1. Introduction
Copper mine tailings are a concerning waste of mining extraction activity. It is the result of the concentration of copper sulfides by separation of copper sulfide ore from gangue ore by flotation methods [
1,
2]. As a result, the gangue is transported as a slurry to its final deposition in tailing dams. Given the current standard conditions in a mill plant concentrator (i.e., copper head grade 1%, copper recovery of 90%, and tailings copper grade of 0.1%), for each ton of copper concentrate produced, approximately thirty tons of tailings are generated. The exposure of tailings to air and water can lead to dangerous risks such as acid mine drainage, toxic chemical exposure, dust contamination and landslides due to tailings dumps failure [
3].
On a global scale, mine tailings are among the biggest streams of industrial waste, with estimates suggesting that around 10 to 14 billion tons are generated each year across all mineral sectors [
4,
5]. The tailings produced from copper mining are especially concerning due to their large volume and unstable geochemical nature. As the world faces declining ore grades and a growing demand for minerals, the volume of generated tailings is expected to increase in the coming decades [
6]. This trend brings with it serious environmental and geotechnical risks, along with increasing challenges for effective long-term mine waste management and sustainable mineral processing practices.
Copper tailings usually have a high presence of crystalline silicates, aluminates, and iron-rich minerals. Also, one can commonly find oxides like calcium oxide (CaO), magnesium oxide (MgO), potassium oxide (K
2O), and sodium oxide (Na
2O), all present in different ratios [
7,
8]. It is well known that copper tailings composition is highly dependent on the mine deposit formation and the milling processes applied [
9,
10]. Mill plant processing can alter the original crystalline structures and the chemical composition of the materials, which can improve the reactivity of the tailings by increasing the surface area and size of crystal structures [
11]. Physicochemical and chemical processes are applied during the concentration process to activate or depress certain ores, because they can modify the mineral surface chemistry and pulp composition, which may lead to different mineralogical and chemical characteristics of the resulting tailings [
12].
On the other hand, the construction materials industry is facing its own challenge: to reduce its CO
2 footprint, since the production of Ordinary Portland Cement (OPC), which the main ingredient of concrete, is the source of roughly 8% of anthropogenic CO
2 emissions worldwide [
13,
14]. This situation highlights the need for new low-carbon construction materials that are not derived from conventional cement production, but rather from the valorization of industrial residues, like copper mine tailings. One promising path in this sense are alkali-activated materials (AAMs).
AAMs are formed from tetrahedral chains of AlO
4 and SiO
4, linked by oxygen atoms [
15]. They are charge-balanced by alkali cations, which are usually potassium, sodium, or calcium that come from an added activating solution. To create them, one needs a precursor that is rich in aluminosilicates, along with a co-activator that provides additional chemical elements necessary for the reaction to complete, plus the addition of an alkaline activator that sets off the polymerization process [
16].
Copper tailings also offer environmental advantages when they are used in AAMs, within which geopolymers represent a specific subgroup characterized by a three-dimensional aluminosilicate network, generally derived from low-calcium precursors such as fly ash, metakaolin, or mine tailings [
17,
18]. The geopolymerization process can contribute to the stabilization and immobilization of toxic heavy metals, such as sulfides from pyrite and chalcopyrite, by encapsulating them within the aluminosilicate matrix gel formed during the alkaline activation [
19]. Previous researchers have also shown that with correct treatment and curing time, copper tailings could have similar mechanical performance to cement [
20,
21].
In previous research [
22], a methodology was proposed to assess the potential of mine tailings as supplementary cementitious materials (SCMs) by following the mass percentage of CaO, SiO
2, and Al
2O3 + Fe
2O
3. Based on this work, the present authors have proposed to change the ternary diagram to handle alkali-activated materials (AAMs) by using three main components that can affect geopolymer performance: Al
2O
3 mass fraction, Fe
2O
3 mass fraction, and CaO + MgO + K
2O mass fraction. This classification allows for the identification of tailings with high geopolymer potential, serving as a foundation for a more detailed analysis of their feasibility as geopolymer precursors.
The CaO, MgO and K
2O diagram has been selected based on previous work in the geopolymerization area. Zhang et al. [
23] indicated that tailings with high contents of CaO, SiO
2, and Fe
2O
3 can substitute natural raw materials of calcium, silicon, and iron, and therefore can be used in the production of an alternative cementitious material. The study by Simonsen et al. (2020) [
22] showed that most of the tailings they catalogued exhibited low to neutral alkalinity (pH 6.7–9.4), suggesting that the addition of a CaO-rich source could help prevent a corrosive environment. Moreover, the inclusion of a calcium source—such as blast furnace slag, steel slag, Portland cement, calcium hydroxide (Ca(OH)
2), or calcium oxide (CaO)—can release additional heat during geopolymer formation, improving mechanical properties [
24]. Also, in the case of high-calcium fly ash, the naturally occurring CaO content can also contribute to the early development of mechanical strength in the geopolymer [
25]. Additionally, the study by [
26] highlights the importance of hydration products in alkali-activated materials, particularly the sodium aluminosilicate hydrate (N-A-S-H) gel and the calcium aluminosilicate hydrate (C-A-S-H) gel. In systems such as those based on tailings containing calcium, aluminum, and silicon simultaneously, both gels may coexist, forming a hybrid network that combines the structural properties of each phase. The simultaneous formation of N-A-S-H and C-A-S-H gels can enhance the mechanical strength of the hardened material and improve its long-term stability. This study also considers the effect of K
2O, as it may enhance the geopolymerization of copper tailings. The K
+ ion tends to promote the formation of larger silicate oligomers, thus playing a more significant role when using solutions with higher silicate contents [
27]. MgO may also influence the mechanical performance of geopolymers produced from copper tailings; therefore, it was included in the ternary classification diagram. MgO is considered an expansive component that can lead to volumetric instability due to delayed expansion [
28], and its content should be controlled to prevent long-term issues related to durability and strength. Furthermore, studies have shown that in tailings with high sulfur content (S ≈ 9%) the MgO can act as an alkaline agent by reacting with ground granulated blast furnace slag (GGBS), activating it and promoting the formation of calcium silicate hydrate (C-S-H) and magnesium silicate hydrate (M-S-H) phases—both of which significantly contribute to the strength of the aggregates [
29].
On the other hand, Al
2O
3 promotes polymerization reactions and Fe
2O
3 contributes to the formation of geopolymer gel [
30]. It is well established that the release of aluminum into the solution from the solid aluminosilicate source governs the rate, stoichiometry, and extent of the reactions occurring in the solution phase [
27]. Moreover, geopolymers can serve as a support matrix for photoactive components, and those synthesized from residues containing Fe
2O
3 have been shown to exhibit intrinsic photoactive behavior [
31].
The strategy of this study consisted in evaluating the potential of copper tailings for geopolymerization by combining chemical classification by mass fraction presence of the compounds of interest—obtained from XRF data—with their compressive strength testing performance. In this sense, one sample (R1) was selected from the “high-geopolymeric potential zone” and another (R3) from the “low-geopolymeric potential zone” for test comparison. This approach aimed to explore whether a chemical composition identification alone could serve as a reliable predictor of mechanical performance.
A second phase of the methodology used mineralogical composition as an additional variable. The use of mineralogy allowed for a more comprehensive understanding of the compressive strength test results.
In summary, this paper proposes a methodology to evaluate the potential of copper tailings to be used as a geopolymer construction material. The methodology has been applied to three different tailings compositions.
2. Materials and Methods
2.1. Tailing Samples
To assess whether the tailings samples used in this study are representative of their respective ore deposits, geological characterization plays a critical role. It is known that mineralogical composition of mine tailings is strongly influenced by the ore deposit type, which controls the formation of ore minerals and associated gangue phases, as well as the processing techniques applied [
10]. Understanding these geological and mineralogical relationships is essential to ensure that the samples analyzed are representative of their respective deposits.
Three samples of copper tailings were obtained from different mining districts in central Chile, with each having distinct ore deposit types: two porphyry Cu-Mo systems (samples R1 and R2) and an iron oxide-copper-gold (IOCG)-style deposit (sample R3).
Sample R1 derives from a tailing pond associated with a large porphyry Cu-Mo system hosted in subvolcanic mafic intrusions affected by intense hydrothermal alteration. The deposit is located in the main Andean Mountain range of central Chile. Mineralization is characteristic of a high-sulfidation state core within a larger porphyry system, with a complex intrusion history and telescoped alteration zonation including potassic, phyllic, and chlorite-sericite assemblages. The main ore minerals in the system are chalcopyrite and bornite, while assemblage of gangue minerals include quartz, anhydrite, biotite, muscovite (sericite), and K-feldspar [
32,
33,
34].
Sample R2 is derived from a tailing pond linked to another porphyry-style deposit, with a prolonged magmatic-hydrothermal evolution and a multiphase intrusive and breccia history. This system exhibits a diversity of alteration types, including overprints of advanced argillic, and is characterized by abundant tourmaline and anhydrite. Ore minerals include chalcopyrite, bornite, molybdenite, and pyrite, while the gangue minerals include quartz, secondary biotite and K-feldspar that defines the early potassic alteration, muscovite (sericite), and aluminosilicate-rich breccias [
35,
36].
Sample R3 came from a tailing pond related to a structurally controlled vein-type copper deposit situated in the Coastal mountain range. This system, associated with iron oxide-copper-gold (IOCG) mineralization, is hosted in dioritic rocks and is characterized by a central mineralized vein which represents a mineralized fragile fault zone (filled with clays or fault gouge) surrounded by a narrow alteration halo, characterized by chlorite-sericite-calcite alteration. The ore association consists of chalcopyrite and bornite, while the gangue minerals include high contents of specular hematite, magnetite, and quartz. These features are typical of IOCG-type systems developed in arc-related settings [
37,
38].
2.2. Effect of the Co-Activator
To evaluate the performance of the tailings as geopolymers, tests were carried out using mixtures containing varying proportions of cement and tailings, reaching up to 100% tailings proportion. Ordinary Portland Cement (OPC) Type I was used in this study, in compliance with the ASTM C150/C150M standard [
39].
Table 1 presents the chemical composition of the cement employed [
40].
2.3. Tailings Group Classification by Ternary Diagrams
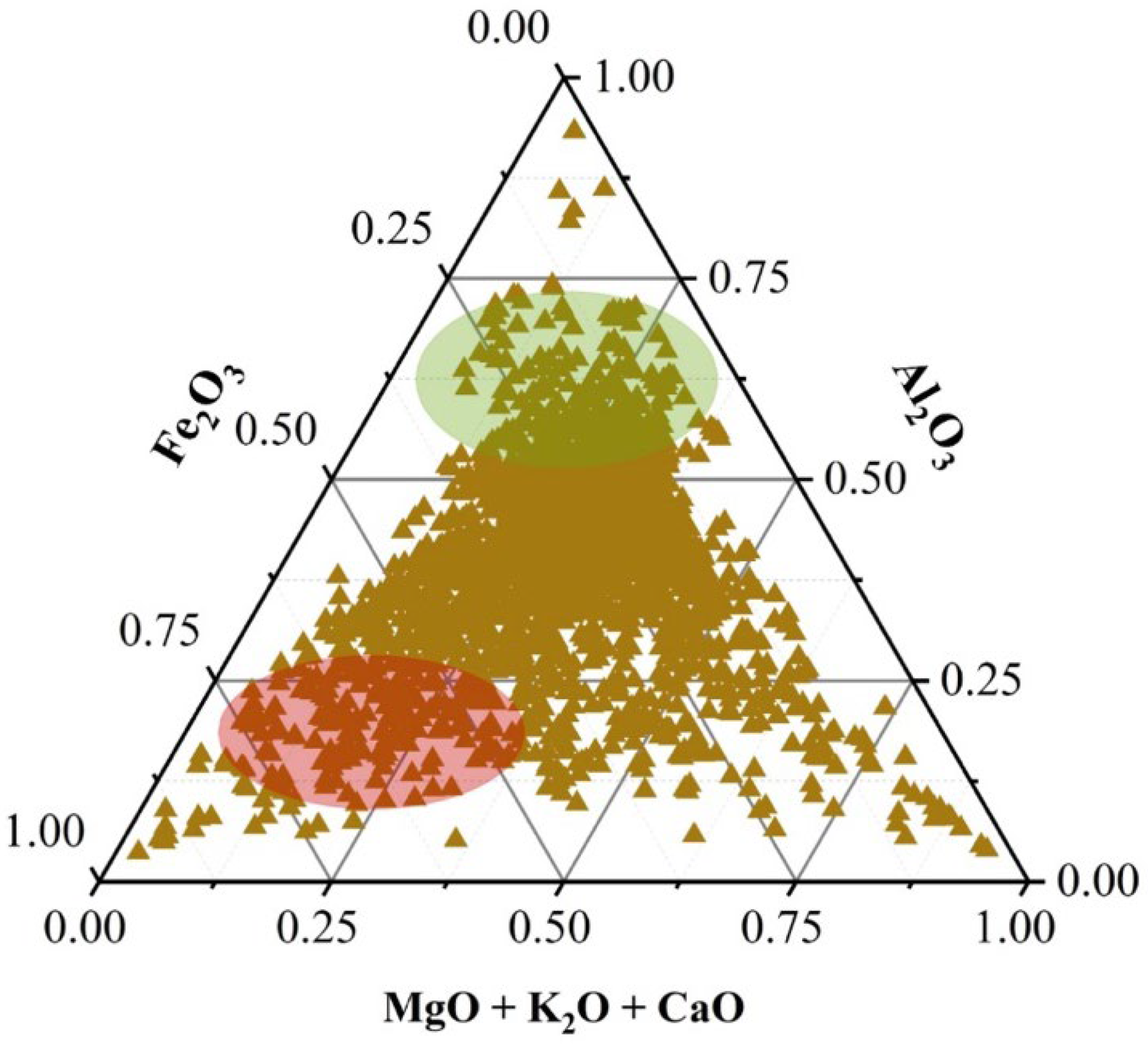
This section presents a ternary diagram based on the selection of compounds that are most relevant to the geopolymerization process. Specifically, three compositional axes were defined: (1) mass fraction of Al2O3, (2) mass fraction of Fe2O3, and (3) summed mass fraction of CaO, K2O, and MgO.
As shown in
Figure 1, the proposed ternary diagram allows for the classification of the tailings into high- or low-geopolymeric-potential zones, depending on their proximity to predefined compositional domains. These reference zones are marked in green (high potential) and red (low potential).
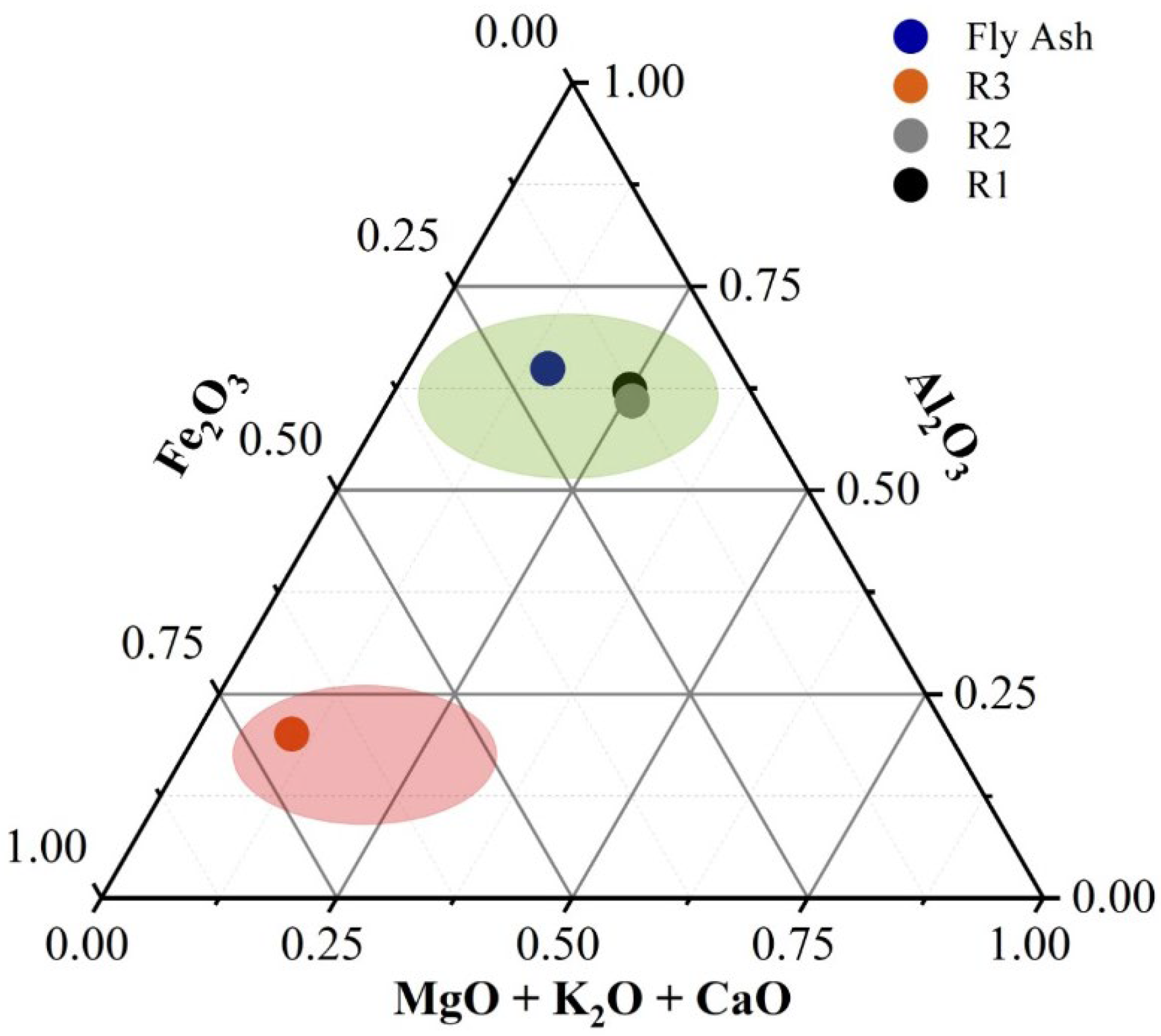
Accordingly, the specific tailings analyzed in this study are presented in
Figure 2. Based on their geochemical composition, R1 and R2 can be grouped together with fly ash as high-potential tailings, whereas R3 is positioned at the opposite end, corresponding to materials with lower theoretical mechanical performance.
2.4. Analytical Equipment
To establish a technical profile for each tailings sample, physicochemical and mineralogical characterization tests were conducted using X-ray fluorescence (XRF) and quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN) analysis methods.
XRF was essential for determining the elemental composition of each sample, focusing on the most abundant oxides: SiO2, Al2O3, Fe2O3, CaO, MgO, and K2O. This information was used to define an initial classification of the samples as potential geopolymer precursors.
The analysis was performed using a wavelength-dispersive sequential spectrometer (Bruker S4 Explorer, AXS GmbH, Karlsruhe, Germany). Each sample was prepared in pellet form using a mixture of 10 g of tailings and 1 g of binder, compacted into tablets with an automatic press at 250 kN. Measurements were conducted using the Fast-Vac34 method, providing elemental results. This method offers a reliable approximation of the elemental composition of the samples.
In addition, QEMSCAN analysis was performed to obtain quantitative mineralogical data, allowing for the identification of mineral phases and the estimation of their relative abundance. For this purpose, 100 g of each tailings sample was analyzed using a QEMSCAN MOBILE system (iDiscover 5.3 and iMeasure 5.3, FEI Company, Hillsboro, OR, USA), applying the Particle Mineralogical Analysis (PMA) technique at an accelerating voltage of 20 kV. The samples were mixed with graphite (supporting matrix), embedded in epoxy resin (for homogenization), ground and polished (to improve imaging quality), and coated with a thin layer of carbon (for protection during measurement). This analysis is critical to understanding which mineral phases may influence the performance of the resulting geopolymer.
2.5. Geopolymer Sample Preparation
For each mix design, three replicate cubic specimens (2 × 2 × 2 cm) were prepared and evaluated independently. The specimens were cured in an oven at 60 °C for 28 days and then tested under uniaxial compression.
To produce geopolymers based on tailings, a particle size below 150 µm was selected to maximize surface area and enhance participation in the geopolymerization reaction, as reported in previous studies [
41,
42]. Detailed particle size distribution (PSD) data obtained from QEMSCAN analyses for all tailings are provided in
Supplementary Table S4.
For the preparation of the cubic specimens, 1000 g of tailings were used, previously dried in an oven at 60 °C for 24 h. All specimens were made from this pre-treated material. Depending on the intended mixture, the required amount of tailings was weighed: for example, to produce a geopolymer with 90% tailings content, 90 g of tailings and 10 g of OPC were used.
Tailings activation was carried out using a 10 M NaOH solution, which has shown good results in previous studies [
30]. The mixing process began with an initial mixing stage using a vertical mixer (SCILOGEX OS40-S, SCILOGEX, LLC, Rocky Hill, CT, USA, 0–2200 rpm range), operating at 1000 rpm. The process started at low speed (500 rpm), followed by the addition of 35 g of 10 M NaOH as the alkaline activator. The speed was then increased to medium (1500 rpm) and maintained for approximately 3 min.
Once mixing was completed, the molding process began using 2 × 2 × 2 cm cube molds. The mixture was poured to fill one-third of the mold and vibrated for 10 s using a JT14 vibrating table (UTEST Material Testing Equipment Inc., Ankara, Turkey). The process was repeated for the second and final thirds, with vibration applied at each stage. After molding, the specimens were placed in an oven at 60 °C for 24 h. Finally, the cubes were demolded and returned to the oven to complete a total curing period of 28 days.
2.6. Experimental Method
The objective of the experimental design in this study was to evaluate the mechanical performance of geopolymeric mixtures—using tailings as the main precursor and OPC as a co-activator—through compressive strength. The test was performed in accordance with ASTM C109/C109M-21 [
43], which specifies the procedure for testing hydraulic cement mortars using cubic specimens, like the ones used in this research.
The aim was to understand the behavior of geopolymers incorporating different levels of OPC in order to identify compositions capable of achieving sufficient strength for potential use as construction materials. In this regard, for each selected tailings sample, five mixtures were prepared with varying cement-to-tailings proportions. The proportions of tailings and cement were calculated based on the total weight of solid materials. All mixtures were prepared under identical experimental conditions to ensure consistency in the results. Mixtures were made of 100%, 97.5%, 95%, 92.5% and 90% tailings, complemented by 0%, 2.5%, 5%, 7.5%, and 10% OPC by weight, respectively.
2.7. Compressive Strength Test
The test specimens were placed in a GeoTac triaxial press (GeoTac, Inc., Arlington, TX, USA), with a 2000 lbs load cell, applying a constant loading rate of 1 mm/min, to determine their maximum compressive strength. This value was used as the primary indicator for comparing the mechanical performance of the different geopolymeric mixtures.
Subsequently, the results obtained from both sets of tests were analyzed and compared to establish relationships between compressive strength and the proportions of tailings used in the mixtures.
3. Results
3.1. Sample Characterization
The results of the XRF technique are presented below in
Table 2.
In general terms, both R1 and R2 samples exhibited similar compositions, characterized by a high Al2O3 content and a low Fe2O3 presence. A high Al2O3 content is essential for the formation of the aluminosilicate oligomer network that constitutes the geopolymer structure. Additionally, the proportions of other oxide species (MgO, CaO, and K2O) were relatively low, suggesting that the material could retain good reactivity without compromising the structural stability of the geopolymer.
In contrast, R3 sample showed a chemical composition with elevated Fe2O3 levels and low Al2O3 content, conditions that are unfavorable for the reactions required in the geopolymerization process.
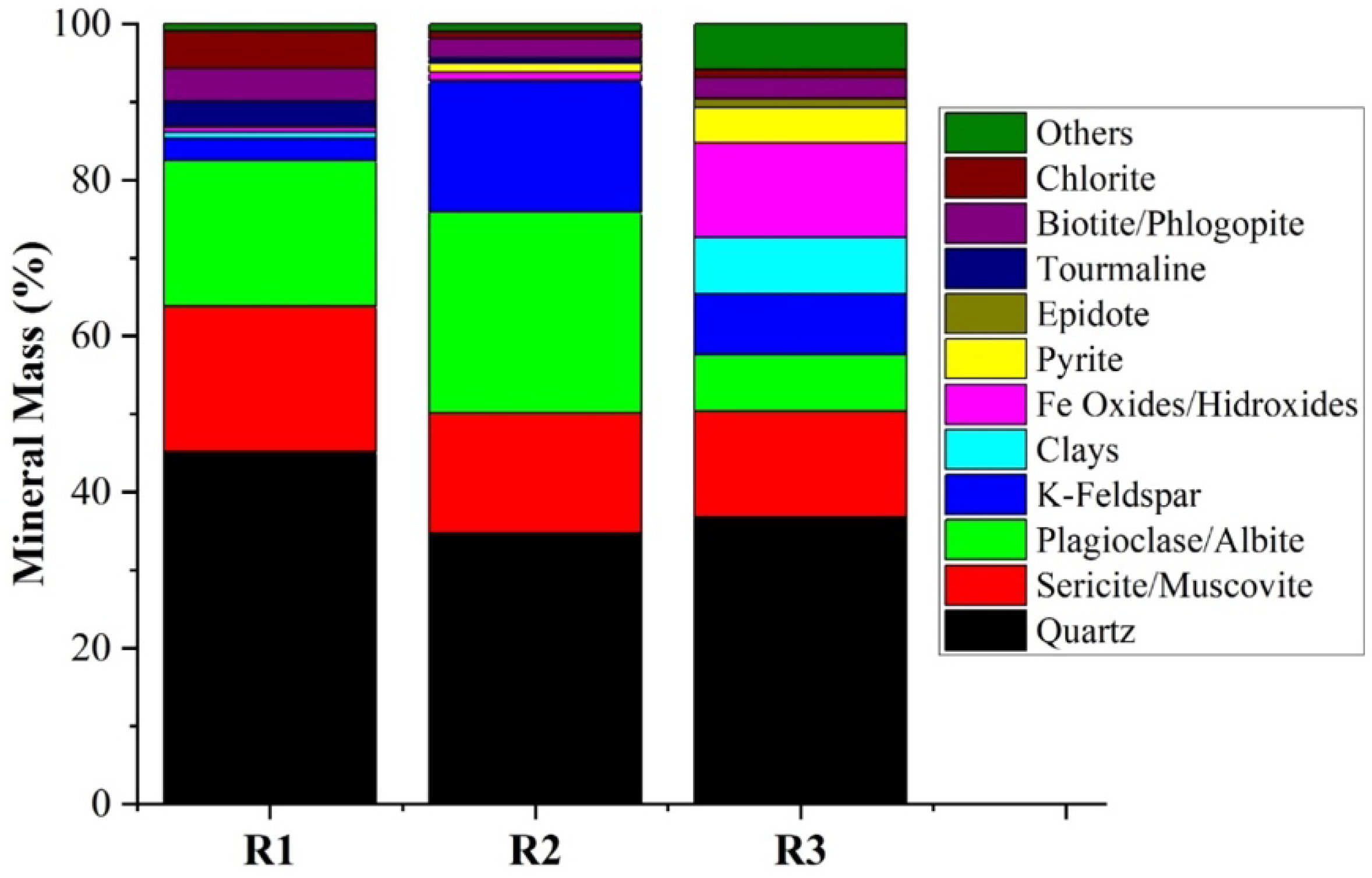
The QEMSCAN results are shown in
Figure 3 and
Table 3. Complete datasets, including mineral associations, occurrence, and particle mapping for all analyzed tailings, are provided in
Supplementary Tables S1–S3. In general, all three analyzed tailings samples showed quartz as the primary mineral phase, along with other aluminosilicate minerals such as feldspars, plagioclase, and chlorite. These minerals are fundamentally important for the geopolymerization reaction, as they supply essential elements such as Si, Al, and Fe.
Sample R1, derived from a porphyry Cu-Mo system within a subvolcanic mafic rock, had the highest quartz content, and higher sericite/muscovite and plagioclase. This composition fits very well with the potassic and phyllic alteration zones that are commonly reported in these systems, in which white micas and quartz represent the main gangue minerals.
Sample R2, also from a porphyry system, with intense brecciation and feldspathic alteration, presented the highest K-feldspar contents, consistent with pervasive feldspathization and the presence of tourmaline-bearing breccias documented in its source.
Sample R3 presented reduced amounts of aluminosilicates and higher iron oxides/hydroxides and pyrite. This reflects the oxidized and Fe-rich character of IOCG systems where specularite, magnetite and sulfides show up in the alteration envelope.
3.2. Compressive Strength Results
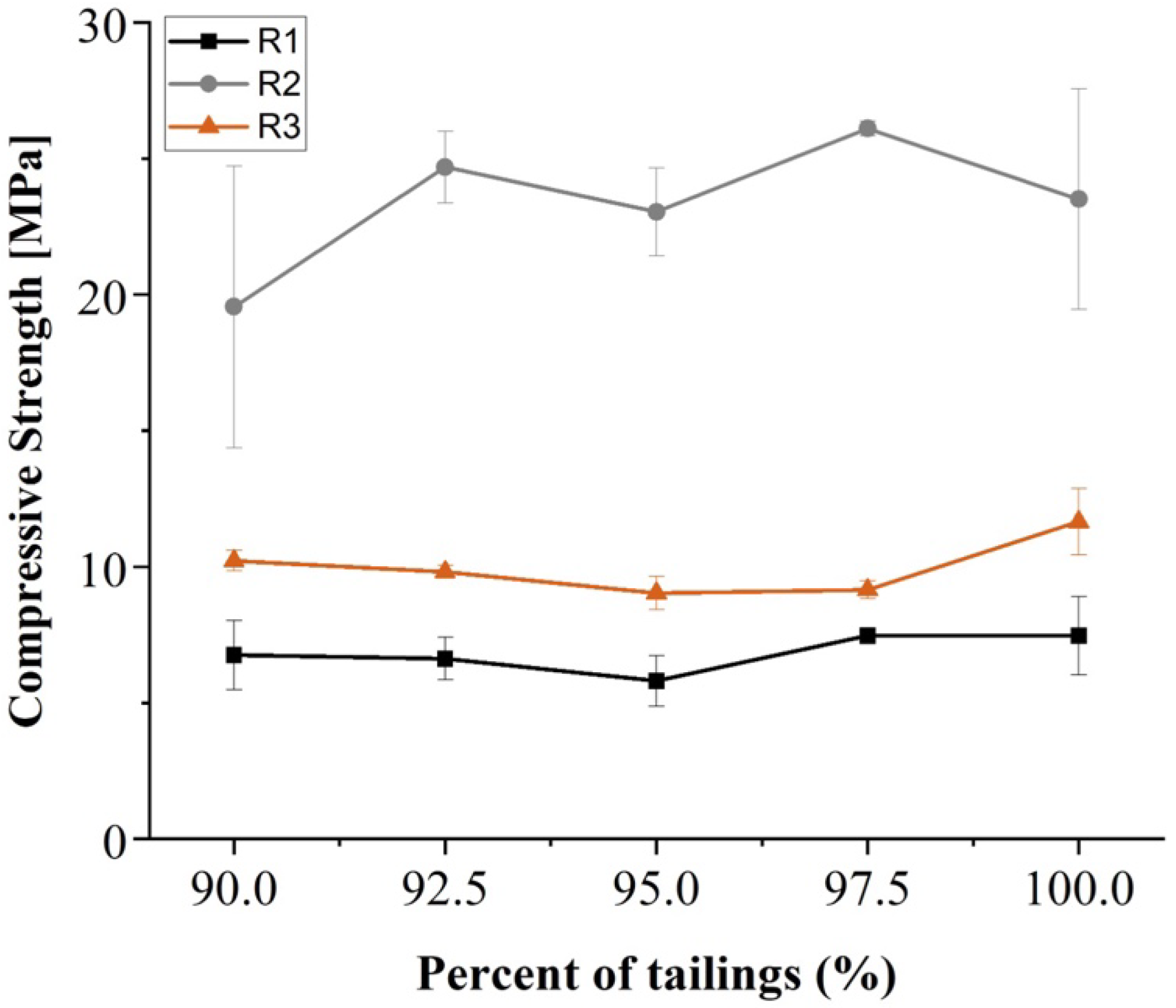
Figure 4 shows the results of the compressive strength tests performed after 28 days of curing for the geopolymers prepared with their respective cement replacement levels. The compressive strength of geopolymeric specimens was assessed after 28 days, following general building materials standards, such as the American Concrete Institute’s (ACI) ACI 318-19 [
44], which establishes 28-day compressive strength as the benchmark for concrete design and quality control. Similarly, in the context of geopolymer research, the study by [
45] demonstrated that kaolin-based geopolymer specimens exhibited substantially higher compressive strength at 28 days compared to earlier ages, confirming the relevance of this testing interval for mechanical characterization.
The results revealed a clear difference in the mechanical performance among the tailings. The R1 sample exhibited the lowest compressive strength values, ranging between 6 and 8 MPa. Moreover, across all mix designs, the mechanical behavior of this material remained relatively consistent. In contrast, the R2 sample achieved the highest compressive strength, within the range of 20 to 26 MPa. This suggests a high chemical reactivity, likely associated with its higher mineralogical concentration of K-feldspar and plagioclase—both part of the feldspar group, primarily alkali feldspars. Additionally, the sample exhibited an acceptable variation in strength as the cement content increased. On the other hand, the R3 tailing sample showed a lower performance than R2 with a compressive strength ranging from 9 to 11 MPa, without a clear trend of improvement or deterioration with varying co-activator dosage.
4. Discussion
To comprehend the results obtained for compressive strength for the different tailings samples, XRF and QEMSCAN results are used to observe tendencies between tailings’ composition and mechanical performance. The XRF method enables the determination of the Si/Al molar ratio. The values of the Si/Al ratios were 2.98 for R1, 3.05 for R2 and 5.39 for R3. As described by [
46], in alkali-activated systems, the compressive strength tends to be better when the Si/Al ratio of the precursor is close to 3.0. This suggests that the high molar ratio of 5.39 as observed in sample R3 may lead to a lower variability of aluminum in the geopolymer matrix, possibly affecting the mechanical strength of the material.
R1 and R2 geopolymerization performance was significantly different even though their Si/Al molar ratios were similar—2.98 and 3.05, respectively. This discrepancy shows that this ratio alone is not enough as predictor of copper tailings potential as a geopolymer, and complementary information is required.
From the QEMSCAN data, it can be noted that the R2 sample is enriched with feldspars (K-feldspar and plagioclase/albite), which are minerals known to react favorably in alkaline environments, leading to the formation of a stronger geopolymeric matrix. Plagioclase contains a quantity of available silicon that is three times greater than that provided by traditional sodium and potassium activators. This higher silicon availability may promote the formation of stronger bonds during the geopolymerization process, resulting in improved mechanical properties and enhanced durability of the geopolymer concrete. Similar results were observed by [
47] when working with iron smelting slag. Indeed, the presence of plagioclase has previously been associated with more active geopolymerization reactions and the formation of crystalline phases [
25].
A further consideration is the low clay content of the sample R2. Higher clay content—as in the R3 sample—may result in strength problems as these clays keep their original crystal lattice and release lower amounts of reactive species. This effect could be avoided by changing the crystal lattice by thermal activation before use. In the absence of thermal activation, the contribution of clays to geopolymerization is known to be low [
48].
The R3 sample also contains a significantly higher amount of iron oxides and hydroxides, which could interfere with geopolymerization. When the Fe
2O
3 content reaches high levels (around 20%), some of the dissolved Fe
3+ can be incorporated into the geopolymer network by substituting Al
3+, forming ferro-sialate structural units (Fe–O–Si–O–Al–O–Si–O). This substitution does not necessarily have a negative effect on the compressive strength of the geopolymer; in fact, it may enhance it [
49]. However, an excess of iron may have adverse effects, as it can induce a slower reactivity phase at the beginning of the geopolymerization process by delaying the dissolution of key elements such as silicon or aluminum. This may be due to the early formation of iron hydroxides, which consume a portion of the available OH
− ions. Additionally, Fe-rich materials have been associated with the development of photoactive properties, opening possibilities for multifunctional applications beyond structural performance [
31].
Additionally, the presence of sulfides, such as pyrite (4.44% in the case of R3), could cause long-term durability issues due to expansive reactions that weaken the material. Prior research has proven that partial dissolution of pyrite occurred when the alkalinity was high, generating leaching of the Fe ions and S ions [
50]. They can react with hydroxyl ions and with chloride ions, which in turn may form further products, that further react with CO
2, O
2, and H
2O, which will ultimately degrade the final product.
XRF analysis proved to be a valuable tool during the initial stages of the methodology proposed in this study, given its versatility, speed, reproducibility, and low cost—particularly when compared to QEMSCAN. This last analysis involves more time-consuming and expensive procedures for sample preparation, data handling, and quality control [
51]. In contrast, XRF provides faster and more efficient results, making it ideal for production environments or early-stage studies, where significant optimization of time and resources is required for preliminary materials characterization [
52].
Also, the mineralogical results from QEMSCAN were consistent with the geological characteristics (gangue minerals) of the source deposits, and the mineralogy is related to the mechanical properties of the alkali-activated materials. The R2 sample, with the highest compressive strength out of the three mixtures, showed the highest content in feldspathic phases (25.75% plagioclase and 16.64% K-feldspar). This suggests that the availability of these aluminosilicate minerals may have played a critical role in enhancing reactivity, because under alkaline activation, feldspars such as K-feldspar and albite provide soluble Si and Al under alkaline conditions, promoting the formation of N-A-S-H and C-A-S-H gels. These gels are both associated with enhanced strength and durability, contributing to the formation of a more compact and mechanically robust matrix [
26]. On the other hand, Sample R1 which possessed the highest contents of quartz (45.11%) and muscovite (18.62%), showed the lowest compressive strength, possibly because of the low chemical reactivity of quartz and mica in alkali-activated systems. Sample R3 shows a higher percentage of clays (7.20%) and iron oxides (12.19%), therefore resulting in low compressive strength.
5. Conclusions
The aim of this paper was to develop a mine tailings characterization procedure that can be applicable for the evaluation of copper tailings, which can be converted into geopolymers based on mineralogical and geochemical characteristics. In this case, the geopolymerization of the R2 sample exhibited the highest compressive strength and the lowest variability, reaching a maximum strength with a mix composed of 97.5% tailings and 2.5% OPC.
Based on the findings of this study, a practical screening methodology is proposed for evaluating copper tailings as geopolymer precursors. This includes (i) geochemical characterization through XRF to assess key oxide contents (e.g., SiO2, Al2O3, Fe2O3, CaO, MgO, and K2O), (ii) classification using a ternary diagram (Al2O3–Fe2O3–CaO+MgO+K2O) to estimate reactivity potential, and (iii) mineralogical analysis using QEMSCAN to confirm the presence of reactive aluminosilicates like plagioclase and K-feldspar. Tailings that combine a suitable chemical profile with abundant reactive minerals are more likely to achieve greater mechanical performance.
The importance of the tailing mineralogy beyond the chemical composition is shown when the R2 and R1 tailing samples are compared. Although both tailings have a similar location in the ternary diagram, they show a significant difference in compressive strength performance. QEMSCAN analysis identified high concentrations of plagioclase and K-feldspar in R2, in contrast to lower concentrations of iron oxides, excess clays, and quartz in R1. The average compressive strength of R2-based geopolymers was approximately 250% higher than that of R1-based geopolymers. This indicated that, in general, geopolymers prepared from the R2-like tailings were more than three times more resistant than when R1 tailings were used. The results of copper tailings evaluation support the notion that feldspathic gangue minerals (especially plagioclase/albite and K-feldspar) directly contribute to the physical performance of alkali-activated tailings-based materials.
This finding highlights the necessity of mineralogical studies in the validation process of high-potential tailings, especially when geochemical compositions are very similar. The sequential holistic approach therefore led to the finding that the exclusive use of XRF-based geochemical characterization cannot be considered as a guarantee for a reliable prediction of the geopolymer performance. Only when mineralogy obtained from QEMSCAN is included as a validation tool can a highly reactive sample of tailings be identified.
The specific influence of iron oxides on geopolymerization mechanisms should be further studied. In particular, the competition between Fe
3+ and Al
3+ for OH
− ions, and the possible early precipitation of iron hydroxides, could interfere with precursor dissolution and hinder gel formation [
49]. Future research should therefore examine in more detail how Fe-rich phases impact the polymerization process and the final performance of alkali-activated tailings.
From the aspect of the circular economy, this study offers a valuable alternative for the valorization of mine tailings. Producing geopolymers from tailings presents a significant opportunity to leverage local resources and reclassify these materials from environmental liabilities to valuable assets. Particularly, this approach enables a considerable reduction in CO
2 emissions compared to traditional cement-based concrete. The results obtained demonstrate that tailings-based geopolymers possess promising technical properties for use in various applications, such as non-structural bricks, fills, pavers, and slabs. This strategy aligns with recent sustainability proposals grounded in applied mineralogy, which promote the use of tailings as a resource rather than as a waste product [
53].
Lastly, while the proposed approach in this study for pretreatment of the tailings is not energy-intensive, a high concentration of alkaline activator itself creates environmental and economical issues. NaOH is an energy-intensive and high-CO2-generating product. To achieve tailings-based geopolymers with higher mechanical strengths and simultaneously higher sustainability, further investigation is required to develop alternative low-carbon alkaline activators. Future research also needs to address the long-term performance and stability of these geopolymers. This includes the evaluation of durability indicators such as sulfate resistance, carbonation, acid attack, and heavy metal leaching (particularly relevant in tailings containing sulfides such as pyrite and elevated SO3 content). These aspects are essential for confirming the suitability of the material in real-world construction and environmental applications.