1. Introduction
The consequences of climate change and the trend of increasing electricity demands in developing countries are driving growing interest in the further development of nuclear energy [
1,
2]. Efforts focused on the design and commissioning of new modular nuclear power plants, both small- and large-scale, featuring compact dimensions and low carbon footprints, can have a significant impact on the reduction in CO
2 emissions worldwide. Uranium is the key element for the sustainable development of nuclear energy across the globe [
3,
4]. Kazakhstan’s uranium mining industry, based on highly efficient in situ recovery (ISR) technology, is capable of making a substantial contribution to meeting the growing demand for uranium resources [
5].
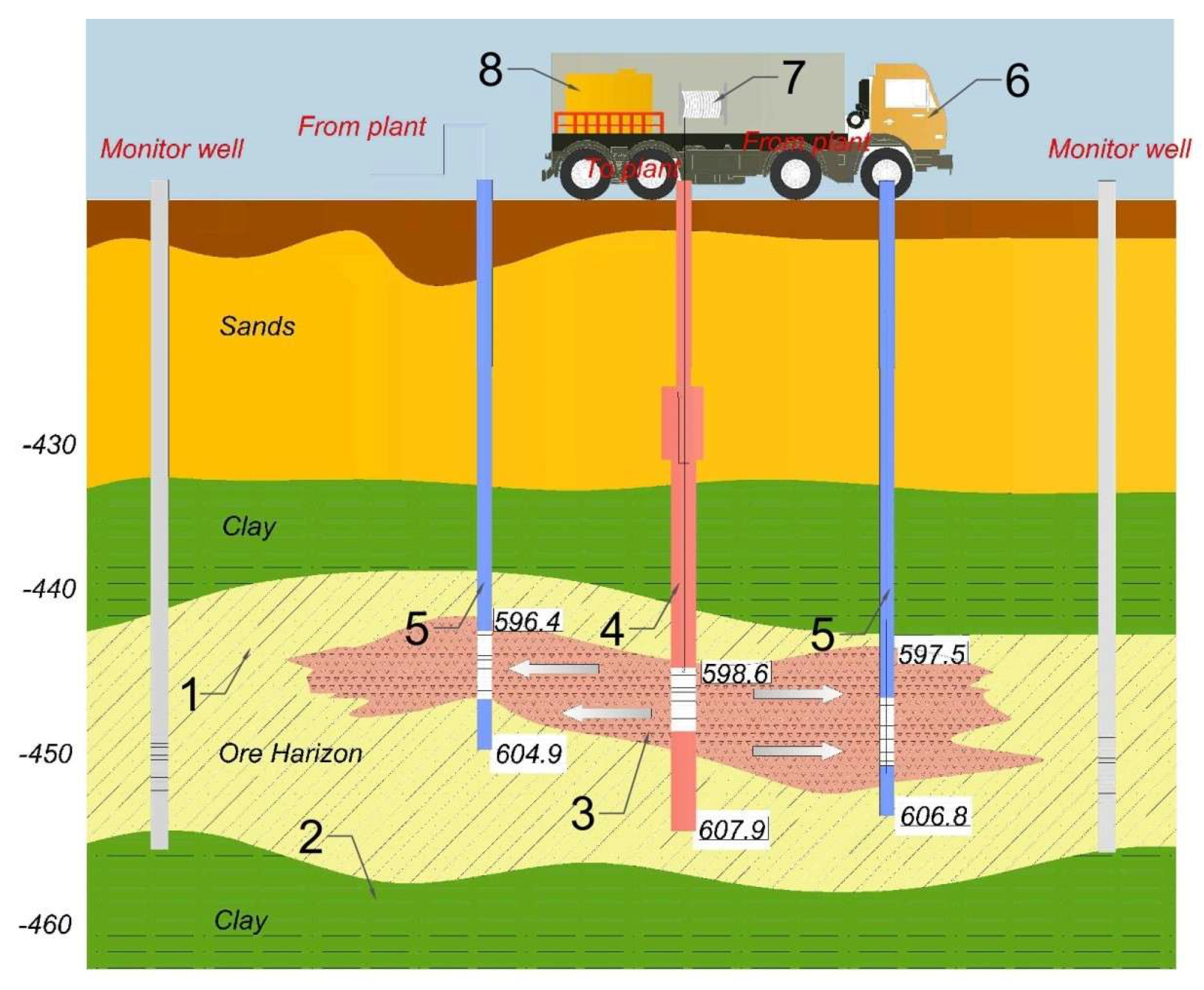
The in situ method of uranium mining is the most economically viable and environmentally safe under conditions of deep ore occurrence (below 300 m), where the useful component in the ore mass has a content below 0.1%–0.2%. This technology is the most efficient and does not involve high capital or operating costs during the development of the deposit [
6]. The ISR technology involves dissolving the useful component in place using a moving stream of leaching solution, followed by the extraction and lifting of the formed compounds to the surface through production wells. The sulfuric acid used as a leaching reagent reacts with carbonate and clay minerals in the host rocks, clogging the productive horizon [
7]. The resulting precipitates increase hydraulic resistance in the formation, reduce the filtration properties of the ores, decrease well productivity, and hinder the uranium in situ leaching processes. As a result, both the productivity and the mean time between failures (MTBFs) of wells are reduced, which lowers the utilization coefficient of the wells and the technological equipment. This increases the operation time of production blocks and, consequently, leads to the increased consumption of sulfuric acid, electricity, and other operating costs for extraction [
8]. Wells in such blocks are often shut down for repair and restoration work (RRW), and there arises a need to perform additional work to restore the permeability of the near-wellbore zone (NWBZ) of the formation [
9].
Well productivity directly depends on the mineralogical characteristics of the ores and the methods used to improve their filtration properties [
10]. The chemical methods applied to restore the permeability of the NWBZ are based on the penetration of the reagent into the NWBZ, the dissolution and removal of the reaction products, the restoration of the original permeability, and the improvement of the well productivity and the mean time between failures [
11]. Traditional chemical methods for restoring the permeability of the productive horizon under complex geological conditions at sites with low filtration properties of ores do not yield positive results. During acid treatments of the production well filter zone containing ore with low filtration characteristics, the injected decolmatizing solution penetrates into the near-filter zone of the formation through the upper part of the filter along the most permeable sections. As a result, the main lower part of the near-filter zone remains untreated, containing impermeable ore zones. The decolmatizing solution, moving through the most permeable areas, reacts with the host rocks and becomes neutralized without interacting with the precipitate formations in the productive horizon. The undissolved precipitates enter the near-filter zone of the well and clog the filter openings, and the well productivity drops sharply. In some cases, comprehensive, multicomponent chemical treatments of wells are required using mineral acids, active additives, and surfactants (SAA). Studying the physicochemical characteristics of ores in the productive horizon will make it possible to develop rational parameters for chemical well treatment under various mining and geological conditions.
The purpose of this study was to improve the efficiency of underground in situ uranium leaching under complex geological conditions of ore occurrence, depending on the filtration properties and clay content of the ores. This involves the development of effective parameters for multifunctional chemical reagent formulations aimed at improving the filtration properties of the ores and preventing precipitation formation during uranium in situ leaching in various mining and geological settings.
The objectives of this study included determining the physicochemical characteristics of ores in the productive horizon and the composition of precipitates; analyzing characteristics and ranking technological blocks and sites in order to develop multifunctional chemical reagent formulations; conducting microscopic laboratory studies and pilot-scale well tests to select rational parameters of multifunctional chemical reagents; substantiating rational parameters for the application of innovative chemical well treatments aimed at the effective dissolution and prevention of precipitation; and developing an effective methodology for selecting optimal parameters for chemical well treatment depending on the physicochemical characteristics of the ores and precipitates in the productive horizon.
3. Discussion of Results
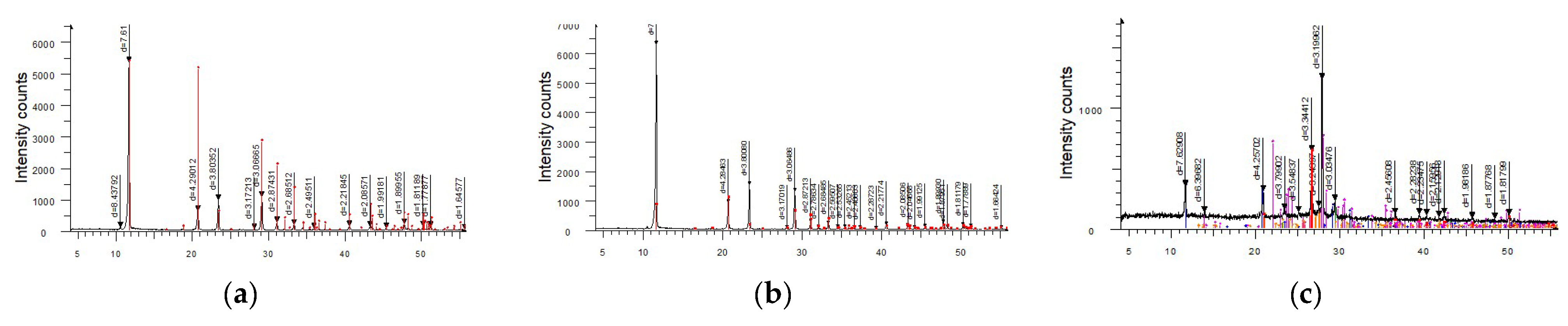
An X-ray diffraction analysis was carried out using the equipment described in the methodology section (Chapter: Methods,
Section 2.1). The content of the major crystalline phases was calculated.
Table 2 presents the results of the semi-quantitative X-ray diffraction analysis of core samples.
Table 3 provides the results of the semi-quantitative X-ray diffraction analysis of precipitate samples.
The results of the X-ray diffraction analysis of core samples from different ore-bearing stratigraphic stages revealed a general similarity in the mineralogical composition of the productive horizons at the North Kharasan deposit. However, the ores exhibited variations in the content of certain minerals across a broader range: quartz varied from 54.7% to 90.8%, potassium feldspar (K-feldspar) varied from 5.7% to 10.1%, and kaolinite varied from 6.7% to 11.6%. Additionally, gypsum (up to 16.4%) in the samples from the Campanian and Maastrichtian stages indicated the formation of ion-exchangeable, mechanical, and chemical precipitates, which hinder the in situ mining process. The quantity of kaolinite governs the scale of ion-exchange colmatation, as it swells upon interaction with sulfuric acid, whereas the presence of gypsum contributes to the development of chemical colmatation. In contrast, ores from the Santonian stage are more homogeneous and show minimal precipitation during in situ leaching.
The swelling of clay minerals and gypsum deposition in solution discharge zones lead to the formation of impermeable areas within the productive horizon. This results in a change in the flow direction of leaching solutions toward barren zones, a reduction in the uranium concentration in the pregnant solution (PLS), and a consequent delay in uranium recovery from the subsurface.
The results of the X-ray diffraction analysis of precipitate samples from wells in the Santonian horizon of the Syrdarya depression indicate that the deposits are single-component and consist entirely (100%) of gypsum, a chemically derived product.
The precipitates from wells in the Maastrichtian stage were similar in composition but differed slightly, containing 88% gypsum, 5.1% quartz, and 6.9% albite. The dominance of gypsum confirmed the chemical nature of the deposits, while the presence of quartz (5.1%) and albite points to the existence of minor mechanical impurities.
The precipitates from wells in the Campanian horizon of the Syrdarya depression were multicomponent and exhibited a complex structure. The presence of quartz (35.6%), albite (33.9%), and potassium feldspar (4.9%) confirmed the predominance of a mechanical clogging mechanism. At the same time, the presence of gypsum (16.7%) and calcite (8.9%) indicated the formation of chemically derived precipitates.
In reference [
20], data on the mineralogical composition of well precipitates relative to the productive horizon are presented. Practical experience in well regeneration confirms that homogeneous precipitates are effectively removed using conventional chemical methods involving hydrochloric acid. However, multicomponent precipitates tend to be denser, are less soluble, and exhibit a reduced responsiveness to standard chemical treatments. Quartz-, gypsum-, and feldspar-based precipitates tend to accumulate in the filter section of wells, reducing the filtration properties of the productive horizon and obstructing the flow paths of the leaching solution. As a result, the solution is diverted to barren zones, reducing the uranium recovery efficiency.
Laboratory experiments enabled the selection of effective chemical reagent compositions and concentrations for the breakdown and prevention of precipitates, depending on their mineralogical characteristics. Microscopic studies allowed for a detailed examination of the surface structure of precipitate samples before and after treatment and facilitated a comparative analysis of the dissolution effectiveness of different chemical reagent formulations.
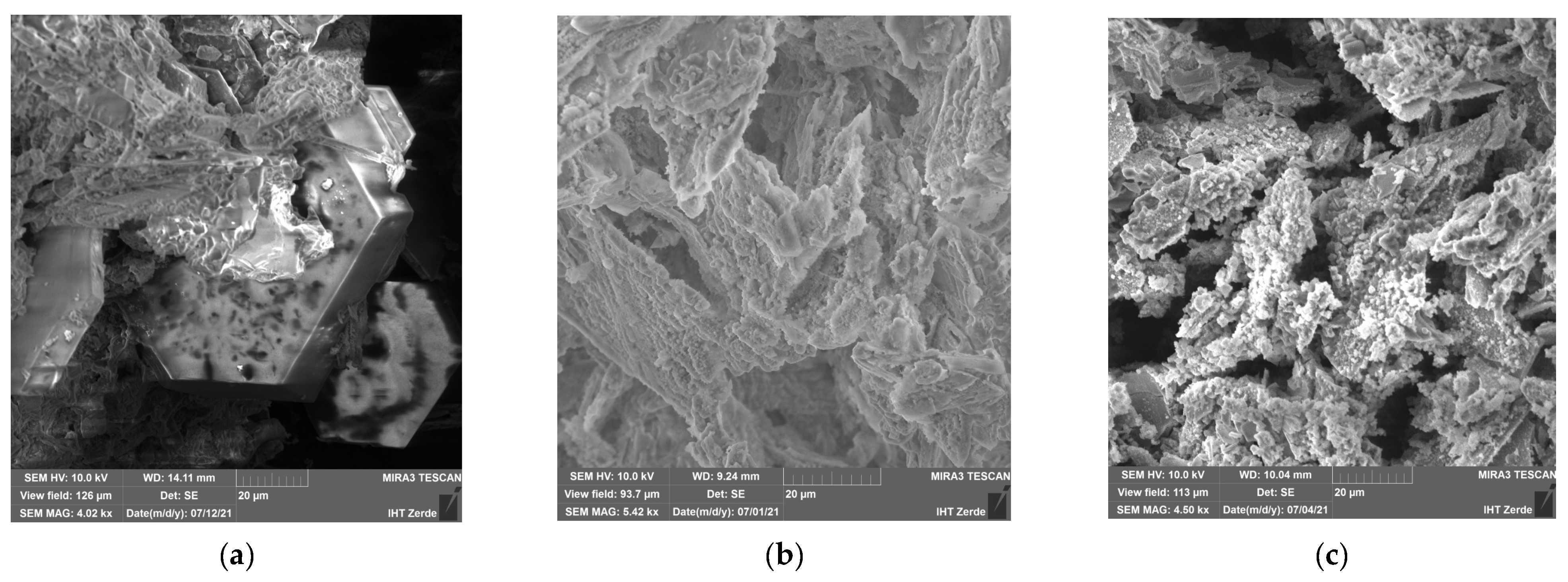
Figure 5 shows scanning electron microscope (SEM) images of precipitates from a well in the Campanian horizon of the Syrdarya depression: (a)—untreated sample; (b)—after treatment in Experiment A1; and (c)—after treatment in Experiment A2.
The image of the untreated sample shown in
Figure 5a reveals a solid structure composed of intergrown hexagonal prisms of various sizes, with overgrowths of small, irregularly shaped crystals bonded together. The hexagonal prisms varied in size, and the smaller elongated crystals were tightly packed, forming a continuous structure with no visible fractures or gaps.
Figure 5b shows that, after treatment with the decolmatant solution from Experiment A1, structural damage and fissures appeared. The hexagonal prism crystals were dissolved, and the material lost its original mechanical strength, taking on a loose and porous texture. Fine crystals were no longer visible, and larger crystals were significantly thinned.
In
Figure 5c, after treatment in Experiment A2, the structural changes were similar to those observed in Experiment A1. The hexagonal crystals were dissolved, with the appearance of cracks and erosional features. However, the remaining crystals were relatively larger and less thinned, and small crystals were still present. The overall structure appeared denser and showed fewer ruptures compared to that in Experiment A1.
The more pronounced dissolution observed after Experiment A1 and the comparatively milder effect in Experiment A2 were attributed to the action of hydrofluoric acid on siliceous, gypsum, aluminosilicate, and carbonate minerals in the precipitate composition. The breakdown of gypsum, aluminosilicate, siliceous, and carbonate deposits in the productive horizon occurs under the influence of hydrofluoric acid (HF), according to Reactions (1)–(4).
As demonstrated by Interaction Equations (1)–(4), the complete dissolution of gypsum, aluminosilicate, siliceous, and carbonate precipitates in the near-filter zone of the wells was achieved through the application of a 2.5%–5% hydrofluoric acid solution in volumes sufficient to penetrate 0.5 to 1.0 m along the entire length of the well filter, thereby creating an acid bath. This approach enables the restoration of filtration properties in the near-wellbore zone of the formation and enhances the productivity of geotechnological wells affected by complex, multicomponent precipitate compositions.
Figure 6 presents scanning electron microscope (SEM) images of a precipitate sample from a well in the Campanian horizon of the Syrdarya depression: (a)—original sample; (b)—after treatment in Experiment B1; and (c)—after treatment in Experiment B2.
As shown in
Figure 6a, the original precipitate sample consisted of large, elongated crystals arranged in a chaotic pattern. The crystal surfaces were smooth and uniformly structured, with no visible fractures or discontinuities, displaying a characteristic skeletal (framework) morphology.
Figure 6b shows a scanning electron microscope (SEM) image of the sample after treatment with the decolmatant solution from Experiment B1. Significant structural degradation was observed: the crystals decreased in size, became thinner, and showed signs of erosion. The surfaces were deformed, and fine flakes formed from dissolved crystals were clearly visible.
Figure 6c presents the SEM image of the sample after treatment with the decolmatant solution from Experiment B2. The surface exhibited partial deformation—some crystals were partially dissolved and reduced in size, while others remained unaffected. A minor presence of flakes from partially dissolved crystals was noted, but the overall structure and density of the sample remained largely unchanged.
The limited dissolution observed after Experiment B1 and the minimal effect seen in Experiment B2 were attributed to the action of hydrochloric acid, which reacted primarily with the gypsum and carbonate minerals, but not with the siliceous or aluminosilicate components present in the precipitate samples. The dissolution of gypsum and carbonate precipitates in the near-filter zone of the formation occurred under the influence of hydrochloric acid according to Reactions (5) and (6).
As shown in Equations (5) and (6), the complete dissolution of gypsum and carbonate deposits in the near-filter zone of the formation was achieved by applying a 2.5%–5.0% hydrochloric acid solution in a volume sufficient to penetrate 0.5–1.0 m from the well filter, thereby forming an acid bath. The restoration of filtration characteristics in wells is ensured only when gypsum and carbonate minerals dominate the precipitate composition.
Determining the ore structure and the composition of precipitate-forming components within the productive horizons of uranium deposits in the Syrdarya depression enables the development of universal methods for intensifying wellfield extraction. These new approaches involve the application of specially formulated solutions tailored to the mineralogical composition of the ores and precipitates in the productive horizon. The developed techniques allow for the effective dissolution and prevention of mineral deposits in the productive zone and significantly increase the solvent capacity of the working reagent (WR) used in well-based uranium leaching operations [
21,
22].
This technology offers several key advantages: it enhances the uranium concentration in the pregnant solution (PLS), restores filtration properties in the productive horizon at deposits in the Syrdarya depression, and reduces the labor intensity, power consumption, and other operating costs associated with uranium extraction.
The selection of the concentration and composition for the multifunctional chemical reagent complex depends on the well productivity, the clay content, and the filtration characteristics of the ore in the productive horizon of the technological block or the specific well being treated. Well productivity is influenced by the type of filter and the lithology of the productive formation and is calculated according to Equation (7).
where
Q—well productivity, m
3/h;
l—filter length, m; and
α—empirical coefficient, dependent on the filtration characteristics of the formation. For fine-grained sand with a filtration coefficient ranging from 2 to 5,
α = 90.
The rational volume of the decolmatant solution is determined based on the composition of the precipitate-forming materials, the productivity of the well, and the effectiveness of previous chemical treatments. The volume is calculated by taking into account the porosity coefficient of the productive horizon rocks and the radial dispersion range of the decolmatant solution from the well filter, which is determined according to Equation (8).
where
= 0.22;
Hₑ—effective thickness of the productive horizon and
r—radial dispersion distance of the decolmatant solutions.
The effectiveness of selecting rational parameters for the multifunctional chemical reagent complex also depends on the filtration characteristics of the rock within the block or well, and is calculated according to Equation (9).
where
R—radius of the depression cone, m;
r—radius of the well, m;
H—thickness of the aquifer, m; and
h—water column in the well after pumping, m.
Experimental studies on the dissolution of precipitates made it possible to determine the optimal concentrations and volumes of multifunctional chemical reagents to enhance the productivity of production wells. To ensure experimental consistency, a single treatment of the filter section was carried out on each of 30 pre-selected wells using a prepared decolmatizing solution with a volume of 3 m3, containing hydrofluoric acid at concentrations ranging from 2.0%, 2.5%, 3.2% and 4.0%, along with the addition of complexing agents, 5–10 wells for each method. The subsequent monitoring and analysis of the well performance and the operational duration before and after treatment allowed for an assessment of the effectiveness of the innovative chemical (RRW) using a multifunctional reagent composition based on hydrofluoric acid and surfactants (SAA). The effectiveness was evaluated by measuring the mean time between failures (MTBF) of the wells.
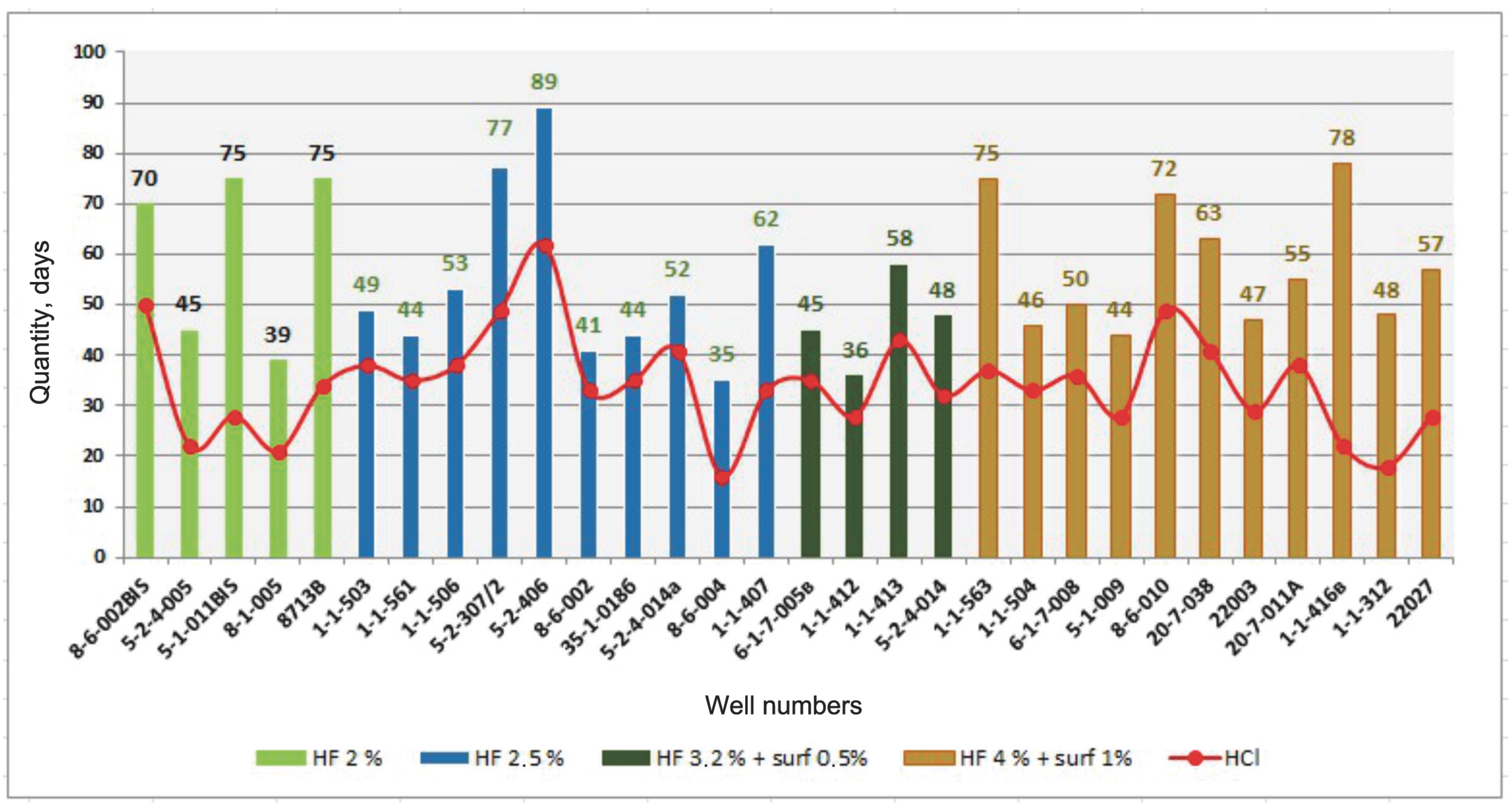
Figure 7 shows a diagram comparing the uninterrupted operation periods of uranium ISR wells before and after the experimental treatments conducted on the wells.
A comparative analysis of the performance of geotechnological wells before and after chemical treatment revealed that the average mean time between failures (MTBF) prior to treatment was 34 days when hydrochloric acid (HCl) was used in quantities of 150, 200, 250, or 300 kg.
As shown in
Figure 7, the application of a multifunctional chemical reagent complex containing hydrofluoric acid (HF at 2.0%) increased the MTBF for wells with a clay content coefficient (
kc) ≤ 12 from 21 to 50 to 45 to 75 days. For wells with
kc ≤ 15, the use of a 2.5% HF solution increased the MTBF from 35 to 62 to 44 to 89 days. In wells with a high clay content (
kc ≤ 18), a multifunctional solution containing 3.2% HF and 0.5% surfactant (SAA) was applied, resulting in an increase in the MTBF from 28 to 43 to 45 to 58 days. For wells with a very high clay content (
kc ≥ 19), a multifunctional reagent solution with 4.0% HF and 1.0% surfactant was used, leading to an MTBF increase from 18 to 36 to 50 to 78 days. To reproduce the research results at sites with similar lithological properties, it is necessary to apply a declogging solution with the described concentrations of hydrofluoric acid, adjusted according to the carbonate content, clay content, or grain size of the productive horizon. The results will help improve the efficiency of restoring filtration properties and ISR well performance, while reducing reagent consumption and labor costs at wells with sufficient or high effectiveness.
The increase in the MTBF was attributed to the reactive capability of hydrofluoric acid, which effectively dissolves precipitates in the near-filter zone of the wells. The presence of surfactants in high-clay wells helps prevent the solution from bypassing through previously flushed areas of the filter, allowing the reagent to reach and dissolve deposits in the lower part of the filter column. Moreover, the surfactant does not negatively affect the filtration properties of the wells, as it is water-soluble and is fully removed during the post-treatment pumping of the calculated solution volume prior to putting the well back into operation.
The lower efficiency observed in areas with a very high clay content can be explained by the even-lower performance of conventional chemical methods under similar conditions.
Hydrofluoric acid effectively destroys the primary compounds in precipitate formations, while the surfactant, especially in complex geological areas, reduces the permeability in the upper washed zones of the filter. This prevents the uncontrolled spreading and dilution of the decolmatant solution within the productive horizon. Consequently, the reagent is distributed more uniformly along the filter column, promoting declogging and activating the lower sections of the well screen.
As confirmed by pilot-industrial trials, the application of the multifunctional chemical reagent composition resulted in more stable well operation and longer MTBF intervals. The wells required fewer repair and restoration interventions, and the effectiveness of subsequent operations increased by approximately 15% compared to previous treatments. However, the use of hydrofluoric acid requires additional safety measures for monitoring and ensuring the quality of specialized clothing, footwear, and protective equipment to safeguard the skin and respiratory organs from accidental splashes.
4. Conclusions
These comparative quantitative and qualitative mineralogical studies demonstrated a predominance of quartz in the ore intervals of the Syrdarya depression: 90.8% in the Santonian stage, 54.7% in the Maastrichtian stage, and 66.3% in the Campanian stage. The second most common mineral across these intervals was potassium feldspar (K-feldspar): 9.2% in the Santonian, 10.1% in the Maastrichtian, and 5.7% in the Campanian horizon. The presence of kaolinite (6.7% in Maastrichtian and 11.6% in Campanian ores), along with gypsum (16.4% in the Campanian ores), indicates the complex and heterogeneous structure of the Maastrichtian and Campanian productive horizons. According to operational data, the well productivity in these blocks remains unstable and requires additional measures to improve the ore filtration characteristics.
An X-ray diffraction analysis of the precipitate samples revealed that, in wells of the Santonian horizon, the dominant precipitate type is chemical gypsum (100%). In the Maastrichtian wells, the precipitates are relatively homogeneous, composed mainly of chemically deposited gypsum (88%) with minor amounts of quartz (5.1%) and albite (6.9%). In contrast, precipitates in the Campanian horizon are multicomponent and consist of quartz (35.6%), albite (33.9%), K-feldspar (4.9%), calcite (8.9%)—formed from mechanical particle transport—and gypsum (16.7%), resulting from chemical reactions. Homogeneous and slightly contaminated precipitates are effectively removed without an additional operational impact, while multicomponent deposits are more difficult to eliminate and require increased effort and resources. In such cases, prolonged well operation and increased maintenance efforts are often necessary.
Microscopic studies of the samples before and after laboratory testing made it possible to select effective chemical compositions and concentrations for well treatment, depending on the geological conditions and precipitate composition. A 2.5% hydrofluoric acid solution effectively destroyed homogeneous and moderately complex precipitates, with noticeable structural degradation and crystal deformation. A 5.0% HF solution showed even stronger effects on multicomponent precipitates, significantly reducing the crystal sizes. A 2.5% hydrochloric acid solution caused only minor changes, with slight deformation and no reduction in crystal size, whereas 5.0% HCl induced moderate damage and changes in structure and size. These findings confirm that selecting the appropriate concentration and composition of chemical reagents can enhance the uranium in situ leaching efficiency and reduce the operational costs and labor.
A preliminary analysis of the geological parameters and block performance enabled the classification of sites and wells based on the filtration coefficient, clay content, and MTBF, supporting the selection of optimal chemical treatment parameters. Zones with kc ≤ 12 or kc ≤ 15 demonstrated stable well operation, requiring only low-concentration HF (2.0%–2.5%) or high-concentration HCl (4.0%). Blocks with kc ≤ 18 are considered moderately challenging and can be treated using 3.2% HF with spot application of surfactants, while HCl-based treatments above 4.0% are ineffective. For areas with kc ≥ 19, complex treatments involving 4.0% HF with surfactants are required, based on previous performance data.
As a result of pilot-industrial tests conducted on geotechnological wells used for in situ uranium leaching, optimal parameters for chemical well treatment under various mining and geological conditions were developed. Monitoring and comparative analysis of well performance before and after experimental treatments with multifunctional chemical reagent solutions demonstrated the high efficiency of the innovative method compared to traditional chemical treatment approaches.
At wells with low clay content (kc ≤ 12), the use of a hydrofluoric acid solution (HF—2.0%) increased the average inter-repair cycle from 31 to 60 days. In areas with a clay coefficient of kc ≤ 15, a declogging solution containing hydrofluoric acid (HF—2.5%) was applied, resulting in an average increase in the inter-repair cycle from 38 to 54 days. For wells with an ore clay coefficient of kc ≤ 18, treatment with a multifunctional solution containing hydrofluoric acid at a concentration of 3.2% and a surfactant (0.5%) increased the average inter-repair cycle from 34 to 46 days. In geotechnological blocks with high clay content in the productive horizon (kc ≥ 19%), the application of multifunctional solutions with a hydrofluoric acid concentration of 4.0% and a surfactant concentration of 1.0% increased the average inter-repair cycle from 32 to 57 days. The increase in the inter-repair cycle of wells enabled a greater extraction of productive solution from the subsurface and the greater injection of leaching solution, due to the reduced downtime for maintenance and restoration activities.
Based on the collected data, the effectiveness of applying multifunctional chemical reagent complexes for well treatment in challenging geological conditions was validated through field trials. The results substantiate the use of tailored chemical treatment parameters based on the ore and precipitate composition. Block and well classification, coupled with parameter selection, improves productivity, extends uninterrupted operation, reduces equipment stress, and enhances environmental and occupational safety. The rational parameters for well treatment, based on the ore clay content, filtration rate, carbonate content, and precipitate composition, increase the ISR efficiency and reduce the chemical consumption and operating costs by 10%–15%.
These studies have generated a large body of data that will guide the future development of improved and novel methods to enhance uranium leaching in low-permeability ores.