodology, P.M., L.S. and C.P.B.; validation, P.M. and L.S.; formal analysis, P.M.; investigation, P.M., L.S. and C.P.B.; resources, P.M. and L.S.; data curation, P.M., L.S. and C.P.B.; writing—original draft preparation, P.M. and L.S.; writing—review and editing, P.M., L.S. and C.P.B.; visualization, P.M. and A.D.; supervision, P.M. and C.P.B.; project administration, P.M. and C.P.B.; funding acquisition, P.M. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Associação Beneficente da Indústria Carbonífera de Santa Catarina (SATC), CGT Eletrosul Usina termoelétrica de Candiota and National Council for Scientific and Technological Development (CNPq).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are very grateful to the Associação Beneficente da Indústria Carbonífera de Santa Catarina (SATC) for making the infrastructure available for this research, to FAPESC for the support, to CGT Eletrosul Usina termoelétrica de Candiota for making the FGD material available and National Council for Scientific and Technological Development (CNPq 403702/2023-2).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feng, Y.; Li, Y.; Cui, L. Critical Review of Condensable Particulate Matter. Fuel 2018, 224, 801–813. [Google Scholar] [CrossRef]
- Song, J.; Lu, S.; Wu, Y.; Zhou, C.; Li, X.; Li, J. Migration and Distribution Characteristics of Organic and Inorganic Fractions in Condensable Particulate Matter Emitted from an Ultralow Emission Coal-Fired Power Plant. Chemosphere 2020, 243, 125346. [Google Scholar] [CrossRef]
- Cruz, M.d.A.; Araújo, O.d.Q.F.; de Medeiros, J.L.; de Castro, R.d.P.V.; Ribeiro, G.T.; de Oliveira, V.R. Impact of Solid Waste Treatment from Spray Dryer Absorber on the Levelized Cost of Energy of a Coal-Fired Power Plant. J. Clean. Prod. 2017, 164, 1623–1634. [Google Scholar] [CrossRef]
- Rodrigues, I.N.; de Medeiros, J.L.; de Queiroz F. Araújo, O. Sulfite Removal from Flue-Gas Desulfurization Residues of Coal-Fired Power Plants: Oxidation Experiments and Kinetic Parameters Estimation. Energy Rep. 2021, 7, 8142–8151. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhu, T.; Jing, P.; Wang, J. Simulation of the Heterogeneous Semi-Dry Flue Gas Desulfurization in a Pilot CFB Riser Using the Two-Fluid Model. Chem. Eng. J. 2015, 264, 479–486. [Google Scholar] [CrossRef]
- Ma, X.; Kaneko, T.; Tashimo, T.; Yoshida, T.; Kato, K. Use of Limestone for SO2 Removal from Flue Gas in the Semidry FGD Process with a Powder-Particle Spouted Bed. Chem. Eng. Sci. 2000, 55, 4643–4652. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K. Energy Industry. Phys. Sci. Rev. 2018, 3, 20180023. [Google Scholar] [CrossRef]
- Yao, X.; Wang, W.; Liu, M.; Yao, Y.; Wu, S. Synergistic Use of Industrial Solid Waste Mixtures to Prepare Ready-to-Use Lightweight Porous Concrete. J. Clean. Prod. 2019, 211, 1034–1043. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of Geopolymerization and Factors Influencing Its Development: A Review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Eleutério, R.V.; Simão, L.; Hotza, D. Alkali-Activated Materials for Catalytic Applications: A State-of-the-Art Review. Mater. Sci. Eng. B 2024, 299, 117007. [Google Scholar] [CrossRef]
- Tochetto, G.A.; Simão, L.; de Oliveira, D.; Hotza, D.; Immich, A.P.S. Porous Geopolymers as Dye Adsorbents: Review and Perspectives. J. Clean. Prod. 2022, 374, 133982. [Google Scholar] [CrossRef]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Vandeperre, L.J.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr Contaminated Clinoptilolite in Geopolymers Produced from Metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar] [CrossRef]
- Davidovits, J. Global Warming Impact on the Cement and Aggregates Industries. World Resour. Rev. 1994, 6, 263–278. [Google Scholar]
- de Oliveira, L.B.; de Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of Geopolymers with Industrial Waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Marvila, M.T.; Fediuk, R.; Vieira, C.M.F.; Azevedo, A.R.G. Development of a Complementary Precursor Based on Flue Gas Desulfurization (FGD) for Geopolymeric Pastes Produced with Metakaolin. J. Mater. Res. Technol. 2023, 22, 3489–3501. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Injorhor, B.; Phoo-ngernkham, T.; Damrongwiriyanupap, N.; Li, L.-Y.; Sukontasukkul, P.; Chindaprasirt, P. Drying Shrinkage, Strength and Microstructure of Alkali-Activated High-Calcium Fly Ash Using FGD-Gypsum and Dolomite as Expansive Additive. Cem. Concr. Compos. 2020, 114, 103760. [Google Scholar] [CrossRef]
- Zhang, M.; He, M.; Zhang, J. Mitigation of Efflorescence for Multi-Componential Geopolymer: Influence of Steel Slag, Flue Gas Desulfurization Gypsum and Pre-Curing Periods. J. Clean. Prod. 2023, 403, 136835. [Google Scholar] [CrossRef]
- Simão, L.; De Rossi, A.; Hotza, D.; Ribeiro, M.J.; Novais, R.M.; Klegues Montedo, O.R.; Raupp-Pereira, F. Zeolites-containing Geopolymers Obtained from Biomass Fly Ash: Influence of Temperature, Composition, and Porosity. J. Am. Ceram. Soc. 2021, 104, 803–815. [Google Scholar] [CrossRef]
- De La Torre, A.G.; Bruque, S.; Aranda, M.A.G. Rietveld Quantitative Amorphous Content Analysis. J. Appl. Crystallogr. 2001, 34, 196–202. [Google Scholar] [CrossRef]
- ABNT NBR 10004; Resíduos Sólidos—Classificação. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Characterization of Pulp and Paper Mill Waste for the Production of Waste-Based Cement. Rev. Int. Contam. Ambient. 2019, 35, 237–246. [Google Scholar] [CrossRef]
- USEPA. Hazardous Waste Test Methods; United States Environmental Protecion Agency: Washington, DC, USA, 2016; p. 295.
- ABNT NBR 10005; Procedimento Para Obtenção de Lixiviado de Resíduos Sólidos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- ABNT NBR 10006:2004; Procedimento Para Obtenção de Extrato Solubilizado de Resíduos Sólidos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- Wu, Q.; Ma, H.; Chen, Q.; Huang, Z.; Zhang, C.; Yang, T. Preparation of Waterproof Block by Silicate Clinker Modified FGD Gypsum. Constr. Build. Mater. 2019, 214, 318–325. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Q.; Pan, Y.; Liu, Z.; Wu, S.; Xu, Y.; Qian, G. Heavy Metals Distribution Characteristics of FGD Gypsum Samples from Shanxi Province 12 Coal-Fired Power Plants and Its Potential Environmental Impacts. Fuel 2017, 209, 238–245. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Du, Z.; Cheng, H.; Cheng, F. Effect of Impure Components in Flue Gas Desulfurization (FGD) Gypsum on the Generation of Polymorph CaCO3 during Carbonation Reaction. J. Hazard. Mater. 2019, 369, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical Investigation of Phase Assemblages of Alkali-Activated Materials in CaO-SiO2-Al2O3 Systems: The Management of Reaction Products and Designing of Precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Chemistry & Applications; Geopolymer Institute: Saint-Quentin, France, 2008; ISBN 9782951482098. [Google Scholar]
- Guo, X.; Shi, H.; Dick, W.A. Utilization of Thermally Treated Flue Gas Desulfurization (FGD) Gypsum and Class-C Fly Ash (CFA) to Prepare CFA-Based Geopolymer. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2013, 28, 132–138. [Google Scholar] [CrossRef]
- Lei, D.-Y.; Guo, L.-P.; Sun, W.; Liu, J.; Miao, C. Study on Properties of Untreated FGD Gypsum-Based High-Strength Building Materials. Constr. Build. Mater. 2017, 153, 765–773. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, F.; Wu, R. Study on the Performance of FGD Gypsum-Metakaolin-Cement Composite Cementitious System. Constr. Build. Mater. 2016, 128, 1–11. [Google Scholar] [CrossRef]
- Zhong, S.; Ni, K.; Li, J. Properties of Mortars Made by Uncalcined FGD Gypsum-Fly Ash-Ground Granulated Blast Furnace Slag Composite Binder. Waste Manag. 2012, 32, 1468–1472. [Google Scholar] [CrossRef]
- Engbrecht, D.C.; Hirschfeld, D.A. Thermal Analysis of Calcium Sulfate Dihydrate Sources Used to Manufacture Gypsum Wallboard. Thermochim. Acta 2016, 639, 173–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity Activation of Waste Coal Gangue and Its Impact on the Properties of Cement-Based Materials—A Review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Acordi, J.; Simão, L.; Faraco, M.N.S.; Borgert, C.H.; Olivo, E.; Montedo, O.R.K.; Raupp-Pereira, F. Waste Valorization of Coal Mining Waste from a Circular Economy Perspective: A Brazilian Case Study Based on Environmental and Physicochemical Features. Resour. Policy 2023, 80, 103243. [Google Scholar] [CrossRef]
- Simão, L.; Souza, M.T.; Ribeiro, M.J.; Klegues Montedo, O.R.; Hotza, D.; Novais, R.M.; Raupp-Pereira, F. Assessment of the Recycling Potential of Stone Processing Plant Wastes Based on Physicochemical Features and Market Opportunities. J. Clean. Prod. 2021, 319, 128678. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z. Investigation the Synergistic Effects in Quaternary Binder Containing Red Mud, Blast Furnace Slag, Steel Slag and Flue Gas Desulfurization Gypsum Based on Artificial Neural Networks. J. Clean. Prod. 2020, 273, 122972. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of Low-Quality Desulfurized Ash from Semi-Dry Flue Gas Desulfurization by Mixing with Hemihydrate Gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Guan, Q.; Hu, Y.; Tang, H.; Sun, W.; Gao, Z. Preparation of α-CaSO4·½H2O with Tunable Morphology from Flue Gas Desulphurization Gypsum Using Malic Acid as Modifier: A Theoretical and Experimental Study. J. Colloid Interface Sci. 2018, 530, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dutta, P.K. Synthesis of Free-Standing Chabazite-Type Films. Microporous Mesoporous Mater. 2000, 38, 151–159. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, D.; Wu, Y.; Yu, H. Synthesis and Characterization of Calcium Aluminate Compounds from Gehlenite by High-Temperature Solid-State Reaction. Ceram. Int. 2018, 44, 13544–13550. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Leiva, C.; Cornejo, A.; Font, O.; Querol, X.; Moeno, N.; Arenas, C.; Fernández-Pereira, C. Potential Utilization of FGD Gypsum and Fly Ash from a Chinese Power Plant for Manufacturing Fire-Resistant Panels. Constr. Build. Mater. 2015, 95, 910–921. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z.H. Solid-State Conversion of Fly Ash to Effective Adsorbents for Cu Removal from Wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef]
- Bhagath Singh, G.V.P.; Subramaniam, K.V.L. Quantitative XRD Study of Amorphous Phase in Alkali Activated Low Calcium Siliceous Fly Ash. Constr. Build. Mater. 2016, 124, 139–147. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, Y.; Lu, J.; Gupta, R.; Pudasainee, D.; Liu, S.; Liu, M.; Lu, J. Thermal Stability, Chemical Speciation and Leaching Characteristics of Hazardous Trace Elements in FGD Gypsum from Coal-Fired Power Plants. Fuel 2018, 231, 94–100. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X. Stabilization of FGD Gypsum for Its Disposal in Landfills Using Amorphous Aluminium Oxide as a Fluoride Retention Additive. Chemosphere 2007, 69, 295–302. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, P.; Zeng, L.; Yu, L.; Li, D. Immobilization of Chromium Ore Processing Residue by Alkali-Activated Composite Binders and Leaching Characteristics. Environ. Sci. Pollut. Res. 2023, 30, 71154–71170. [Google Scholar] [CrossRef]
- Falayi, T. Sustainable Solidification of Ferrochrome Slag through Geopolymerisation: A Look at the Effect of Curing Time, Type of Activator and Liquid Solid Ratio. Sustain. Environ. Res. 2019, 29, 21. [Google Scholar] [CrossRef]
- Maqbool, Q.; Mobili, A.; Blasi, E.; Sabbatini, S.; Ruello, M.L.; Tittarelli, F. Sustainable Alkali-Activated Mortars for the Immobilization of Heavy Metals from Copper Mine Tailings. ACS Sustain. Resour. Manag. 2024, 1, 154–164. [Google Scholar] [CrossRef]
- Sasaki, K.; Kurumisawa, K.; Ibayashi, K. Effect of Retarders on Flow and Strength Development of Alkali-Activated Fly Ash/Blast Furnace Slag Composite. Constr. Build. Mater. 2019, 216, 337–346. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to Control the Geopolymer Setting Time with the Alkaline Silicate Solution. J. Non-Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength Development and Durability of Alkali-Activated Fly Ash Mortar with Calcium Carbide Residue as Additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Phiangphimai, C.; Intarabut, D.; Hanjitsuwan, S.; Damrongwiriyanupap, N.; Li, L.; Chindaprasirt, P. Low Cost and Sustainable Repair Material Made from Alkali-Activated High-Calcium Fly Ash with Calcium Carbide Residue. Constr. Build. Mater. 2020, 247, 118543. [Google Scholar] [CrossRef]
- Eleutério, R.V.; Simão, L.; Lemes, P.; Hotza, D. Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers. Minerals 2023, 13, 1158. [Google Scholar] [CrossRef]
- Hua, M.; Wang, B.; Chen, L.; Wang, Y.; Quynh, V.M.; He, B.; Li, X. Verification of Lime and Water Glass Stabilized FGD Gypsum as Road Sub-Base. Fuel 2010, 89, 1812–1817. [Google Scholar] [CrossRef]
- Yip, C.K.; van Deventer, J.S.J. Microanalysis of Calcium Silicate Hydrate Gel Formed within a Geopolymeric Binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The Coexistence of Geopolymeric Gel and Calcium Silicate Hydrate at the Early Stage of Alkaline Activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Khadka, S.D.; Jayawickrama, P.W.; Senadheera, S.; Segvic, B. Stabilization of Highly Expansive Soils Containing Sulfate Using Metakaolin and Fly Ash Based Geopolymer Modified with Lime and Gypsum. Transp. Geotech. 2020, 23, 100327. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Zhang, E.Q.; Ren, J. Shrinkage Behaviour, Early Hydration and Hardened Properties of Sodium Silicate Activated Slag Incorporated with Gypsum and Cement. Constr. Build. Mater. 2020, 248, 118687. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Ai, T.; Huang, B.; Shu, X.; He, Q. Strength Properties of Geopolymers Derived from Original and Desulfurized Red Mud Cured at Ambient Temperature. Constr. Build. Mater. 2016, 125, 905–911. [Google Scholar] [CrossRef]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Quantitative Analysis of C–S–H in Hydrating Alite Pastes by in-Situ XRD. Cem. Concr. Res. 2013, 53, 119–126. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H Gels with Various Ca/Si and Al/Si Ratio: Microstructural and Structural Characteristics with DTA/TG, XRD, FTIR and TEM Analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).