1. Introduction
Kazakhstan ranks 10th in the world in terms of titanium mineral resource reserves [
1]. The territory of the Republic hosts nearly all the major industrial-genetic types of titanium deposits: magmatogenic, metamorphogenic and exogenic [
2]. Titanium reserves are primarily concentrated in three geologically distinct industrial deposits. Ilmenite sands from the Satpayevskoye deposit have been mined since 2002 by the mining and processing enterprise of the same name (TOO SGOP) [
3,
4]. Ilmenite concentrate obtained by gravity-magnetic separation of hard-to-process raw materials with a high (50%) content of clay minerals, especially kaolinite (40%), is delivered by road to the Ust-Kamenogorsk Titanium and Magnesium Plant (JSC UK TMK). To increase the plant’s production capacity, a second processing plant was built and put into operation in 2021.
Extraction of ilmenite sands has been carried out since 2002 through operations conducted at the Satpayev deposit by the eponymous mining and processing enterprise (LLP “SGOP”) [
3,
4]. The ilmenite concentrate, obtained by gravity–magnetic separation of difficult-to-process raw materials with a high (50%) content of clay minerals, especially 40% kaolinite, is transported by road to the Ust-Kamenogorsk Titanium–Magnesium Plant (JSC “UK TMK”). To increase the plant’s production capacity, a second beneficiation plant was commissioned and built in 2021.
UK TMK occupies a leading position in the global market for premium titanium sponge, which has been rapidly progressing in recent years [
5]. The plant’s products, certified by all aircraft manufacturers in the world, titanium sponge, titanium ingots and alloys, are exported to the USA, Great Britain, Russia, France, South Korea, India and China. The share of Kazakhstan titanium in the aerospace industry is 18%.
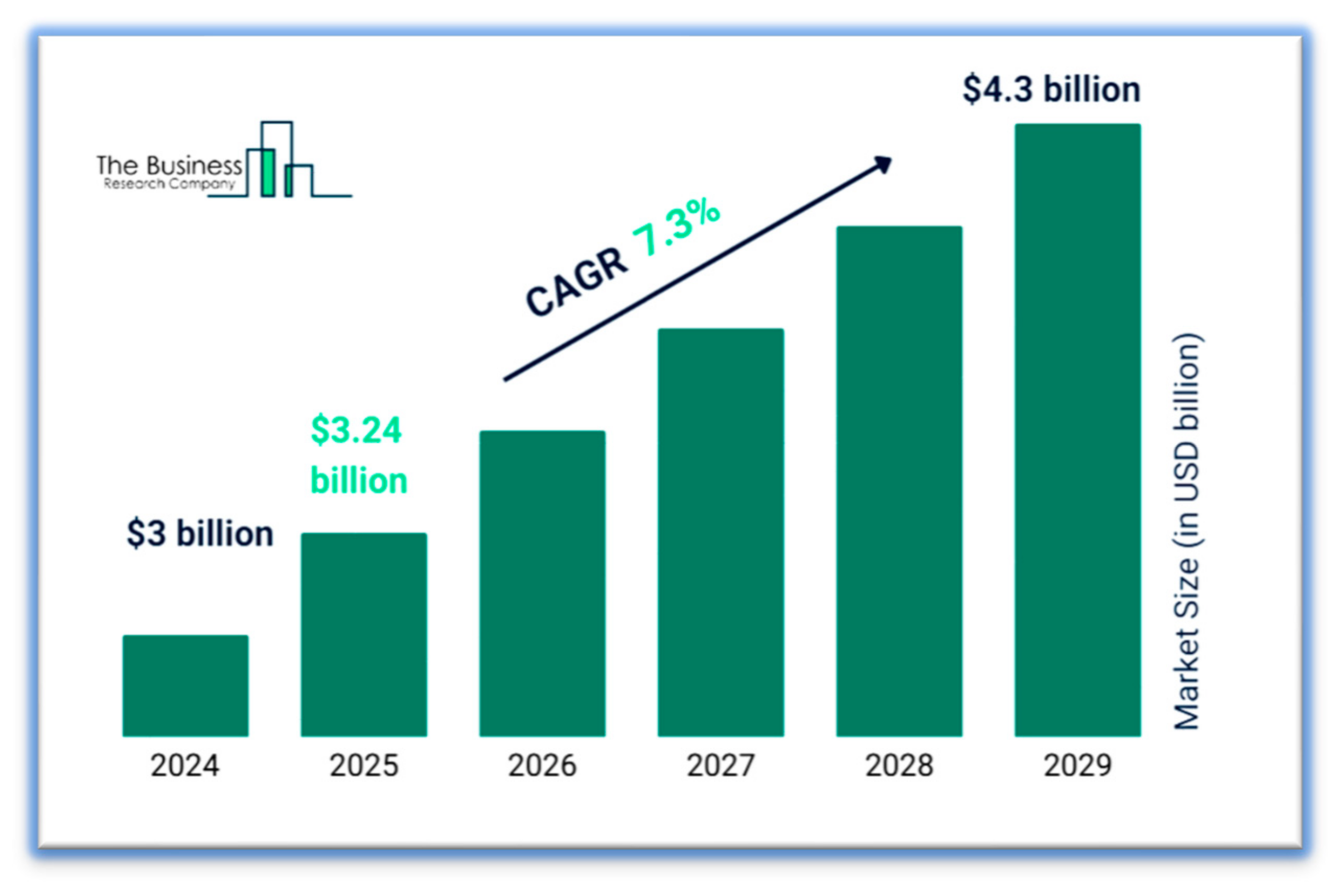
According to the leading marketing firm Business Research Company, the global aerospace titanium market reached USD 3.24 billion in 2025 [
6]. The projected compound annual growth rate (CAGR) from 2026 to 2033 is 7.3%, with revenue expected to reach USD 4.3 billion by 2029 (see
Figure 1).
Titanium sponge is a high-tech semi-finished product used for the production of titanium ingots, alloys, rolled products and structural products. Titanium alloys, which are lightweight, exceptionally strong in a wide temperature range (from cryogenic to +500–600 and higher), corrosion-resistant in aggressive environments, biocompatible and unique in other ways, are key—and in many cases, the only—materials in aircraft, rocket, ship and automobile manufacturing, nuclear power engineering, chemical, petrochemical and defense industries, medicine and electronics. The best raw materials for the production of titanium sponge are natural and synthetic rutile and electrothermal titanium slag. The reserves of the most economically valuable sand rutile ores and high-quality ilmenite sands are limited in volume. However, polymineral titanium raw materials of very complex material composition are widespread in many countries of the world. The bulk (90%) of the mined titanium raw materials is used to obtain titanium dioxide pigment, which is consumed mainly by the paint, varnish, polymer, pulp and paper industries. About 6% of the raw materials are used to produce compact metallic titanium and its alloys; the remaining 4% are used by the electrode industry [
7]. According to the information and analytical company Artikol, global consumption of titanium dioxide in 2022 decreased by 6.2%, and titanium sponge increased by about 10%. The escalation of the geopolitical situation around Ukraine caused an even greater increase in energy prices, a rush demand for titanium sponge and feverish purchases by titanium metal producers, aggravated by a shortage of high-quality raw materials. In this regard, titanium slag on the US market rose in price by 27%. Prices for titanium sponge in Europe increased by 1.9% compared to the average cost in 2021; in China, they increased by 12%, which stimulated an increase in its production in China by 25%. In the first quarter of 2023, the increase in demand for titanium metal in the world continued both from the civil aircraft and shipbuilding industries and the defense industry. In 2024, the price of one metric ton of Australian alluvial rutile containing at least 95% TiO
2 was approximately USD 1310, and ilmenite was USD 340 [
8]. The leading producers of titanium raw materials are China, Mozambique, Australia, South Africa and Canada, who accounted for 73% of the world’s production of titanium in concentrates in 2022. Russia has one of the world’s largest titanium raw material bases, accounting for 14.5% of the world’s metal reserves. At the same time, the Russian Federation’s contribution to the world production of titanium concentrates is only 0.03% [
7]. China is the absolute leader in the production of ilmenite concentrates, thanks to the giant ilmenite–titanomagnetite deposits of titanium concentrated in Panzhihua in Sichuan Province. Almost all of the mined titanium raw materials are used domestically. The produced concentrate, with an average content of 47.5% TiO
2, is used mainly for the production of titanium dioxide by the environmentally hazardous and very expensive sulfate method and is partially processed into titanium slag. China is also the main consumer of high-quality ilmenite concentrates, supplied (45% of world imports) mainly from Mozambique, Kenya and Vietnam, and a producer of pigment titanium dioxide and titanium sponge using chloride technology. The production volume of pigment titanium dioxide is 52% of the world output, titanium sponge—61%, and titanium products—64%. The production of compact metallic titanium obtained from titanium sponge is an energy-intensive, multi-stage and lengthy process, which explains the small volumes and high cost of its production, despite the availability of widespread natural polymineral raw materials.
The initial operation of titanium sponge production is the carbothermic smelting of ilmenite concentrates to produce titanium slag, processed by chlorination, rectification and magnesium thermal reduction of refined titanium tetrachloride (Kroll method), followed by vacuum distillation [
9].
The largest producers of titanium slag are South Africa and Canada, and of synthetic rutile—Australia and India. South Africa produced about 900.000 metric tons of titanium slag in 2022, and Canada about 800.000 metric tons [
10].
The Canadian company QIT-Fer & Titane Inc., by processing at the Sorel-Tracy smelter ilmenite concentrate with a low (34.5 wt.%) TiO
2 content and high sulfur due to the presence of pyrite (FeS
2), produces titanium slags with the trademark SOREL SLAG, UGS, RTCS and high-purity, malleable iron, Sorelmetal. The primary product SOREL SLAG, with a content of about 80% TiO
2, sold to pigment manufacturers by the sulfate method, is obtained by desulfurization by oxidative roasting in an air atmosphere at a temperature of 1090 °C of ilmenite concentrate, removal of non-ferrous impurities (mainly SiO
2, Al
2O
3, CaO) by high-intensity dry magnetic separation of concentrate cinder, carbothermic melting of ferromagnetic material at a temperature of about 1700 °C and acid leaching of impurities [
11]. The modified UGS product, containing about 95% TiO
2 and sold mainly to titanium dioxide producers by the chloride process and to titanium metal producers, is produced by redox refining of the Sorel slag, leaching the trace iron, magnesium, aluminum, manganese, calcium, vanadium and chromium impurities under high pressure at moderate temperature with a solution of regenerated azeotropic hydrochloric acid, washing, drying and calcining. The intermediate RTCS product, containing about 90% TiO
2, is sold mainly to titanium pigment producers by the chloride process.
The South African company Richards Bay Minerals, by processing fine-grained ilmenite concentrate with a high content of chromium oxide Cr
2O
3 using Canadian adapted technology, created and first tested for the enrichment of coarse-grained ilmenite concentrate, produces titanium slag with a content of 85% TiO
2, 94% synthetic rutile, zirconium dioxide and pig iron [
12].
The ERMS SR technology of the Australian company Austpac Resources, combining the energy-efficient processes of redox roasting and magnetic separation (ERMS), continuous leaching and enhanced acid regeneration (EARS), allows for the production of super-pure (>97% TiO
2) synthetic rutile at a competitive price for the production of white pigment titanium dioxide and metallic titanium by the chloride method [
13]. However, the energy-efficient, two-stage oxidation–reduction roasting, currently considered the preferred process, eliminates the possibility of producing cast iron.
For the production of premium titanium sponge, high-quality titanium slag at UK TMK is obtained by single-stage carbothermic melting at a temperature of 1600 °C of coal–ilmenite charge, with the predominant weight mass of conditioned imported concentrate mixed with domestic Satpayev concentrate. The traditional method of carbothermic melting without the use of high-grade raw materials is unacceptable for processing ilmenite concentrate of SGOP LLC, which forms a non-stratified refractory melt even at very high temperatures.
Detailed information on world titanium resources, methods of processing difficult-to-enrich ilmenite concentrates with very complex material composition, achievements and innovations are covered by us in the review paper [
14]. In order to become involved in the processing of the non-realizable and highly problematic ilmenite concentrate of the Obukhovskoye deposit with chrome–spinelide mineralization (over 8% Cr
2O
3) [
15], our previous studies have developed a less-expensive alternative technology for single-stage oxidation–soda conversion of raw materials without magnetic separation compared to the Australian two-stage oxidation–reduction ERMS process with the production of synthetic rutile of 98% purity [
16]. Below is presented the soda technology we have developed for low-temperature carbothermic smelting of thermally activated clay ilmenite concentrate of the Satpayevskoye deposit with the production of titanium slag, alloyed cast iron and synthetic rutile [
17].
2. Materials and Methods
2.1. Raw Materials
Scientific and applied research was carried out with a representative batch of ilmenite concentrate from sand–alumina placers of the Satpayevskoye deposit, provided by JSC UK TMK.
Anthracite with an ash content of 3.87% and a moisture content of 3.03% was used as a solid carbonaceous reducing agent.
The flux additives used were high-grade technical soda ash of the OKP 21 3111 0220 (granulated) and OKP 21 3111 0120 (powdered) brand with a mass fraction of sodium carbonate (Na
2CO
3) of at least 99.4%, iron (in terms of Fe
2O
3) of no more than 0.003% and trace content of other impurities, in accordance with GOST 5100-85 (State Standard of Russia) [
18].
Molasses (a by-product of the sugar industry) was used as a binder in an amount of 3% of the total mass of the fluxed coal–ilmenite batch.
2.2. Main Equipment and Technological Conditions
Thermal activation of ilmenite concentrate loaded into porcelain crucibles was carried out by oxidative firing in an air atmosphere in a Nabertherm muffle furnace (Laupheim, Germany) at a temperature of 950 °C for 60 min.
The thoroughly mixed batch with the optimal mass ratio of ilmenite concentrate cinder, coal and soda ash equal to 1 ÷ 0.05 ÷ 0.5 with a binder was briquetted in molds and compacted on a Metallkraft WPP 50M hydraulic press (Guangzhou, China) with a compression gain of 100 to 50,000 kg.
Carbothermic melting of briquettes of carbon-soda charge of ilmenite concentrate cinder, placed in graphite crucibles with tightly closing graphite lids, was carried out in a Nabertherm muffle furnace at a temperature of 1300 °C for 90 min.
Magnetic separation of sinter of briquettes, cooled in the ambient atmosphere and crushed, was carried out using a laboratory magnetic separator of NPP “Prodecologia” (Kyiv, Ukraine), with manual adjustment of the magnetic field strength from 80 to 900 T.
The primary titanium slag was refined with water, then with 20% hydrochloric acid at L:S = 3:1, temperature 80 °C for 4 h with the impeller speed of the mechanical stirrer ES of Velp-Scientifica (Usmat, Italy) 500 rpm in thermostatted glasses heated through the outer jacket with hot water of the circulation thermostat LT-108 of LOIP (Budapest, Hungary).
The alkaline and acidic pulp of hydrothermal refining of titanium slag with water and hydrochloric acid was filtered with a water-jet vacuum pump through a Buchner funnel with a paper filter “blue ribbon”.
2.3. Physicochemical Investigations
The fractional composition of the ilmenite concentrate was determined by classification on a vibratory shaker with a set of calibration sieve cloths with different cell sizes.
The material composition of the concentrate, intermediate and final products was studied by X-ray fluorescence analysis on a Venus 200 PANalyical B.V. wave dispersion spectrometer (Eindhoven, The Netherlands); X-ray phase analysis of the mineral composition of each concentrate sample of different sizes, which made it possible to determine the presence of clay minerals in them, was carried out by shooting on a BRUKER D8 ADVANCE diffractometer (Freiburg, Germany) with copper radiation at an accelerating voltage of 36 kW and a current of 25 mA, with initial and final shooting angles from 7 to 90 degrees, a shooting step of 0.02 degrees and a delay time of each shooting step of 0.75–1.2 s. The results were decoded by identifying various phases of the diffraction pattern using the DiffRac. Eva v5.2 application; IR spectroscopic analysis (IRS) of samples of products prepared with KBr on an infrared Fourier spectrometer “Avatar 370” (Thermo Fisher Scientific, Waltham, MA, USA) in the spectral range of 4000–400 cm−1 with the Avatar Diffuse Reflectance attachment; mineragraphic and crystal-optical analysis of polished sections under an optical polarizing microscope of the LEICA DM 2500 P brand. The concentration of regenerated HCl before reuse was determined by the standard titrimetric method of analyzing three aliquot samples of the same aqueous solution. The acidity of the solution was calculated taking into account its weight and the volume of 1 N sodium hydroxide solution spent until a non-disappearing pink color of the alcoholic solution of phenolphthalein appeared.
The technological indicators of the key operations of the created soda-carbothermic technology were verified by 10 multiple tests in the selected optimal mode at the pilot plant of the experimental shop of the Institute of Metallurgy and Ore Beneficiation (OEMC JSC “IMOB”).
3. Results and Discussions
3.1. Characteristics of the Raw Materials
Using sieve analysis of the granulometric composition, we established that the ilmenite concentrate of the Satpayevskoye deposit is a finely dispersed raw material with a low (0.26%) content of dust fraction (
Table 1).
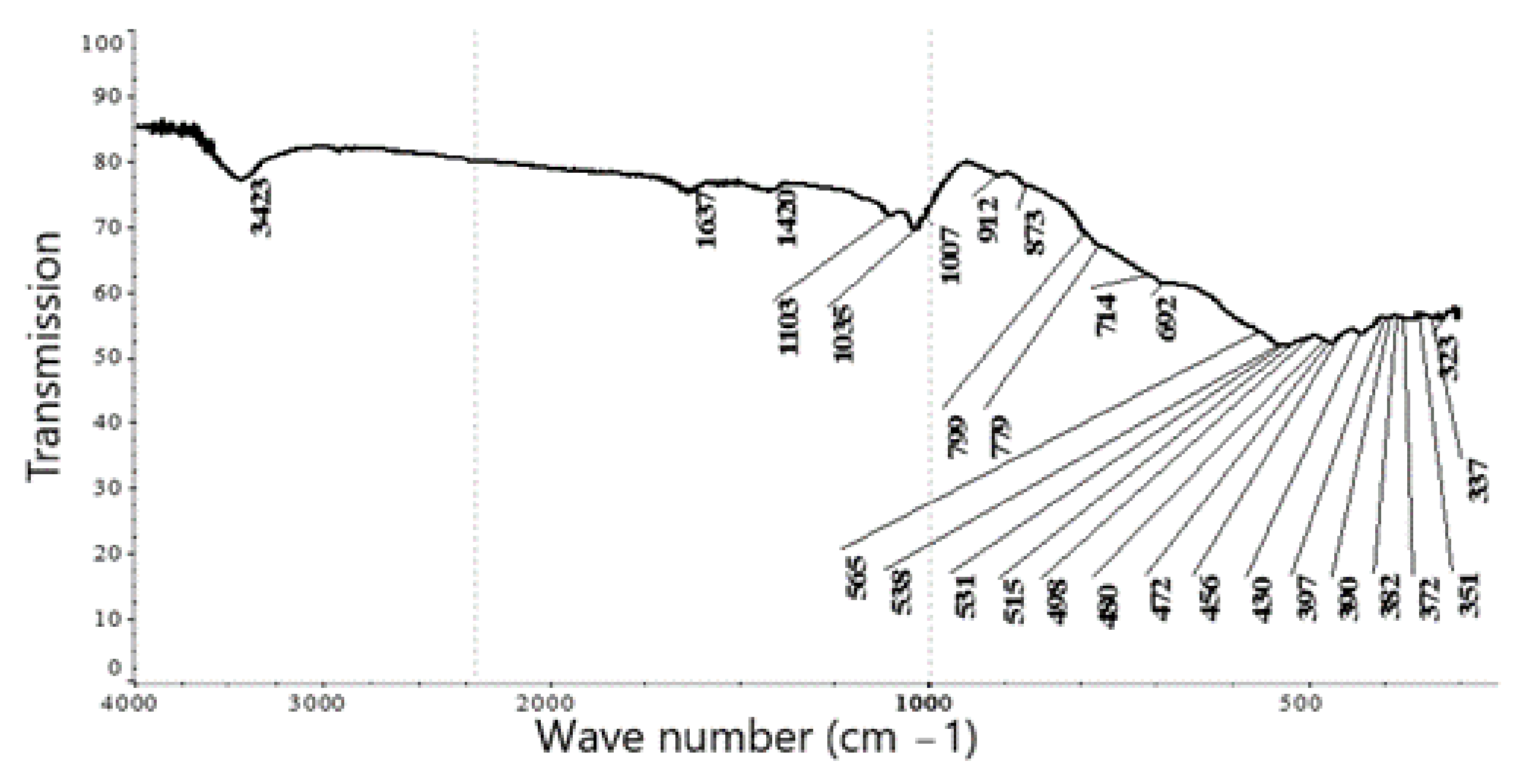
The study of the material composition of the prepared concentrate samples by IR spectroscopic analysis of the wave numbers of the infrared absorption bands, compared with the reference spectra, identified the presence of ilmenite FeTiO
3—metatitanate of divalent iron; its leucoxene variety—pseudobrookite Fe
2TiO
5; polymorphic modifications of titanium dioxide TiO
2—rutile, anatase and brookite; kaolinite Al
4[(OH)
8|Si
4O
10], which is the main component of many clay minerals from the group of hydrous aluminum silicates, formed during the weathering and hydrothermal alteration of feldspar rocks; quartz SiO
2 and calcite CaCO
3 (
Figure 2,
Table 2).
Further research revealed that the presence of rutile, soluble only in hydrofluoric acid, excludes the possibility of processing the ilmenite concentrate of the Satpayevskoye deposit using the traditional method of sulfuric acid decomposition.
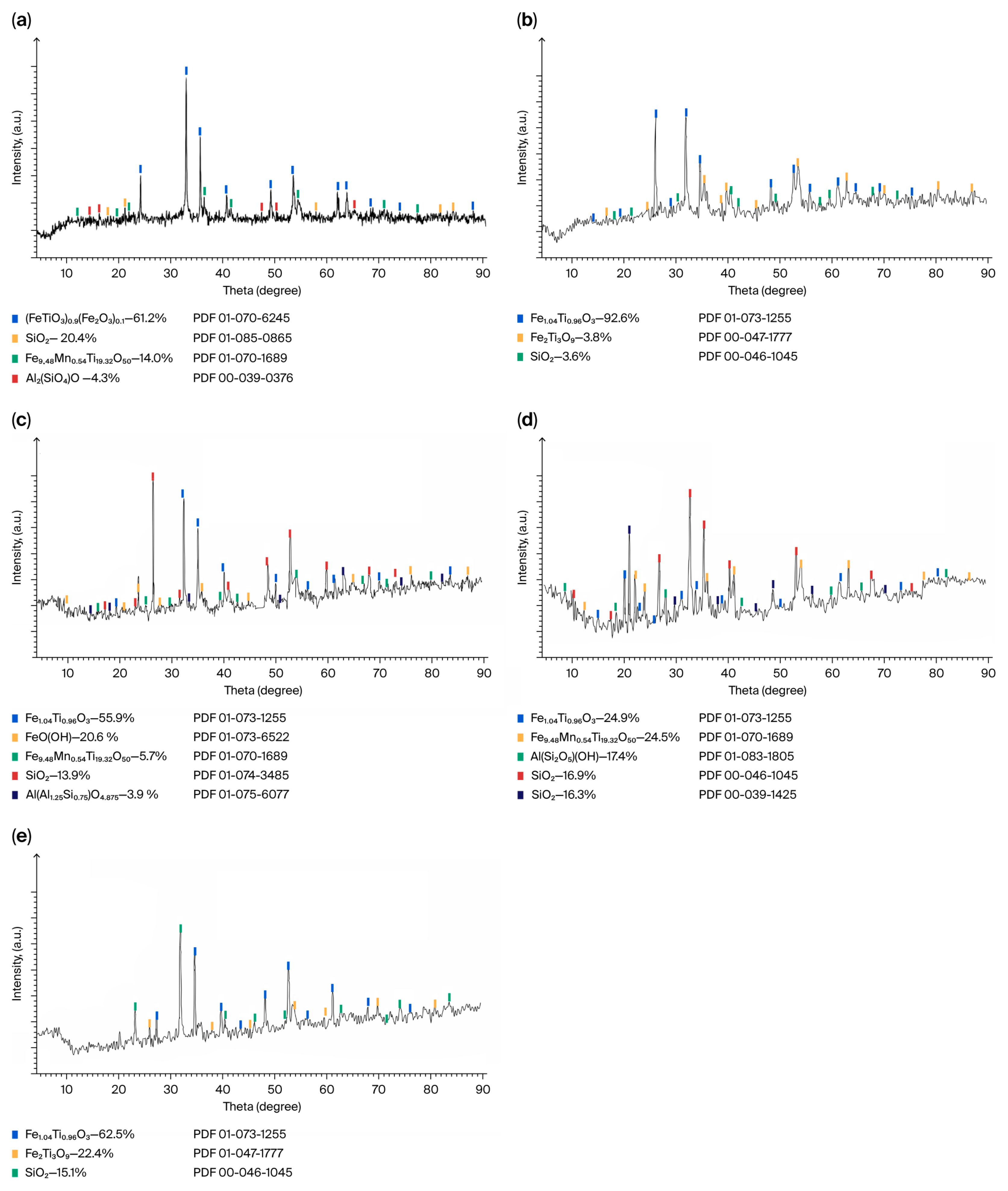
X-ray phase analysis of the material composition of the original and classified concentrate established that the mineral base of the raw material enriched by gravity-magnetic separation is primary ilmenite FeTiO
3 with a mass fraction of over 60% (
Figure 3).
The titanium–iron oxide base of the concentrate is supplemented by pseudorutile of non-stoichiometric composition Fe9.48Mn0.54Ti19.32O50, which is another leucoxene variety of primary ilmenite. In addition to kaolinite, the aluminous association is represented by kyanite Al2(SiO4)O, one of the most common anisotropic aluminosilicate minerals; metamict mullite (syn) Al(Al1.25Si0.75)O4.875, a rare aluminosilicate mineral formed during contact metamorphism of clay minerals; and pyrophyllite Al(Si2O5)(OH), a layered aluminum hydrosilicate mineral capable of splitting into thin sheets when heated.
The silica mineralization is diversified by cristobalite (syn) SiO2—a mineral of high-temperature polymorphic modification of refractory (1713–1728 °C), forming a highly viscous quartz melt. Goethite FeO(OH), a product of weathering in natural conditions at normal temperature and pressure of siderite, magnetite, pyrite and other iron-containing minerals, is manifested in the material composition of the classified concentrate.
It was found that natural primary ilmenite is inferior in strength to the clay mineral kyanite, concentrated in proportion to the grain size in the largest class of concentrate granulometric composition from −1.0 to +0.25 mm. Primary ilmenite and pseudorutile, unlike rock-forming and iron oxide minerals, are present in all classes of concentrate size. The process of leucoxenization of primary ilmenite is accompanied by grinding of its grains and, accordingly, a significant increase in the mass fraction of fine-grained pseudorutile. Aluminosilicate and siliceous minerals and iron (III) oxyhydroxide are unevenly distributed in the material composition of the raw material, the localization of which in a certain class of raw material size is limited by the size of their grains (
Table 3).
Based on grain size, these minor minerals are grouped in the following sequence: kyanite > mullite, goethite, quartz > quartz, pyrophyllite, cristobalite.
Cementation of titanium minerals by fine-dispersed clayey associates hinders the reduction and separation of metallic iron, which is why ilmenite concentrate cannot be processed by carbothermic smelting.
Using the pyrometallurgical enrichment method in an electric arc ore-thermal furnace at a temperature of 1600 °C at UK TMK, we previously established that the ilmenite concentrate of the Satpayevskoye deposit is not suitable for processing by carbothermic smelting due to the formation of a refractory, highly viscous melt, which complicates the recovery, agglomeration and separation of metallic iron from titanium slag.
The sharp disruption of the process technological parameters is explained by the fact that the specified clay minerals, except for kyanite, have a high melting point.
Kyanite, as is known, does not melt, but decomposes at approximately 1100 °C into quartz glass (SiO2) and mullite. The melting point of mullite is 1810–1830, pyrophyllite about 1700 °C, kaolinite 1670–1730 °C, quartz glass 1500–1600 °C. The increase in the viscosity of the melt of clay minerals is facilitated by the temperature in a parabolic dependence and the components of their chemical composition, Al2O3 and SiO2.
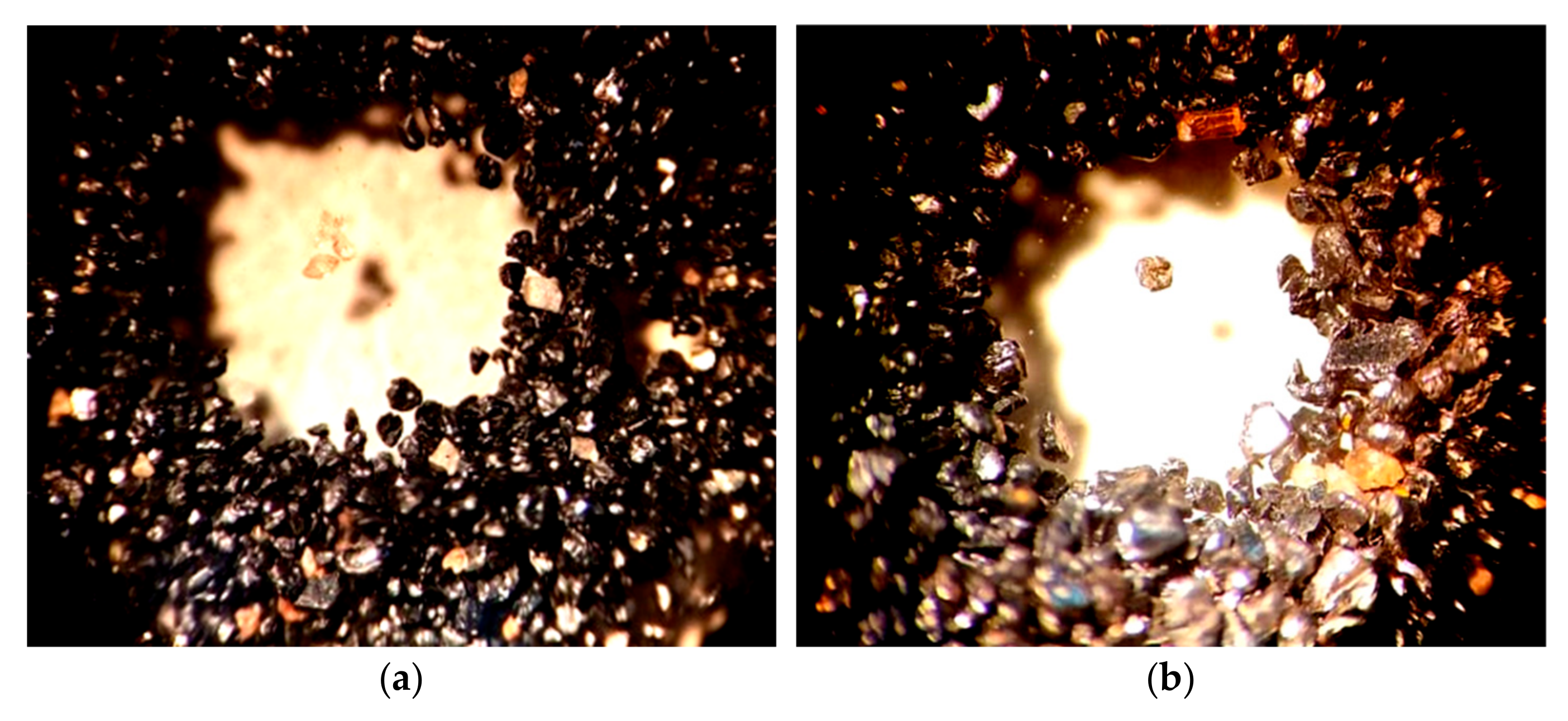
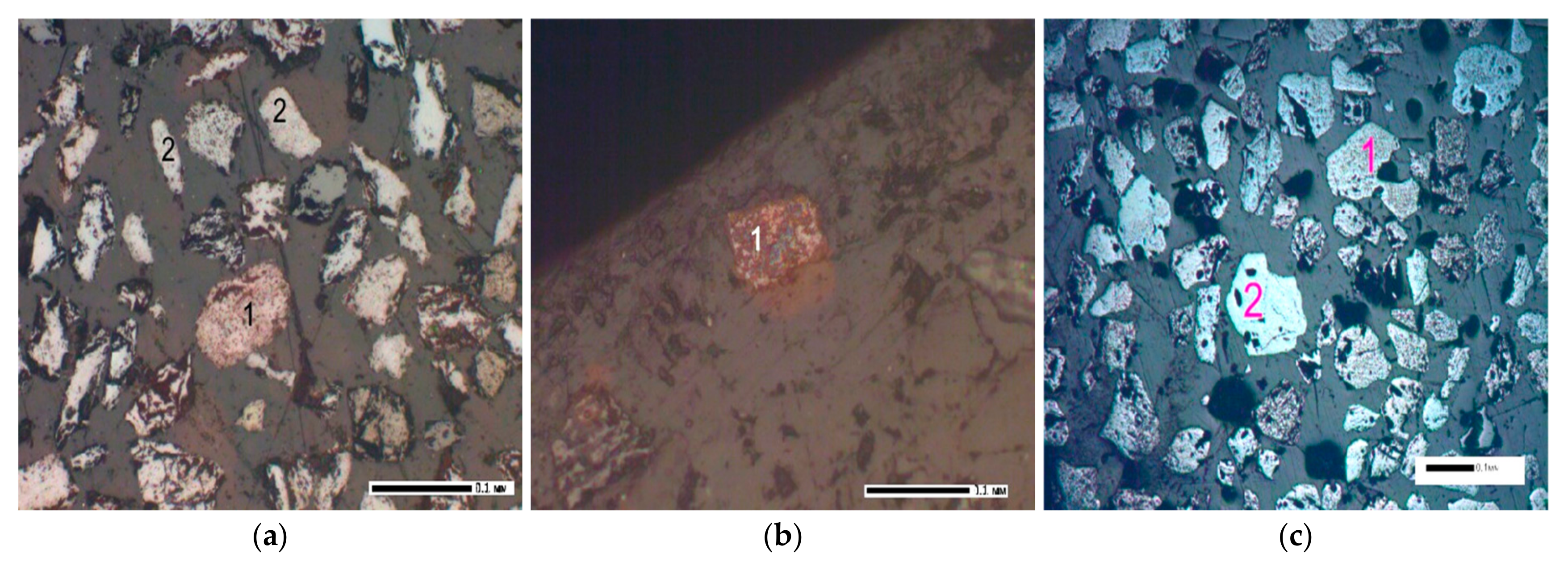
Mineographic analysis revealed the presence of another very refractory (melting point about 2550 °C) accessory mineral of the subclass of island silicates—zircon ZrSiO
4, which differs in the size and configuration of light transparent grains from ilmenite crystals (
Figure 4). The content of uranium, thorium and rare earth elements (REE) in zircon is known to vary depending on its place of origin. For example, in zircons from the Malmyzh granitoids of the Lower Amur Region (Far East), the average uranium content is 89–112 g/t, and thorium content is 58–76 g/t. In some varieties of zircon, REEs can be found in significant quantities, for example, up to 15.89% [
25].
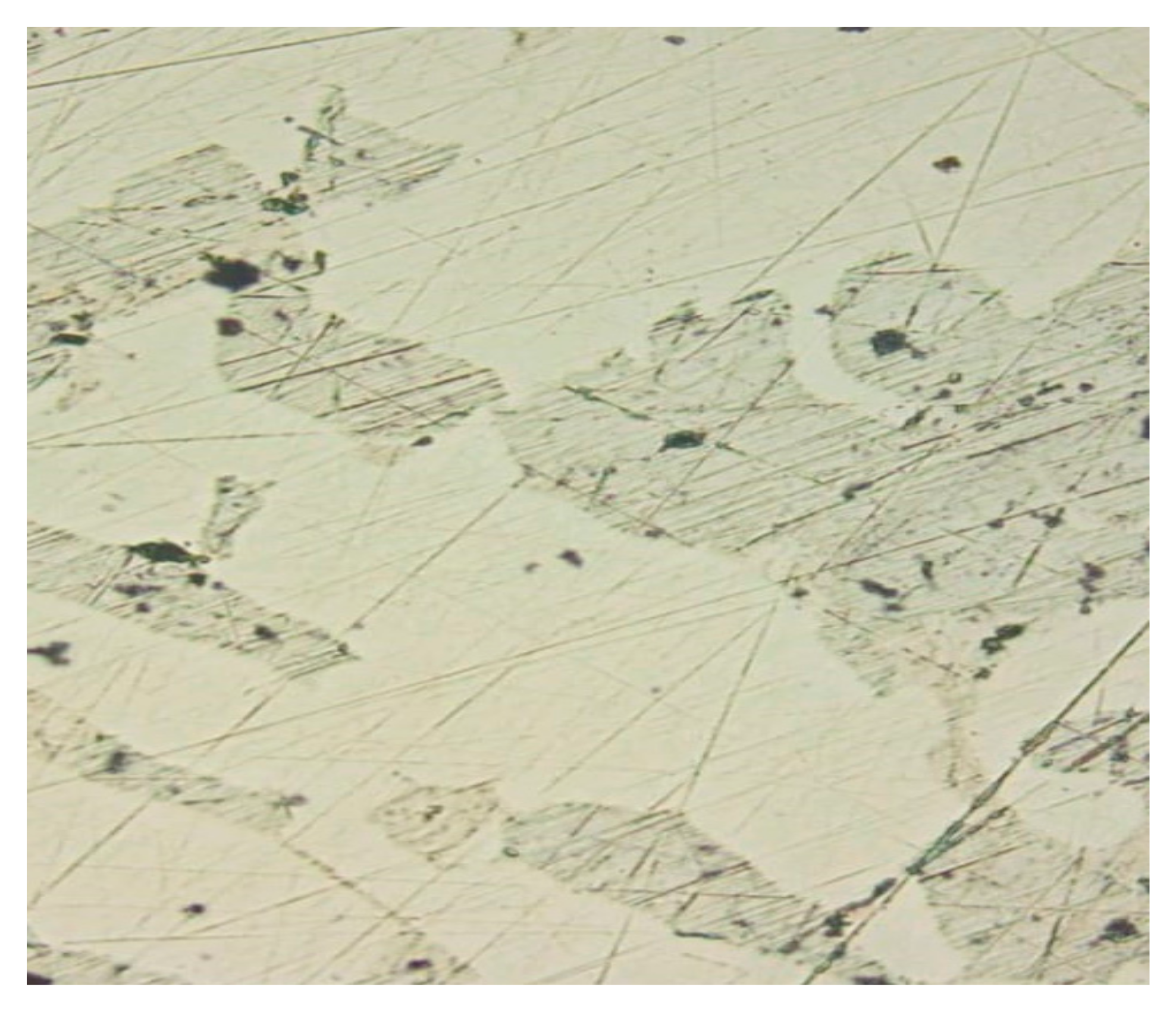
Crystal-optical analysis confirmed the presence of rutile, ilmenite and pseudorutile and revealed another mineral that makes up the material composition of the concentrate, a refractory, widespread, opaque mineral from the class of natural iron oxides (II, III)—magnetite FeO∙Fe
2O
3, decomposing at a temperature of 1591–1597 °C (
Figure 5).
Analysis of the morphology of titanium minerals established that gray with a noticeable pinkish-brownish tint, opaque with a dusty surface, oval and geometrically irregular in configuration, ilmenite crystals, as a result of leucoxenization, when crushed, acquire a light transparent surface, a round or acute-angled, fine-grained, pseudorutile form.
The elemental composition of valuable ore minerals ilmenite, pseudorutile, pseudobrookite, rutile, goethite and magnetite determines the high content of titanium and iron oxides, which makes the concentrate commercially attractive. However, the unacceptably high total concentration, more than 8%, of difficult-to-recover impurity components SiO
2, Al
2O
3, MnO, MgO, CaO and Cr
2O
3 predetermines its low quality. The presence of zirconium oxide has no practical significance due to its small mass fraction (
Table 4).
3.2. Technological Scheme
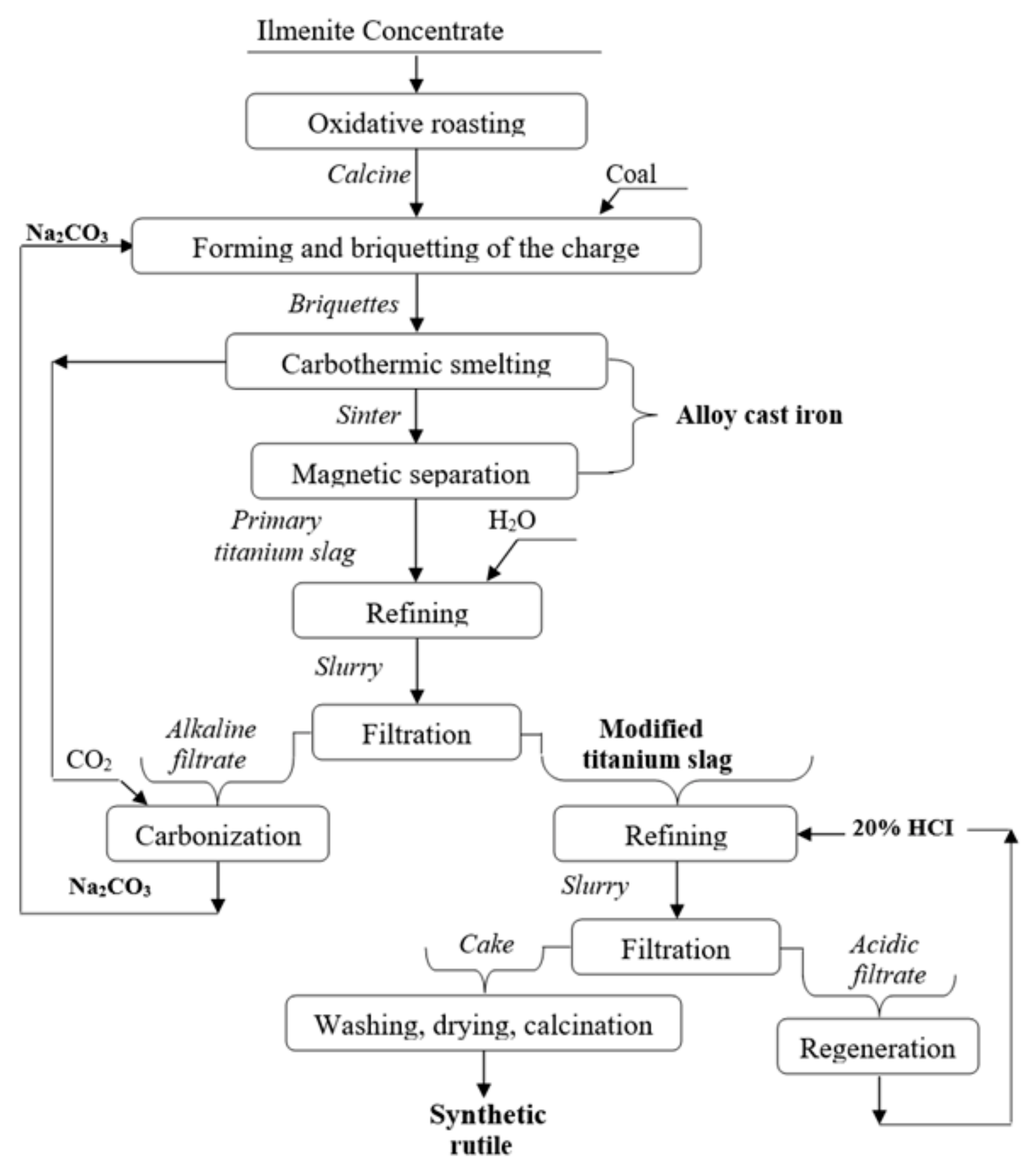
The technology developed by selecting cost-effective and environmentally safe conditions for raw material conversion, schematically shown in
Figure 6, includes oxidative roasting of ilmenite concentrate in an air atmosphere; formation and briquetting of carbon-soda charge of concentrate cinder; carbothermic smelting of briquettes of soda-fluxed cinder; magnetic separation and refining of titanium slag with water, then hydrochloric acid and regeneration of reagents.
Oxidative roasting ensures disintegration of intergrowths and destruction of grains of ore and rock-forming minerals of ilmenite concentrate under the influence of temperature and atmospheric oxygen. According to the results of X-ray phase analysis of cinder, the material composition of the concentrate is transformed due to oxidation of divalent iron and disintegration of clay associates, decomposing with the formation of a weakly crystalline aqueous aluminosilicate clay mineraloid—allophane (Al
2O
3∙2SiO
2∙3H
2O) (
Figure 7).
Briquetting of carbon-soda concentrate cinder is an auxiliary operation intended to minimize carryover and eliminate losses of finely dispersed fraction of concentrate and alkaline reagent with exhaust gases.
Carbothermic melting of soda fluxed cinder briquettes is intended to reduce iron oxides and obtain titanium-enriched primary slag and cast iron.
Magnetic separation is used to remove small iron beads from primary titanium slag.
Refining primary titanium slag with water provides the production of modified slag suitable for the production of premium titanium sponge. Modified slag with hydrochloric acid provides the production of synthetic rutile with multifunctional application.
The regeneration of reagents by carbonization of alkaline filtrate of water refining pulp of primary titanium slag with carbon dioxide released during decomposition of Na2CO3 during carbothermic smelting of concentrate allows the recycling of soda for repeated use; by dehydration in an evaporator of hydrochloric acid filtrate of modified titanium slag pulp—hydrogen chloride.
It is worth noting that the carbonization process is used in various industries, for example in alumina production for the decomposition of aluminate solutions with the release of aluminum hydroxide Al(OH)
3 [
25]. The regeneration of hydrochloric acid is carried out in many countries of the world from spent pickling solutions of metal products, i.e., cleaning from corrosion products (scale, rust, oxide films) in installations with a large-capacity counter-current reactor of the spray type from Rutner (Austria) at a relatively low temperature of 480–520 °C; spraying in a turbulent reactor with an extremely small capacity from Otto Havig (Germany) at a high temperature of 600 °C; fine spraying in a fluidized bed reactor at a temperature of 800 °C by Lurgi (Germany), Keramhemi (Canada), Nippon Steel and Threads Etu Chemical Engineering (Japan) [
26].
Adding soda ash to the batch of oxidized concentrate cinder prevents the formation of refractory lower titanium oxides, accelerates reduction and facilitates agglomeration and separation of metallized iron from the liquid titanium slagmelt (
Figure 8).
The accelerating effect on the reduction of iron is exerted by the melting gas CO, which easily penetrates into the cracks and pores of minerals and is formed upon contact with carbon of soda ash, which decomposes in the initial period of carbothermic melting with the formation of sodium oxide and carbon dioxide CO2—the Deville process.
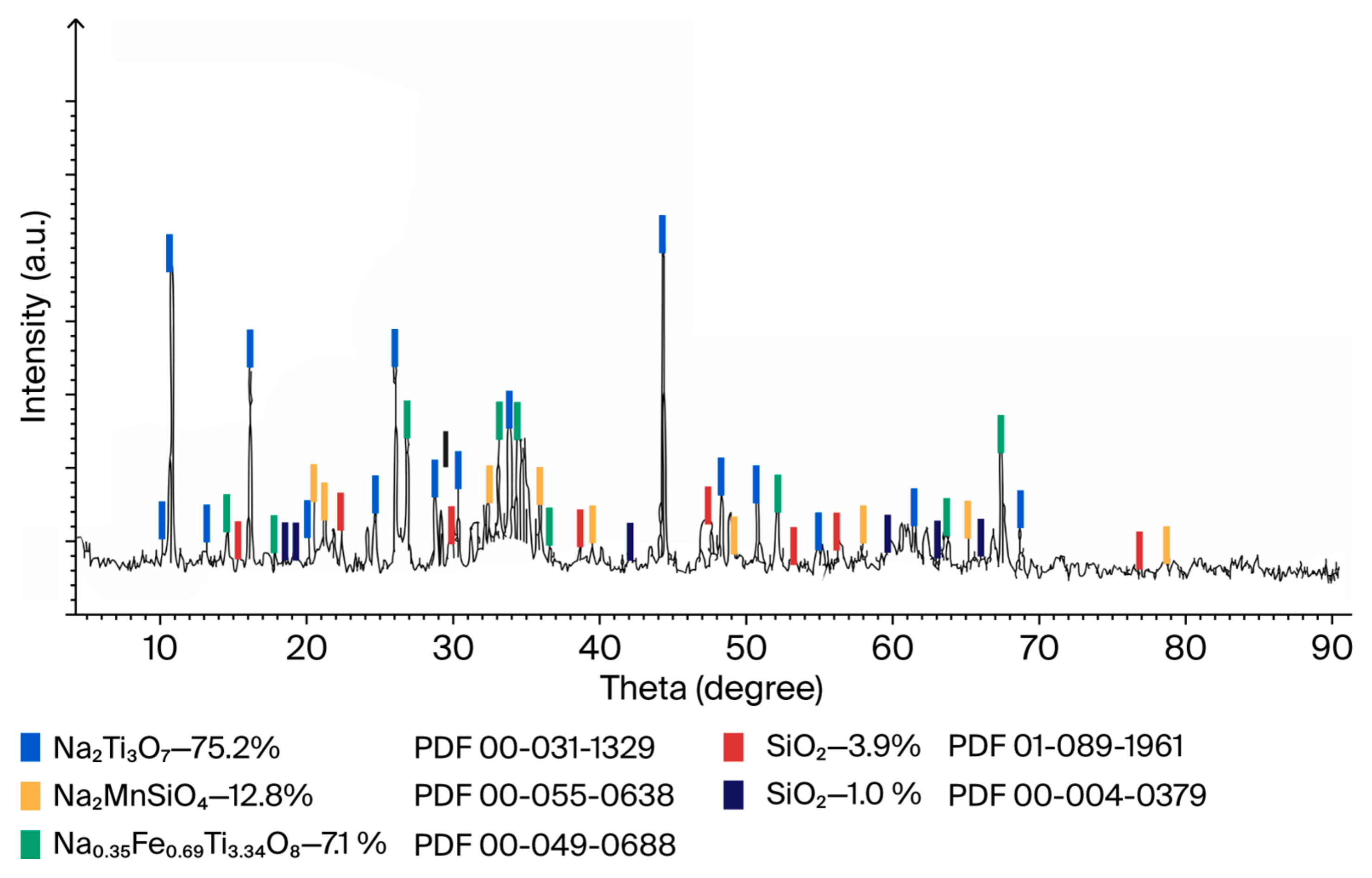
The strongly basic sodium oxide of decomposing soda, binding titanium dioxide into low-melting sodium trititanate Na2Ti3O7 (melting point 1128 °C), reduces the viscosity of the melt and prevents the formation of refractory titanium sesquioxide Ti2O3 (melting point 2130 °C), due to which the energy costs and duration of the carbothermic melting process are significantly reduced.
The phase base of the primary titanium slag, purified by magnetic separation from small beads of metallic iron, is a mixture of electrode materials formed in the process of soda-carbothermic smelting of ilmenite concentrate: Na
2Ti
3O
7 (75%), Na
2MnSiO
4 (13%) and Na
0.35Fe
0.69Ti
3.34O
8 (7%) (
Figure 9).
Solid-phase synthesis of electrode and sorption materials directly from cheap natural raw materials is of great practical importance for large-scale production of battery energy storage systems and purification of nuclear wastewater from radioactive isotopes.
It should be clarified that sodium titanate Na
2Ti
3O
7, obtained using the expensive precursors titanium dioxide TiO
2, titanium metal powder, titanic acid, tetrabutoxytitanate (C
4H
9O)
4Ti and titanium chloride TiCl
3 [
27], is an ideal anode material for sodium-ion batteries, possessing excellent corrosion resistance, durability, lightness, non-toxicity, good electrical conductivity, the ability to reversibly intercalate two Na
+ ions at an average potential of 0.3 V (rel. Na/Na
+) and a theoretical capacity of about 200 mAh/g [
28].
Sodium silicomanganate Na
2MnSiO
4 is not only a promising cathode material for sodium-ion batteries, possessing a high discharge capacity of 210 mAh/g at an average voltage of 3 V and a current of 0.1 C and excellent stability during long-term cycling (500 cycles at a current of 0.1 C) [
29], but also a selective (SMSO) sorbent of trace amounts of strontium ions (90Sr
2+) in a wide pH range from 3 to 12. The degree of Sr
2+ removal, due to the high specific surface area (~559.86 m
2/g) and adsorption capacity (249.0 mg/g), reaches over 98% [
30].
Titanium iron oxide sodium intercalate Na
0.35Fe
0.69Ti
3.34O
8 is a composite anode material [
31] and a synthetic analogue of the rare natural primary mineral freudenbergite, found in samples of hyperalkaline syenites of volcanic rocks in southwestern Germany in Katzenbuckel, containing pseudobrookite, ilmenite, hematite, magnetite and secondary freudenbergite formed as a result of the destruction of pseudobrookite and ilmenite [
32].
The quantitative and qualitative composition of electrode and sorption materials depends to a large extent on the process conditions of their production from ilmenite raw materials. Using low-temperature carbothermic smelting of coal charge of unoxidized ilmenite concentrate with soda ash and diatomite, we have for the first time determined the possibility of obtaining titanium slag, which is a mixture of electrode materials Na2Ti3O7 (48.2%), Na0.23TiO2 (22.0%), Na2TiSiO5 (11%) and Na0.67Al0.1Mn0.9O2 (8.5%), which are transformed during water refining of primary titanium slag into single-phase titanium dioxide with intercalated sodium Na0.23TiO2.
Thermodynamic analysis of the thermal effects of chemical reactions of the interaction of the initial substances and the resulting products has scientifically substantiated the role of silicon and sodium oxides, carbon, oxygen and water in the formation of various electrode materials in the process of carbothermic flux conversion of ilmenite concentrate and water refining of titanium slag [
33].
3.3. Technological Indicators
The reproduction of the results of physical, chemical and experimental studies is confirmed by balance tests of material costs by measuring the weight of processed and obtained products and evaluating the technological indicators of each key operation.
The yield of ilmenite concentrate cinder, titanium slag, alloyed cast iron and synthetic rutile and the degree of extraction of target metals, taking into account the chemical composition of the obtained products, are presented, based on 10 kg of processed ilmenite concentrate, in
Table 5 and
Table 6.
Oxidative roasting allows to reduce the mass of ilmenite concentrate cinder due to dehydration and evaporation of moisture of clay and other minerals and to significantly increase the content of target metal oxides (TiO2 and Fe2O3) in it to 14%. Soda-carbothermic melting of briquetted cinder allows enriching the primary slag with titanium dioxide by 86% compared to the TiO2 content in the cinder due to the reduction and separation of almost the entire mass of metallized iron. Regeneration of primary titanium slag with water improves the quality of modified slag, enriched with titanium dioxide by 66.7% relative to the TiO2 content in the primary slag, by cleaning sodium salts of impurity elements from it. Regeneration of modified slag with hydrochloric acid will remove residual impurities and increase the concentration of titanium dioxide in synthetic rutile.
The optimal mode of key operations ensures complete extraction of titanium dioxide and iron into ilmenite concentrate cinder, titanium dioxide into titanium slags and synthetic rutile, and iron into alloyed cast iron.
The modified titanium slag meets the standard requirements of JSC “UK TMK” in terms of TiO
2 content, and in terms of the residual mass fraction of iron oxide, it surpasses first-class products obtained for the production of premium titanium sponge (
Table 7).
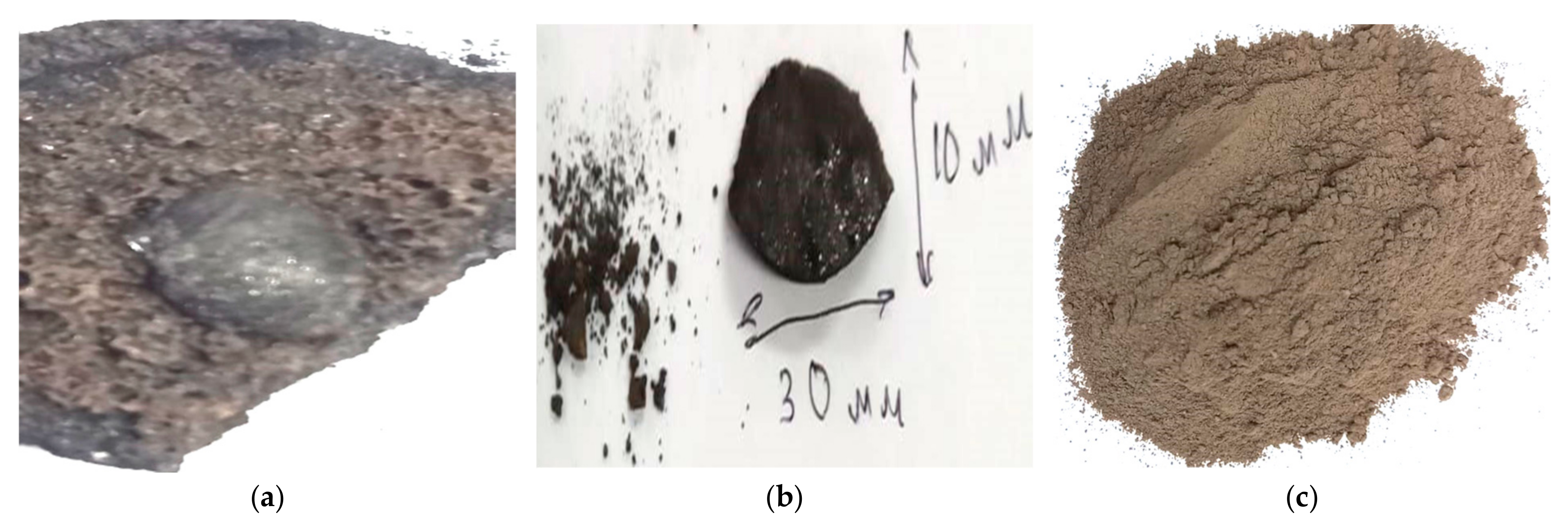
Alloyed cast iron obtained by soda-carbothermic conversion of oxidized ilmenite concentrate meets the standard requirements of ST 73-1917-AO-4-05 of JSC UK TMK (
Table 8) and has high wear resistance due to the standard hardness of 210 HB, determined by pressing a steel ball into the smoothly polished surface of a cast iron ingot (
Figure 10) using the Brunell method according to GOST 9012-59 [
34].
The production of cast iron with such mechanical properties is of great importance for the manufacture of castings or high-strength products for mechanical engineering and machine tool manufacturing.
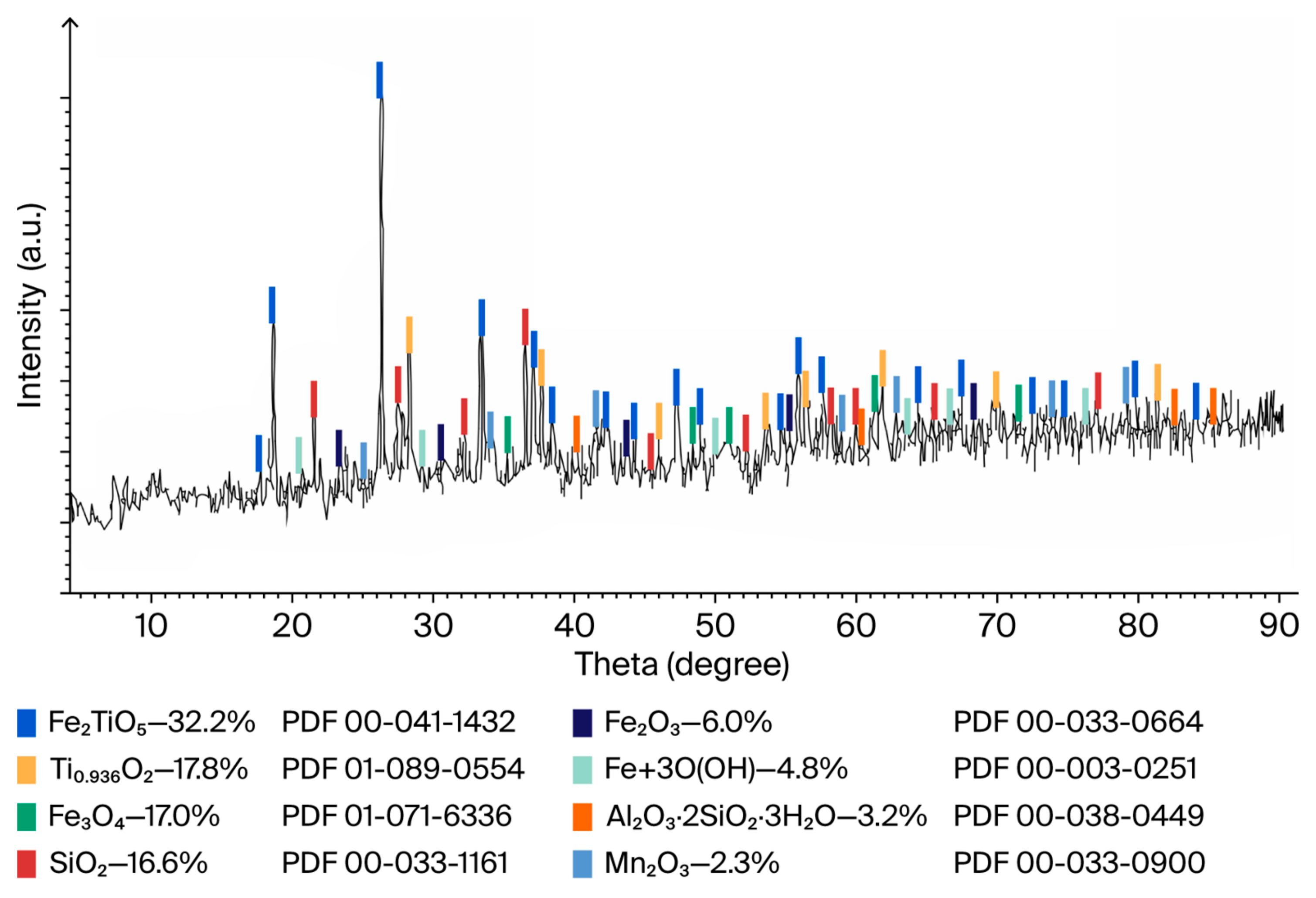
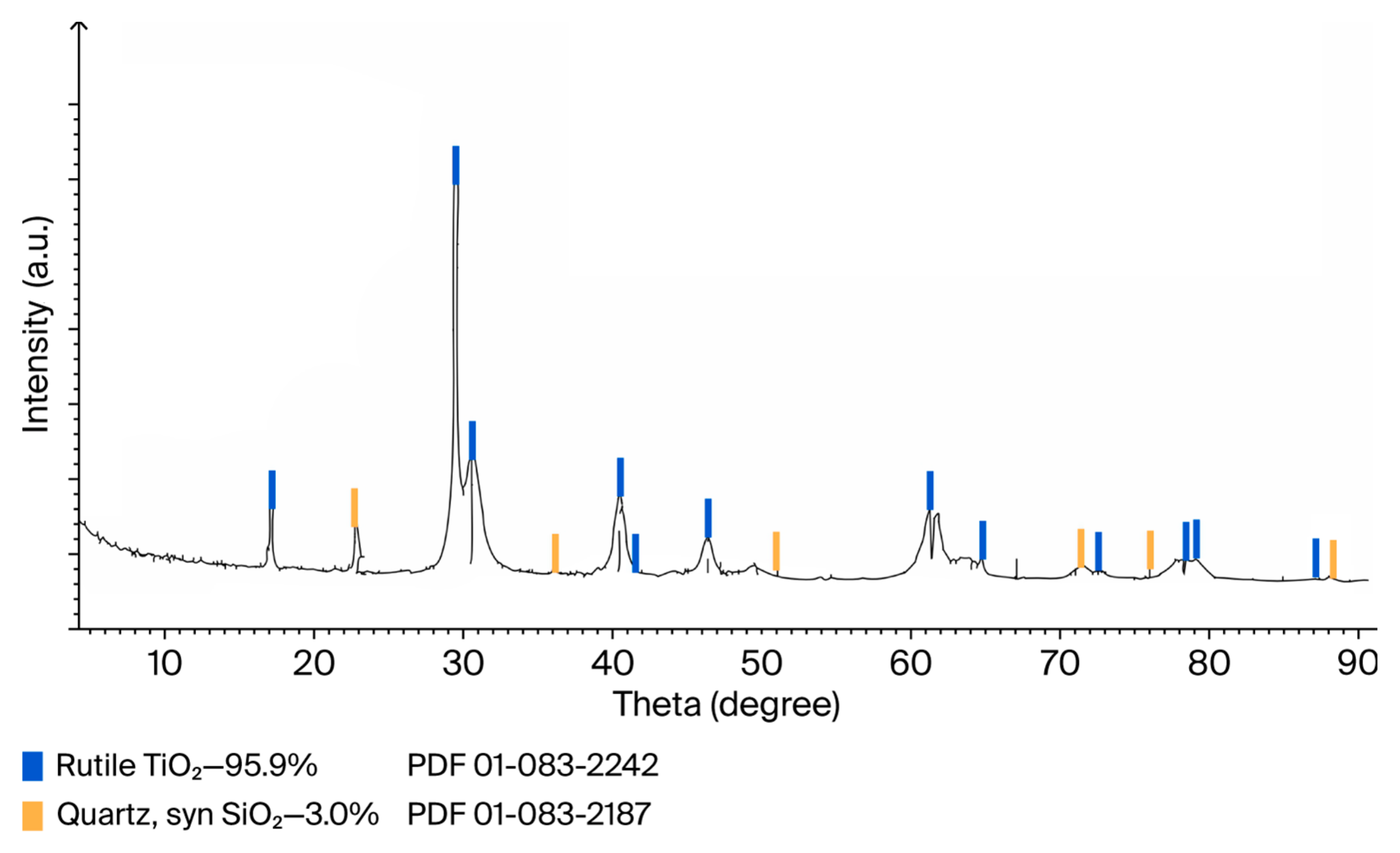
An assessment of the quality using X-ray fluorescence and X-ray phase analysis (
Figure 11) established that the obtained synthetic rutile is not inferior in terms of the content of the main substance (96 wt.% TiO
2) to the branded products of titanium pigments from the American company DuPont (
Table 9).
The trace content of heavy metals in synthetic rutile is wt.%: ThO2—0.047; WO3—0.041; PbO—0.008; CuO—0.019; ZnO—0.016.
The high efficiency of key operations indicates the possibility of obtaining a multiplier effect from the implementation of the created technology, the practical application of which can be carried out in stages on the industrial equipment of LLP “SGOP” and JSC “UK TMK”.
The initial implementation of the oxidizing roasting operation at the enrichment plant of LLC “SGOP” will significantly improve the quality and facilitate further processing of domestic ilmenite concentrate together with high-quality, imported raw materials using the technology used at UK TMK.
The subsequent implementation of the soda-carbothermic melting operation of thermally activated ilmenite concentrate will eliminate the consumption of imported raw materials and energy costs. The implementation of the refining operation will improve the quality of titanium slag and expand the range of commercial titanium products for multifunctional applications.
4. Conclusions
Ilmenite concentrate from the Satpayevskoye deposit is not suitable for processing by commercial hydro- and pyrometallurgical enrichment methods due to the presence of rutile, soluble only in hydrofluoric acid, and many refractory clay associates cementing titanium ore minerals in its material composition.
Innovative soda technology for oxidation–reduction conversion of highly resistant mineral raw materials with regeneration and recycling of chemical reagents ensures the production of first-class titanium slag for the production of premium titanium sponge, alloyed cast iron for the manufacture of high-strength and wear-resistant products for mechanical engineering and machine tool building, and high-quality synthetic rutile, superior to the branded products of titanium pigments from the American company DuPont.
Soda-carbothermic melting is a promising method for solid-phase synthesis of various electrode materials for sodium-ion batteries directly from cheap natural raw materials.
The implementation of the created technology at the Ust-Kamenogorsk Titanium Magnesium Plant, which solves the problem of import substitution, energy saving and environmental protection, will reduce the cost of production of premium titanium sponge, expand the range of commercial titanium products with high added value and reduce the anthropogenic impact of the enterprise on the ecosystem.