1. Introduction
Lithium-ion rechargeable batteries have become an increasingly critical technology in our modern world. They are widely used in portable devices, such as smartphones, laptops, cameras, and electric vehicles, due to their ability to efficiently and reliably store and deliver energy. Lithium-ion batteries have a high energy density, allowing them to store more energy per unit of weight and volume, making them an ideal choice for portable devices with limited space and weight. Additionally, their rechargeability makes them cost-effective and environmentally friendly compared to disposable batteries, reducing waste generated by discarded batteries [
1]. Nonetheless, the generation of waste from end-of-life batteries increases year after year. The total amount of batteries, solely from electric vehicles, worldwide from 2021 to 2030 will reach 12.85 million tons [
2]. These batteries contain valuable metals such as cobalt, nickel, copper, and lithium that can be extracted and reused in new battery production or for other industrial purposes. Recycling not only reduces the environmental impact of mining these metals but also conserves natural resources for future generations and reduces the cost of battery production. Moreover, proper disposal of end-of-life batteries reduces the risk of environmental pollution and health hazards associated with toxic waste. Therefore, developing efficient and sustainable recycling methods for lithium-ion batteries is crucial for a cleaner and more sustainable future.
The main components in Li-ion batteries are the cathode, the anode, the electrolyte, the separator, and the casing. Active material is at the cathode, attached by a PVDF-based binder to an Al foil. Common active cathode materials are lithium manganese oxide (LiMn
2O
4), lithium cobalt oxide (LiCoO
2), lithium nickel manganese cobalt oxide (LiNiMnCoO
2), and lithium nickel cobalt aluminum oxide (LiNiCoAlO
2) [
3]. In the anode, a copper sheet is coated by graphite or carbon black. These components work together to enable the transfer of lithium ions between the cathode and the anode, allowing the charging and discharging of batteries [
4].
Currently, the most prevalent technologies used for extracting metals from discarded batteries are hydrometallurgy and pyrometallurgy. However, these methods are costly and environmentally harmful. Hydrometallurgy employs large quantities of harsh chemicals, while pyrometallurgy operates at high temperatures, leading to excessive energy consumption, and generates toxic gases that require further treatment. A significant limitation of the pyrometallurgical process is its inefficiency in extracting lithium, as lithium is highly volatile at the high temperatures used, causing it to vaporize and escape. Additionally, lithium forms stable compounds within the slag that are difficult to reduce or separate, and the process parameters are optimized for more common metals like copper and nickel, not lithium. Consequently, alternative methods are often necessary for effective lithium extraction [
5,
6].
Nowadays, the utilization of microorganisms for extracting metals from electronic waste is widely studied. It is regarded as a promising and environmentally friendly approach to recover valuable metals from urban solid waste, such as printed circuit boards and other electronic waste. Biohydrometallurgy has demonstrated through various studies that bacteria, such as
A. ferrooxidans, can effectively extract copper from PCBs, achieving recovery rates above 85% [
7,
8,
9].
A. ferrooxidans oxidizes Fe
2+ to Fe
3+. Then, Fe
3+ is used as a metal leaching agent. After leaching, Fe
3+ is reduced to Fe
2+ and recirculated back to the bioreactor to be oxidized to Fe
3+ again by the bacteria. Bioleaching technology accounts for more than 30% of global copper production, as well as 5% of gold production, with small quantities of cobalt, nickel, uranium, and zinc being bioleached [
6]. Moreover, bioleaching has the potential to effectively reduce the carbon footprint by utilizing carbon dioxide as the carbon source for chemoautotrophic microorganisms. This approach offers a sustainable method for recovering valuable metals from urban mining operations [
10,
11,
12].
The use of bioleaching technology for the extraction of valuable metals from lithium-ion batteries (LIBs) is not as developed as it is for mining and other electronic waste. In the bioleaching of LIBs, microorganisms and their metabolites are utilized to dissolve the metals present in the electrodes. These metals exist in two different forms: elemental metals, such as copper (Cu) and aluminum (Al), found in the electron collectors, and oxides, such as lithium (Li), nickel (Ni), cobalt (Co), zinc (Zn), and manganese (Mn), which are present as active cathode materials [
13,
14,
15,
16,
17].
However, the bioleaching of LIBs presents significant engineering challenges due to the slow kinetics involved. This not only impacts processing costs and cycle times but also imposes limitations on the practical application of this technology in large-scale or industrial operations.
Waste battery streams are commonly subjected to mechanical and/or chemical pre-treatment steps to facilitate subsequent processing and improve the efficiency of metal recovery. Key pre-treatment methods include dismantling, crushing, screening, thermal treatment, mechanochemical techniques, and dissolution processes. These pre-treatment stages are essential for the effective separation and recovery of cathode and anode materials, improving accessibility to valuable components while reducing the energy consumption and processing time required in downstream operations, thereby enhancing the overall economic viability of the recycling process [
5,
18,
19].
Moreover, these preparatory methods significantly contribute to minimizing environmental impact by reducing the need for aggressive chemicals and extreme operational conditions during later stages of treatment [
20,
21].
Certain techniques, such as thermal treatment, ultrasonic cleaning, and solvent dissolution using organic reagents, are particularly effective for the removal of organic binders. For example, thermal processes like vacuum pyrolysis, typically conducted at temperatures exceeding 300 °C, effectively eliminate organic binders such as polyvinylidene fluoride (PVDF). However, the thermal decomposition of such materials can release hazardous and toxic emissions, including hydrogen fluoride (HF) and flue gases contaminated with heavy metals. Therefore, the implementation of advanced gas management systems, including condensers, cooling units, bag filters, and activated carbon filters, is necessary to capture and remove these emissions. This requirement significantly increases the complexity and cost of the processing equipment [
22].
Alternatively, solvent-based dissolution techniques are employed to disrupt the adhesion between the metallic substrate (aluminum or copper foil) and the active cathode material by dissolving the binder. Common solvents such as N-methyl-2-pyrrolidone (NMP) and dimethylformamide (DMF) are highly effective at dissolving PVDF. Nevertheless, these solvents are often expensive and pose substantial risks to both human health and the environment [
5,
17,
18,
23].
Ultrasonic-assisted separation is another treatment process used to remove the active cathode material from the aluminum foil due to the cavitation effect generated by ultrasonic sound waves [
19,
24]. However, this method requires a significant capital investment and generates noise pollution during operation.
Extensive research has focused on pre-treatment processes, but practical challenges persist. The use of various pre-treatment techniques is hindered by disorganized and less effective classification of spent batteries, as well as complex disassembly and dismantling processes, leading to inefficient extraction of valuable metals such as Co, Ni, Li, and Mn [
24]. Furthermore, in most studies, the battery is manually disassembled, and the battery casing is separated from the electrodes [
25,
26,
27]. In some cases, even the cathode and anode are separated, performing the bioleaching process only with the cathode [
28,
29]. These manual interventions complicate the industrial application of the process and hinder the treatment of large quantities of waste at an industrial scale. Therefore, it is essential to combine pre-treatment processes with physicochemical procedures to efficiently recycle the valuable metals present in spent batteries.
The objective of this study is to develop a multi-stage process for the efficient extraction of valuable metals from crushed lithium-ion battery (LIB) black mass (BM), using minimal pre-treatment of raw material. For the first time, one metric ton of end-of-life batteries, without prior separation or manual handling, was processed through bioleaching techniques. This work explores whether simple particle size fractionation of BM can enhance metal recovery. Each fraction’s extraction strategy is adapted based on its specific metal composition, determined through analytical characterization. Two biogenic leaching agents are evaluated: Fe (III), produced via Acidithiobacillus ferrooxidans bio-oxidation, and H2SO4, generated by A. thiooxidans through sulfur oxidation. A key advantage of this process is its operation at ambient temperature and without the need for pH values below 1.5, emphasizing environmental sustainability and energy efficiency. The novelty and main contribution of this study lie in implementing bioleaching on minimally pre-treated LIB waste. This simplified approach enhances industrial scalability by reducing processing time, operational complexity, and reagent consumption, thus promoting a more economical and eco-friendly recycling pathway.
2. Materials and Methods
2.1. Spent LIBs: Sample Preparation and Metal Characterization
One ton of end-of-life lithium-ion batteries from electric scooters, bicycles, and motorcycles was supplied by Recuperacions Marcel Navarro i Fills S.L. (Llagostera, Spain). In order to ensure safe processing, batteries were discharged of any residual charge by submerging them in a 1% sodium chloride solution for 24 h. The shredding was carried out using a UNTHARS 40-45 knife mill, (Untha Shredding Technology Gmbh, Kuchl, Austria) equipped with a 5 mm screen. The process was operated in semi-continuous mode to ensure safe handling of potentially reactive materials. Feed batches of approximately 30–50 kg were sequentially loaded, allowing for intermediate temperatures below 70 °C. The key operating parameters were a rotor speed of 35–45 rpm and torque control via automatic reverse rotation. The total processing time to complete 1 ton was approximately 6.5 h, including downtime for safety checks and cooling between batches.
After crushing, part of the black mass obtained was separated into two fractions by sieving through a 500-micron sieve. The coarse fraction (CBM), above 500 microns, constituted 86% of the unsorted black mass (UBM) and showed great visual heterogeneity. The thin fraction (FBM) was 14% in weight, below 500 microns, and appeared as a more homogeneous black powder (
Figure 1).
Each fraction was quartered to obtain a representative sample. The samples were then digested using aqua regia (HCl/HNO3 1:3, v/v). Cu, Ni, Co, Li, Mn, and Al concentrations were determined by atomic absorption spectroscopy (AAS). For this purpose, 0.1 g of the UBM was added to 10 mL of aqua regia and placed in a 4-step microwave digester Start D (Milestone S.r.l., Milan, Italy) for 30 min at 150 °C. The digested samples were filtered through a 0.45-micron membrane and diluted with deionized water. The entire determination was carried out in triplicate. The same digestion procedure was performed for the other fractions, CBM and FBM.
2.2. Bacterial Strains and Growth Conditions
Two bacterial strains were used in this study: A. ferrooxidans (ATCC23270), kindly provided by the Department of Chemical Engineering at the University of the Basque Country (Bilbao, Spain), and A. thiooxidans (DMS-14882), obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms. A. thiooxidans was grown in 0 K medium ((NH4)2SO4 (3 g/L), MgSO4·7H2O (0.50 g/L), K2HPO4 (0.5 g/L), KCl (0.1 g/L), Ca (NO3.4H2O (0.014 g/L)) supplemented with 1% (w/v) of sulfur and initially adjusted to pH = 3 with 10% (w/w) H2SO4. Acidithiobacillus ferrooxidans was cultivated in a 2 L stirred-tank bioreactor containing 6K mineral mediu. The pH was maintained at 2 by the periodic addition of 10% (w/w) H2SO4, and agitation was set at 200 rpm. The temperature was controlled at 30 °C using a thermostatic bath connected to the reactor jacket. Aeration was provided via compressed air, sparged through a submerged ceramic diffuser at a flow rate of 50 NL/h, regulated by a rotameter. When the oxidation–reduction potential (Eh) reached 600 mV, complete iron oxidation was assumed. At that point, 20% of the total medium volume was replaced with fresh 6K medium to support further microbial growth.
For subsequent tests, 90 mL of 6K mineral medium was transferred to 250 mL Erlenmeyer flasks and inoculated with 10% (v/v) of active culture from the bioreactor. The flasks were incubated at 30 °C with agitation at 110 rpm in an orbital shaker. A. ferrooxidans growth was monitored over time by measuring redox potential (Eh), pH, and the concentrations of Fe (II) and Fe (III). To maintain a stable pH, 10% (w/w) H2SO4 was periodically added as needed. A decrease in pH was used as an indirect indicator of A. thiooxidans activity, reflecting sulfur oxidation to sulfuric acid by the microorganisms.
The role of A. thiooxidans in this study was to biologically generate sulfuric acid by oxidizing elemental sulfur. The biogenic H2SO4 thus produced was used to evaluate its effectiveness in metal leaching as compared to conventional mineral sulfuric acid. This approach allows exploring the potential of sustainable alternatives in leaching processes. Although obtaining biogenic acid from industrial sulfur waste is not part of this study, positive results could support its consideration in future work.
2.3. Bioleaching of Coarse Black Mass Fraction >500 Microns
A total of 1 g of coarse black mass > 500 microns (CBM) was added into 100 mL of a six-day culture of A. ferrooxidans in a 200 mL Erlenmeyer flask. As a control test, 1 g of the CBM > 500 microns was leached with 100 mL of H2SO4 solution with a pH of 1.5. Flasks were placed on a magnetic stirrer to keep the CBM in suspension during the experiment. Leaching was carried out at a room temperature of 27 °C, and pH was periodically adjusted to 1.5 by adding 10% H2SO4 (w/w). Samples of 2 mL of leachate were collected at various time intervals, filtered through a 0.45-micron membrane to eliminate any potential impurity, and analyzed for Cu, Al, Ni, Co, Li, and Mn concentrations by atomic absorption spectrometry (AAS). All assays were performed in triplicate.
2.4. Bioleaching of Fine Black Mass Fraction < 500 Microns
The leaching test with the FBM was conducted following the procedure described in
Section 2.3. Additionally, the extraction efficiency of biogenic acid produced via sulfur bio-oxidation by
A. thiooxidans was compared to those obtained with mineral H
2SO
4 acid.
2.5. Bioleaching of Unsorted Black Mass
In these experiments, we used the unsorted black mass (UBM) that was obtained immediately after crushing and quartering. The leaching conditions remained consistent with those described in
Section 2.3 of the previous leaching tests. Triplicate experiments were conducted.
2.6. Analytical Methods
The pH and oxidation potential reduction (ORP) were periodically measured using a precise pH meter, pH7/mV + DHS LabProcess, XS (Xylem Analytics, Weilheim, Germany).
The concentrations of Cu, Al, Ni, Co, Li, and Mn were analyzed using an atomic absorption spectrophotometer (AAS) PinAAcle 500, (Perkin Elmer, Waltham, MA, USA). The samples were diluted with distilled water to attain the suitable concentration. All measurements were performed in triplicate.
The ferric ions and total iron determinations were conducted using the colorimetric method proposed by Karamanev et al., 2002 [
30], based on the use of 5-sulfosalicylic acid (SSA) and ammonia. The spectrophotometer utilized was a Lambda 25 Perkin Elmer. The concentration of Fe (II) ions was calculated by subtracting the Fe (III) content from the total iron measurement. All measurements were performed in triplicate.
Sulfate ions were determined by Sulfaver® 4 using the turbidimetric method (Hach®, Düsseldorf, Germany).
Back-scattered electron images of fine black mass were obtained using a Hitachi TM-1000 Tabletop Scanning Electron Microscope, SEM (High-Technologies Corporation, Tokyo, Japan), equipped with an energy-dispersive X-ray spectrometer (EDS).
Statistical analyses were performed using Minitab® 21.4.
3. Results and Discussion
3.1. BM Metal Characterization
Understanding the precise metal composition of various black mass fractions is crucial for accurately selecting leaching agents and efficiently optimizing their extraction process.
Table 1 shows the weight percentage of the CBM and the FBM, as well as the metal contents in these two fractions, expressed as a percentage (
w/
w); intervals are obtained as the mean plus or minus one standard deviation. The UBM values are calculated from the weighted average, considering the weight fraction of the CBM and FBM.
Six metals (Cu, Ni, Co, Mn, Al, and Li) were determined in the black mass from batteries. As can be seen, the separation process into two fractions has mainly concentrated Cu and Al in the coarse fraction. A total of 99% of the total Cu in the UBM remains in the coarse fraction, while 98% of the total Al can be found in the CBM. This is probably since these two metals are present in the UBM in their elemental form as large, thin sheets. These laminar structures are difficult to crush by the blade mill, so copper and aluminum do not pass through the 500-micrometer sieve and remain mainly in the coarse fraction. On the other hand, the rest of the metals are present as oxides that can be easily crushed; therefore, Co, Ni, Mn, and Li are quite evenly distributed between the two fractions, although the FBM fraction is slightly more concentrated in Ni (24.7%) than in the other metals.
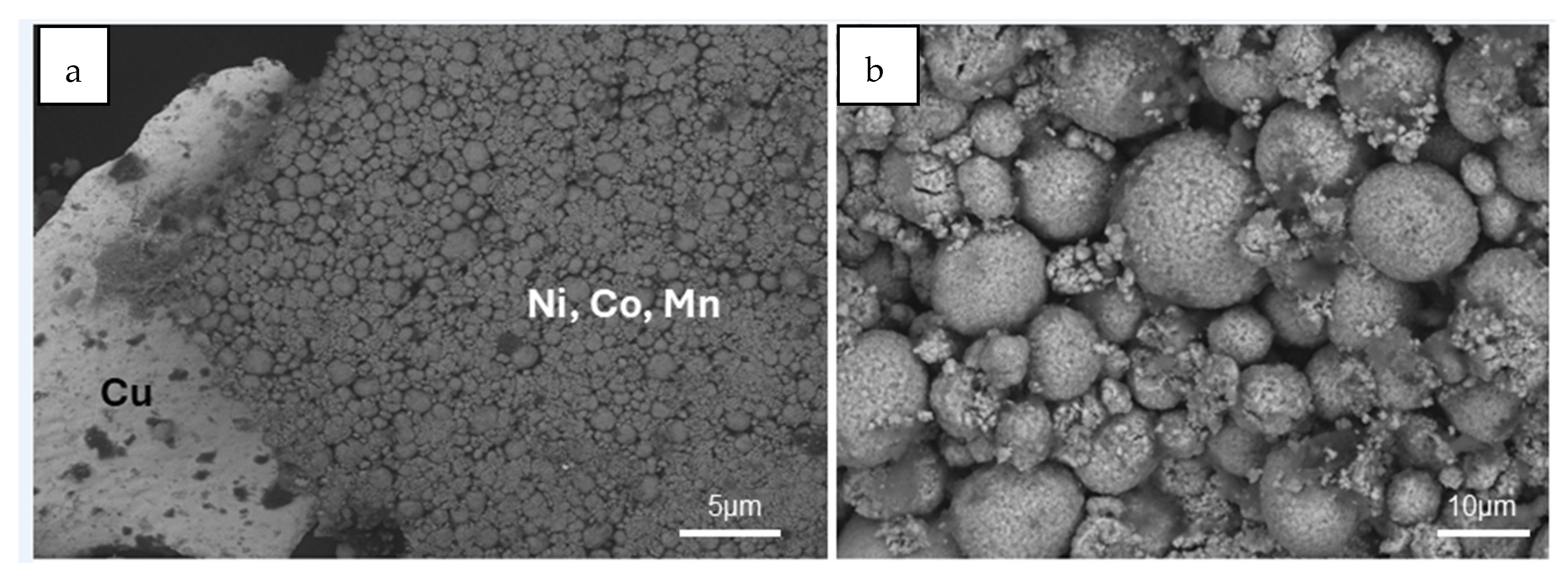
As shown in
Figure 2a, this fraction of the black mass is primarily composed of two components. On one hand, botryoidal aggregates of Ni, Co, and Mn oxides represent the most abundant component, a detail that is further highlighted in the magnified view shown in
Figure 2b. On the other hand, small amounts of copper are also present, appearing in the form of thin lamellae. These findings are consistent with the results obtained by atomic absorption spectroscopy, further confirming the composition of the sample.
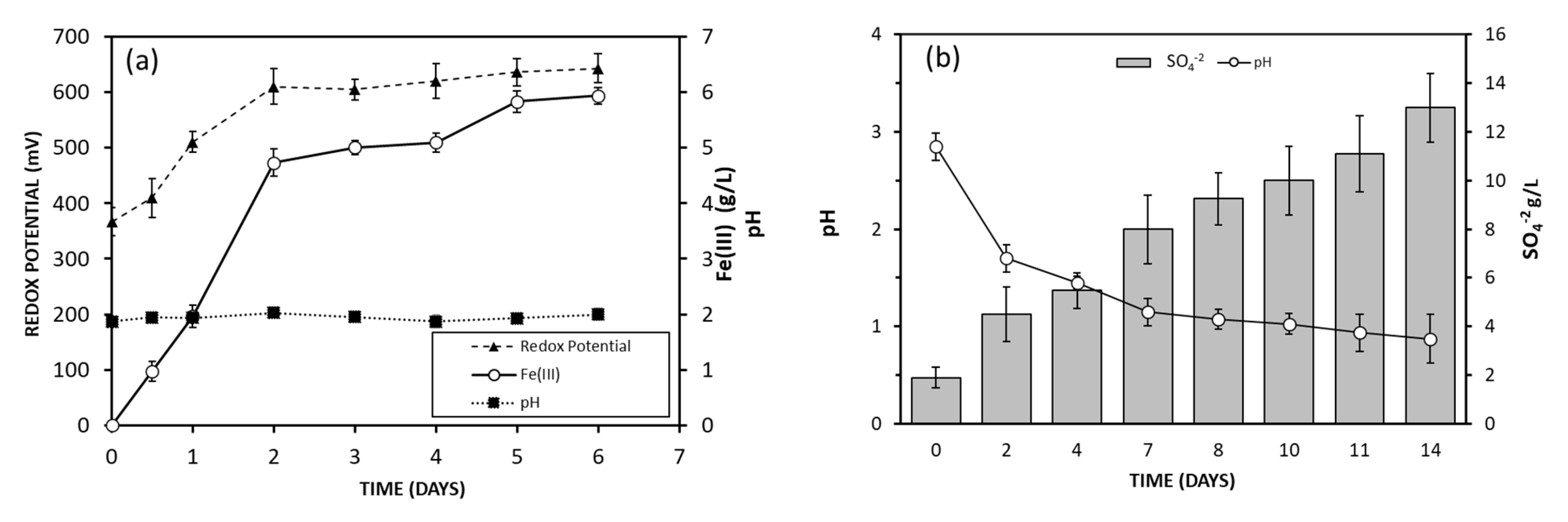
3.2. Fe (III) and Acid Production by Microorganisms
Figure 3a,b show the Fe (III) and the acid production as a function of time in
A. ferrooxidans and
A. thiooxidans cultures, respectively. The pH of the
A. ferrooxidans culture was maintained constant by periodically adding 10% (
w/
w) H
2SO
4 as needed. After 2 or 3 days of incubation, the culture took on a reddish color, indicating the formation of Fe (III). As can be seen in
Figure 3a, after 6 days, all Fe (II) was oxidized.
In
A. thiooxidans culture, a decrease in pH was observed due to the production of sulfuric acid from the sulfur oxidation carried out by the microorganism. After 7 days, the pH of the culture decreased from 3.0 to 1.2, and sulfate concentration significantly increased from 1500 ppm to 13,000 ppm. This culture was used for further leaching experiments. Results are shown in
Figure 3b.
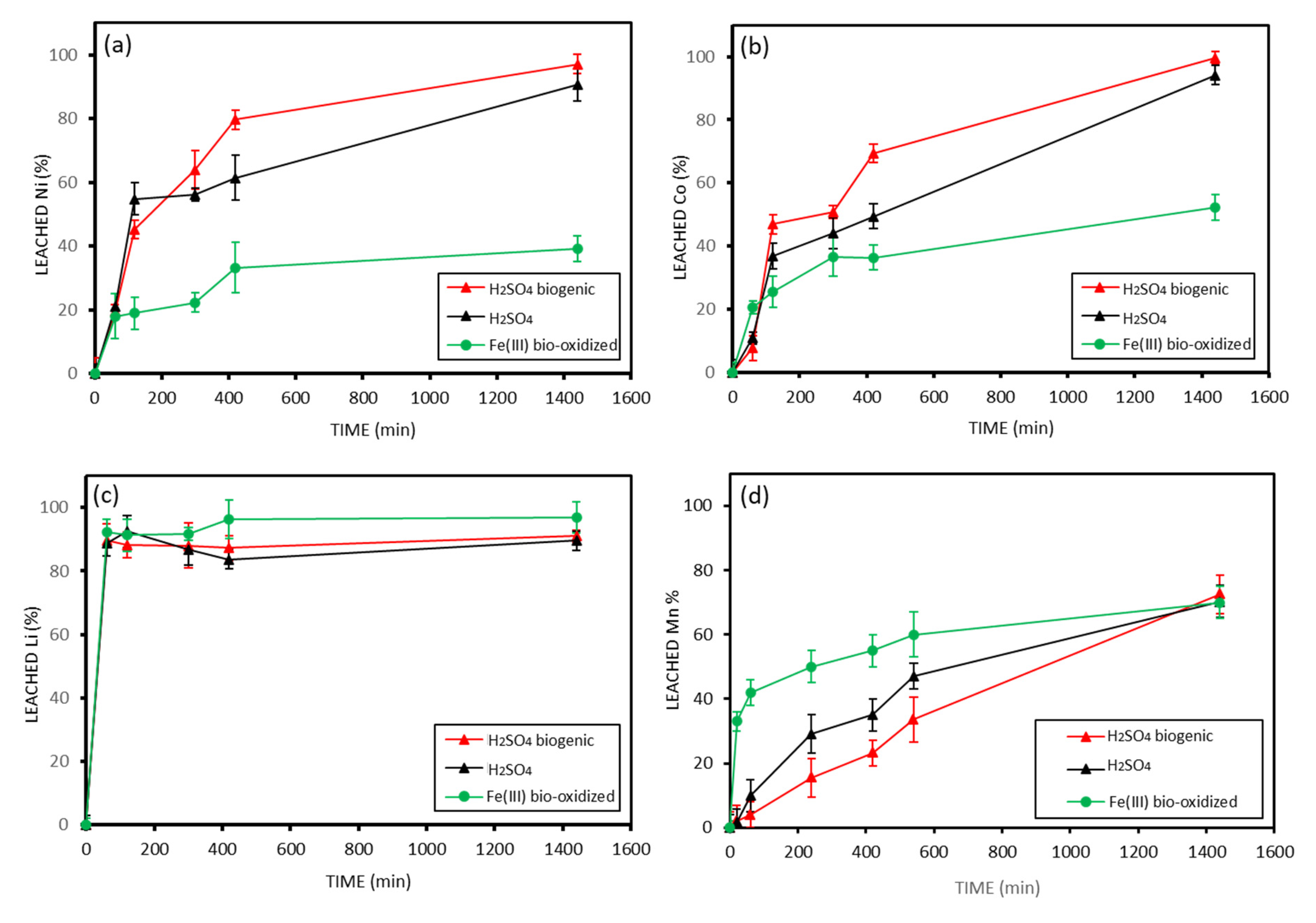
3.3. Metal Leaching from Coarse Black Mass >500 Microns
Figure 4 shows the extraction percentage of Cu, Al, Ni, Mn, Co, and Li over time using Fe (III) obtained from
A. ferrooxidans activity and using a solution of mineral H
2SO
4. Leaching with biogenic H
2SO
4 was not conducted on the coarse black mass due to its lower content of oxidized metals (Co, Mn, Ni, Li). Based on the literature and preliminary assessments, low extraction yields were expected. Therefore, Fe (III)-mediated bioleaching was selected as the most suitable approach for this fraction.
Two different behaviors were observed: Fe (III) produced by bio-oxidation extracts nearly 100% of Cu and Al within 20 min, whereas H
2SO
4 only dissolves approximately 5% of these metals (
Figure 4a,b). On the other hand, Ni, Mn, Co, and Li (
Figure 4c–f) have a different behavior. A high extraction of these metals is obtained by the Fe (III) solution. However, significant metal solubilization, exceeding 50%, is also observed with the sulfuric acid solution. Moreover, the extraction is much slower than those obtained for Cu and Al. A total of 100%, 90%, 90%, and 70% of Ni, Co, Li, and Mn are extracted, respectively, in 10 h using Fe (III), while the H
2SO
4 solution extracts around 70% of Ni, Co, and Li and 50% of Mn during the same period.
The observed outcomes can be attributed to the diverse extraction mechanisms depending on the speciation form of metals in the waste. On one hand, Cu and Al are in their elemental state, and they can be extracted by Fe (III) that is produced by the microorganism’s metabolic activity.
Acidithiobacillus ferrooxidans is a chemolithoautotrophic bacterium that derives energy by oxidizing Fe (II) present in the culture medium to Fe (III), as described in reaction 1 [
27]. The resulting ferric ion then acts as an oxidizing agent, facilitating the oxidation of elemental metals such as copper and aluminum found in the black mass. Through this reaction, metallic copper is converted into soluble copper ions (Cu
2+), which can subsequently be recovered from the leachate (reaction 2). The Fe (II) produced is re-oxidized by
A. ferrooxidans to Fe (III), thereby regenerating the oxidizing agent and sustaining the leaching cycle. A similar oxidative mechanism applies to elemental aluminum (reaction 3) [
7,
8,
9,
27].
To confirm these mechanisms, the Fe (III) concentration was monitored throughout the leaching process. A clear decrease of approximately 3200 ppm in Fe (III) concentration was recorded after 120 min of bioleaching, indicating its consumption via redox reactions with Cu and Al. This experimental evidence supports that the oxidation of these elemental metals occurred concurrently with the reduction of Fe (III), thereby validating the proposed mechanism.
On the other hand, transition metals such as Co, Ni, and Mn, as well as Li, are present in their oxide form, and they can be leached under acidic conditions [
14]. According to several authors, leaching of these transition metals can be accelerated by reducing agents because the reduced ionic species are more soluble than the oxidized ones [
5,
16,
18]. Then, protons may attack metal oxides when a metal oxide comes into contact with an acidic medium (Equation (4)). This results in the dissolution of metal oxide [
3,
14].
where M is a divalent metal present in the oxide in this generic reaction.
However, even though solubilization of metals in the form of oxides is very effective with the acidic solution, extraction with Fe (III) is better for all metals. This is due in part to the action of sulfuric acid, which was added to maintain a pH of 1.5 during the growth of microorganisms, but the presence of Cu and Al probably also plays a role in it. Fe (III), in the presence of elemental copper and elemental aluminum, which are two good reducing agents, is reduced to Fe (II). Fe (II), in turn, can reduce the metals Mn (IV), Ni (III), and Co (III) to their most reduced forms, Mn (II), Ni (II), and Co (II), which, according to many authors, are more soluble than the oxidized forms [
3,
5,
16,
28].
It can also be observed in
Figure 4 that metals in the form of oxides (LiNiMnCoO
2, LiCoO
2, LiMn
2O
4, etc.) leach slower than metals in their elemental form, both with Fe (III) and with the acid solution. This may be attributed to the presence of the binder in the sample. Let us recall that the waste under study was obtained by crushing the entire battery, and the binder was not removed (see
Section 2.1). The particles of metal oxides here are attached to the binder, and this may limit the accessibility of the leaching agent to metal oxides, delaying their dissolution rates.
3.4. Leaching Results of Fine Black Mass <500 Microns
Results of the leaching performed with the FBM (<500 microns) using biogenic acid produced by
A. thiooxidaans, mineral H
2SO
4 solution, and Fe (III) produced by bio-oxidation are shown in
Figure 5. It is worth noting that the fine fraction does not contain Cu nor Al because these two metals remain in the coarse fraction (
Section 3.1). Ni and Co have a similar behavior (
Figure 5a and
5b, respectively). As it can be seen, better extractions were achieved with acidic solutions than with the Fe (III). In 24 h, 98% of Ni and 100% of Co were extracted using biogenic H
2SO
4, and similar percentages of these two metals were obtained with the mineral H
2SO
4 solution. However, the extraction using Fe (III) produced by bio-oxidation as a leaching agent is much lower for the two metals; only 40% of the Ni and 50% of the Co were extracted. In addition, the test carried out with Fe (III) provides slower extractions for both metals. These results are in accordance with those obtained by other authors that extract these metals in acidic conditions, as acidic conditions facilitate the extraction of transition metals in the form of oxides [
3,
14]. Please refer to Equation (4) for the general reaction.
Very different behavior was shown by Li and Mn (
Figure 5c and
5d, respectively). Regarding Li, its extraction was extremely rapid. About 100% of the Li was solubilized in just 20 min with the three leaching agents. In the case of Mn, percentage extractions were lower than those obtained for the other three metals. By utilizing Fe (III) generated through bio-oxidation, approximately 69% of Mn was leached within a 24-h period. With the use of both mineral and biogenic H
2SO
4, the extraction outcomes exhibit similarities, with a slower initial progress but reaching around 67% extraction within 24 h. This is probably since Mn (IV) is a poorly soluble speciation form and needs strong acid solutions as well as strong reducing agents to be leached [
19,
32]. The absence of effective reducing agents such as Cu and Al, which remained in the CBM fraction, hinders the reduction of Mn (IV) to Mn (II) and consequently its dissolution. Therefore, extraction yields of Mn are lower than those obtained from the CBM.
It is worth noting that, regardless of the extraction percentages, the yields obtained for the four metals using the two types of sulfuric acid (biogenic and mineral) are very similar, indicating that biogenic acid is just as effective as mineral acid. This was statistically confirmed by two-sample t-tests assuming equal variances, which yielded p-values > 0.05 for all four metals (Ni, Co, Li, Mn), indicating no significant difference between the two acid types. Statistical analyses were performed using Minitab® 21.4.
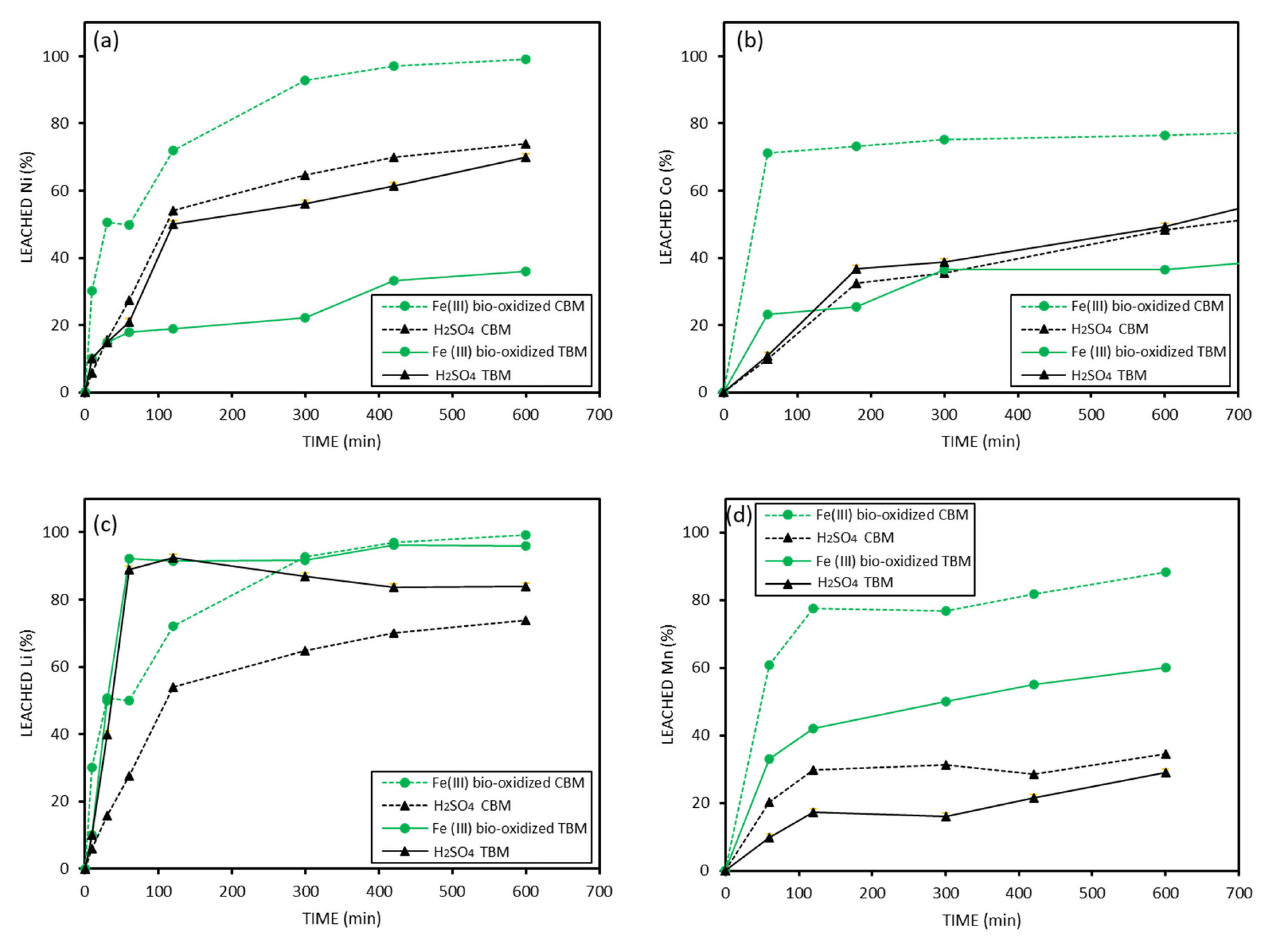
Although the particle size of the CBM is larger than the FBM, and one would expect leaching to occur more slowly in this fraction due to a smaller surface area, when comparing the extractions obtained from the CBM to those of the FBM (
Figure 6), it can be observed that significantly faster and more efficient extraction was obtained from the CBM for Ni, Co, and Mn. This can be attributed to the presence of reducing metals (Cu and Al) in the CBM. Cu and Al are elements capable of reducing the quite insoluble forms Ni (III), Co (III), and Mn (IV) to their reduced forms, Ni (II), Co (II), and Mn (II), which are much more soluble, and thus accelerating the metals’ leaching. On the contrary, in the case of Li, the extraction was faster with the FBM. This is probably because Li-ion is highly soluble, and it is not affected by the presence of reductants. A total of 100% of Li is extracted from the FBM in just 40 min (see
Figure 6c). The extraction results of Ni, Co, and Mn using H
2SO
4 as a leaching agent are lower in all cases than the extraction results of the CBM with Fe (III) produced by bio-oxidation.
3.5. Leaching Results of Unsorted Black Mass
In light of the results obtained in
Section 3.3 and
Section 3.4, a new set of experiments using the unsorted black mass (UBM) (directly obtained from the industrial crushing process, without any further sieving treatment) was carried out. The leaching results are shown in
Figure 7. As can be seen, the extraction of Cu and Al using Fe (III) is fast and efficient; almost 100% of Cu and Al were extracted in 20 min (
Figure 7a,b). These results are consistent with those obtained for copper and aluminum in the CBM. However, the extraction of these metals is very low, less than 10%, when acids are used as leaching agents.
Regarding Ni, 95% was extracted in 20 min using Fe (III) as a leaching agent, whereas no significant differences were found when using mineral H
2SO
4 and biogenic H
2SO
4, extracting a smaller amount at a slower rate (
Figure 7c). However, the extraction with Fe (III) is faster in the UBM than in the FBM and CBM separately.
This superior performance of the UBM can be attributed to its composition. This fraction is rich in elemental Al and Cu compared to the FBM. Ni, Co, and Mn are in the black mass as oxides. In these oxides, the metals are found in their highest oxidation state (III). It is well known that oxides of metals in reduced states (e.g., II) exhibit greater solubility than those in higher oxidation states and that the reduction of oxidized forms significantly enhances metal extraction [
33,
34]. In this context, the presence of copper and aluminum in their elemental form in the UBM facilitates the reduction of Co, Ni, and Mn into the oxidation species (II). Conversely, the limited presence of these reducing agents in the FBM fraction hinders this reduction process, thereby resulting in lower solubilization of these metals.
The UBM contains the reducing agents (Cu and Al) as well as the fine fraction with a smaller particle size and therefore a larger contact surface with the leaching agent. This would explain the faster extraction process with the UBM than with the CBM. The same behavior was observed for Co, Mn, and Li. Thus, it can be concluded that the most efficient leaching process involves the use of the UBM and Fe (III) obtained by bio-oxidation as a leaching agent.
Table 2 presents a comparison of the key conditions, results, and efficiencies of previous studies with those of this research. The novelty of this study lies in the preparation process of the residue. Unlike previous bioleaching studies that often relied on manual manipulation, the residue in this study was obtained using standard production equipment, moving away from manual dismantling processes that are impractical for industrial applications. Furthermore, the preparation of one ton of batteries in this study represents a significant industrial quantity, suitable for production scale-up trials.
The particle size of the sample used in this study is considerably larger compared to those in previous bioleaching studies. Here, the particle size is less than 5 mm, while other studies typically use fine powder residues ranging from 75 to 200 microns (see
Table 2). This larger particle size reduces both the grinding time and the energy required for processing.
Importantly, our study does not require the removal of the binder, thereby eliminating the need for high-temperature pyrolysis processes, the treatment of hazardous gases, or the use of specific chemicals. The leaching conditions in our work were also designed to minimize energy consumption, with the process being conducted at room temperature (27 °C). In contrast, other bioleaching studies typically operate at higher temperatures, ranging from 30 °C to 42 °C.
In terms of pH, our study aligns with previous research, maintaining a pH of 1.5 throughout the entire bioleaching process. The pulp density in our study was 1%, with the potential to increase this concentration by raising the levels of Fe (III) stoichiometrically bio-oxidized from the leaching agent.
Regarding efficiencies, our study achieved recovery rates of 97% for Cu, 96% for Al, 98% for Ni, 92% for Co, 90% for Li, and 80% for Mn within just 30 min at a pulp density of 1%. In comparison, the fastest methods in previous leaching studies took an entire day and yielded lower efficiencies (
Table 2). This highlights the significant time and efficiency advantages of our approach.
4. Conclusions
The results of this study highlight several key contributions to the field of metal recovery from lithium-ion battery black mass using bioleaching. These contributions can be summarized as follows:
1. Technical Breakthrough and Simplified Pre-treatment
This study introduces a novel bioleaching process that utilizes bio-oxidized Fe (III) and biogenic H2SO4 as effective leaching agents for extracting metals from different size fractions of lithium-ion battery black mass. A major innovation lies in the use of black mass directly derived from the production grinding process, eliminating the need for manual dismantling, binder removal, or harsh pre-treatment.
2. High Recovery Efficiency and Time Performance
The proposed method achieved rapid and efficient metal recovery. The highest metal extraction rates were achieved using the unsorted black mass (UBM), including up to 99% of Cu and Al, 95% of Ni and Li, and 96% of Co within 20–30 min. This performance ranks among the highest reported to date, enabled by the synergistic effect of Fe (III)-mediated bioleaching and the intrinsic reducing capacity of metals such as Cu and Al, which are highly abundant in the black mass.
3. Industrial Feasibility and Operational Advantages
The bioleaching process operates at room temperature and avoids extreme pH conditions, reducing energy demands and safety risks. This study demonstrates a clear pathway toward industrial scalability and integration into existing production workflows by using residue from standard LIB production equipment and processing one ton of material.
4. Sustainability and Environmental Impact
This method avoids the use of hazardous chemicals, high temperatures, and gas treatments, aligning with sustainable and environmentally friendly recovery strategies for critical battery metals. These findings establish a strong foundation for advancing bioleaching as a practical, efficient, and green solution for large-scale recovery of valuable metals from lithium-ion battery waste.