A Cl-Dominant Analogue of Annite Occurs at the Eastern Edge of the Oktyabrsky Cu-Ni-PGE Deposit, Norilsk, Russia
Abstract
1. Introduction
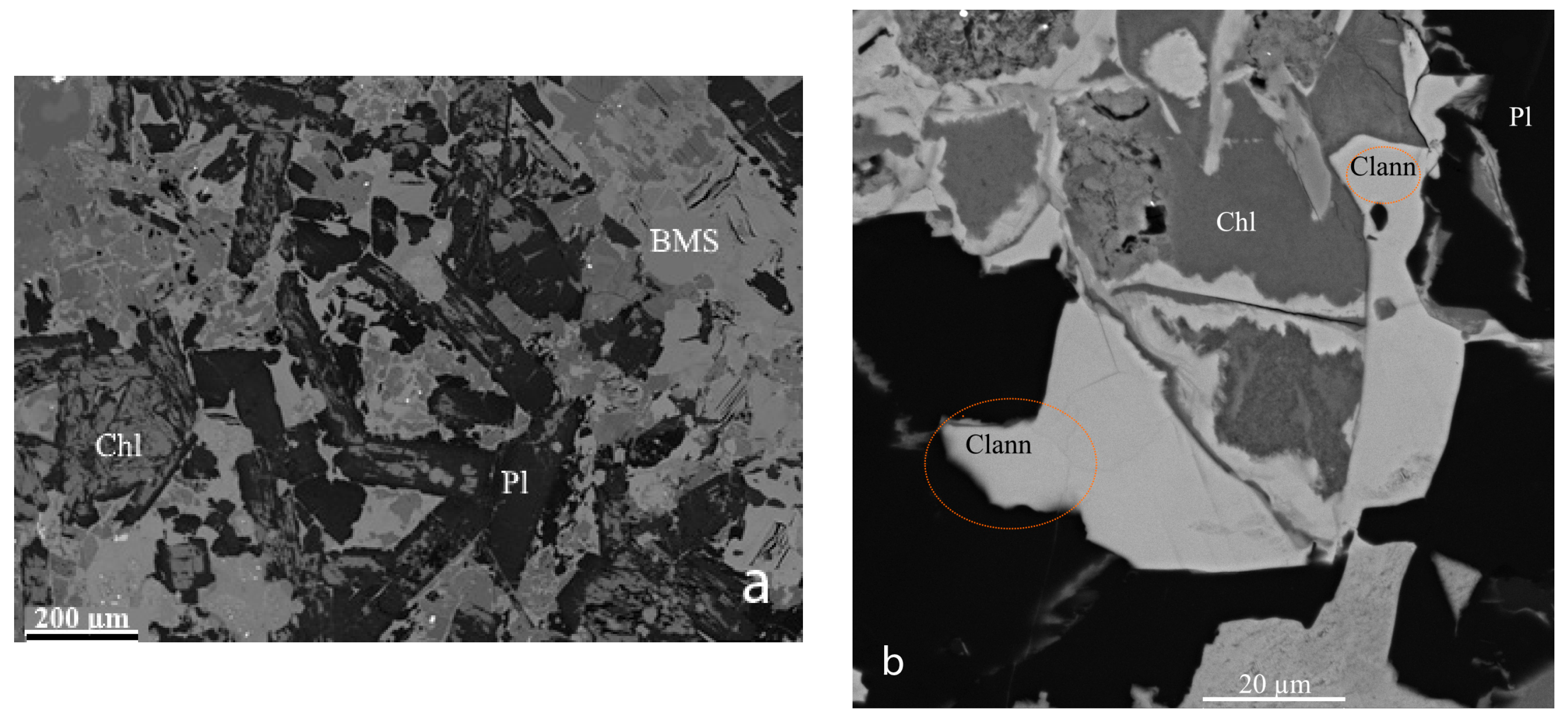
2. Occurrence, Geological Setting, and Mineral Association
3. Physical and Optical Properties
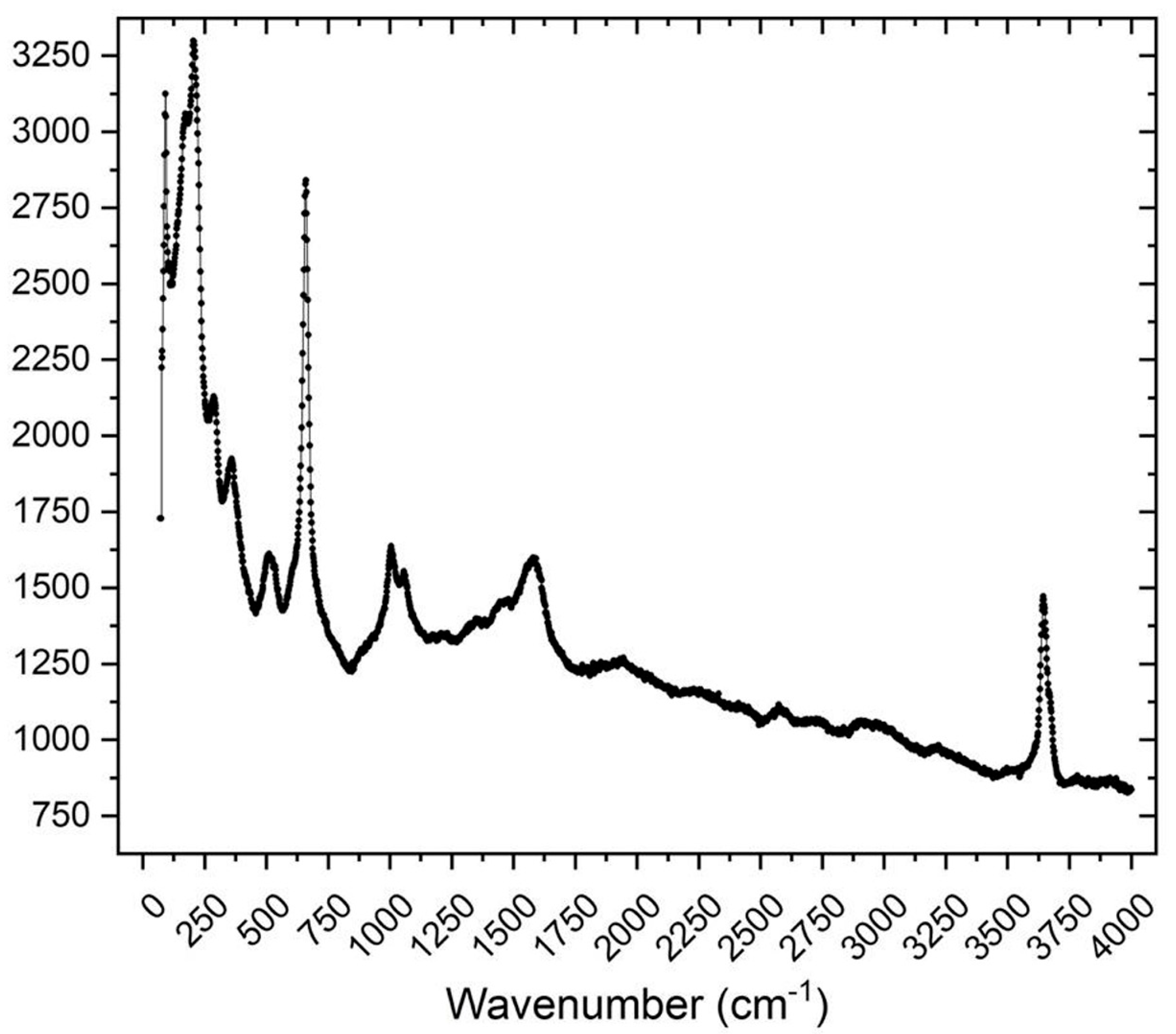
4. Raman Spectroscopy
5. Chemical Composition
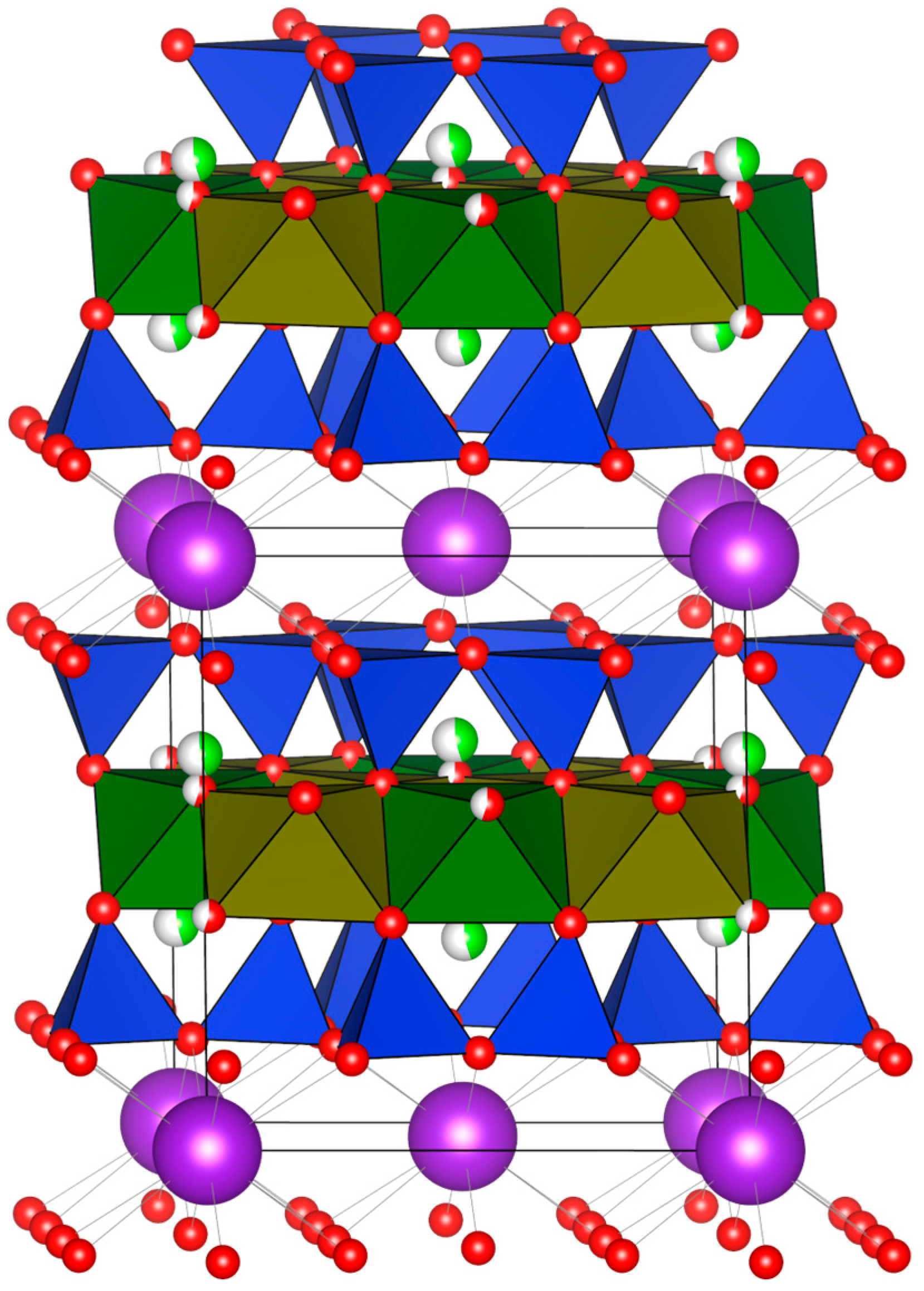
6. Crystallography
| Cl-Rich Annite | Synthetic Annite * | |
|---|---|---|
| Whole layer | ||
| ΔTM (Å) | 0.441 | −8.948 |
| βideal | 100.120 | 100.037 |
| intralayer shift | −0.358a | −0.337a |
| Interlayer | ||
| VK-O12 (Å3) | 63.99 | 68.85 |
| tint (Å) | 3.362 | 3.504 |
| ΔK-O (Å) | 0.044 | 0.126 |
| ECON | 11.97 | 11.79 |
| Tetrahedral sheet | ||
| α (°) | 0.08 | 3.57 |
| Δz (Å) | −0.011 | −0.103 |
| τ (°) | 110.07 | 108.37 |
| TAV | 0.4865 | 4.6046 |
| TQE | 1.0001 | 1.0017 |
| BLD | 0.211 | 1.521 |
| VT (Å3) | 2.35 | 2.34 |
| ttet (Å) | 2.234 | 2.242 |
| Octahedral sheet | ||
| VM1 (Å3) | 12.18 | 12.18 |
| VM2 (Å3) | 12.08 | 12.08 |
| ψM1 (°) | 59.18 | 58.46 |
| ψM2 (°) | 56.07 | 58.46 |
| OAVM1 | 43.0311 | 28.6575 |
| OAVM2 | 42.3758 | 30.4012 |
| OQEM1 | 1.0131 | 1.0086 |
| OQEM2 | 1.0128 | 1.0096 |
| BLDM1 | 0.507 | 0.364 |
| BLDM2 | 0.286 | 1.533 |
| ELDM1 | 5.248 | 4.516 |
| ELDM2 | 5.153 | 4.557 |
| ShiftM2 (Å) | −0.046 | 0.000 |
| tM(O3) (Å) | 2.231 | 2.153 |
| tM(O4) (Å) | 2.012 | 2.314 |
| tM(O3-O4) (Å) | 2.158 | 2.207 |
| w | 0.110 | −0.080 |
| K | M1 | M2 | T | ∑ O/Cl | |
|---|---|---|---|---|---|
| O1 | 0.046×4↓, 0.043×4↓ | 0.922, 0.894 | 1.905 | ||
| O2 | 0.050×2↓, 0.040×2↓ | 0.912×2→ | 1.914 | ||
| O3 | 0.363×4↓ | 0.372×2↓, 0.372×2↓ | 0.908 | 2.015 | |
| O4,F (×0.45) | 0.170×2↓ | 0.170×2↓×2→ | 0.510 | ||
| Cl (×0.55) | 0.040×2↓ | 0.216×2↓ | 0.190×2↓×2→ | 0.636 | |
| 0.614 | 2.226 | 2.208 | 3.635 |
7. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkov, A.Y.; Nikulin, I.I.; Nikiforov, A.A.; Lobastov, B.M.; Silyanov, S.A.; Martin, R.F. Atypical mineralization involving Pd–Pt, Au–Ag, REE, Y, Zr, Th, U, and Cl–F in the Oktyabrsky deposit, Norilsk Complex, Russia. Minerals 2021, 11, 1193. [Google Scholar] [CrossRef]
- Rieder, M.; Cavazzini, G.; D’yakonov, Y.S.; Frank-Kamenetskii, V.A.; Gottardi, G.; Guggenheim, S.; Koval, P.W.; Müller, G.; Neiva, A.M.R.; Radoslovich, E.W.; et al. Nomenclature of the micas. Clays Clay Miner. 1998, 46, 586–595. [Google Scholar] [CrossRef]
- Barnes, S.J.; Malitch, K.N.; Yudovskaya, M.A. Introduction to a special issue on the Norilsk−Talnakh Ni–Cu–platinum group element deposits. Econ. Geol. 2020, 115, 1157–1172. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Yakovenchuk, V.N.; Yanicheva, N.Y.; Pakhomovsky, Y.A.; Shilovskikh, V.V.; Bocharov, V.N.; Krivovichev, S.V. Crystal chemistry of ivanyukite-group minerals, A3–xH1+x[Ti4O4(SiO4)3](H2O)n (A = Na, K, Cu), (n = 6–9, x = 0–2): Crystal structures, ion-exchange, chemical evolution. Mineral. Mag. 2021, 85, 607–619. [Google Scholar] [CrossRef]
- Tlili, A.; Smith, D.C.; Beny, J.-M.; Boyer, H. A Raman microprobe study of natural micas. Mineral. Mag. 1989, 53, 165–179. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.36.20; Data Collection and Processing Software for Agilent X-Ray Diffractometers; Agilent Technologies: Tokyo, Japan, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Hazen, R.M.; Burnham, C.W. The crystal structures of one-layer phlogopite and annite. Am. Mineral. 1973, 58, 889–900. [Google Scholar]
- Redhammer, G.J.; Beran, A.; Schneider, J.; Amthauer, G.; Lottermoser, W. Spectroscopic and structural properties of synthetic micas on the annite-siderophyllite binary: Synthesis, crystal structure refinement, Mössbauer, and infrared spectroscopy. Am. Mineral. 2000, 85, 449–465. [Google Scholar] [CrossRef]
- Ďurovič, S. OD-Charakter, Polytypie und Identifikation von Schichtsilikaten. Fortschritte Mineral. 1981, 59, 191–226. [Google Scholar]
- Nazzareni, S.; Comodi, P.; Bindi, L.; Safonov, O.G.; Litvin, Y.A.; Perchuk, L.L. Synthetic hypersilicic Cl-bearing mica in the phlogopite–celadonite join: A multi-methodical characterization of the missing link between di- and tri-octahedral micas at high pressures. Am. Mineral. 2008, 93, 1429–1436. [Google Scholar] [CrossRef]
- Lepore, G.O.; Bindi, L.; Pedrazzi, G.; Conticelli, S.; Bonazzi, P. Structural and chemical variations in phlogopite from lamproitic rocks of the Central Mediterranean region. Lithos 2017, 286, 191–205. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Henry, D.J.; Daigle, N.M. Chlorine incorporation into amphibole and biotite in high-grade iron-formations: Interplay between crystallography and metamorphic fluids. Am. Mineral. 2018, 103, 55–68. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Guggenheim, S. Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. Rev. Mineral. Geochem. 2002, 46, 1–97. [Google Scholar] [CrossRef]
- Mercier, P.H.; Rancourt, D.G.; Redhammer, G.J.; Lalonde, A.E.; Robert, J.L.; Berman, R.G.; Kodama, H. Upper limit of the tetrahedral rotation angle and factors affecting octahedral flattening in synthetic and natural 1M polytype C2/m space group micas. Am. Mineral. 2006, 91, 831–849. [Google Scholar] [CrossRef][Green Version]
- Lepore, G.O.; Bindi, L.; Zanetti, A.; Ciriotti, M.E.; Medenbach, O.; Bonazzi, P. Balestraite, KLi2VSi4O10O2, the first member of the mica group with octahedral V5+. Am. Mineral. 2015, 100, 608–614. [Google Scholar] [CrossRef]
- Cruciani, G.; Zanazzi, P.F. Cation partitioning and substitution mechanisms in 1M phlogopite: A crystal chemical study. Am. Mineral. 1994, 79, 289–301. [Google Scholar]
- Gianfagna, A.; Scordari, F.; Mazziotti Tagliani, S.; Ventruti, G.; Ottolini, L. Fluorophlogopite from Biancavilla (Mt. Etna, Sicily, Italy): Crystal structure and crystal chemistry of a new F-dominant analog of phlogopite. Am. Mineral. 2007, 92, 1601–1609. [Google Scholar] [CrossRef]
- Schingaro, E.; Lacalamita, M.; Scordari, F.; Brigatti, M.F.; Pedrazzi, G. Crystal chemistry of Ti-rich fluorophlogopite from Presidente Olegario, Alto Paranaíba igneous province, Brazil. Am. Mineral. 2011, 96, 732–743. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Nikulin, I.I.; Martin, R.F.; Nikiforov, A.A.; Silyanov, S.A.; Lobastov, B.M. Ore assemblages, platinum-group minerals and behavior of Cl in low-sulfide zones of the Vologochan–Pyasinskiy suites, Norilsk complex, Russia. Can. J. Mineral. Petrol. 2024, 62, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Malitch, K.N. Forecasting criteria for sulphide PGE−Cu−Ni deposits of the Noril’sk province. Lithosphere 2021, 21, 660–682. [Google Scholar] [CrossRef]

| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| SiO2 wt.% | 36.57 | 36.88 | 36.81 | 35.73 | 34.64 | 34.33 | 33.84 |
| TiO2 | 0.04 | 0.06 | 0.12 | 0.20 | 0.37 | 0.46 | 0.50 |
| Al2O3 | 6.92 | 7.14 | 7.67 | 8.61 | 9.72 | 9.90 | 10.19 |
| Cr2O3 | 0.01 | 0.04 | 0.03 | 0.09 | 0.14 | 0.14 | 0.13 |
| FeO | 39.75 | 39.48 | 39.83 | 39.69 | 39.48 | 39.11 | 39.21 |
| MnO | 0.74 | 0.67 | 0.65 | 0.49 | 0.25 | 0.21 | 0.22 |
| MgO | 0.44 | 0.58 | 0.62 | 0.77 | 0.88 | 0.99 | 0.95 |
| CaO | 0.09 | 0.08 | 0.06 | 0.04 | 0.03 | 0.04 | 0.04 |
| Na2O | 0.27 | 0.29 | 0.26 | 0.29 | 0.11 | 0.10 | 0.15 |
| K2O | 6.18 | 6.30 | 6.71 | 7.42 | 8.51 | 8.52 | 8.61 |
| F | 0.21 | 0.16 | 0.09 | 0.14 | 0.20 | 0.31 | 0.33 |
| Cl | 7.75 | 7.53 | 7.49 | 7.07 | 6.55 | 6.26 | 6.05 |
| Subtotal | 98.97 | 99.22 | 100.34 | 100.54 | 100.88 | 100.37 | 100.22 |
| F≡O | 0.09 | 0.07 | 0.04 | 0.06 | 0.09 | 0.13 | 0.14 |
| Cl≡O | 1.75 | 1.70 | 1.69 | 1.60 | 1.48 | 1.41 | 1.36 |
| Total | 97.13 | 97.45 | 98.61 | 98.88 | 99.31 | 98.82 | 98.72 |
| Si apfu | 3.237 | 3.237 | 3.194 | 3.099 | 2.993 | 2.974 | 2.939 |
| Ti | 0.003 | 0.004 | 0.008 | 0.013 | 0.024 | 0.030 | 0.033 |
| Al | 0.722 | 0.739 | 0.784 | 0.880 | 0.990 | 1.011 | 1.043 |
| Cr | 0.001 | 0.003 | 0.002 | 0.006 | 0.010 | 0.010 | 0.009 |
| Fe2+ | 2.943 | 2.898 | 2.891 | 2.879 | 2.853 | 2.834 | 2.848 |
| Mn | 0.055 | 0.050 | 0.048 | 0.036 | 0.018 | 0.015 | 0.016 |
| Mg | 0.058 | 0.076 | 0.080 | 0.100 | 0.113 | 0.128 | 0.123 |
| Ca | 0.009 | 0.008 | 0.006 | 0.004 | 0.003 | 0.004 | 0.004 |
| Na | 0.046 | 0.049 | 0.044 | 0.049 | 0.018 | 0.017 | 0.025 |
| K | 0.698 | 0.705 | 0.743 | 0.821 | 0.938 | 0.942 | 0.954 |
| F | 0.059 | 0.044 | 0.025 | 0.038 | 0.055 | 0.085 | 0.091 |
| Cl | 1.163 | 1.120 | 1.102 | 1.039 | 0.959 | 0.919 | 0.890 |
| OH (calc) | 0.778 | 0.836 | 0.873 | 0.923 | 0.986 | 0.996 | 1.019 |
| Mg# | 1.90 | 2.51 | 2.65 | 3.32 | 3.79 | 4.30 | 4.12 |
| Cl-Rich Annite | Synthetic Annite * | |
|---|---|---|
| Crystal size (mm) | 0.017 × 0.009 × 0.007 | |
| Cell setting | Monoclinic | Monoclinic |
| Space group | C2/m | C2/m |
| a (Å) | 5.3991(4) | 5.3899(8) |
| b (Å) | 9.3586(6) | 9.337(1) |
| c (Å) | 10.242(1) | 10.309(1) |
| β (°) | 100.873(9) | 100.16(1) |
| V (Å3) | 508.22(7) | 510.6(1) |
| Z | 2 | 2 |
| Data collection and refinement | ||
| Radiation, wavelength (Å) | MoKα, λ = 0.71073 | |
| Temperature (K) | 293 | |
| 2θ max (°) | 66.70 | |
| Unique reflections | 4593 | |
| Reflections with F > 4σF | 3720 | |
| Rint | 0.0497 | |
| Range of h, k, l | −8 ≤ h ≤ 7, −13 ≤ k ≤ 13 | |
| −15 ≤ l ≤ 15 | ||
| R [F > 4σF] | 0.0747 | |
| R (all data) | 0.0855 | |
| wR (on F2) | 0.2179 | |
| GooF | 1.085 | |
| Number of least-squares parameters | 63 | |
| Maximum residual peak (e Å−3) | 3.22 (at 1.15 Å from C1) | |
| Minimum residual peak (e Å−3) | −1.25 (at 0.75 Å from M2) |
| hkl | dcalc (Å) | Icalc | hkl | dcalc (Å) | Icalc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1 | 10.0582 | 100 | 2 | 0 | 2.0251 | 4 | |||||
| 0 | 0 | 2 | 5.0291 | 2 | 1 | 3 | 3 | 2.0060 | 8 | ||||
| 0 | 2 | 0 | 4.6793 | 2 | 1 | 3 | 1.9310 | 2 | |||||
| 1 | 1 | 0 | 4.6132 | 2 | 1 | 3 | 1.6901 | 8 | |||||
| 1 | 1 | 1 | 3.9545 | 2 | 2 | 0 | 4 | 1.6736 | 4 | ||||
| 1 | 1 | 3.7170 | 5 | 0 | 6 | 0 | 1.5598 | 5 | |||||
| 0 | 2 | 2 | 3.4258 | 7 | 3 | 3 | 1.5588 | 10 | |||||
| 0 | 0 | 3 | 3.3527 | 17 | 2 | 0 | 1.5556 | 2 | |||||
| 1 | 1 | 2 | 3.1517 | 10 | 3 | 3 | 1.5431 | 2 | |||||
| 1 | 1 | 2.9523 | 10 | 1 | 3 | 5 | 1.5415 | 4 | |||||
| 0 | 2 | 3 | 2.7254 | 7 | 0 | 6 | 1 | 1.5413 | 2 | ||||
| 1 | 3 | 2.6618 | 25 | 3 | 3 | 0 | 1.5377 | 2 | |||||
| 2 | 0 | 0 | 2.6511 | 13 | 2 | 0 | 1.3767 | 2 | |||||
| 1 | 1 | 3 | 2.5224 | 3 | 1 | 3 | 6 | 1.3651 | 3 | ||||
| 0 | 0 | 4 | 2.5146 | 4 | 4 | 0 | 1.3459 | 2 | |||||
| 1 | 3 | 2.4714 | 26 | 2 | 6 | 0 | 1.3443 | 4 | |||||
| 2 | 0 | 1 | 2.4521 | 12 | 3 | 3 | 1.3317 | 2 | |||||
| 2 | 2 | 2.3332 | 2 | 4 | 0 | 1.3208 | 2 | ||||||
| 2 | 2 | 0 | 2.3066 | 2 | 2 | 6 | 1 | 1.3161 | 3 | ||||
| 2 | 0 | 2.3014 | 2 | 2 | 6 | 1.2912 | 2 | ||||||
| 1 | 3 | 2 | 2.2821 | 5 | 2 | 6 | 4 | 1.1410 | 2 | ||||
| 1 | 3 | 2.2029 | 13 | 2 | 6 | 1.1014 | 2 | ||||||
| 2 | 0 | 2 | 2.1816 | 6 | 3 | 9 | 0.9003 | 2 | |||||
| Atom | Wyckoff | s.o.f. | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|---|
| A | 2a | K1.00 | 0 | 0 | 0 | 0.0472 (11) |
| M1 | 2d | Fe0.88(2)Mg0.12(2) | 0 | ½ | ½ | 0.0142 (7) |
| M2 | 4h | Fe0.92(2)Mg0.08(2) | 0 | 0.82843 (12) | ½ | 0.0140 (5) |
| Si | 8j | Si1.00 | 0.5728 (3) | 0.16615 (15) | 0.2240 (2) | 0.0144 (5) |
| O1 | 8j | O1.00 | 0.8042 (8) | 0.2498 (5) | 0.1677 (5) | 0.0223 (11) |
| O2 | 4i | O1.00 | 0.5504 (12) | 0 | 0.1666 (7) | 0.0228 (15) |
| O3 | 8j | O1.00 | 0.6301 (8) | 0.1650 (4) | 0.3891 (5) | 0.0143 (10) |
| O4 | 4i | O0.55(3) | 0.126 (3) | 0 | 0.400 (3) | 0.014 (3) |
| Cl4 | 4i | Cl0.45(3) | 0.1114 (10) | 0 | 0.3390 (13) | 0.018 (3) |
| Cl-Rich Annite | Synthetic Annite * | |
|---|---|---|
| M1 | ||
| O3 (×4) | 2.114(4) | 2.115(15) |
| O4 (×2) | 2.09(1) | 2.098(10) |
| <M1-O> | 2.106 | 2.110 |
| Cl (×2) | 2.412(9) | |
| M2 | ||
| O3 (×2) | 2.103(4) | 2.065(13) |
| O3’ (×2) | 2.104(4) | 2.104(16) |
| O4 (×2) | 2.09(2) | 2.157(14) |
| <M2-O> | 2.099 | 2.109 |
| Cl (×2) | 2.456(9) | |
| T | ||
| O1 | 1.655(4) | 1.62(3) |
| O1’ | 1.667(5) | 1.65(3) |
| O2 | 1.659(3) | 1.661(13) |
| O3 | 1.661(6) | 1.710(13) |
| <T-O> | 1.661 | 1.659 |
| A | ||
| O1inner (×4) | 3.173(5) | 3.174(16) |
| O2inner (×2) | 3.135(6) | 3.137(16) |
| O1outer (×4) | 3.197(5) | 3.312(15) |
| O2outer (×2) | 3.220(7) | 3.24(2) |
| <A-O>inner | 3.160 | 3.162 |
| <A-O>outer | 3.205 | 3.288 |
| <A-O> | 3.183 | 3.224 |
| Cl | 3.41(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkov, A.Y.; Lepore, G.O.; Bindi, L.; Martin, R.F.; Panikorovskii, T.; Nikulin, I.I.; Silyanov, S.A. A Cl-Dominant Analogue of Annite Occurs at the Eastern Edge of the Oktyabrsky Cu-Ni-PGE Deposit, Norilsk, Russia. Minerals 2025, 15, 640. https://doi.org/10.3390/min15060640
Barkov AY, Lepore GO, Bindi L, Martin RF, Panikorovskii T, Nikulin II, Silyanov SA. A Cl-Dominant Analogue of Annite Occurs at the Eastern Edge of the Oktyabrsky Cu-Ni-PGE Deposit, Norilsk, Russia. Minerals. 2025; 15(6):640. https://doi.org/10.3390/min15060640
Chicago/Turabian StyleBarkov, Andrei Y., Giovanni Orazio Lepore, Luca Bindi, Robert F. Martin, Taras Panikorovskii, Ivan I. Nikulin, and Sergey A. Silyanov. 2025. "A Cl-Dominant Analogue of Annite Occurs at the Eastern Edge of the Oktyabrsky Cu-Ni-PGE Deposit, Norilsk, Russia" Minerals 15, no. 6: 640. https://doi.org/10.3390/min15060640
APA StyleBarkov, A. Y., Lepore, G. O., Bindi, L., Martin, R. F., Panikorovskii, T., Nikulin, I. I., & Silyanov, S. A. (2025). A Cl-Dominant Analogue of Annite Occurs at the Eastern Edge of the Oktyabrsky Cu-Ni-PGE Deposit, Norilsk, Russia. Minerals, 15(6), 640. https://doi.org/10.3390/min15060640











