The B-Zone 4611 Silver-Rich Pod—An Unusual Ag-Ge-Sb-As-Ni Assemblage Within the Irish-Type Zn-Pb Silvermines Deposit, County Tipperary, Ireland
Abstract
1. Introduction
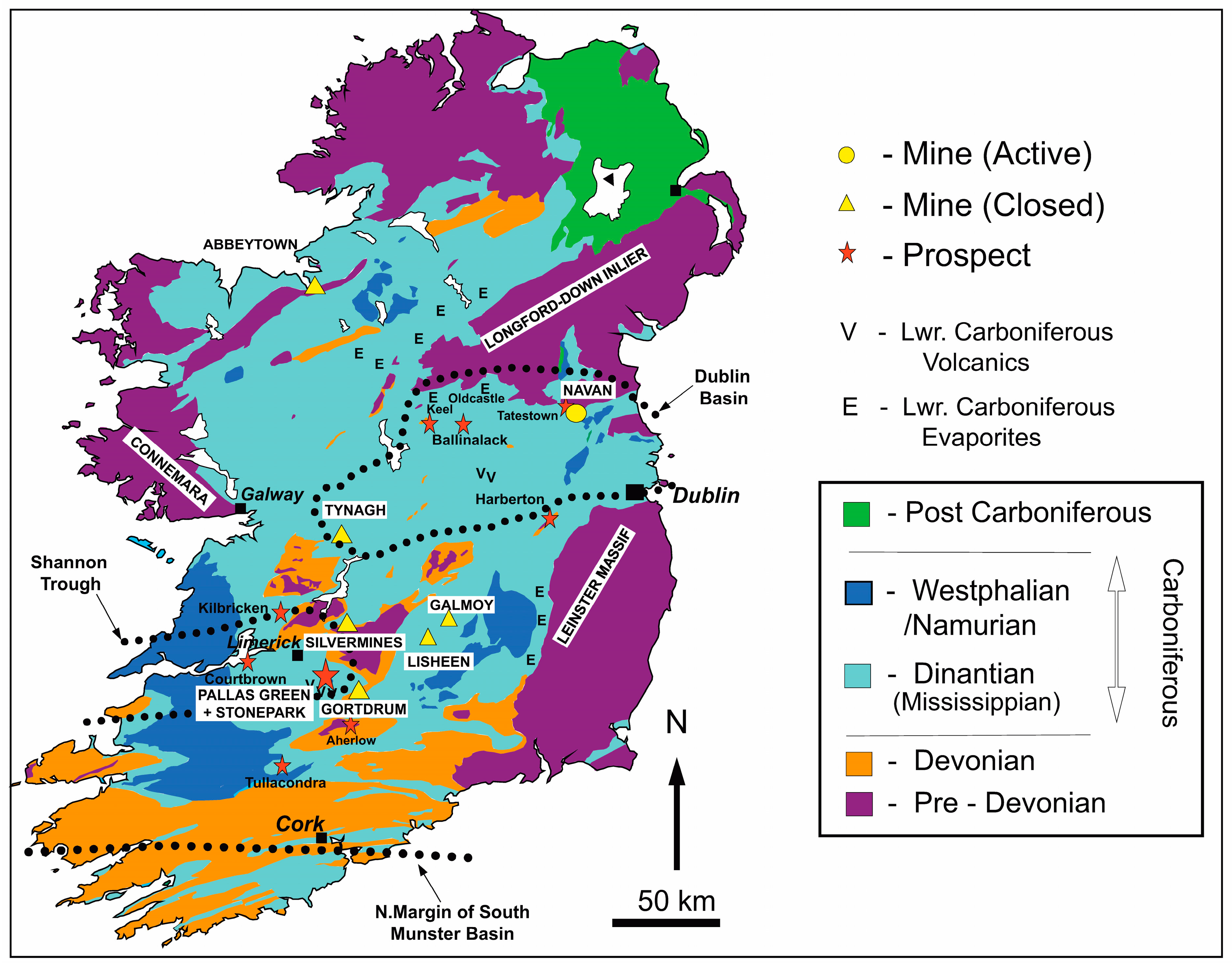
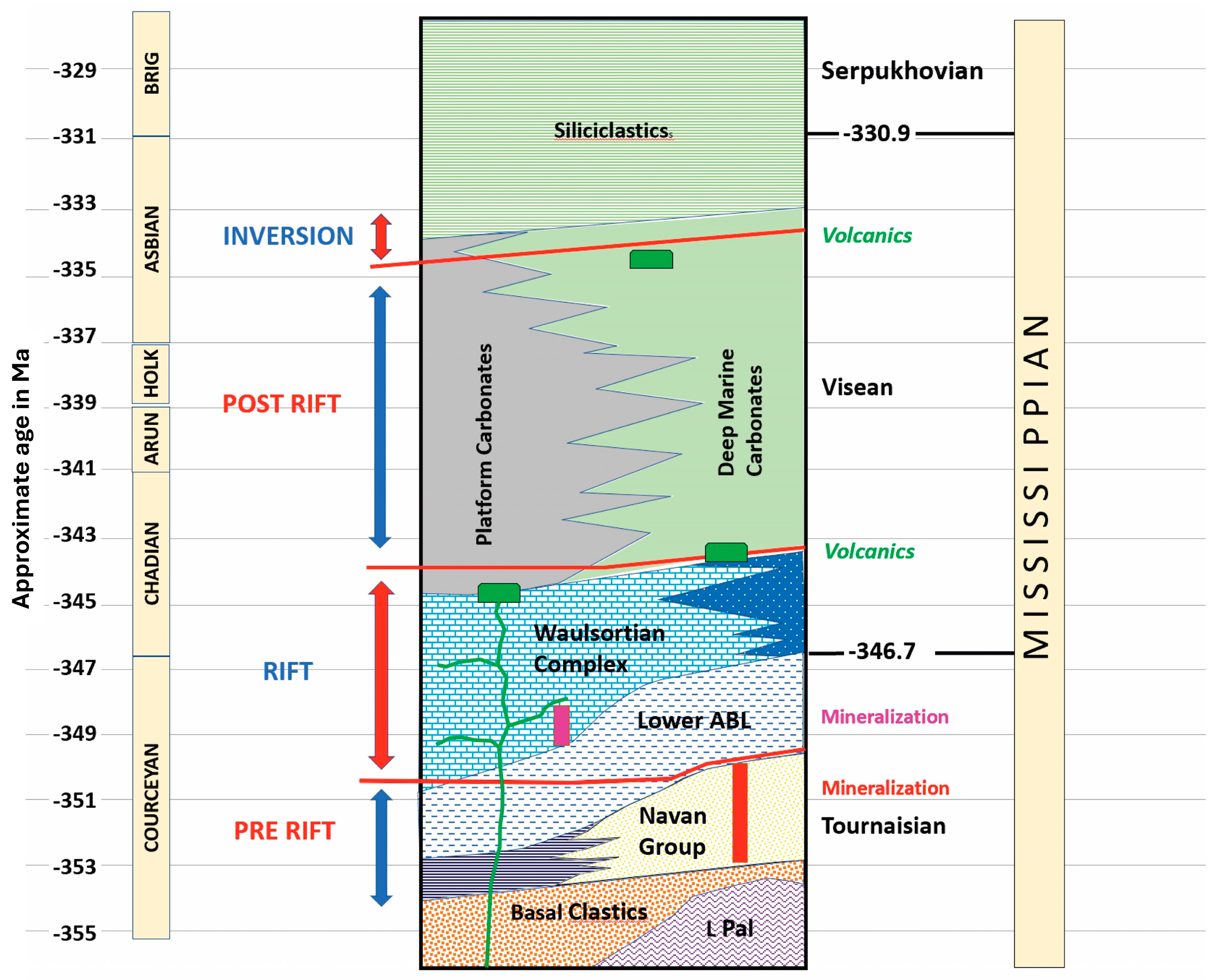
2. Regional Geological Setting and Metallogenesis
3. Introduction to Silver Mineralization in the Silvermines Deposits
4. Previous Work
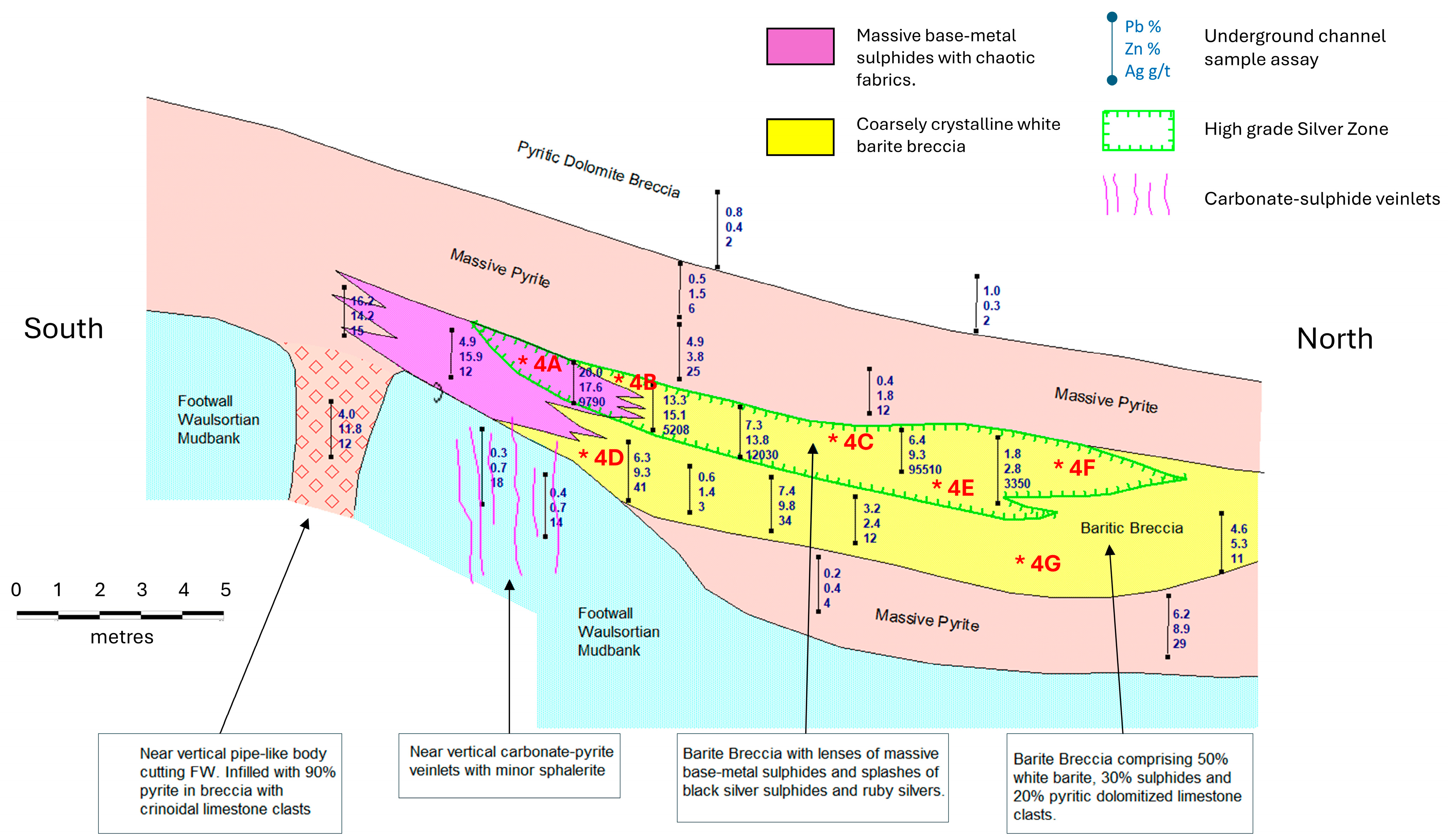
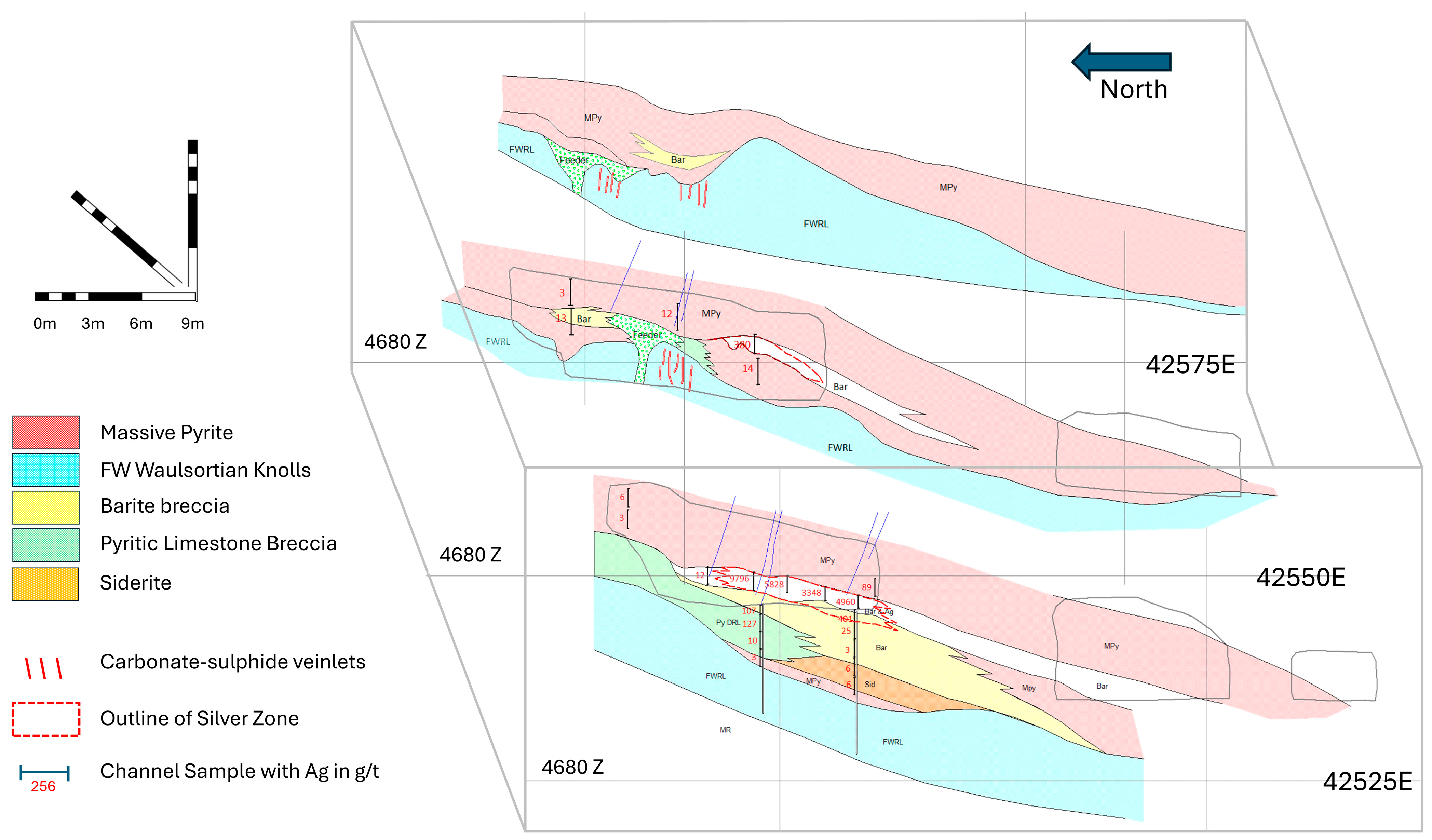
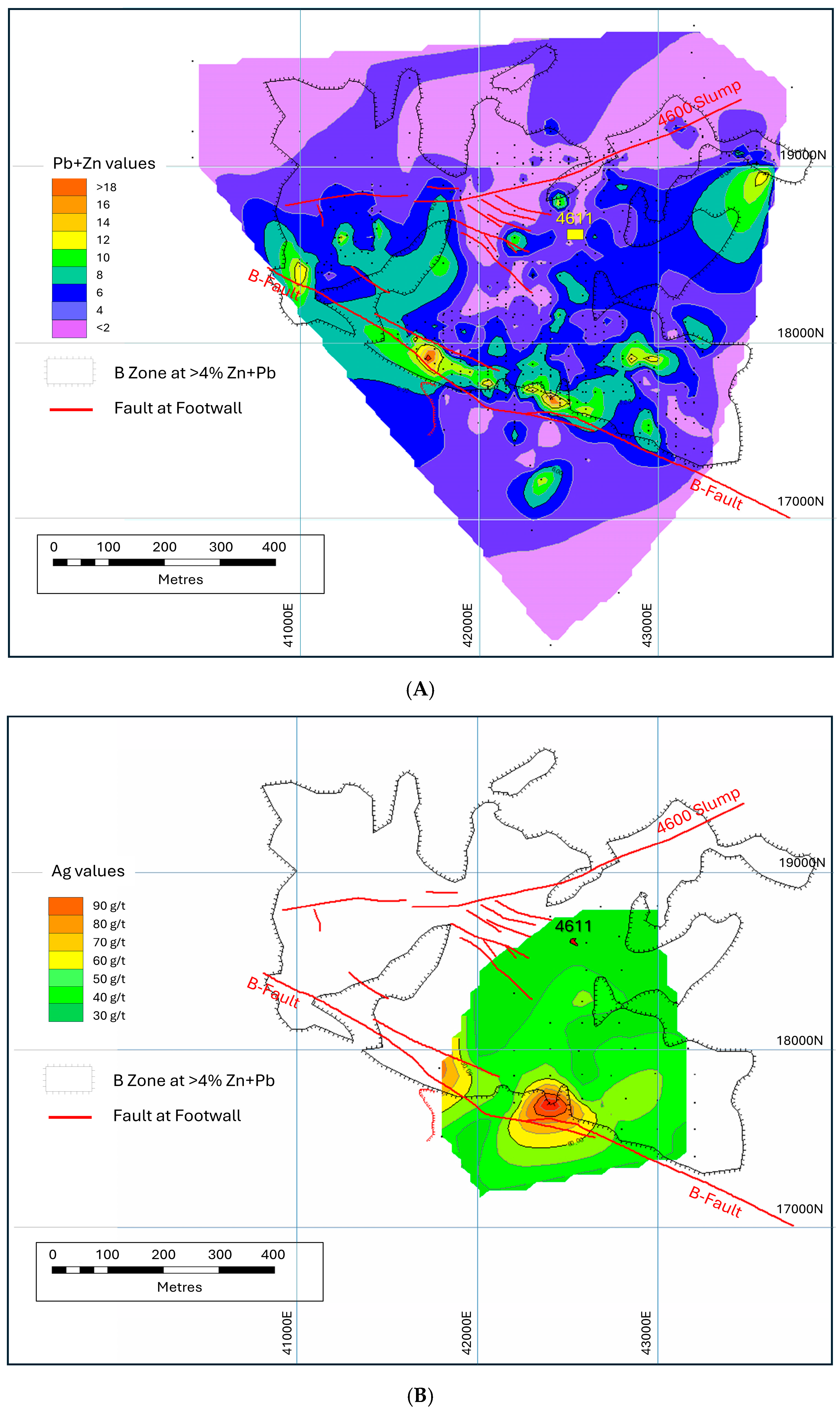
5. The B-Zone
6. The 4611 Pod
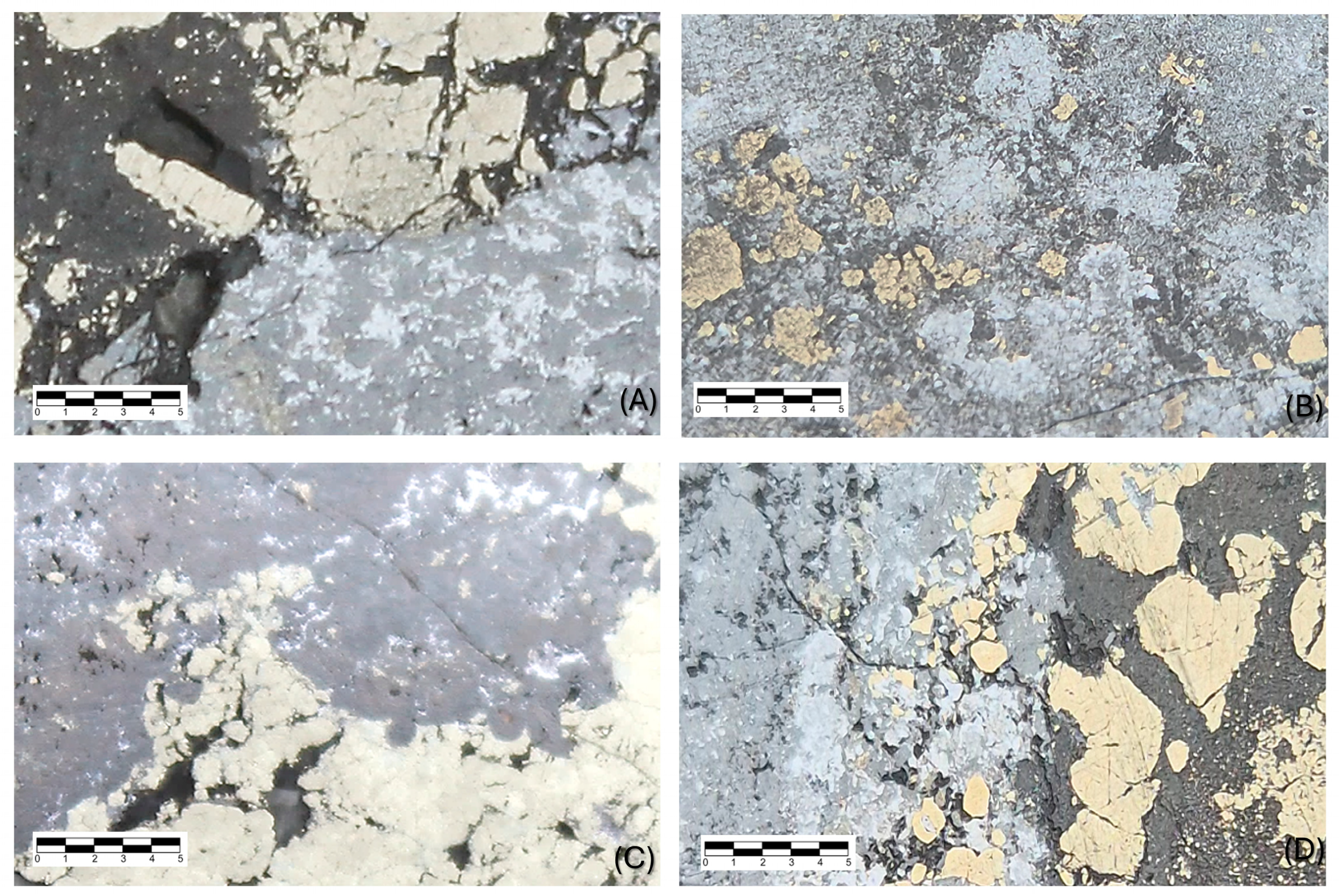
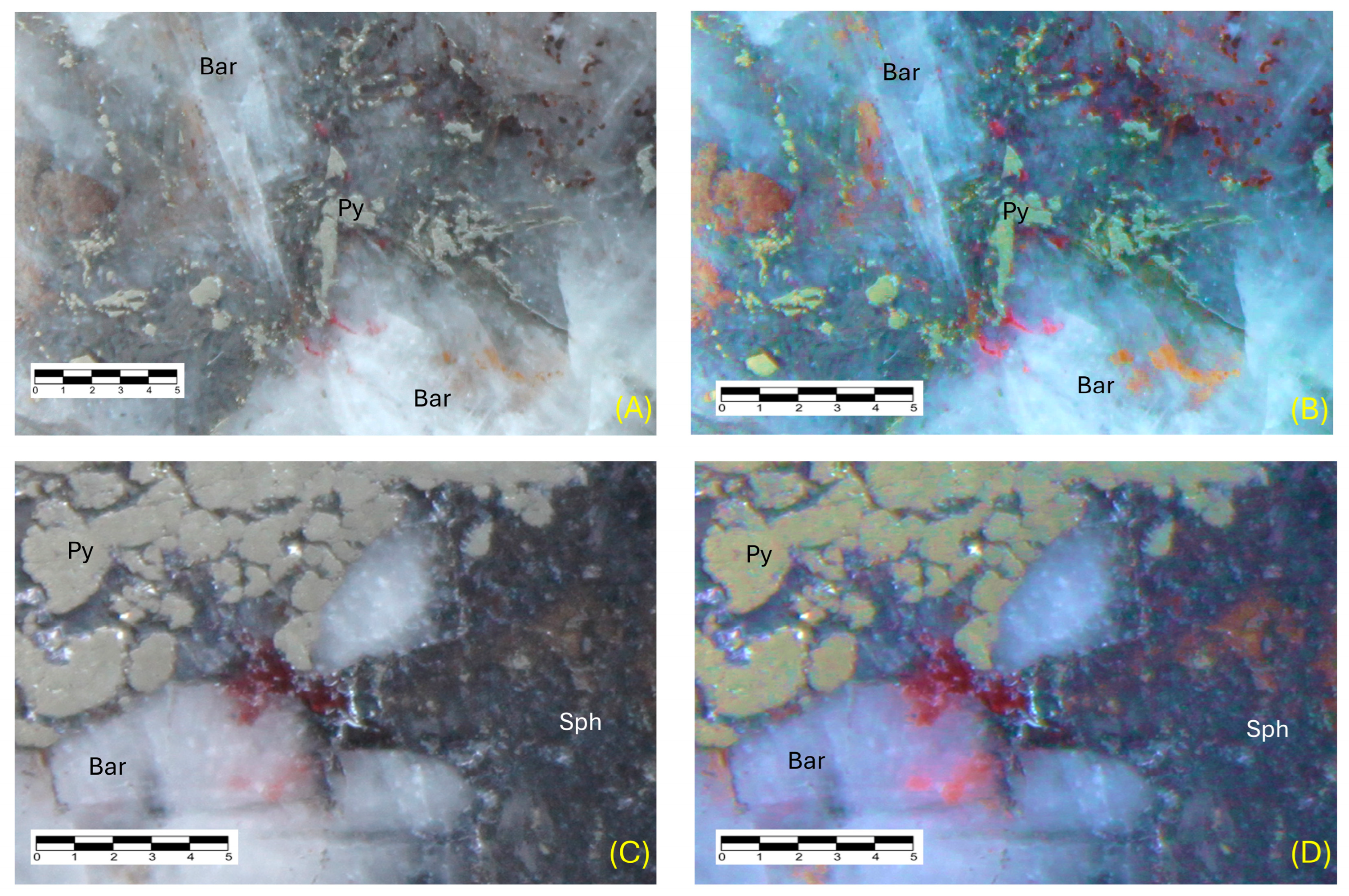
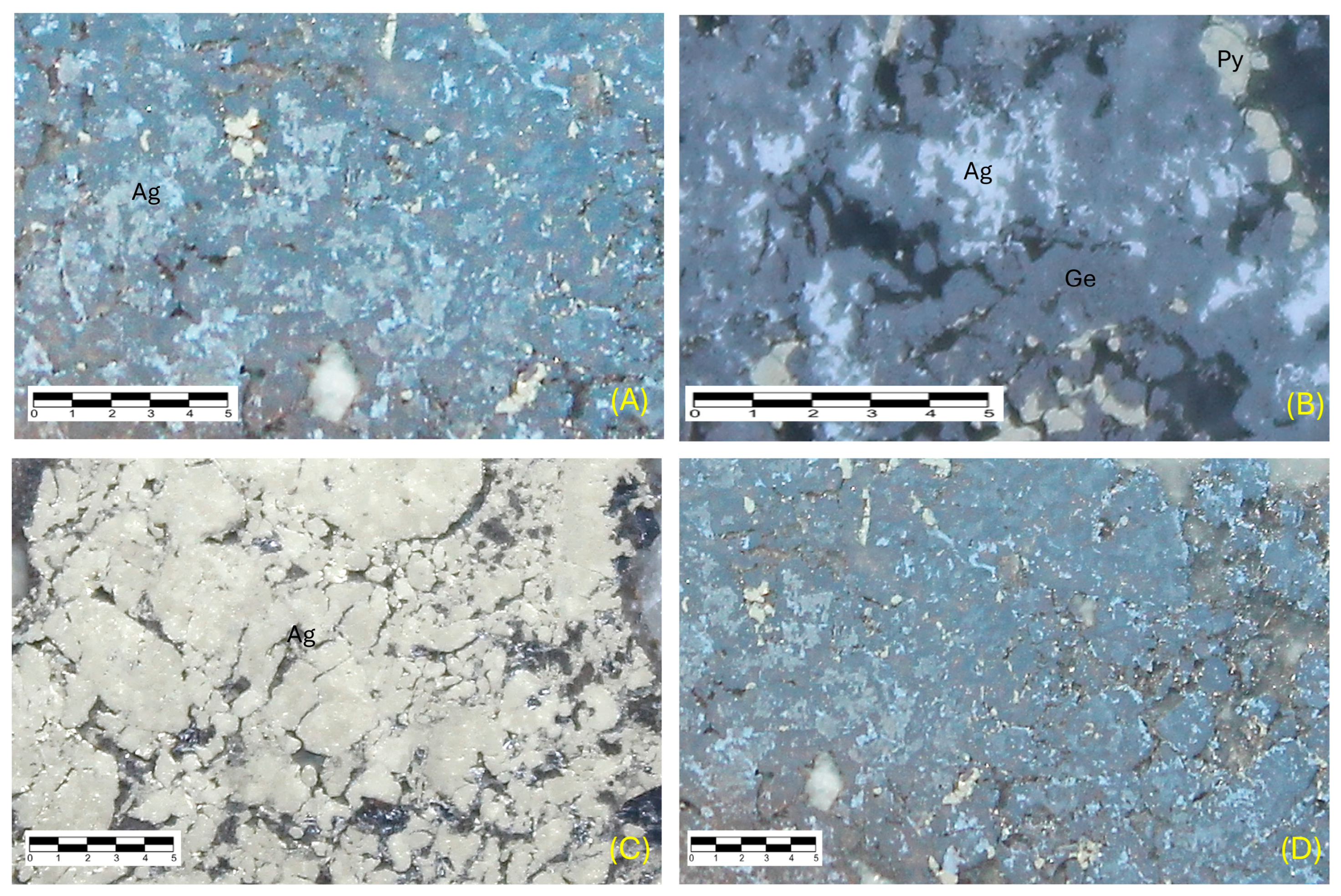
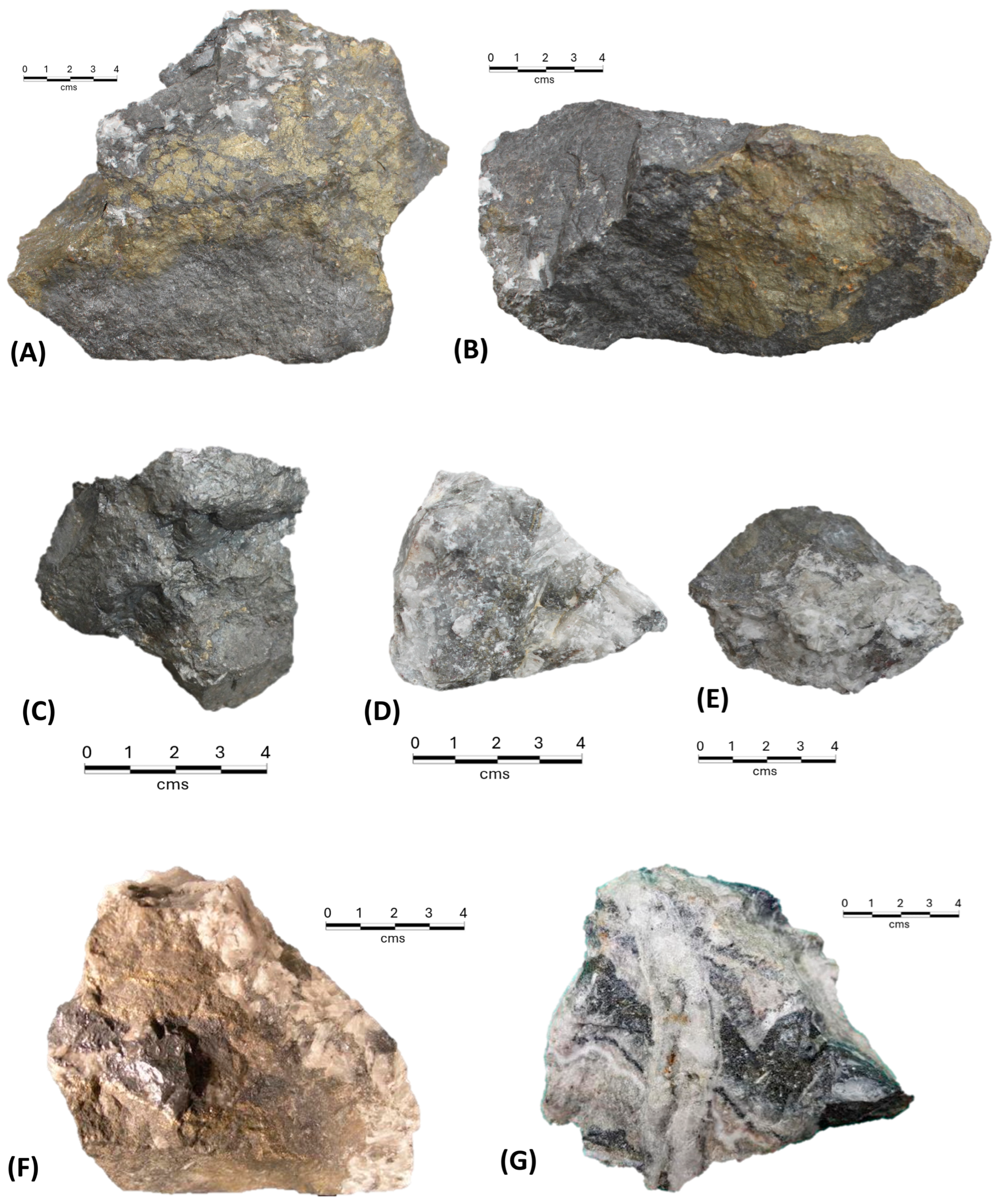
7. Mineralogy
8. Paragenesis
9. Discussion
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Description of Minerals
| Argyrodite | |||||||
| Ag | Ge | S | Total | Ag/Ge | |||
| SVF 1Z | 77.34 | 6.50 | 16.26 | 100.1 | 11.9 | ||
| SVF 1Y | 82.62 | 5.25 | 13.16 | 101.3 | 15.7 | ||
| SVF lX | 80.87 | 4.67 | 12.19 | 97.71 | 17.3 | ||
| SVF 1U | 79.83 | 5.04 | 13.41 | 98.28 | 15.8 | ||
| SVF 1V | 80.19 | 5.46 | 13.86 | 99.51 | 14.7 | ||
| SVF 1U | 81.71 | 2.81 | 11.49 | 96.01 | 29.1 | ||
| SVF 1V | 77.05 | 6.28 | 17.19 | 100.52 | 12.3 | ||
| SVF 1T | 82.38 | 1.73 | 12.15 | 96.26 | 47.6 | ||
| Ideal Composition | 76.51 | 6.44 | 17.05 | 100.00 | 11.9 | ||
| Proustite–Pyrargyrite | |||||||
| Ag | Sb | As | S | Total | As/(As+Sb) | ||
| SVF 1D | 66.78 | 2.51 | 11.86 | 18.07 | 99.22 | 0.83 | |
| SVF lE | 65.74 | 3.57 | 11.39 | 17.85 | 98.55 | 0.76 | |
| SVF IF | 66.24 | 2.56 | 12.38 | 17.88 | 99.06 | 0.83 | |
| SVF 1G | 66.39 | 2.38 | 12.01 | 18.06 | 98.84 | 0.83 | |
| W6 CD | 62.66 | 17.34 | 3.19 | 17.05 | 100.24 | 0.16 | |
| W6 CA | 61.41 | 18.11 | 2.31 | 16.65 | 98.48 | 0.11 | |
| SVF 1ZC | 64.79 | 6.29 | 9.84 | 17.84 | 98.76 | 0.61 | |
| SVF 1ZD | 65.06 | 4.87 | 10.64 | 17.97 | 98.54 | 0.69 | |
| SVF Z1Z | 66.75 | 5.17 | 10.84 | 18.02 | 100.78 | 0.68 | |
| SVF Z1Y | 65.92 | 5.45 | 9.62 | 18.22 | 99.21 | 0.64 | |
| SVF Z1X | 67.06 | 4.89 | 10.31 | 18.19 | 100.45 | 0.68 | |
| SVF Z1Y | 67.12 | 4.59 | 10.44 | 18.19 | 100.34 | 0.69 | |
| SVF Z1T | 67.23 | 4.71 | 10.06 | 18.00 | 100.00 | 0.68 | |
| SVF Z1S | 66.97 | 4.67 | 10.02 | 17.91 | 99.57 | 0.68 | |
| Zak 11 | 61.10 | 11.00 | 7.80 | 18.30 | 98.20 | 0.41 | |
| Zak 12 | 61.50 | 10.90 | 8.10 | 18.50 | 99.00 | 0.43 | |
| Zak 13 | 61.40 | 11.20 | 8.20 | 18.60 | 99.40 | 0.42 | |
| Ideal Composition | 65.42 | 0 | 15.14 | 19.44 | 100.00 | ||
| Diaphorite | |||||||||
| Cu | Ag | Pb | Sb | S | Total | ||||
| SF 5D | 0.59 | 24.81 | 30.92 | 26.91 | 17.69 | 100.82 | |||
| SF 5F | 0.58 | 24.62 | 31.58 | 26.60 | 17.69 | 101.07 | |||
| W6CC | 0 | 25.03 | 29.43 | 26.76 | 18.33 | 99.55 | |||
| W6CB | 0 | 24.86 | 29.63 | 26.18 | 18.31 | 98.98 | |||
| Geocronite | |||||||||
| Pb | Sb | As | S | Total | |||||
| SVF 1H | 69.26 | 6.39 | 5.81 | 16.67 | 98.13 | ||||
| SVF 1K | 68.31 | 6.48 | 5.71 | 17.63 | 98.13 | ||||
| SVF 1L | 68.31 | 6.70 | 5.67 | 18.08 | 98.76 | ||||
| Zak 8 | 69.40 | 7.70 | 5.50 | 17.20 | 99.80 | ||||
| Zak 9 | 70.50 | 6.90 | 6.00 | 17.20 | 100.60 | ||||
| Zak 10 | 70.20 | 6.70 | 5.90 | 17.30 | 100.10 | ||||
| Boulangerite | |||||||||
| Ag | Zn | Pb | Sb | As | S | Total | |||
| SVF5 B1 | 0.33 | 0 | 58.30 | 24.75 | 0.20 | 17.08 | 100.33 | ||
| SVF5 B2 | 0.93 | 0 | 58.03 | 24.15 | 0.22 | 16.67 | 99.07 | ||
| SVF5 B3 | 1.27 | 0 | 58.10 | 23.90 | 0.28 | 16.45 | 98.73 | ||
| SVF5 E1 | 0.42 | 0 | 58.34 | 24.51 | 0.24 | 17.33 | 100.42 | ||
| SVF5 E2 | 0.25 | 0 | 58.54 | 24.54 | 0.00 | 17.17 | 100.25 | ||
| SVF5 H | 0.63 | 0 | 57.86 | 25.15 | 0.20 | 17.42 | 100.63 | ||
| SVF5 L | 2.98 | 0.89 | 55.79 | 25.10 | 0.00 | 16.58 | 98.36 | ||
| SVF5 L | 2.91 | 0.70 | 55.23 | 24.38 | 0.00 | 17.14 | 97.45 | ||
| W6A C | 0.89 | 0.00 | 56.58 | 24.12 | 0.93 | 17.48 | 99.11 | ||
| W6A B | 0.47 | 0 | 57.13 | 24.94 | 0.68 | 17.72 | 100.47 | ||
| W9B A | 0.96 | 0 | 56.07 | 24.62 | 0.37 | 17.98 | 99.04 | ||
| SVF5 Z | 1.80 | 0 | 56.17 | 24.73 | 0.31 | 16.99 | 98.20 | ||
| SVF5 Y | 1.02 | 0 | 56.34 | 24.75 | 0.27 | 17.62 | 98.98 | ||
| SVF5 X | 1.45 | 0 | 56.17 | 24.64 | 0.29 | 17.45 | 98.55 | ||
| Tetrahedrite | |||||||||
| Cu | Ag | Zn | Fe | Sb | As | S | Total | ||
| SVF IS | 18.68 | 26.5 | 4.36 | 2.55 | 23.70 | 2.42 | 21.37 | 99.58 | |
| Zak 1 | 23.30 | 20.60 | 4.04 | 3.54 | 14.90 | 9.20 | 23.10 | 98.68 | |
| Zak 2 | 22.90 | 22.00 | 5.57 | 3.02 | 14.60 | 8.60 | 23.30 | 99.99 | |
| Zak 3 | 23.90 | 21.10 | 4.79 | 2.88 | 14.90 | 8.30 | 23.40 | 99.27 | |
| Zak 4 | 22.10 | 23.90 | 5.05 | 3.01 | 14.70 | 8.30 | 22.70 | 99.76 | |
| Zak 5 | 22.60 | 22.50 | 4.22 | 2.83 | 14.70 | 9.10 | 22.90 | 98.85 | |
| Zak 6 | 37.50 | 2.90 | 5.00 | 2.43 | 22.40 | 4.90 | 24.40 | 99.53 | |
| Zak 7 | 40.00 | 1.70 | 4.93 | 2.87 | 13.30 | 11.20 | 26.80 | 100.80 | |
| Intergrowth Gersdorffite, Geocronite and Galena | |||||||||
| Ag | Fe | Ni | Pb | As | Sb | S | Total | ||
| SF 1ZL | 0.86 | 3.20 | 12.61 | 45.79 | 26.00 | 0.00 | 11.06 | 99.52 | |
| SF 1ZM | 0.88 | 3.76 | 13.35 | 44.58 | 27.84 | 0.84 | 12.12 | 103.37 | |
| SF 1ZN | 0.81 | 4.48 | 14.28 | 36.76 | 29.88 | 0.00 | 12.95 | 99.16 | |
| SF 1Z P | 0.57 | 7.15 | 14.09 | 39.61 | 28.58 | 0.00 | 14.64 | 104.64 | |
| Zak 22 | 0.00 | 1.35 | 3.00 | 60.40 | 11.60 | 6.30 | 16.90 | 99.55 | |
| Zak 23 | 0.05 | 1.89 | 5.00 | 68.80 | 9.50 | 0.20 | 14.10 | 99.54 | |
| Gersdorffite | |||||||||
| Fe | Ni | Cd | As | S | Total | ||||
| SVF 1ZA. | 16.60 | 18.99 | 0.42 | 38.77 | 24.21 | 98.99 | |||
| SVF 1ZB | 15.09 | 21.06 | 0 | 41.56 | 22.67 | 100.38 | |||
| SVF lZW | 14.32 | 22.03 | 0 | 45.35 | 19.92 | 101.62 | |||
| SVF 1ZU | 14.07 | 22.29 | 0 | 45.63 | 19.20 | 101.19 | |||
| Zak 14 | 14.30 | 19.50 | 0 | 43.50 | 20.20 | 97.50 | |||
| Zak 15 | 8.60 | 25.90 | 0 | 45.60 | 19.50 | 99.60 | |||

References
- Derry, D.R.; Clark, G.R.; Gillatt, N. The Northgate Base-Metal Deposit at Tynagh, County Galway, Ireland—A Preliminary Geological Study. Econ. Geol. 1965, 60, 1218–1237. [Google Scholar] [CrossRef]
- Ashton, J.H.; Andrew, C.J.; Hitzman, M.W. Irish-type Zn-Pb deposits—What are they and can we find more? In Irish-Type Deposits Around the World; Andrew, C.J., Hitzman, M.W., Stanley, G., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 2023; pp. 95–146. [Google Scholar] [CrossRef]
- Exploration and Mining Division. Zinc and Lead in Ireland; Department of Communications, Climate Action and Environment: Dublin, Ireland, 2016; 6p.
- Hitzman, M.W.; Large, D. Carbonate-hosted base metal deposits. In Geology and Genesis of Mineral Deposits in Ireland; Andrew, C.J., Crowe, R.W.A., Finlay, S., Pennell, W.M., Pyne, J.F., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 1986; pp. 217–238. [Google Scholar]
- Andrew, C.J. Irish Zn-Pb deposits—A review of the evidence for the timing of mineralization. Constraints of stratigraphy and basin development. In Irish-Type Deposits Around the World; Andrew, C.J., Hitzman, M.W., Stanley, G., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 2023; pp. 169–210. [Google Scholar] [CrossRef]
- Phillips, W.E.A.; Stillman, C.J.; Murphy, T. A Caledonian plate tectonic model. J. Geol. Soc. Lond. 1976, 132, 579–609. [Google Scholar] [CrossRef]
- Beamish, D.; Smythe, D.K. Geophysical images of the deep crust: The Iapetus suture. J. Geol. Soc. Lond. 1986, 143, 489–497. [Google Scholar] [CrossRef]
- Freeman, B.; Klemperer, S.L.; Hobbs, R.W. The deep structure of northern England and the Iapetus Suture zone from BIRPS deep seismic reflection profiles. J. Geol. Soc. Lond. 1988, 145, 727–740. [Google Scholar] [CrossRef]
- Chadwick, R.A.; Holliday, D.W. Deep crustal structure and Carboniferous basin development within the Iapetus convergence zone, northern England. J. Geol. Soc. Lond. 1991, 148, 41–53. [Google Scholar] [CrossRef]
- Todd, S.P.; Murphy, F.C.; Kennan, P.S. On the trace of the Iapetus suture in Ireland and Britain. J. Geol. Soc. Lond. 1991, 148, 869–880. [Google Scholar] [CrossRef]
- Vaughan, A.; Johnston, J.D. Structural constraints on closure geometry across the Iapetus suture in eastern Ireland. J. Geol. Soc. Lond. 1992, 149, 65–74. [Google Scholar] [CrossRef]
- McConnell, B.; Riggs, N.; Fritschle, T. Tectonic history across the Iapetus suture zone in Ireland. In Pannotia to Pangaea: Neoproterozoic and Paleozoic Orogenic Cycles in the Circum-Atlantic Region; Murphy, J.B., Strachan, R.A., Quesada, C., Eds.; Special Publication 503; Geological Society: London, UK, 2020; pp. 333–345. [Google Scholar]
- Lees, A.; Miller, J. Waulsortian banks. In Carbonate Mudmounds; Monty, C.L.V., Bosence, D.W.J., Bridges, P.H., Pratt, B.R., Eds.; International Association of Sedimentologists Special Publication 23; Blackwell Science: Oxford, UK, 1995; pp. 191–271. [Google Scholar]
- Wilkinson, J.J.; Hitzman, M.W. The Irish Zn-Pb Orefield: The View from 2014. In Current Perspectives on Zinc Deposits; Archibald, S.M., Piercey, S.J., Eds.; Special Publication; Irish Association of Economic Geology: Dublin, Ireland, 2015; pp. 59–72. [Google Scholar]
- Gagnevin, G.; Boyce, A.J.; Barie, C.D.; Menuge, J.F.; Blakeman, R.J. Zn, Fe and S fractionation in a large hydrothermal system. Geochim. Cosmochim. Acta 2012, 88, 183–198. [Google Scholar] [CrossRef]
- Wilkinson, J.J.; Eyre, S.L.; Boyce, A.J. Ore-forming processes in Irish-type carbonate-hosted Zn–Pb deposits: Evidence from mineralogy, chemistry, and isotopic composition of sulfides at the Lisheen Mine. Econ. Geol. 2005, 100, 63–86. [Google Scholar] [CrossRef]
- Taylor, S.; Andrew, C.J. Silvermines orebodies, Co. Tipperary, Ireland. Trans. Inst. Min. Metall. Sect. B-Appl. Earth Sci. 1978, 87, B111–B124. [Google Scholar]
- Andrew, C.J. The tectono-stratigraphic controls to mineralization in the Silvermines area, County Tipperary, Ireland. In Geology and Genesis of Mineral Deposits in Ireland; Andrew, C.J., Crowe, R.W.A., Finlay, S., Pennell, W., Pyne, J.F., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 1986; pp. 377–417. [Google Scholar] [CrossRef]
- Taylor, S. Structural and palaeotopographic controls of lead-zinc mineralization in the Silvermines orebodies. Repub. Irel. Econ. Geol. 1984, 79, 529–548. [Google Scholar] [CrossRef]
- Rutty, J. An Essay Towards a Natural History of the County of Tipperary; W. Sleater: Dublin, Ireland, 1772; Volume 2. [Google Scholar]
- Wynne, A.B.; Kane, G.H. Memoir to Accompany 1” Sheet No. 154; Memoir Geological Survey of Ireland: Dublin, Ireland, 1861; 52p. [Google Scholar]
- Apjohn, J. On the occurrence of electric calamine at the Silver Mines, County of Tipperary. J. Geol. Soc. Dublin 1860, 8, 157. [Google Scholar]
- Rhoden, N.H. Structure and Economic Mineralization of the Silvermines District, Co. Tipperary, Eire. Trans. Inst. Min. Metall. 1959, 68, 67–94. [Google Scholar]
- Boland, M.B.; Clifford, J.A.; Meldrum, A.H.; Poustie, A. Residual Base Metal and Barite Mineralization at Silvermines, Co. Tipperary, Ireland. In The Irish Minerals Industry 1980–1990; Bowden, A.A., Earls, G., O’Connor, P.G., Pyne, J.F., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 1992; pp. 247–260. [Google Scholar]
- Lee, M.J.; Wilkinson, J.J. Cementation, hydrothermal alteration, and Zn-Pb mineralization of carbonate breccias in the Irish Midlands: Textural evidence from the Cooleen Zone, near Silvermines, County Tipperary. Econ. Geol. 2002, 97, 653–662. [Google Scholar] [CrossRef]
- Kyne, R.; Torremans, K.; Güven, G.; Doyle, D.; Walsh, J. 3-D Modeling of the Lisheen and Silvermines Deposits, County Tipperary, Ireland: Insights into Structural Controls on the Formation of Irish Zn-Pb Deposits. Econ. Geol. 2019, 114, 93–116. [Google Scholar] [CrossRef]
- Graham, R.A.F. The Mogul Base Metal Deposits, Co. Tipperary, Ireland. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 1970. Unpublished. [Google Scholar]
- Carson, D. Mogul of Ireland Silver Recoveries; Internal Memo, Mogul of Ireland Ltd.: Park City, UT, USA, 1978; p. 4. [Google Scholar]
- Carson, D. Mogul of Ireland Silver Recoveries; Internal Memo, Mogul of Ireland Ltd.: Park City, UT, USA, 1979; p. 6. [Google Scholar]
- Moreton, S. The Silvermines District—Co Tipperary, Ireland: The Mineralogical Record; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 99–106. [Google Scholar]
- Zakrzewski, M.A. Members of the freibergite-argentotennantite series and associated minerals from Silvermines, County Tipperary, Ireland. Mineral. Mag. 1989, 53, 293–298. [Google Scholar] [CrossRef]
- Gasparrini, C. Identification and Description of the Silver-Bearing Minerals in Ten Sulphide Samples from Ireland; Confidential Report Prepared for Noranda Mines Ltd.: Toronto, ON, Canada; MinMet Scientific: Toronto, ON, Canada, 1978. [Google Scholar]
- Hall, A.J.; Pattrick, R.A.; Russell, M.J. Economic Implications of the Origin of the Silver-Rich Mass in ‘B Zone’ (Room 4611) Silvermines: Confidential Report Prepared for Mogul of Ireland Ltd. by Dept. of Applied Geology; University of Strathclyde, Scotland: Glasgow, UK, 1978. [Google Scholar]
- Pattrick, R. Internal Correspondence to Mogul of Ireland Dated 21 October 1981, 6 pages.
- Ashton, J.H. A Geological Study of the B Zone Base Metal Deposit at the Mogul of Ireland Mine, Silvermines, Eire. Bachelor’s Thesis, University of London, London, UK, 1975. Unpublished. [Google Scholar]
- Reed, C.P.; Wallace, M.W. Zn–Pb mineralization in the Silvermines district, Ireland: A product of burial diagenesis. Miner. Depos. 2004, 39, 87–102. [Google Scholar] [CrossRef]
- Andrew, C.J. Investigation into anomalous silver values in head grade: Mogul of Ireland Internal Memo, 15th July 1978.
- Rajabi, A.; Mahmoodi, P.; Alfonso, P.; Canet, C.; Andrew, C.; Azhdari, S.; Rezaei, S.; Alaminia, Z.; Tamarzadeh, S.; Yarmohammadi, A.; et al. Barite Replacement as a Key Factor in the Genesis of Sediment-Hosted Zn-Pb±Ba and Barite-Sulfide Deposits: Ore Fluids and Isotope (S and Sr) Signatures from Sediment-Hosted Zn-Pb±Ba Deposits of Iran. Minerals 2024, 14, 671. [Google Scholar] [CrossRef]
- Eyre, S.L. Geochemistry of Dolomitization and Zn–Pb Mineralization in the Rathdowney Trend, Ireland. Ph. D. Thesis, University of London, London, UK, 1998; 418p. Unpublished. [Google Scholar]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Tamas, C.G.; Bailly, L.; Ghergari, L.; O’Connor, G.; Minut, A. New occurrences of tellurides and argyrodite in Rosia Montana, Apuseni Mountains, Romania, and their metallogenetic significance. Can. Mineral. 2006, 44, 367–383. [Google Scholar] [CrossRef]
- Johan, Z.; Oudin, E. Présence de grenats, Ca3Ga2(GeO4)3, Ca3Al2((Ge, Si)O4)3 et d’un équivalent ferrifère, germanifère et gallifère de la sapphirine, Fe4(Ga, Sn, Fe)4(Ga, Ge)6O20, dans la blende des gisements de la zone axiale pyrénéenne. Cond. Form. Phases Ger. Gall. Comptes Rendus L’acad. Des Sci. Ser. IIA Earth Planet. Sci. 1986, 303, 811–816. [Google Scholar]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- So, C.S.; Yun, S.T.; Choi, S.G. Occurrence and geochemistry of argyrodite, a germanium-bearing mineral (Ag8Ge6) from the Weolyu Ag-Au hydrothermal vein deposits. Korean Soc. Nat. Resour. Environ. Geol. Resour. Environ. Geol. 1993, 26, 117–127. [Google Scholar]
- Zhuravkova, T.V.; Palyanova, G.A.; Kravtsova, R.V. Physicochemical formation conditions of silver sulfoselenides at the Rogovik deposit, Northeastern Russia. Geol. Ore Depos. 2015, 57, 313–330. [Google Scholar] [CrossRef]
- Pažout, R.; Sejkora, J.; Šrein, V. Ag-Pb-Sb Sulfosalts and Se-rich Mineralization of Anthony of Padua Mine near Poličany—Model Example of the Mineralization of Silver Lodes in the Historic Kutná Hora Ag-Pb Ore District, Czech Republic. Minerals 2019, 9, 430. [Google Scholar] [CrossRef]
- Martin, D.K. Die Himmelsfürst-Fundgrube hinter Erbisdorf bei Freiberg/Sachsen. Aufschluss 1970, 21, 332–337. [Google Scholar]
- Burisch, M.; Hartmann, A.; Bach, W.; Krolop, P.; Krause, J.; Gutzmer, J. Genesis of hydrothermal silver-antimony-sulfide veins of the Bräunsdorf sector as part of the classic Freiberg silver mining district, Germany. Miner. Depos. 2019, 54, 263–280. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Wei, J.; Ulrich, T. Mineralization processes at the Daliangzi Zn-Pb deposit, Sichuan-Yunnan-Guizhou metallogenic province, SW China: Insights from sphalerite geochemistry and zoning textures. Ore Geol. Rev. 2023, 161, 105654. [Google Scholar] [CrossRef]
- Samson, I.M.; Russell, M.J. Fluid inclusion data from the Silvermines Zn + Pb + BaSO4 deposits, Ireland. Inst. Min. Metall. Trans. 1983, 92, 67–71. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrew, C.J.; Ashton, J.H. The B-Zone 4611 Silver-Rich Pod—An Unusual Ag-Ge-Sb-As-Ni Assemblage Within the Irish-Type Zn-Pb Silvermines Deposit, County Tipperary, Ireland. Minerals 2025, 15, 540. https://doi.org/10.3390/min15050540
Andrew CJ, Ashton JH. The B-Zone 4611 Silver-Rich Pod—An Unusual Ag-Ge-Sb-As-Ni Assemblage Within the Irish-Type Zn-Pb Silvermines Deposit, County Tipperary, Ireland. Minerals. 2025; 15(5):540. https://doi.org/10.3390/min15050540
Chicago/Turabian StyleAndrew, Colin J., and John H. Ashton. 2025. "The B-Zone 4611 Silver-Rich Pod—An Unusual Ag-Ge-Sb-As-Ni Assemblage Within the Irish-Type Zn-Pb Silvermines Deposit, County Tipperary, Ireland" Minerals 15, no. 5: 540. https://doi.org/10.3390/min15050540
APA StyleAndrew, C. J., & Ashton, J. H. (2025). The B-Zone 4611 Silver-Rich Pod—An Unusual Ag-Ge-Sb-As-Ni Assemblage Within the Irish-Type Zn-Pb Silvermines Deposit, County Tipperary, Ireland. Minerals, 15(5), 540. https://doi.org/10.3390/min15050540






