Abstract
An alternative to conventional methods for mine tailings disposal is stabilization with alkali-activated binders (AABs), developed from agro-industrial waste. Despite increasing interest in this topic, there is still a lack of studies focusing on the stabilization of iron ore tailings (IOTs) using AABs, particularly those that combine the characterization of cementitious gels with an evaluation of leaching behavior. This study assessed the strength, mineralogy, and leaching performance of IOTs stabilized with AABs formulated from rice husk ash (RHA) and hydrated eggshell lime (HEL), using sodium hydroxide as the alkaline activator. Tests included unconfined compressive strength (UCS), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and metal leaching analyses. The IOT–AAB mixture with the highest AAB content and dry unit weight achieved an average UCS of 2.14 MPa after 28 days of curing. UCS increased with AAB content, followed by dry unit weight and curing time, the latter showing a non-linear influence. The formation of C–S–H gel was confirmed after 28 days, while N–A–S–H gel was detected as early as 7 days of curing. The cemented IOT–AAB mixtures showed no metal toxicity and effectively encapsulated barium originating from the RHA.
1. Introduction
The mining industry is a significant global employer, directly supporting approximately 35 million jobs worldwide [1]. In Brazil, this sector sustains around 1 million industrial jobs and contributes 4% to the gross domestic product of the country [2,3]. According to the Brazilian Mining Institute [2], mineral production in 2022 reached 1 billion tons, with iron ore accounting for 59.6% of total output. Globally, iron mining generates an estimated 2 billion tons of tailings annually [4]. In Brazil, where tailings constitute roughly 40% of the total processed mass [5], iron ore tailing (IOT) production amounts to approximately 234 million tons per year. IOT typically exhibits an aqueous slurry consistency and is conventionally disposed of in tailings dams, which present geotechnical stability risks and potential environmental hazards due to structural failures [6]. Following the catastrophic failures in Mariana and Brumadinho in Brazil, regulatory measures were implemented in the country, including the prohibition of upstream tailings dam construction and the mandated decommissioning of existing structures [7].
As an alternative to conventional disposal methods, dry stacking—involving the filtration and compaction of IOTs into unsaturated piles—has emerged as a promising solution, offering enhanced geotechnical safety and reduced environmental impact [8]. Franks et al. [9] report that this method results in less than half the incidence of stability issues compared to other disposal techniques, while also mitigating contaminant leakage and minimizing land use. However, maintaining tailings in an unsaturated state poses operational challenges, particularly in handling and stack formation due to variable moisture content [10]. To address these limitations, binders such as Portland cement can be incorporated into IOT prior to compaction, improving cohesion and mechanical performance [6,11]. Nevertheless, Portland cement production is energy-intensive, relies on non-renewable resources, and contributes substantially to global CO2 emissions—accounting for up to 8% of the total [12].
A further concern is the potential leaching of hazardous contaminants from mining tailings. Studies by Bao et al. [13] and An et al. [14] have examined heavy metal leaching in stabilized materials, emphasizing the role of chemical speciation and composite binders in contaminant immobilization. Khoeurn et al. [15] and Bao et al. [13] highlight that tailings from gold, silver, copper, cobalt, zinc, and iron sulfide mining can release metals, posing risks to water resources. To mitigate this problem, solidification/stabilization techniques—particularly alkaline activation—have been studied to reduce leaching and repurpose waste materials as cement substitutes [16,17,18,19,20,21].
Alkali-activated binders (AABs) are synthesized through the reaction of an aluminosilicate-rich precursor with an alkaline activator [22]. The inclusion of calcium sources can reduce activator concentration and facilitate ambient-temperature curing. These binders offer a sustainable alternative for IOT stabilization, exhibiting cementitious properties comparable to conventional cement while minimizing environmental impact [5,8,23,24]. Additionally, alkali activation enhances physical encapsulation, effectively immobilizing heavy metals and improving material durability [16,18,25,26,27].
Potential precursors for alkali activation include natural materials (e.g., calcined clays, natural pozzolans) and industrial by-products (e.g., fly ash, blast furnace slag, glass waste, mining tailings, and ceramic waste) [25]. Agro-industrial residues such as rice husk ash (RHA) and eggshell-derived lime have also been investigated. RHA, rich in amorphous silica, demonstrates pozzolanic reactivity [8,28,29], while eggshells provide a sustainable calcium source due to their high CaCO3 content [25,30].
Although prior studies have explored AABs incorporating RHA and HEL and the stabilization of IOT using alkali-activated cements, limited research has addressed the combined use of RHA and HEL as a binder system specifically for IOT. Furthermore, few studies integrate the analysis of mechanical performance, mineralogical development of cementitious gels, and leaching behavior in a single framework. To address these gaps, this study aims to evaluate, under laboratory conditions, the mechanical strength, mineralogical evolution, and environmental safety of IOTs stabilized with an RHA-HEL-based AAB. The findings provide a foundation for future investigations and potential field-scale applications in dry tailings stack construction.
2. Materials and Methods
2.1. Research Approach
This study evaluates the stabilization of IOTs using an alkali-activated binder based on RHA and HEL. Through an experimental design, different curing times (7, 17.5, and 28 days), AAB contents in mass (15%, 20%, and 25%), and dry unit weights (13.3, 14.3, and 15.3 kN/m3) were assessed. Tests were conducted to determine the unconfined compressive strength (UCS), mineralogical composition, and metal leaching of the IOT–AAB mixtures in order to observe the characteristics of the cemented mixtures, including cementitious gel formation and toxicity.
2.2. Materials
The IOTs used in this study were sourced from a mining industry located in the state of Minas Gerais, Brazil, and were subjected to drying at 50 °C for 48 h. The rice husk ash (RHA) originated from a thermoelectric power plant in the state of Rio Grande do Sul, Brazil, where the husks are burned at temperatures ranging from 800 °C to 1000 °C for energy generation, and then slowly cooled under ambient conditions. The preparation of the ash involved drying in an oven at 50 °C for 48 h, followed by sieving through a 200 mesh (75 μm). The eggshell lime (HEL) was produced in the laboratory following a series of steps: (a) Collection and washing of eggshells; (b) Drying in a ventilated oven at 50 °C for 12 h; (c) Grinding in a knife mill; (d) Calcination in a muffle furnace at 1050 °C for 6 h; (e) Hydration in distilled water for 48 h; and (f) Sieving through a 200 mesh (75 μm) screen [31].
The alkaline activator used was NaOH in micro pearls with 98% purity [25]. The AAB was prepared with an RHA/HEL ratio of 80/20, using a 1M NaOH activating solution (Na2O of 2.61% mass) and a water/binder rate equal to 1 as previously developed by Pompermaier et al. [32].
IOTs are primarily composed of iron oxides (49.3%), silicon (35.1%), and aluminum (8.48%), with a loss on ignition of 4.61% [24], with a specific weight of 31.3 kN/m3. RHA has a specific weight of 23.1 kN/m3 and is composed of silicon oxides (87.6%), potassium oxides (2.87% mass), calcium oxides (0.88%), and a loss on ignition of 6.41%, making it a good source of silica [33]. HEL is a rich source of calcium, comprising 72.9% mass of CaO and a total loss on ignition of 25.14% [30], with a specific gravity of 2.24.
IOTs exhibit a particle size distribution predominantly consisting of 48.95% fine sand and 30.72% silt. RHA is primarily composed of silt-sized particles (90.13%). HEL mainly consists of particles corresponding to silt size (93.01%). Regarding Atterberg limits, all three materials are considered non-plastic [24].
The mineralogical composition of RHA reveals the formation of cristobalite (SiO2) and quartz (SiO2) crystals. The presence of silica in the form of these minerals indicates that RHA is a waste material containing both amorphous and crystalline phases [33]. The reactivity of the amorphous phases is inferred through the pozzolanic activity of the RHA, which is 724.6 mg Ca(OH)2/g [28]. The mineralogical composition of HEL includes portlandite (Ca(OH)2) and calcite (CaCO3) [30]. The mineralogy of IOTs is composed of kaolinite (Al2Si2O5(OH)4), goethite (Fe3+O(OH)), quartz (SiO2), and hematite (Fe2O3) [24]. IOTs are classified as Class II-B non-hazardous inert [24], and the RHA as Class II-A non-hazardous non-inert [33], as they exceed the limit established in Annex G of NBR 10004 [34] for Mn.
2.3. Experimental Program
For the evaluation of the unconfined compressive strength (UCS) of IOT–AAB mixtures a full factorial design was applied, utilizing 3 factors and combinations conducted in duplicates. The design included combinations of 8 factorial points, 6 face centered axial points with α = 1 and 4 central points, resulting in a total of 36 combinations. This analysis aimed to assess the influence of controllable factors on a nonlinear response surface [35]. The evaluated factors included curing time, binder content, specific weight and moisture content (Table 1). Curing periods of 7 and 28 days were selected to examine the mechanical behavior over time, considering the satisfactory mechanical behavior in terms of durability and resistance obtained in previous studies with alkali-activated binders [24,32]. The binder content levels (15% and 25% mass), moisture content (22.8%), and specific weight (13.3 and 15.3 kN/m3) were determined based on compaction tests conducted in previous studies with IOTs and other AABs produced from sugarcane bagasse ash and hydrated eggshell lime [24]. These parameters were chosen to better understand their impact on the properties of the IOT–AAB mixtures.

Table 1.
Experimental program.
2.4. Molding and Curing Procedures
The molding procedures started by mixing the dry materials (IOTs, RHA, and HEL) with the NaOH solution, and water until homogenization was achieved. Subsequently, the material was statically compacted in three layers inside a cylindrical mold with a diameter of 50 mm and a height of 100 mm to achieve the desired dry unit weight. The material was compacted in three layers to ensure a homogeneous distribution of density and moisture throughout the specimen, minimizing layering effects and promoting uniform compaction. Afterward, the specimens were removed from the cylindrical molds, and their masses, diameters, and heights were measured with an accuracy of 0.01 g and 0.1 mm, respectively. The specimens were then placed in airtight bags and stored in a humid chamber under controlled conditions (23 ± 2 °C and 95 ± 2% moisture) for the curing period.
2.5. Unconfined Compressive Strength (UCS) Test
To minimize suction effects during the final stages of curing, the specimens were submerged in water 24 h prior to testing, as recommended by [36]. After curing, unconfined compressive strength (UCS) tests were conducted in accordance with ASTM D2166-16 [37]. The tests were performed using an automatic hydraulic press equipped with a 50 kN load cell (5 N resolution) and a constant loading rate of 1 mm/min.
2.6. Microstructural Analysis
The mixtures that resulted in the best mechanical performance for both curing times were further evaluated for their mineralogical composition using X-ray diffraction (XRD) and Fourier-transform infrared (FTIR) spectroscopy. XRD analysis was conducted using a Rigaku diffractometer, model Miniflex® 300, from the Liverpool, United Kingdom, (θ-2θ configuration), with a fixed Cu anode tube (wavelength λ = 1.5406 Å), operating at 40 kV and 25 mA. FTIR spectra were obtained using a Perkin Elmer spectrometer, model Spectrum 100S (Shelton, CT, USA), covering the range from 4000 to 400 cm−1, with a resolution of 4 cm−1.
2.7. Leaching Test
The leaching behavior of the cemented material was evaluated using the IOT–AAB mixtures that demonstrated the best UCS results. After the curing period, the specimens were broken and sieved through a 9.5 mm mesh. The leaching tests were then performed following the guidelines of NBR 10005 [38]. Leachate extracts were analyzed for inorganic elements (metals) using an inductively coupled plasma atomic emission spectrometer (ICP-OES, Shimadzu, model ICPE-9800), with a multi-element ICP Certipur standard solution (Merck), from Kyoto, Japan. The results were compared with the limits specified in Annex F of NBR 10004 [34] and EPA [39] regarding metal toxicity, as well as water quality standards: CONAMA 460 [40], the Dutch List [41], and EPA [42].
3. Results
3.1. Mechanical Behavior
The analysis of unconfined compressive strength (UCS) (Figure 1) and the Pareto chart (Figure 2) enables a detailed assessment of the strength development of IOT/AAB mixtures, as well as the interactions and significance of the three factors evaluated (curing time, AAB content, and specific weight). The Pareto chart was constructed by ranking categories in descending order of frequency and plotting their cumulative percentages, allowing the identification of the most significant contributors based on the Pareto principle (80/20 rule).
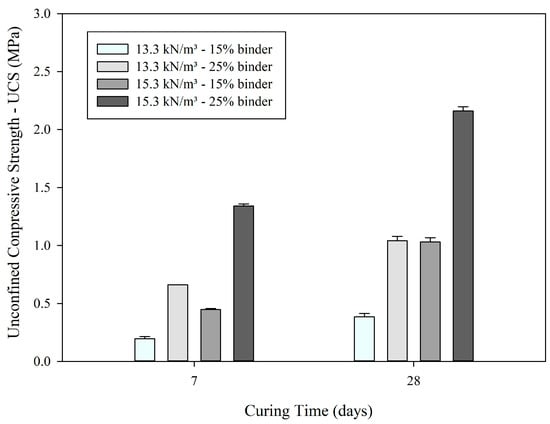
Figure 1.
Average UCS values.
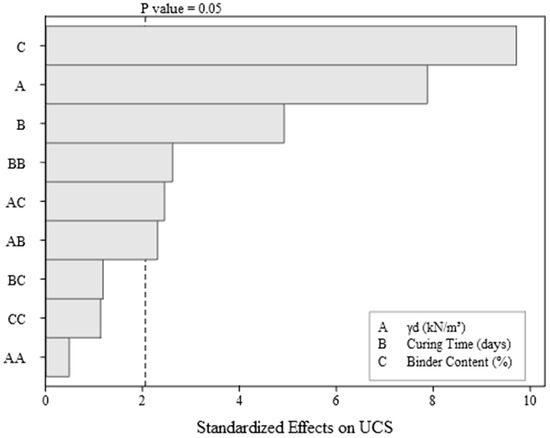
Figure 2.
Pareto chart for UCS.
In general, the results and their replicates have low variability, as observed by the standard error bars (Figure 1), and suggest a clear trend of strength improvement as curing time progresses, along with increased binder content and specific weight, achieving a compressive strength of 1.34 MPa and 2.14 MPa after 7 and 28 days of curing, respectively, at a specific weight of 15.3 kN/m3 and 25% AAB.
It is observed that the samples with 25% binder content exhibit higher strength at both the 7-day and 28-day curing periods, compared to the 15% binder samples. This indicates that the binder content plays a crucial role in the formation of a more robust matrix over time, promoting greater cohesion between the IOT particles.
As shown in the Pareto chart (Figure 2), the binder content (C) is the variable with the most significant impact on the UCS gain of the IOT–AAB mixture. In studies on the stabilization/solidification of tailings, it is common for binder content to play a predominant role by providing more reactive materials (such as aluminosilicates or calcium compounds) for hydration and alkaline activation reactions, reducing the relative proportion of inert or less reactive phases (such as aggregates or low-reactivity materials), and increasing the proportion of hydrated or polymerized products that contribute to the development of cementing gels such as C-S-H and/or C-A-S-H/N-A-S-H. These compounds are responsible for the increased mechanical strength of the mixture [17,24,43]. It is also evident that the samples compacted with a higher specific weight (15.3 kN/m3) exhibit superior UCS results compared to the samples with a specific weight of 13.3 kN/m3, at both binder contents.
The specific weight (A) (Figure 2) is a significant factor in the UCS gain, a result that aligns with studies indicating that greater compaction (or specific weight) of the material leads to a better distribution of the binder and a reduction in voids, thereby increasing mechanical strength [24,43].
Although curing time (B) is less influential than binder content and specific weight, it is still an important factor in the development of the chemical and mechanical properties of IOT–AAB. All treatments showed a significant increase in strength between 7 and 28 days, indicating that the curing process of the binder contributes to the strength gain over time. This increase is more pronounced in the samples with 25% binder content and a specific weight of 15.3 kN/m3, which achieve the highest UCS values at 28 days. Levandoski et al. [26] investigated the strength of IOTs stabilized/solidified with an alkali-activated binder, highlighting that prolonged curing periods contribute to increased UCS, due to the progressive development of chemical bonds in the mixture. However, the positioning of this factor on the Pareto chart suggests that, for this specific system, curing has a lower magnitude compared to binder content and specific weight.
The presence of significant interactions (BB, AC, and AB) confirms the close relationship between the controllable factors in the stabilization/solidification processes, where the final response is influenced by specific combinations of these factors and by the non-linear effect of curing time (BB). For instance, the relevant interaction AC suggests that the effect of binder content depends on the specific weight conditions. The lesser magnitude quadratic interactions (BC, AA, and CC) indicate that increasing the levels of these factors does not follow a strong linear trend. This may suggest that, for these factors, increases beyond a certain point may not yield proportional additional benefits.
Figure 3 was generated based on the results of the experimental design conducted through unconfined compressive strength (UCS) tests. The controllable factors, namely binder content and specific weight, exhibit a positive and approximately linear influence on the strength development of the IOT–AAB mixtures. This suggests that increasing the levels of these variables tends to enhance mechanical performance, with binder content demonstrating a more pronounced effect on UCS.
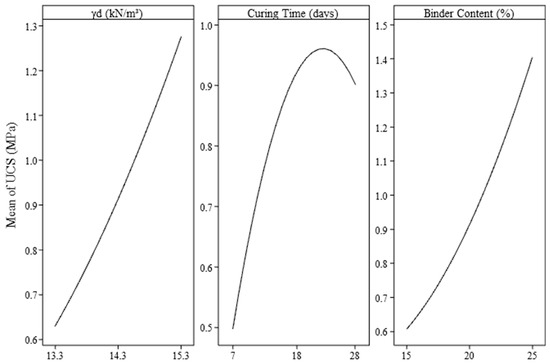
Figure 3.
Main effects of IOT–AAB mixtures as a function of the studied variables.
The curing time exhibits a nonlinear effect on UCS, initially leading to an increase followed by a subsequent decline. This behavior suggests that, as curing time progresses, the rate of UCS improvement diminishes gradually, potentially reaching a point of stabilization. Such a pattern may be attributed to the chemical reaction and hydration processes of the binder, which are likely to reach a peak in efficiency after a certain period [26,44]. In the Pareto chart, curing time was found to be less influential compared to the other factors, and this observed trend further corroborates that, although curing time plays a significant role, its contribution is comparatively less pronounced.
The obtained UCS value of 1.34 MPa and 2.14 MPa after 7 and 28 days of curing, respectively, with a density of 15.3 kN/m3 and 25% binder content, indicates that the material is capable of withstanding structural loads and mitigating risks of sliding or collapse. This demonstrates its potential for use in compacted filtered tailings stacks [5,45,46] and as a base material in infrastructure projects within mining areas, such as roads, platforms, and foundations. Furthermore, the UCS of the IOT–AAB mixture meets the strength requirement of treated soil for pavements [47]. Finally, the application of this study contributes to the recycling of waste materials and supports the principles of a circular economy [48].
3.2. Mineralogical Behavior
The IOT–AAB mixtures demonstrating the most favorable mechanical performance (25% AAB and 15.3 kN/m3 dry unit weight) at 7 and 28 days display a mineralogical composition consisting of both semicrystalline and crystalline phases (Figure 4). These phases include quartz (SiO2), originating from IOTs and RHA; cristobalite (SiO2), derived from RHA; hematite (Fe2O3), goethite (FeO(OH)), and kaolinite (Al2Si2O5(OH)4), all from IOTs; and portlandite (Ca(OH)2), sourced from HEL.
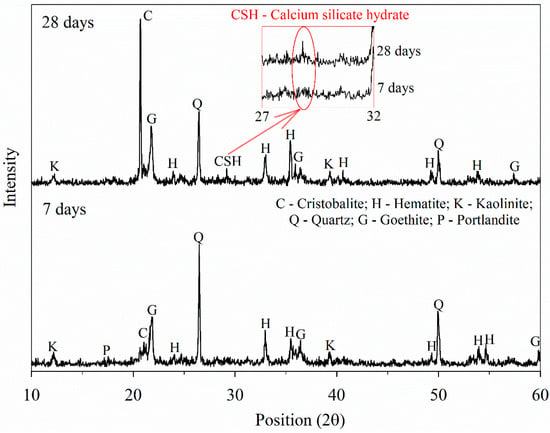
Figure 4.
XRD results of the selected mixtures.
Portlandite (Ca(OH)2) is gradually consumed over time (from 7 to 28 days), whereas a distinct peak and an amorphous hump are observed in the 28-day sample within the 2θ range of 27° to 32°. The emergence of this peak can be correlated with the formation of calcium silicate hydrate (C–S–H) gels [49,50,51]. However, in alkali-activated systems, the intensification of amorphous phases may also indicate the formation of calcium (aluminum) silicate hydrate (C–(A)–S–H) gels (peaks at 2θ = 28–30°) and sodium aluminosilicate hydrate (N–A–S–H) gels (peaks at 2θ = 20–35°) [52,53]. The presence of these gels supports the increase in strength observed in the mechanical performance of the IOT–AAB mixtures from 7 to 28 days.
Through qualitative FTIR analysis (Figure 5), the chemical compounds present in the IOT–AAB mixtures were identified. The main minerals constituting the IOTs were observed, including kaolinite (Si–O–Al bond stretching at 913 cm−1), goethite (Fe–O–H bond stretching vibrations at 795 and 469 cm−1), and hematite (lower Fe–O–H stretching bands at 619 and 517 cm−1) [26,54,55]. The peaks at 3695 and 3619 cm−1 correspond to O–H bond stretching modes related to residual kaolinite and goethite, including asymmetric and symmetric axial Al–O–H modes and tetrahedral Al–O–H modes, which are characteristic of mixtures containing IOTs [26,56].
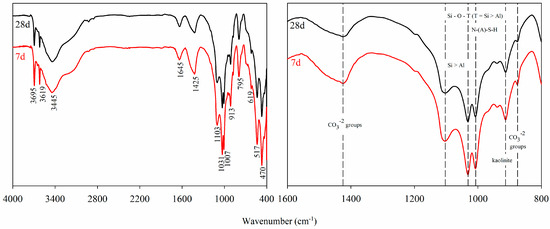
Figure 5.
FTIR spectra of the selected mixtures.
The bands at 3445 and 1645 cm−1 are associated with water molecules, corresponding to H–O–H stretching and bending vibrations, respectively [57]. An observable reduction in the shoulder of the bands at 1418 and 876 cm−1 from 7 to 28 days indicates the presence of carbonate groups (CO32−), typically found in both calcined and untreated geopolymer specimens. These carbonates result from reactions between alkali metal oxides (NaOH) and atmospheric CO2 [44,56]. This process is likely associated with excess calcium ions that are not fully consumed during the early stages of alkali activation (7 days). Over time, these calcium ions are gradually incorporated into the reaction products, reducing their availability at later stages (28 days) and correlating with the observed increase in UCS.
The asymmetric stretching of Si–O–T bonds (where T = Al or Si) is responsible for the bands observed at 1103 and 1031 cm−1. This silica-rich phase, with low aluminum content, originates from precursor oxides—specifically RHA—that did not fully participate in the alkali activation reactions. As a result, a silicate network with a lower degree of aluminum substitution is formed, in contrast to the more cross-linked N–A–S–H or C–A–S–H gels [44,56]. This behavior is consistent with the high SiO2 and low Al2O3 content characteristic of RHA, which leads to a predominance of silicon over aluminum (Si > Al) in the reactive environment and, consequently, to the formation of silicate-rich structures with limited Al incorporation.
The band at 1007 cm−1, typically observed in alkali-activated systems with lower concentrations of alkaline activators, is also attributed to the asymmetric stretching of Si–O–T bonds (T = Al or Si), and is characteristic of N–A–S–H gel formation [26,58,59,60]. This band further reflects the influence of the RHA precursor, reinforcing the silica-dominated nature of the system and supporting the identification of aluminosilicate gels with a lower degree of cross-linking due to the limited availability of aluminum.
3.3. Leaching Behavior
The chemical composition of the leachate extracts from IOT, RHA, and the IOT+AAB mixtures at 7 and 28 days (Table 2) shows that all concentrations remained below the limits established by NBR 10004 [34] and the U.S. EPA (1992) [39]. This indicates that both the raw residues and their cemented mixtures do not exhibit metal toxicity.

Table 2.
Leaching results.
However, the IOT+AAB mixtures showed leaching of two metals when compared to more restrictive limits—specifically, those set for groundwater quality and drinking water standards, as detailed in Table 2. Both cemented mixtures exhibited cadmium (Cd) concentrations that exceeded the maximum limits defined by the Dutch List [41], CONAMA Resolution 460 [40], and EPA (2022) [42]. Additionally, chromium (Cr) leaching surpassed the groundwater quality limit set by the Dutch List [41].
Although IOTs and RHA did not present Cd leaching individually, one or both materials may contain small amounts of insoluble cadmium ions (Cd2⁺) that become mobilized when incorporated into alkali-activated systems. This mobilization may be due to the highly alkaline environment created by the alkaline activator (e.g., NaOH) and HEL, which can promote the formation of Cd-containing compounds that are susceptible to leaching as pH decreases during testing [61]. Nonetheless, over time, Cd may become encapsulated within the cemented IOT–AAB matrix, as the gels formed in alkali-activated systems—such as calcium silicate hydrate (C–S–H) and calcium aluminosilicate hydrate (C–A–S–H)—are known to immobilize heavy metals, including Cd [25,62].
Chromium (Cr) exhibits amphoteric leaching behavior, with solubility increasing under both acidic and alkaline conditions [63,64]. Although the individual materials did not show detectable Cr leaching, the formation of a highly alkaline environment in the IOT–AAB mixtures—resulting from the addition of NaOH and HEL—may have facilitated Cr dissolution into the leachate during testing. Despite this, Cr can be stabilized over time via chemical precipitation, particularly in the presence of calcium (as present in HEL), leading to the formation of poorly soluble compounds or solid solutions. This reduces both the mobility and environmental impact of Cr. Such behavior has been documented by Cornelis et al. [65] and Mahedi et al. [64], who emphasize precipitation as a natural mitigation mechanism in leaching systems. Furthermore, in alkali-activated materials, Cr can also be structurally incorporated into the C–S–H gel network, further reducing its bioavailability [66].
During both curing periods, the IOT–AAB mixture effectively encapsulated barium (Ba), originally present in the RHA. This metal, known for its toxicity at elevated concentrations, was completely immobilized within the alkali-activated matrix, complying with both toxicity thresholds and water quality standards. The encapsulation is attributed to the formation of cementitious phases—such as calcium aluminosilicate hydrate (C–A–S–H) and sodium aluminosilicate hydrate (N–A–S–H)—which develop into a dense and chemically stable three-dimensional network. This structure restricts metal ion mobility through mechanisms such as adsorption, precipitation, and the formation of insoluble complexes [32]. These results highlight the potential of IOT–AAB systems as a sustainable strategy to mitigate the environmental impact of mining and agro-industrial residues, while simultaneously adding value to materials otherwise classified as waste.
4. Conclusions
The incorporation of an alkali-activated binder (AAB), composed of rice husk ash (RHA) and hydrated eggshell lime (HEL), into iron ore tailings (IOTs) resulted in a mixture exhibiting satisfactory mechanical strength (2.16 MPa after 28 days of curing). The strength was positively influenced by the AAB content, followed by the dry unit weight, and curing time, with the latter showing a nonlinear effect. Additionally, the IOT–AAB mixtures demonstrated the formation of cementitious gels, such as C–S–H at 28 days and N–A–S–H from 7 days onwards. The mixtures also showed no metal leaching exceeding toxicity limits and successfully encapsulated barium (Ba) present in the RHA.
While the availability of RHA and HEL may not match the total volume of IOT generated on a national scale, their use as components of alkali-activated binders can be highly effective in regional or site-specific contexts, particularly in agricultural areas where these residues are abundant. This approach reinforces the importance of decentralized and locally adapted strategies for mine tailing stabilization.
A key limitation of this study is the use of laboratory-scale testing, which provides valuable preliminary data but may not fully represent the behavior of stabilized IOTs in large-scale field conditions. External factors such as ambient temperature, humidity, and long-term environmental exposure could influence the performance of alkali-activated binders and should be investigated in future studies. Additionally, while this study focused on mechanical strength and leaching behavior, other factors such as durability under wetting–drying cycles, freeze–thaw cycles, and biological degradation resistance could also impact the long-term stability of tailings piles and should be explored in future research.
Future studies should consider large-scale field testing to validate laboratory results and assess the long-term performance of alkali-activated IOT binders under real-world conditions. Research on optimizing the proportions of RHA and HEL in the binder system may help improve performance and economic viability. Furthermore, evaluating other locally available agro-industrial residues as precursors for alkali activation could further enhance the sustainability of this solution.
Additionally, the variation in feedstock composition, particularly in the RHA and HEL sources, can impact the performance of the alkali-activated binder. The chemical composition, particle size distribution, and impurities present in these agro-industrial residues can affect the binder properties and, ultimately, the stability of the IOT mixture. Future research should investigate the influence of feedstock variability on the binder’s performance, ensuring more consistent and reliable results across different geographic locations and sources of residue.
Author Contributions
Conceptualization, W.M.K.L., S.T.F., G.J.B. and E.P.K.; methodology, W.M.K.L., S.T.F., G.J.B. and E.P.K.; validation, W.M.K.L., J.D.M., C.M., S.T.F., G.J.B. and E.P.K.; formal analysis, W.M.K.L., S.T.F. and G.J.B.; investigation, W.M.K.L., J.D.M. and C.M.; resources, E.P.K.; data curation, W.M.K.L., J.D.M., C.M. and S.T.F.; writing—original draft preparation, W.M.K.L., S.T.F. and G.J.B.; writing—review and editing, J.D.M., C.M. and E.P.K.; visualization, G.J.B. and E.P.K.; supervision, G.J.B. and E.P.K.; project administration, E.P.K.; funding acquisition, E.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil—CAPES], by [Conselho Nacional de Desenvolvimento Científico Tecnológico—CNPq] grant number [305910/2023-0], by [Financiadora de Estudos e Projetos—FINEP] and the APC was funded by [MDPI].
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IOT | Iron Ore Tailings |
| ABB | Alkali-activated Binders |
| HEL | Hydrated Eggshell Lime |
| UCS | Unconfined Compressive Strength |
| XRD | X-ray Diffraction |
| FTIR | Fourier-transform Infrared |
References
- Nishijima, M.; Rocha, F.F. An Economic Investigation of the Dengue Incidence as a Result of a Tailings Dam Accident in Brazil. J. Environ. Manag. 2020, 253, 109748. [Google Scholar] [CrossRef] [PubMed]
- IBRAM. Instituto Brasileiro de Mineração Mineração Em Números; IBRAM: Belo Horizonte, Brazil, 2023. [Google Scholar]
- Senado Federal Eco Senado. Available online: https://www12.senado.leg.br/tv/programas/ecosenado/2023/10/responsavel-por-4-do-pib-mineracao-encontra-maneiras-de-ser-mais-sustentavel-no-brasil (accessed on 11 August 2024).
- Nadig, S. Managing Mining’s Environmental Waste. Available online: https://www.mining-technology.com/features/environmental-waste-management/?cf-view (accessed on 11 August 2024).
- Saldanha, R.B.; Caicedo, A.M.L.; de Araújo, M.T.; Scheuermann Filho, H.C.; Moncaleano, C.J.; Silva, J.P.S.; Consoli, N.C. Potential Use of Iron Ore Tailings for Binder Production: A Life Cycle Assessment. Constr. Build. Mater. 2023, 365, 130008. [Google Scholar] [CrossRef]
- Consoli, N.C.; Vogt, J.C.; Silva, J.P.S.; Chaves, H.M.; Filho, H.C.S.; Moreira, E.B.; Lotero, A. Behaviour of Compacted Filtered Iron Ore Tailings–Portland Cement Blends: New Brazilian Trend for Tailings Disposal by Stacking. Appl. Sci. 2022, 12, 836. [Google Scholar] [CrossRef]
- Leão, S.R.; dos Santos Santiago, A.M. Tailings Dam Scenario: Knowing to Avoid New Catastrophes. Ambiente Soc. 2022, 25, 1–20. [Google Scholar] [CrossRef]
- Servi, S.; Lotero, A.; Silva, J.P.S.; Bastos, C.; Consoli, N.C. Mechanical Response of Filtered and Compacted Iron Ore Tailings with Different Cementing Agents: Focus on Tailings-Binder Mixtures Disposal by Stacking. Constr. Build. Mater. 2022, 349, 128770. [Google Scholar] [CrossRef]
- Franks, D.M.; Stringer, M.; Torres-Cruz, L.A.; Baker, E.; Valenta, R.; Thygesen, K.; Matthews, A.; Howchin, J.; Barrie, S. Tailings Facility Disclosures Reveal Stability Risks. Sci. Rep. 2021, 11, 5353. [Google Scholar] [CrossRef]
- Oldecop, L.A.; Rodari, G.J. Unsaturated Mine Tailings Disposal. Soils Rocks 2021, 44, 1–12. [Google Scholar] [CrossRef]
- Schatzmayr Welp Sá, T.; Oda, S.; Karla Castelo Branco Louback Machado Balthar, V.; Dias Toledo Filho, R. Use of Iron Ore Tailings and Sediments on Pavement Structure. Constr. Build. Mater. 2022, 342, 128072. [Google Scholar] [CrossRef]
- de Oliveira, M.; de Oliveira, G.V.; Moura, B.F. Monitoramento Através de Sensores Das Emissões de Gases Do Efeito Estufa Na Indústria Cimenteira: Uma Revisão. Braz. J. Prod. Eng. 2023, 9, 51–59. [Google Scholar] [CrossRef]
- Bao, J.; Wang, L.; Xiao, M. Changes in Speciation and Leaching Behaviors of Heavy Metals in Dredged Sediment Solidified/Stabilized with Various Materials. Environ. Sci. Pollut. Res. 2016, 23, 8294–8301. [Google Scholar] [CrossRef]
- An, X.; Zuo, D.; Wang, F.; Liang, C. Investigation on Stabilization/Solidification Characteristics of Lead-Contaminated Soil Using Innovative Composite Model of Cement and Soda Residue. Environ. Earth Sci. 2022, 81, 508. [Google Scholar] [CrossRef]
- Khoeurn, K.; Sasaki, A.; Tomiyama, S.; Igarashi, T. Distribution of Zinc, Copper, and Iron in the Tailings Dam of an Abandoned Mine in Shimokawa, Hokkaido, Japan. Mine Water Environ. 2019, 38, 119–129. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; Levandoski, W.M.K.; Ferrazzo, S.T.; Korf, E.P.; Saldanha, R.B.; Consoli, N.C. Leaching Assessment of Cemented Bauxite Tailings through Wetting and Drying Cycles of Durability Test. Environ. Sci. Pollut. Res. 2022, 29, 59247–59262. [Google Scholar] [CrossRef]
- Pereira dos Santos, C.; Bruschi, G.J.; Mattos, J.R.G.; Consoli, N.C. Stabilization of Gold Mining Tailings with Alkali-Activated Carbide Lime and Sugarcane Bagasse Ash. Transp. Geotech. 2022, 32, 100704. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, C.; Li, Y.; Yang, C. Solidification/Stabilization of Gold Ore Tailings Powder Using Sustainable Waste-Based Composite Geopolymer. Eng. Geol. 2022, 309, 106793. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Alaba, P.A.; Rashedi, A. Selected Performance of Alkali-Activated Mine Tailings as Cementitious Composites: A Review. J. Build. Eng. 2022, 50, 104154. [Google Scholar] [CrossRef]
- He, X.; Yuhua, Z.; Qaidi, S.; Isleem, H.F.; Zaid, O.; Althoey, F.; Ahmad, J. Mine Tailings-Based Geopolymers: A Comprehensive Review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Z.; Zhang, T.; Su, X. Effect of Carbonation on Leaching Behavior, Engineering Properties and Microstructure of Cement-Stabilized Lead-Contaminated Soils. Environ. Earth Sci. 2017, 76, 724. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effects of Alkaline-Activated Fly Ash and Portland Cement on Soft Soil Stabilisation. Acta Geotech. 2013, 8, 395–405. [Google Scholar] [CrossRef]
- Levandoski, W.M.K.; Ferrazzo, S.T.; Bruschi, G.J.; Consoli, N.C.; Korf, E.P. Mechanical and Microstructural Properties of Iron Mining Tailings Stabilized with Alkali-Activated Binder Produced from Agro-Industrial Wastes. Sci. Rep. 2023, 13, 15754. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzo, S.T.; Tonini de Araújo, M.; Bruschi, G.J.; Korf, E.P.; Levandoski, W.M.K.; Pereira dos Santos, C.; Consoli, N.C. Metal Encapsulation of Waste Foundry Sand Stabilized with Alkali-Activated Binder: Batch and Column Leaching Tests. J. Environ. Manag. 2023, 348, 119287. [Google Scholar] [CrossRef] [PubMed]
- Levandoski, W.M.K.; Ferrazzo, S.T.; Piovesan, M.A.; Bruschi, G.J.; Consoli, N.C.; Korf, E.P. Long-Term Performance: Strength and Metal Encapsulation in Alkali-Activated Iron Ore Tailings. Environ. Sci. Pollut. Res. 2024, 31, 47071–47083. [Google Scholar] [CrossRef]
- Kiventerä, J.; Sreenivasan, H.; Cheeseman, C.; Kinnunen, P.; Illikainen, M. Immobilization of Sulfates and Heavy Metals in Gold Mine Tailings by Sodium Silicate and Hydrated Lime. J. Environ. Chem. Eng. 2018, 6, 6530–6536. [Google Scholar] [CrossRef]
- Pelisser, G.; Ferrazzo, S.T.; Mota, J.D.; dos Santos, C.P.; Pelisser, C.; Rosa, F.D.; Korf, E.P. Rice Husk Ash-Carbide Lime as an Alternative Binder for Waste Foundry Sand Stabilization. Environ. Sci. Pollut. Res. 2023, 30, 42176–42191. [Google Scholar] [CrossRef]
- Chen, R.; Congress, S.S.C.; Cai, G.; Duan, W.; Liu, S. Sustainable Utilization of Biomass Waste-Rice Husk Ash as a New Solidified Material of Soil in Geotechnical Engineering: A Review. Constr. Build. Mater. 2021, 292, 123219. [Google Scholar] [CrossRef]
- Consoli, N.; Saldanha, R.; Lotero, A.; Scheuermann Filho, H.C.; Moncaleano, C. Eggshell Produced Limes: Innovative Materials for Soil Stabilization. J. Mater. Civ. Eng. 2020, 32, 06020018. [Google Scholar] [CrossRef]
- Tonini de Araújo, M.; Tonatto Ferrazzo, S.; Jordi Bruschi, G.J.; Consoli, N.C. Mechanical and Environmental Performance of Eggshell Lime for Expansive Soils Improvement. Transp. Geotech. 2021, 31, 100681. [Google Scholar] [CrossRef]
- Pompermaier, C.L.; Ferrazzo, S.T.; Levandoski, W.M.K.; Bruschi, G.J.; Prietto, P.D.M.; Korf, E.P. Stabilization of Waste Foundry Sand with Alkali-Activated Binder: Mechanical Behavior, Microstructure and Leaching. Constr. Build. Mater. 2024, 444, 137772. [Google Scholar] [CrossRef]
- Reis, J.B.; Pelisser, G.; Levandoski, W.M.K.; Ferrazzo, S.T.; Mota, J.D.; Silveira, A.A.; Korf, E.P. Experimental Investigation of Binder Based on Rice Husk Ash and Eggshell Lime on Soil Stabilization under Acidic Attack. Sci. Rep. 2022, 12, 7542. [Google Scholar] [CrossRef]
- ABNT NBR 10004; Resíduos Sólidos—Classificação. Associação Brasileira de Normas Técnicas (ABNT): Rio Janeiro, Brazil, 2004.
- Montgomery, D.; St, C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; ISBN 9781119113478 (PBK)/9781119299455 (EVALC). [Google Scholar]
- Consoli, N.C.; Rosa, A.D.; Saldanha, R.B. Parameters Controlling Strength of Industrial Waste-Lime Amended Soil. Soils Found. 2011, 51, 265–273. [Google Scholar] [CrossRef]
- ASTM D2166-16; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2016.
- ABNT NBR 10005; Procedimento Para Obtenção de Extrato Lixiviado de Resíduos Sólidos. Associação Brasileira de Normas Técnicas (ABNT): Rio Janeiro, Brazil, 2004; Volume 16.
- USEPA. 1992 Method 1311: Toxicity Characteristic Leaching Procedure; US Environmental Protection Agency: Boston, MA, USA, 1992.
- CONAMA—CONSELHO NACIONAL DE MEIO AMBIENTE. Resolução nº 460, de 30 de Dezembro de 2013. Altera a Resolução no 420, de 28 de Dezembro de 2009, do Conselho Nacional do Meio Ambiente-CONAMA, que Dispõe Sobre Critérios e Valores Orientadores de Qualidade do solo Quanto à Presença de Substâncias Químicas e dá Outras Providências. Diário Oficial da República Federativa do Brasil, Brasília, DF, 31 dez. 2013. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=676 (accessed on 5 February 2025).
- Van Volkshuisvesting, M. Circular on Target Values and Intervention Values for Soil Remediation-Dutch Target and Intervention Values; Netherlands Government Gazette: Amsterdam, The Netherlands, 2000.
- USEPA. 2022 Ground Water and Drinking Water: National Primary Drinking Water Regulations [WWW Document]. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 4 February 2025).
- Bruschi, G.J.; dos Santos, C.P.; Tonini de Araújo, M.; Ferrazzo, S.T.; Marques, S.F.V.; Consoli, N.C. Green Stabilization of Bauxite Tailings: Mechanical Study on Alkali-Activated Materials. J. Mater. Civ. Eng. 2021, 33, 06021007. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. An Overview of the Chemistry of Alkali-Activated Cement-Based Binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 19–47. ISBN 9781782422884. [Google Scholar]
- Carvalho, J.V.d.A.; Wagner, A.C.; Scheuermann Filho, H.C.; Chaves, H.M.; Silva, J.P.S.; Delgado, B.G.; Consoli, N.C. Evaluation of Strength Parameters for Application in Cemented Iron Ore Tailings Stacks. Indian Geotech. J. 2023, 53, 775–788. [Google Scholar] [CrossRef]
- Consoli, N.C.; Collatto, D.; de Azambuja Carvalho, J.V.; Wagner, A.C.; de Sousa Silva, J.P.; Marçal de Sousa, G.; Scheuermann Filho, H.C. Resilience of Compacted Iron Ore Tailings-Binder Blends for Dry Stacking. Geotech. Geol. Eng. 2025, 43, 164. [Google Scholar] [CrossRef]
- U.S. Department of Defense. UFC 3-250-11: Soil Stabilization and Modification for Pavements; U.S. Department of Defense: Washington, DC, USA, 2020. Available online: https://www.wbdg.org/FFC/DOD/UFC/ufc_3_250_11_2020.pdf (accessed on 6 February 2025).
- Ferrazzo, S.T.; de Araújo, M.T.; Bruschi, G.J.; Chaves, H.M.; Korf, E.P.; Consoli, N.C. Mechanical and Environmental Behavior of Waste Foundry Sand Stabilized with Alkali-Activated Sugar Cane Bagasse Ash-Eggshell Lime Binder. Constr. Build. Mater. 2023, 383, 131313. [Google Scholar] [CrossRef]
- Ferreira, F.A.; Desir, J.M.; de Lima, G.E.S.; Pedroti, L.G.; Franco de Carvalho, J.M.; Lotero, A.; Consoli, N.C. Evaluation of Mechanical and Microstructural Properties of Eggshell Lime/Rice Husk Ash Alkali-Activated Cement. Constr. Build. Mater. 2023, 364, 129931. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, Y.; Li, R.; Xiao, H. Treating Sulfate-Bearing Soil by Using Sodium Silicate and NaOH-Activated Ground Granulated Blast-Furnace Slag. Acta Geotech. 2024, 19, 3129–3138. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Fernández-Jimenez, A.; Palomo, A.; Macphee, D.E. Effect on Fresh C-S-H Gels of the Simultaneous Addition of Alkali and Aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Zhou, G.; Li, H.; Ozturk, I.; Ullah, S. Shocks in Agricultural Productivity and CO2 Emissions: New Environmental Challenges for China in the Green Economy. Econ. Res.-Ekon. Istraz. 2022, 35, 5790–5806. [Google Scholar] [CrossRef]
- Queiroz, L.; Batista, L.; Souza, L.; Lima, M.; Danieli, S.; Bruschi, G.; Bergmann, C. Alkali-Activated System of Carbide Lime and Rice Husk for Granular Soil Stabilization. Proc. Inst. Civ. Eng. Ground Improv. 2022, 176, 279–294. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 9400771282. [Google Scholar]
- Kaze, R.C.; Beleuk à Moungam, L.M.; Fonkwe Djouka, M.L.; Nana, A.; Kamseu, E.; Chinje Melo, U.F.; Leonelli, C. The Corrosion of Kaolinite by Iron Minerals and the Effects on Geopolymerization. Appl. Clay Sci. 2017, 138, 48–62. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M.; Vitola, L.; Alami, J.; Hakkou, R. Recycling of Phosphate Mine Tailings for the Production of Geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR Study of the Sol–Gel Synthesis of Cementitious Gels: C–S–H and N–A–S–H. J. Solgel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.S.; Zhang, Z.; Wang, H. Effect of Fly Ash to Slag Ratio and Na2O Content on Leaching Behaviour of Fly Ash/Slag Based Alkali Activated Materials. Constr. Build. Mater. 2023, 383, 131234. [Google Scholar] [CrossRef]
- Sun, K.; Ali, H.A.; Xuan, D.; Poon, C.S. Sulfuric Acid Resistance Behaviour of Alkali-Activated Slag and Waste Glass Powder Blended Precursors. Cem. Concr. Compos. 2024, 145, 105319. [Google Scholar] [CrossRef]
- Nkwaju, R.Y.; Nouping, J.N.F.; Bachirou, S.; Abo, T.M.; Deutou, J.G.N.; Djobo, J.N.Y. Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties. Materials 2023, 16, 7605. [Google Scholar] [CrossRef]
- Feng, Y.S.; Zhou, S.J.; Xia, W.Y.; Du, Y.J. Solidify/Stabilise a Heavy Metal-Contaminated Soil Using a Novel Steel Slag-Based Binder. Environ. Geotech. 2020, 10, 303–318. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Aydilek, A.H.; Benson, C.H.; Edil, T.B. Effects of PH on the Leaching Mechanisms of Elements from Fly Ash Mixed Soils. Fuel 2015, 140, 788–802. [Google Scholar] [CrossRef]
- Mahedi, M.; Cetin, B.; Dayioglu, A.Y. Effect of Cement Incorporation on the Leaching Characteristics of Elements from Fly Ash and Slag Treated Soils. J. Environ. Manag. 2020, 253, 109720. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Gerven, T.V.; Vandecasteele, C. Leaching Mechanisms of Oxyanionic Metalloid and Metal Species in Alkaline Solid Wastes: A Review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Giels, M.; Iacobescu, R.I.; Cappuyns, V.; Pontikes, Y.; Elsen, J. Understanding the Leaching Behavior of Inorganic Polymers Made of Iron Rich Slags. J. Clean. Prod. 2019, 238, 117736. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).