Selective Recovery of Palladium (II) from Acidic Solutions Using Dithio- and Benzimidazolylthio-Functionalized Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Synthesis Methods
2.3.1. Functionalization of Merrifield Resin with 1,2-Ethanedithiol and 1,2-Benzenedithiol
2.3.2. Functionalization of Merrifield Resin with 2-Benzimidazolylthio Acetic Acid
2.3.3. Acid Stability
2.4. Batch Adsorption
Selectivity Study
3. Results
3.1. Characterization of Materials
3.2. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum group metals: A review of resources, production and usage with a focus on catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Gulliani, S.; Volpe, M.; Messineo, A.; Volpe, R. Recovery of metals and valuable chemicals from waste electric and electronic materials: A critical review of existing technologies. RSC Sustain. 2023, 1, 1085–1108. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 100, 64–90. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Abd Majid, Z.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Lee, J.C.; Hong, H.J.; Chung, K.W.; Kim, S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep. Purif. Technol. 2020, 246, 116896. [Google Scholar] [CrossRef]
- Siu, M.; Yaylayan, V.A.; Bélanger, J.M.; Paré, J.J. Microwave-assisted immobilization of β-cyclodextrin on PEGylated Merrifield resins. Tetrahedron Lett. 2005, 46, 3737–3739. [Google Scholar] [CrossRef]
- Zviagin, I.M.; Khimchenko, S.V.; Blank, T.A.; Shcherbakov, I.K.; Bryleva, E.Y.; Bunina, Z.Y.; Sofronov, D.S.; Belikov, K.N.; Chebanov, V.A. Merrifield resin-linked polyazole-based sorbent for heavy metal ions extraction from water. Functional Materials. Funct. Mater. 2018. [Google Scholar] [CrossRef]
- Fontenot, S.A.; Carter, T.G.; Johnson, D.W.; Addleman, R.S.; Warner, M.G.; Yantasee, W.; Warner, C.L.; Fryxell, G.E.; Bays, J.T. Nanostructured Materials for Selective Collection of Trace-Level Metals from Aqueous Systems. Trace Anal. Nanomater. 2010, 191–221. [Google Scholar]
- Fernández-Puig, S.; Luaces-Alberto, M.D.; Vallejo-Becerra, V.; Teran, A.O.; Chávez-Ramírez, A.U.; González, A.V. Modified Merrifield’s resin materials used in capturing of Pb(II) ions in water. Mater. Res. Express 2019, 6, 115104. [Google Scholar] [CrossRef]
- Yu, X.; Hao, J.; Xi, Z.; Liu, T.; Lin, Y.; Xu, B. Investigation of low concentration SO2 adsorption performance on different amine-modified Merrifield resins. Atmos. Pollut. Res. 2019, 10, 404–411. [Google Scholar] [CrossRef]
- Pustam, A.N.; Alexandratos, S.D. Engineering selectivity into polymer-supported reagents for transition metal ion complex formation. React. Funct. Polym. 2010, 70, 545–554. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ogunlaja, A.S.; Kempgens, P.F.; Antunes, E.; Torto, N.; Nyokong, T.; Tshentu, Z.R. Adsorption and separation of platinum and palladium by polyamine functionalized polystyrene-based beads and nanofibers. Miner. Eng. 2013, 53, 256–265. [Google Scholar] [CrossRef]
- Kang, T.; Park, Y.; Yi, J. Highly selective adsorption of Pt2+ and Pd2+ using thiol-functionalized mesoporous silica. Ind. Eng. Chem. Res. 2004, 43, 1478–1484. [Google Scholar] [CrossRef]
- Liu, J.L.; Sun, M.; Shi, Y.H.; Zhou, X.M.; Zhang, P.Z.; Jia, A.Q.; Zhang, Q.F. Functional modification, self-assembly and application of calix [4] resorcinarenes. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 201–233. [Google Scholar] [CrossRef]
- Tofan, L.; Wenkert, R. Chelating polymers with valuable sorption potential for development of precious metal recycling technologies. Rev. Chem. Eng. 2022, 38, 167–183. [Google Scholar] [CrossRef]
- Wu, F.; Ye, G.; Yi, R.; Sun, T.; Xu, C.; Chen, J. Novel polyazamacrocyclic receptor decorated core–shell superparamagnetic microspheres for selective binding and magnetic enrichment of palladium: Synthesis, adsorptive behavior and coordination mechanism. Dalton Trans. 2016, 45, 9553–9564. [Google Scholar] [CrossRef]
- Kancharla, S.; Sasaki, K. Selective extraction of precious metals from simulated automotive catalyst waste and their conversion to carbon supported PdPt nanoparticle catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131179. [Google Scholar] [CrossRef]
- Mildan, E.; Gülfen, M. Equilibrium, kinetics, and thermodynamics of Pd (II) adsorption onto poly (m-aminobenzoic acid) chelating polymer. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of palladium (II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009, 162, 874–879. [Google Scholar] [CrossRef]
- Krakovský, I.; Pleštil, J.; Almásy, L. Structure and swelling behaviour of hydrophilic epoxy networks investigated by SANS. Polymer 2006, 47, 218–226. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shimazaki, H.; Miyamoto, Y.; Shimada, K.; Haga, F.; Yamada, Y.; Miyazawa, H.; Nishiwaki, K.; Kashimura, S. Simple and convenient synthesis of esters from carboxylic acids and alkyl halides using tetrabutylammonium fluoride. J. Oleo Sci. 2014, 63, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Santini, R.; Griffith, M.C.; Qi, M. A measure of solvent effects on swelling of resins for solid phase organic synthesis. Tetrahedron Lett. 1998, 39, 8951–8954. [Google Scholar] [CrossRef]
- Majavu, A.; Ogunlaja, A.S.; Tshentu, Z.R. Separation of rhodium(III) and iridium(IV) chlorido complexes using polymer microspheres functionalised with quaternary diammonium groups. Sep. Sci. Technol. 2017, 52, 71–80. [Google Scholar] [CrossRef]
- Dardouri, M.; Ammari, F.; Meganem, F. Aminoalkylated Merrifield resins reticulated by tris-(2-chloroethyl) phosphate for cadmium, copper, and iron(II) extraction. Int. J. Polym. Sci. 2015, 2015, 782841. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Zhang, Y.; Ren, K.; Wang, L.; Wang, G. Recyclable Merrifield resin-supported organocatalysts containing pyrrolidine unit through A3-coupling reaction linkage for asymmetric Michael addition. Chirality 2010, 22, 432–441. [Google Scholar] [CrossRef]
- Boruah, J.J.; Das, S.P.; Ankireddy, S.R.; Gogoi, S.R.; Islam, N.S. Merrifield resin supported peroxomolybdenum (VI) compounds: Recoverable heterogeneous catalysts for the efficient, selective and mild oxidation of organic sulfides with H2O2. Green Chem. 2013, 15, 2944–2959. [Google Scholar] [CrossRef]
- Chen, G. Surface Modification with Polymers Using Living Radical Polymerisation and Click Chemistry. Ph.D. Thesis, University of Warwick, Coventry, UK, 2007. [Google Scholar]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.C.; Fouquet, E.; Felpin, F.X. Recyclable heterogeneous palladium catalysts in pure water: Sustainable developments in Suzuki, Heck, Sonogashira and Tsuji–Trost reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef]
- Huang, Y. Novel Applications of Surface-Modified Sporopollenin Exine Capsules. Ph.D. Thesis, University of Hull, Yorkshire, UK, 2013. [Google Scholar]
- Edo, G.I.; Ndudi, W.; Ali, A.B.; Yousif, E.; Zainulabdeen, K.; Onyibe, P.N.; Ekokotu, H.A.; Isoje, E.F.; Essaghah, A.E.; Agmed, D.S.; et al. Poly(vinyl chloride) (PVC): An updated review of its properties, polymerization, modification, recycling, and applications. J. Mater. Sci. 2024, 59, 21605–21648. [Google Scholar] [CrossRef]
- Pisk, J.; Agustin, D.; Poli, R. Organic salts and Merrifield resin supported [PM12O40] 3−(M= Mo or W) as catalysts for adipic acid synthesis. Molecules 2019, 24, 783. [Google Scholar] [CrossRef]
- Boruah, J.J.; Das, S.P. Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum(VI) complex with H2O2 as an oxidant. RSC Adv. 2018, 8, 34491–34504. [Google Scholar] [CrossRef] [PubMed]
- Maurya, M.R.; Chaudhary, N.; Avecilla, F. Polymer-grafted and neat vanadium(V) complexes as functional mimics of haloperoxidases. Polyhedron 2014, 67, 436–448. [Google Scholar] [CrossRef]
- Rajesh Krishnan, G.; Sreekumar, K. Catalysis by Polymer Supported Dendrimers, Their Metal Complexes and Nanoparticle Conjugates. Ph.D. Thesis, Cochin University of Science and Technology, Kochi, India, 2008. [Google Scholar]
- Dalla Valle, C. Novel Mesoporous Polymers and Their Application in Heterogeneous Catalysis. Ph.D. Thesis, University of Padua, Padua, Italy, 2017. [Google Scholar]
- Biernat, J.F.; Konieczka, P.; Tarbet, B.J.; Bradshaw, J.S.; Izatt, R.M. Complexing and chelating agents immobilized on silica gel and related materials and their application for sorption of inorganic species. Sep. Purif. Methods 1994, 23, 77–348. [Google Scholar] [CrossRef]
- Lapinte, V.; Montembault, V.; Houdayer, A.; Fontaine, L. Surface initiated ring-opening metathesis polymerization of norbornene onto Wang and Merrifield resins. J. Mol. Catal. A Chem. 2007, 276, 219–225. [Google Scholar] [CrossRef]
- Kappert, E.J.; Raaijmakers, M.J.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Membr. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef]
- Das, D.; Sivaramakrishna, A.; Brahmmananda Rao CV, S.; Sivaraman, N.; Vijayakrishna, K. Phosphoramidate-functionalized Merrifield resin: Synthesis and application in actinide separation. Polym. Int. 2018, 67, 374–379. [Google Scholar] [CrossRef]
- Seyhan, S.; Çolak, M.; Merdivan, M.; Demirel, N. Solid phase extractive preconcentration of trace metals using p-tert-butylcalix [4] arene-1, 2-crown-4-anchored chloromethylated polymeric resin beads. Anal. Chim. Acta 2007, 584, 462–468. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hossain, M.S.; Ahmed, S.; Mobarak, M.B. Characterization and adsorption performance of nano-hydroxyapatite synthesized from Conus litteratus waste seashells for Congo red dye removal. RSC Adv. 2024, 14, 38560–38577. [Google Scholar] [CrossRef]
- Cao, M.; Wang, J.; Liu, X.; Pei, Y.; Gao, M.; Wang, W.; Yang, H. Bio-inspired adsorbent with ultra-uniform and abundance sites accelerate breaking the trade-off effect between adsorption capacity and removal efficiency. Chem. Eng. J. 2023, 465, 142790. [Google Scholar] [CrossRef]
- Alias, M.F.; Obed, S.M.; Jassim, A.H. Synthesis and characterization of the heavy metals; Au(III), Pd(II), Pt(IV) Rh(III) complexes of s–propynyl 2–thiobenzimidazole. Baghdad Sci. J. 2007, 4, 95–101. [Google Scholar]
- Sharma, R.K.; Kumar, H.; Kumar, A. A highly efficient and magnetically retrievable functionalized nano-adsorbent for ultrasonication assisted rapid and selective extraction of Pd2+ ions from water samples. RSC Adv. 2015, 5, 43371–43380. [Google Scholar] [CrossRef]
- Petrova, P.; Chochkova, M.; Karadjov, M. Adsorption of Pd(II) on N- and S-modified silica sorbents. J. Chem. Technol. Metall. 2022, 57, 962–970. [Google Scholar]
- Elsayed, S.A.; Badr, H.E.; di Biase, A.; El-Hendawy, A.M. Synthesis, characterization of ruthenium(II), nickel(II), palladium (II), and platinum (II) triphenylphosphine-based complexes bearing an ONS-donor chelating agent: Interaction with biomolecules, antioxidant, in vitro cytotoxic, apoptotic activity and cell cycle analysis. J. Inorg. Biochem. 2021, 223, 111549. [Google Scholar]
- Kumar, S.B.; Solanki, A.; Kundu, S. Copper(II) and palladium(II) complexes with tridentate NSO donor Schiff base ligand: Synthesis, characterization and structures. J. Mol. Struct. 2017, 1143, 163–167. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Meng, X.; Xian, M.; Xu, C. Adsorption behavior study and mechanism insights into novel isothiocyanate modified material towards Pd2+. Sep. Purif. Technol. 2021, 277, 119514. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Qi, Q.; Qiu, J.; Li, Z.; Wang, H.; Wang, J. Tailoring delicate pore environment of 2D Covalent organic frameworks for selective palladium recovery. Chem. Eng. J. 2022, 446, 136823. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Hidalgo, M.; Salvado, V. The selective adsorption of gold (III) and palladium (II) on new phosphine sulphide-type chelating polymers bearing different spacer arms: Equilibrium and kinetic characterisation. React. Funct. Polym. 2001, 46, 283–291. [Google Scholar] [CrossRef]
- Boda, A.; Sahu, P.; Deb, A.S.; Ali, S.M. DFT, MD simulations and experimental analysis of adsorptive complexation and isotope separation of gadolinium ion with macrocyclic crown ether embedded polymeric resin. Sep. Purif. Technol. 2022, 289, 120709. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of π-complexation. J. Hazard. Mater. 2017, 325, 198–213. [Google Scholar] [CrossRef]
- Liu, H.; Mao, Y. Graphene oxide-based nanomaterials for uranium adsorptive uptake. ES Mater. Manuf. 2021, 13, 3–22. [Google Scholar] [CrossRef]
- Scheufele, F.B.; Módenes, A.N.; Borba, C.E.; Ribeiro, C.; Espinoza-Quiñones, F.R.; Bergamasco, R.; Pereira, N.C. Monolayer–multilayer adsorption phenomenological model: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2016, 284, 1328–1341. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, A.; Zhang, F.; Liu, J. Preparation and characterization of a novel macroporous silica-bipyridine asymmetric multidentate functional adsorbent and its application for heavy metal palladium removal. J. Hazard. Mater. 2017, 337, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Zhang, L.; Fan, Y.; Xu, L.; Ma, Z.; Wen, Z.; Wang, H.; Cheng, N. Adsorption of Pd(II) ions by electrospun fibers with effective adsorption sites constructed by N, O atoms with a particular spatial configuration: Mechanism and practical applications. J. Hazard. Mater. 2023, 458, 132014. [Google Scholar] [CrossRef]
- Lin, S.; Bediako, J.K.; Cho, C.W.; Song, M.H.; Zhao, Y.; Kim, J.A.; Choi, J.W.; Yun, Y.S. Selective adsorption of Pd(II) over interfering metal ions (Co(II), Ni(II), Pt(IV)) from acidic aqueous phase by metal-organic frameworks. Chem. Eng. J. 2018, 345, 337–344. [Google Scholar] [CrossRef]
- Uheida, A.; Iglesias, M.; Fontàs, C.; Hidalgo, M.; Salvadó, V.; Zhang, Y.; Muhammed, M. Sorption of palladium (II), rhodium (III), and platinum (IV) on Fe3O4 nanoparticles. J. Colloid Interface Sci. 2006, 301, 402–408. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ogunlaja, A.S.; Antunes, E.; Nyokong, T.; Tshentu, Z.R. The development of palladium (II)-Specific amine-functionalized silica-based microparticles: Adsorption and column separation studies. Sep. Sci. Technol. 2015, 50, 1497–1506. [Google Scholar] [CrossRef]
- Moleko-Boyce, P.; Makelane, H.; Ngayeka, M.Z.; Tshentu, Z.R. Recovery of platinum group metals from leach solutions of spent catalytic converters using custom-made resins. Minerals 2022, 12, 361. [Google Scholar] [CrossRef]

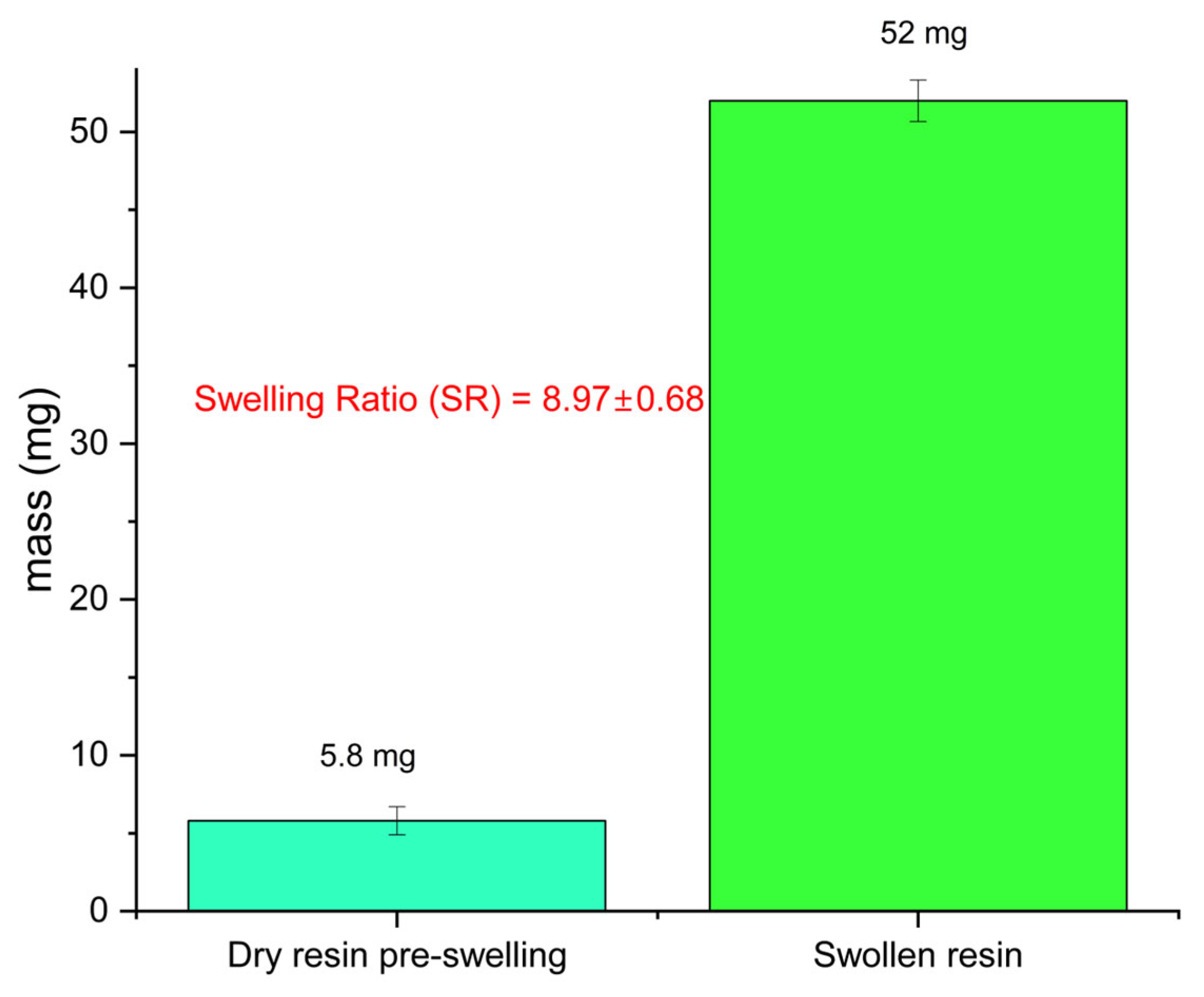
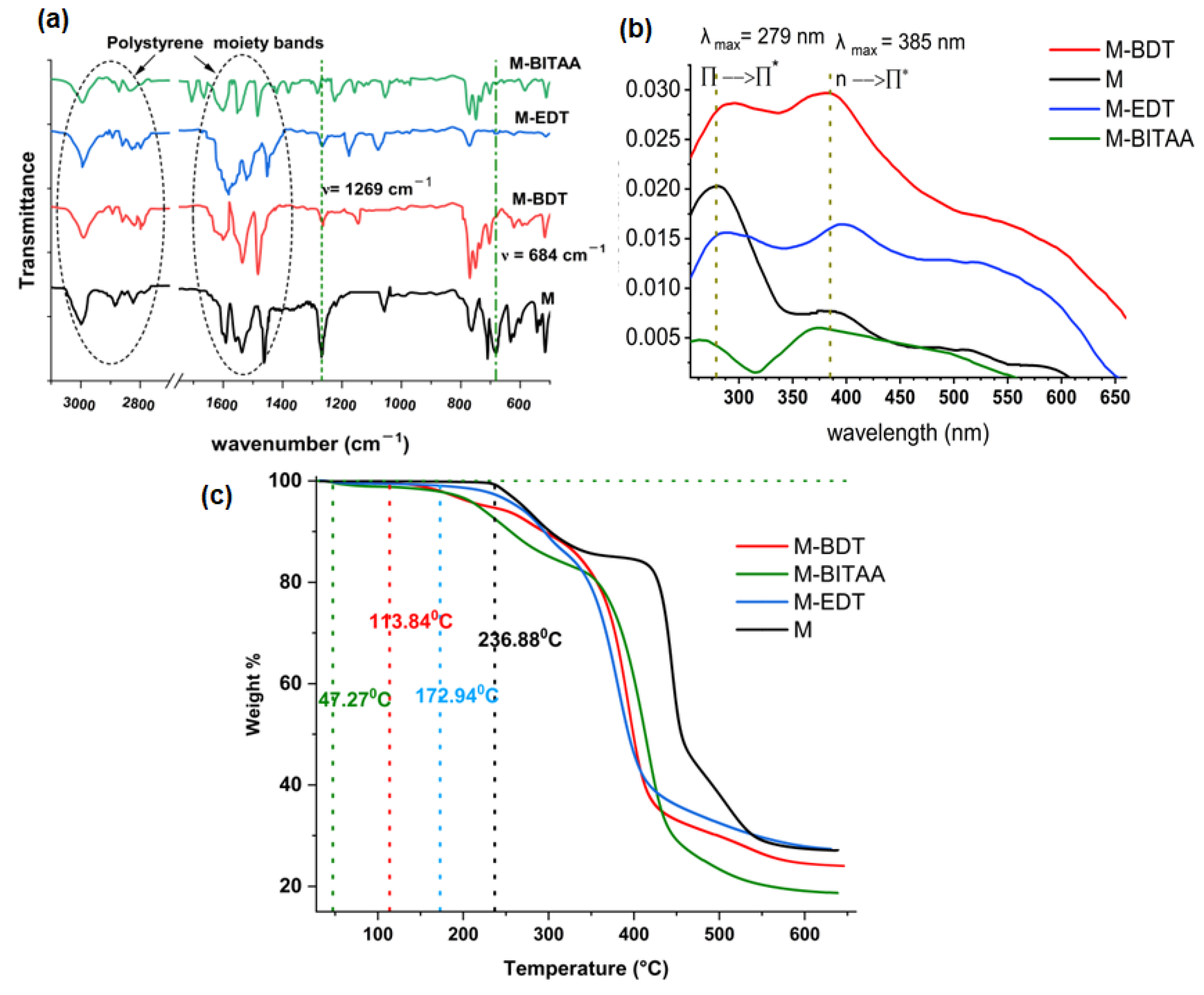

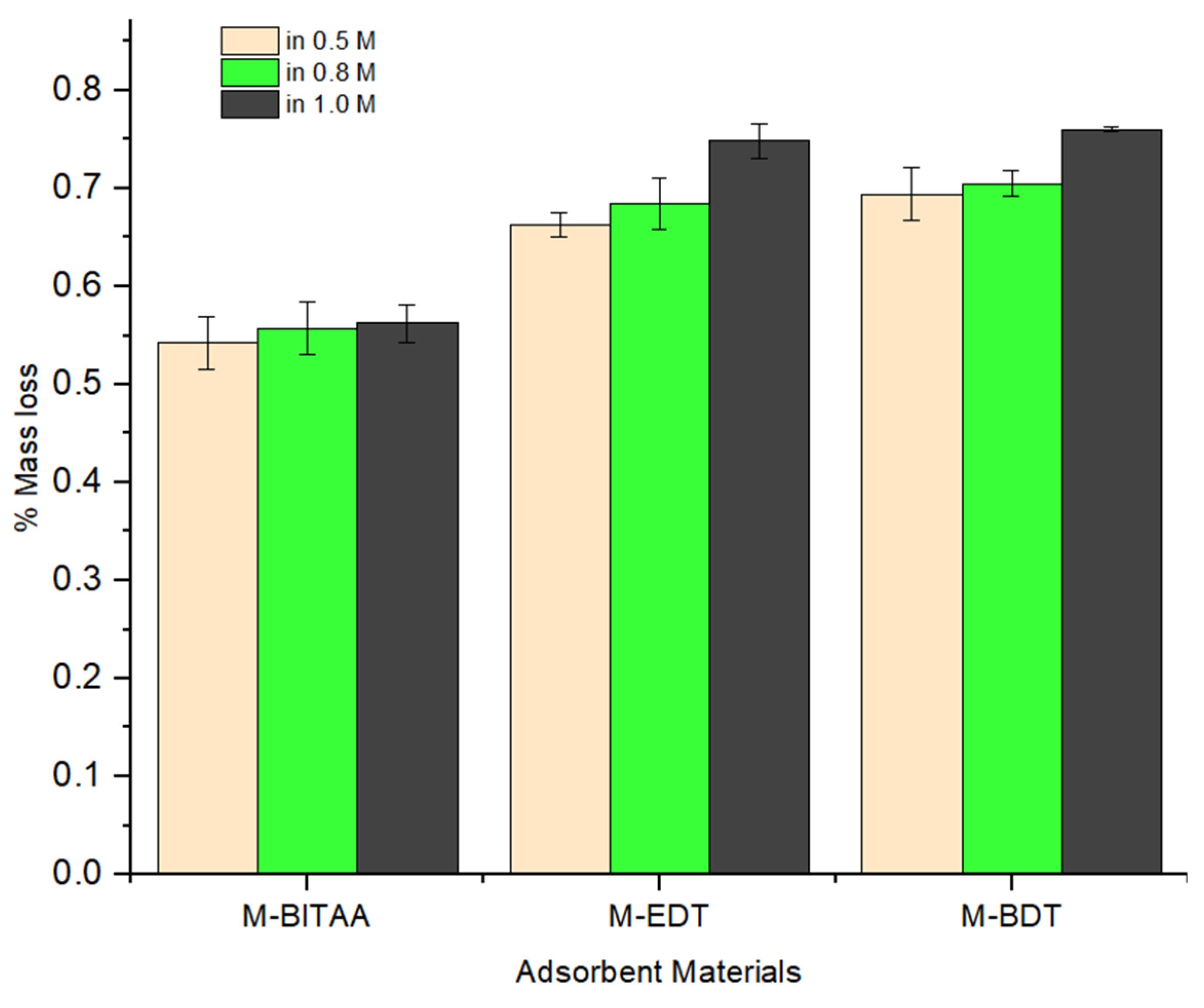
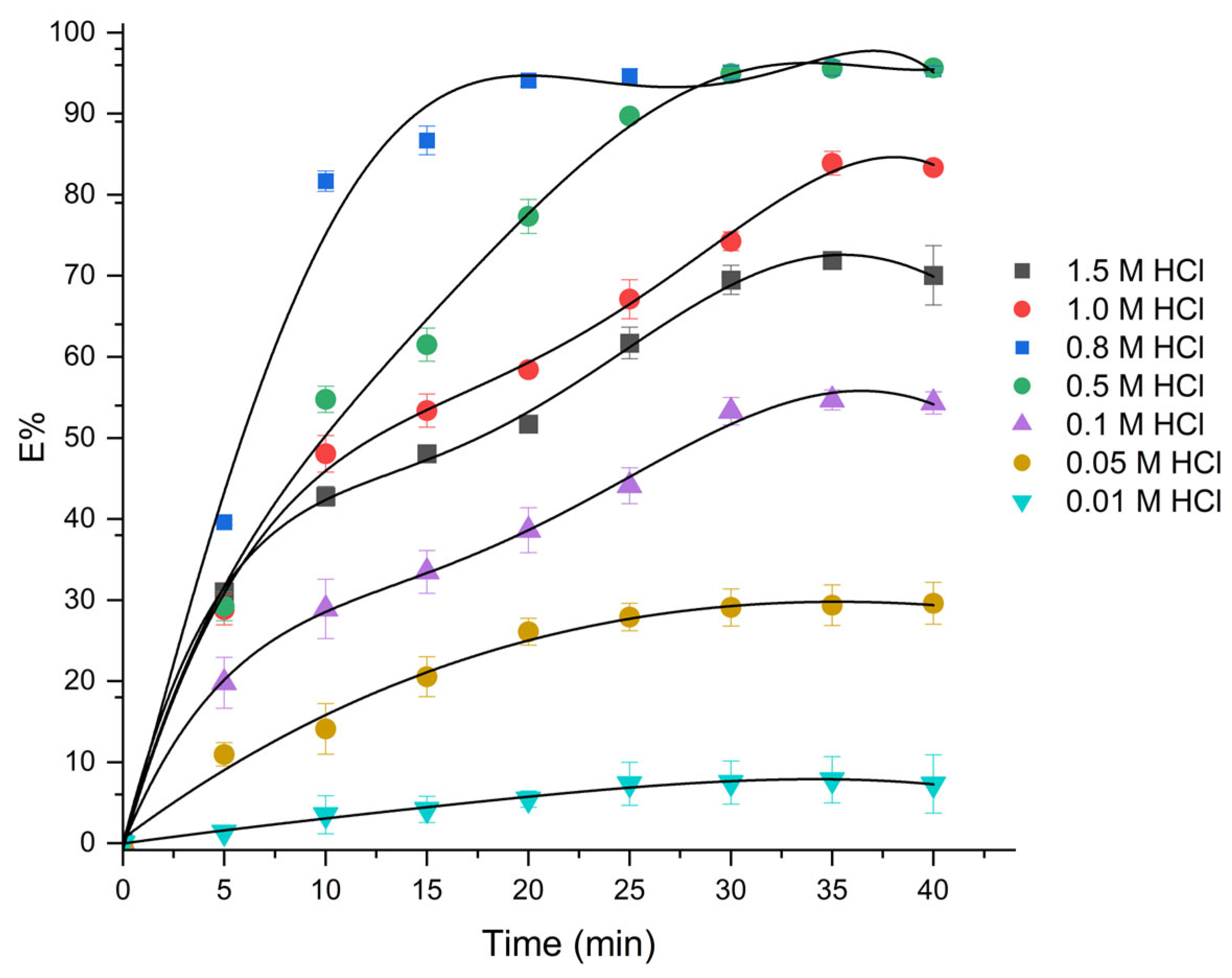
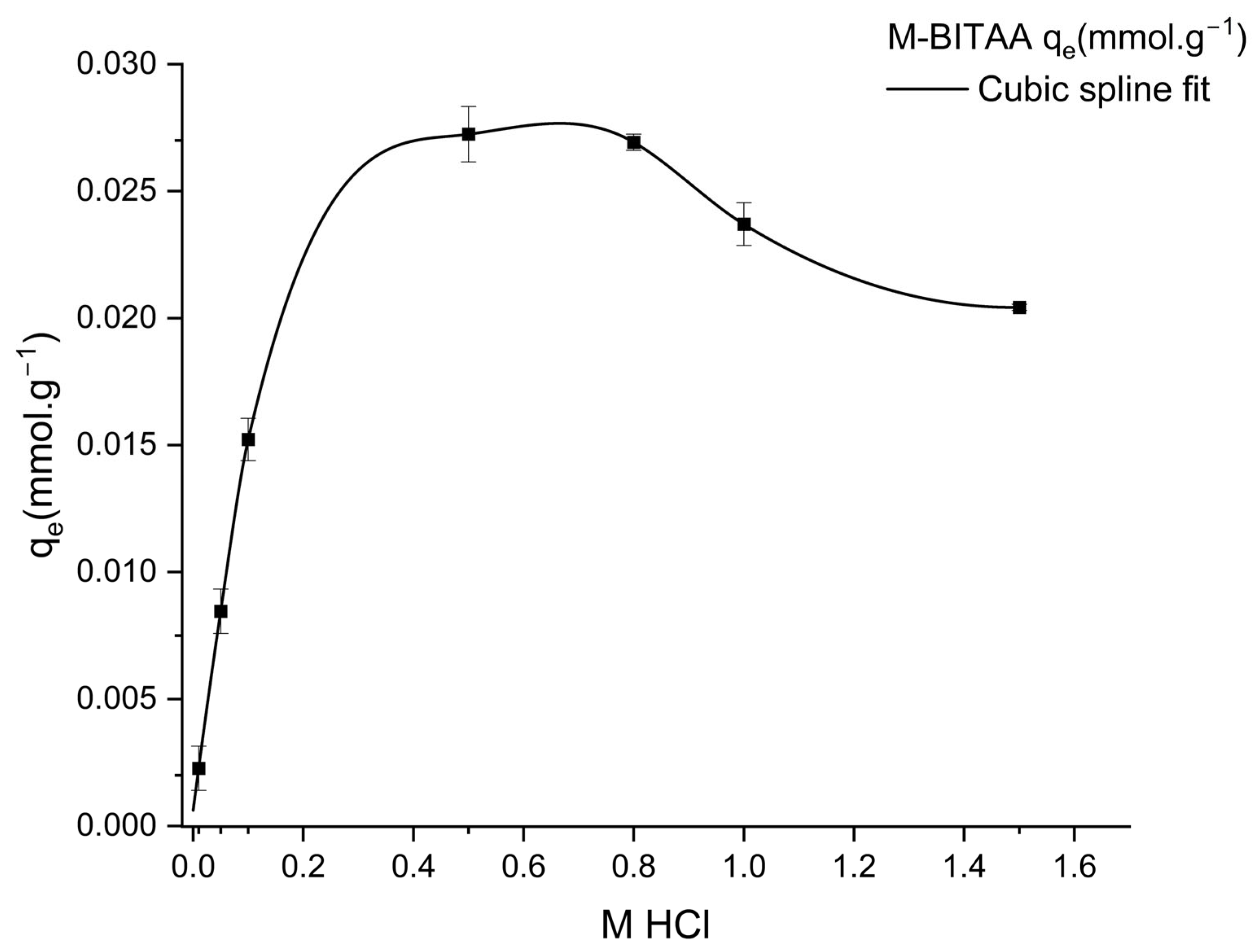
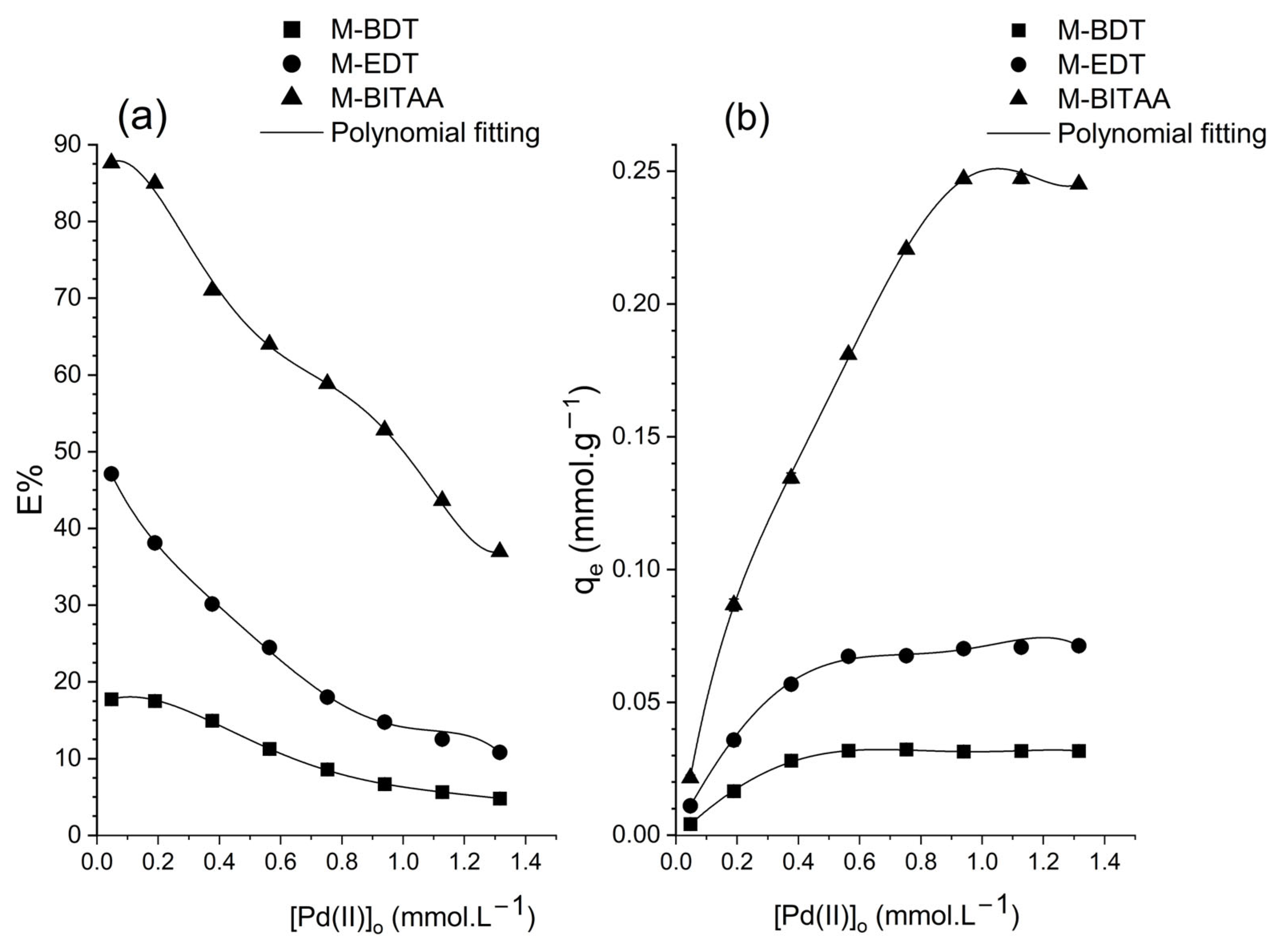

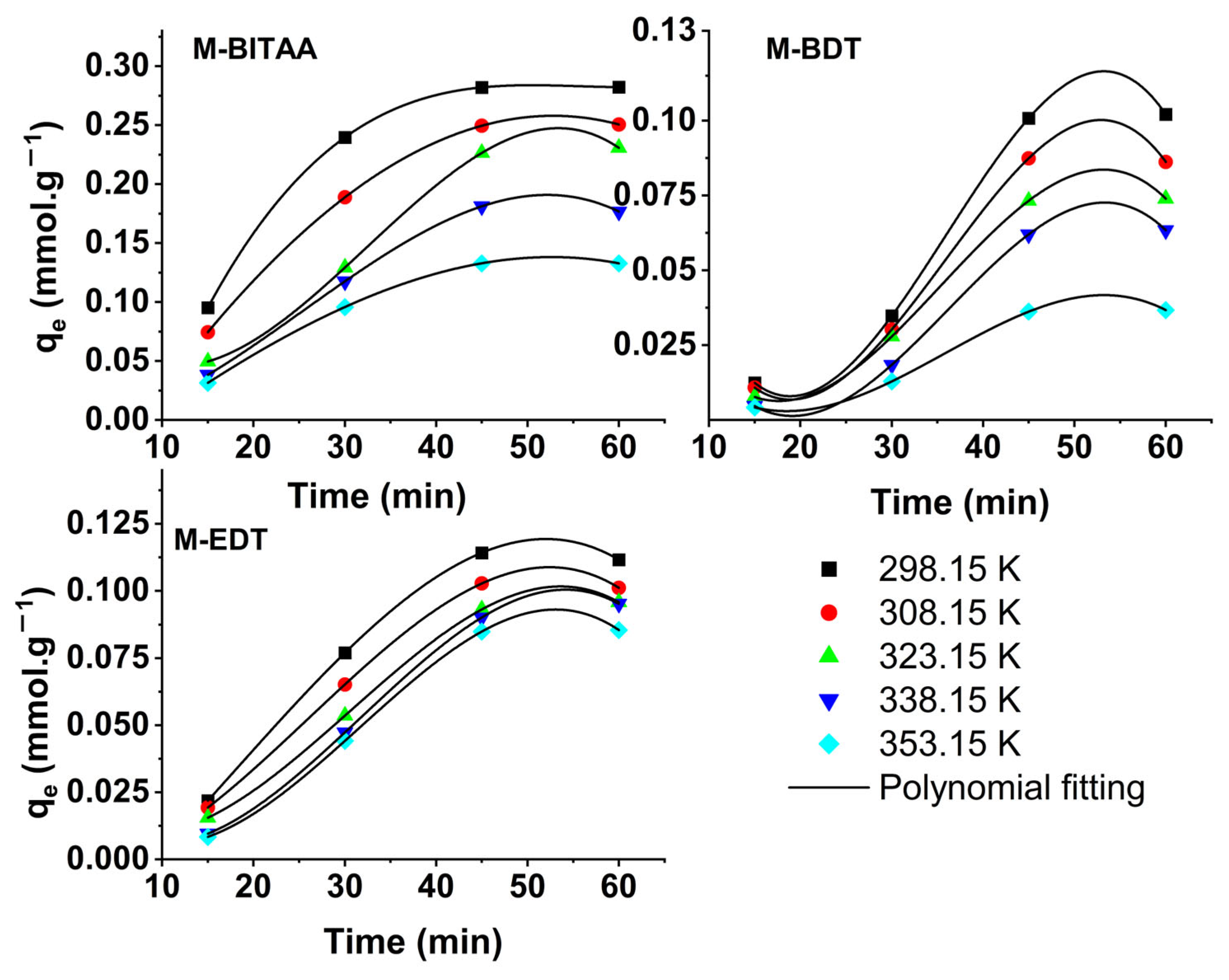
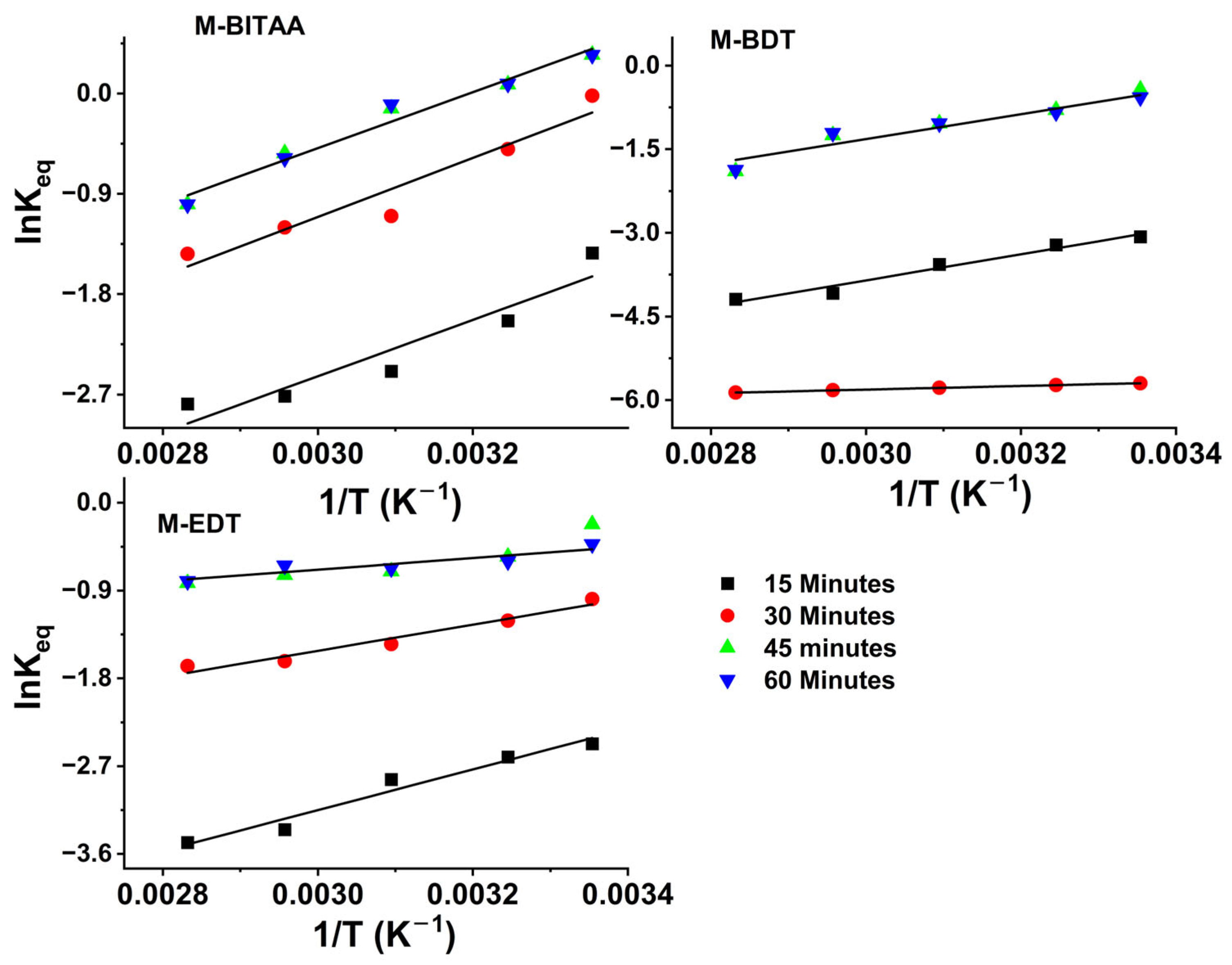
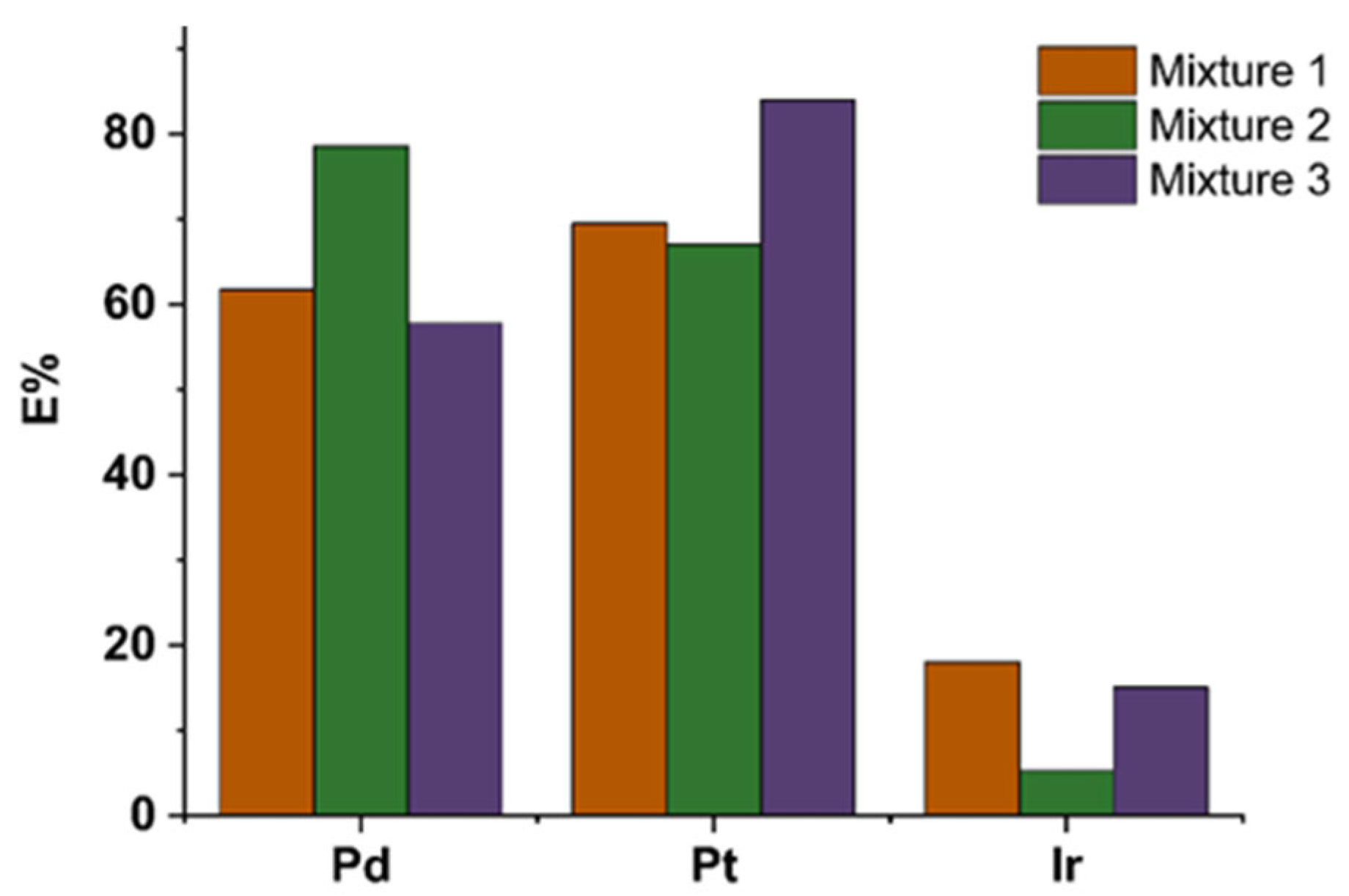
| Pd (mmol·L−1) | Pt (mmol·L−1) | Ir (mmol·L−1) | |
|---|---|---|---|
| Mixture 1 | 0.047 | 0.047 | 0.047 |
| Mixture 2 | 0.094 | 0.047 | 0.047 |
| Mixture 3 | 0.047 | 0.094 | 0.094 |
| Resins | C | H | N | S | Ligand Loading (mmol·g−1) |
|---|---|---|---|---|---|
| M (Pristine) | 79.00 | 5.00 | 0.50 | 0.00 | N/A |
| M-EDT | 79.00 | 6.00 | 0.70 | 0.03 | 0.0022 |
| M-BDT | 78.00 | 6.00 | 0.80 | 0.01 | 0.0018 |
| M-BITAA | 76.00 | 7.00 | 4.00 | 0.04 | 0.020 |
| Adsorbent Name | qe (mmol·g−1) | Adsorbent Dosage (mg) | [M] (mmol·L−1) | pH/ [Acid] | Refs |
|---|---|---|---|---|---|
| M-BITAA | 0.247 | 10 | 0.0467–1.316 | 0.8 M | This work |
| M-EDT | 0.0674 | 10 | 0.0467–1.316 | 0.8 M | This work |
| M-BDT | 0.0319 | 10 | 0.0467–1.316 | 0.8 M | This work |
| Triisobutyl phosphine sulfide functionalized resins | 0.117 | 20 | 4.70 | 1.0 M | [52] |
| Poly (m-aminobenzoic acid) chelating polymer | 0.227 | 300 | 0.282–0.705 | pH 2.0 | [18] |
| Moss (Racomitrium lanuginosum) biomass | 0.886 | 100 | 0.235–2.819 | pH 5 | [19] |
| Polyamine/polystyrene-based beads and nanofibers | 0.040 nanofibers 0.00188 microbeads | 150 | 0.0235–0.0584 | 1.0 M | [22] |
| Rwrt Pt(IV) | Rwrt Ir(III) | |
|---|---|---|
| Mixture 1 | 0.81 | 3.58 |
| Mixture 2 | 2.04 | 28.92 |
| Mixture 3 | 0.32 | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahleba, M.M.; Mukaba, J.-L.; Tshentu, Z.R. Selective Recovery of Palladium (II) from Acidic Solutions Using Dithio- and Benzimidazolylthio-Functionalized Resins. Minerals 2025, 15, 589. https://doi.org/10.3390/min15060589
Mahleba MM, Mukaba J-L, Tshentu ZR. Selective Recovery of Palladium (II) from Acidic Solutions Using Dithio- and Benzimidazolylthio-Functionalized Resins. Minerals. 2025; 15(6):589. https://doi.org/10.3390/min15060589
Chicago/Turabian StyleMahleba, Masivuye M., Jean-Luc Mukaba, and Zenixole R. Tshentu. 2025. "Selective Recovery of Palladium (II) from Acidic Solutions Using Dithio- and Benzimidazolylthio-Functionalized Resins" Minerals 15, no. 6: 589. https://doi.org/10.3390/min15060589
APA StyleMahleba, M. M., Mukaba, J.-L., & Tshentu, Z. R. (2025). Selective Recovery of Palladium (II) from Acidic Solutions Using Dithio- and Benzimidazolylthio-Functionalized Resins. Minerals, 15(6), 589. https://doi.org/10.3390/min15060589









