Abstract
The ammonium bicarbonate precipitation method has become one of the main ways to extract rare earths from rare earth leaching solutions due to its advantages of simple operation, mature technology, and low cost. Therefore, the objective of this study was to explore the precipitation of rare earth carbonate (REC) from a concentrated rare earth and magnesium sulfate solution by ammonium bicarbonate. The terminal pH to precipitate rare earth was determined. The effects of factors including the feeding rate, temperature, ammonium bicarbonate concentration, seed dosage, and stirring rate on the rare earth precipitation efficiency, the Mg and SO42− amounts in the obtained REC, and the median particle sizes of the REC were investigated. The effects of these factors on the Mg and SO42− amounts in the REC and the median particle sizes of the REC were compared by one-way analysis of variance (ANOVA). The results showed that at a terminal pH of 6.8, a feeding rate of 0.5 mL/min, a temperature of 25 °C, an ammonium bicarbonate concentration of 0.6 mol/L, a crystal seed dosage of 7.5%, and a stirring speed of 300 r/min, without aging, the contents of rare earth oxide (REO), MgO, and SO42− in the REC were 57.69%, 0.23%, and 1.68%, respectively, superior to the requirements of the current Chinese national standard (GB/T 16479-2020) for REC. The rare earth precipitation efficiency was 99.78%. Among the investigated factors, the pH was the main factor affecting the rare earth precipitation efficiency. The seed dosage significantly changed the median particle sizes of the REC and the Mg content of the REC. For SO42−, its content was fluctuant and could be controlled at a relatively low value by the seed dosage and specific stirring rate.
1. Introduction
Rare earth elements (REES) are important strategic resources and play a unique role in high-tech and new material industries. In industrial applications, REES are typically classified into three categories: light, medium, and heavy [1,2,3]. In particular, medium and heavy REES are characterized by limited reserves, high demand, and lack of substitutes, receiving widespread attention. Ion-adsorbed rare earth ore, wherein rare earth minerals occur in a simple trivalent cationic state adsorbed onto clays and can be extracted via a simple ion-exchange leaching process, is a significant source of medium and heavy rare earth resources, supplying more than 80% of the world’s total medium and heavy REEs [4,5,6].
The mining of ion-adsorbed rare earth ore has, for an extended period, been conducted predominantly with ammonium salt [7,8]. However, this process is associated with significant environmental problems, including the contamination of water sources with ammonia–nitrogen eutrophication. In addition, the subsequent treatment of the ammonia–nitrogen leaching tail solution represents a significant financial burden for production enterprises [9,10]. In light of these issues, Chinese researchers have developed a variety of non-ammonium leaching agents, including MgSO4, CaCl2, and Al2(SO4)3, to achieve efficient and green mining of ion-adsorbed rare earth minerals, effectively solving the problem of ammonia–nitrogen pollution [11,12,13]. Among them, magnesium salt (MgSO4) has a significant advantage in terms of the cost, a wide range of sources, and being friendly to the environment, and it has been used in industry. The resulting rare earth leaching solution, with a low concentration of REEs and impurities, should be purified and enriched step by step to finally prepare a concentrated and pure rare earth solution for solvent extraction and separation of REEs [14,15,16].
During the purification and enrichment process, precipitating REEs is the most important and simplest method of turning low-concentration REEs into high-concentration REEs. Oxalic acid, carbonate, alkali, sodium sulfate and phosphate have been used to precipitate REEs [17,18]. Among them, oxalic acid and ammonium bicarbonate are mainly used in industry applications, since other types of rare earth precipitate such as sodium and rare earth double salt are difficult to treat. The precipitation rate of rare earth oxalate can reach more than 98% when the pH is 1.5–3, and REEs can be selectively precipitated and separated from impurities, which can further improve the purity of rare earth oxalate [19,20]. However, there are several problems with this method. First, the oxalate reagent is an expensive substance that has a certain impact on the environment due to its toxicity and strong corrosion. In addition, after the precipitation, excess oxalic acid remains in the mother solution, necessitating further treatment [21]. Compared with oxalic acid precipitation, ammonium bicarbonate precipitation has the advantages of low cost, nontoxicity, a high rare earth precipitation rate, and easy treatment. Meanwhile, the excess ammonium in the solution can be recycled and reused in the distillation process.
In the traditional ionic rare earth enrichment process, ammonium bicarbonate is often directly reacted with the low-concentration rare earth leaching solution from mining. Although the operation is simple, there are certain limitations. First of all, the production efficiency is low: the low concentration of rare earth leads to the kinetic hysteresis of the rare earth separation reaction, the precipitation time is prolonged, and the equipment utilization is insufficient, which increases the energy consumption and investment cost. Secondly, the waste of resources is serious: excessive dilution of water and repeated addition of ammonium bicarbonate cause the reagent utilization rate to be less than 50%. Thirdly, it increases the cost of water treatment: a large amount of low-concentration ammonia and nitrogen wastewater is generated, increasing the water treatment volume. Finally, REC is unstable in quality: slow precipitation exacerbates the co-precipitation of impurities such as Fe3+, Al3+, and Mg2+, and it is prone to fine particles or fluffy flocs, leading to difficulties in filtration [22,23,24]. In contrast, the use of ammonium bicarbonate to precipitate REC from a high-concentration rare earth solution can effectively solve the above outstanding problems, increase the production efficiency of REC, solve the problem of water consumption, and improve the quality of REC products.
In our previous study, the enrichment of REEs from a low-concentration rare earth leaching solution from mining by MgO precipitation was performed and sulfuric acid leaching of the rare earth enrichment followed by a neutralization and aluminum removal process was investigated to obtain a concentrated and purified ionic rare earth solution [25]. However, except for the REEs, the obtained rare earth solution contains a large amount of Mg2+ and SO42−. The effect of the precipitation process on the purity of REC remains to be explored. Therefore, in this study, the thermodynamic species distribution and precipitation trends of the main REEs (La, Y, Nd) and Mg in the high-concentration rare earth leaching solution were discussed. In particular, the influence of various parameters on the median particle size of REC, the impurity content of REC, and the rare earth precipitation efficiency were investigated. Meanwhile, the effects of factors on the Mg and SO42− amount and the median particle size of REC were compared by one-way ANOVA. The objective of this study was to obtain high-purity REC with large particles from a concentrated magnesium sulfate solution, providing guidance for industrial applications.
2. Experiment
2.1. Materials
The raw material used in this study was rare earth leaching solution from ionic rare earth enrichment, which was provided by a rare earth smelting enterprise in Ganzhou City, Jiangxi Province, China after the acid leaching and aluminum removal process [25]. The initial pH of the rare earth leaching solution was about 4.7. The main chemical composition is shown in Table 1, and the distribution of REEs is shown in Table 2. Ammonium bicarbonate (99.9%, Macklin reagent, Shanghai, China) was used in the experiments.

Table 1.
Composition of the rare earth leaching solution (g/L).

Table 2.
The partitioning of REEs in the rare earth leaching solution (%/TREO).
2.2. Methods
In this study, the approach of parallel flow feeding was used to precipitate REC, as shown in Figure 1. First, 200 mL of rare earth leaching solution and a certain volume of ammonium bicarbonate solution were, respectively, prepared and added by a peristaltic pump at a pre-set feeding rate when the reaction started. The feeding rate of the rare earth leaching solution was one of the factors to be investigated, while the feeding rate of the ammonium bicarbonate solution was changed with changes to the pH of the reaction solution, because the ammonium bicarbonate solution was seen as a pH regulator to make sure that the pH in the reaction solution reached the pre-set stable value. Before the reaction, 20 mL of deionized water was put into a 500 mL beaker as the bottom water to help the rare earth leaching solution and ammonium bicarbonate solution mix completely during the initial reaction period. The beaker was placed in a constant-temperature water bath at a pre-set temperature. Except for the experiment involving the stirring rate, the rotational speed was maintained at 300 rpm for the other experiments, and the agitation was stopped when the reaction solution reached the pre-set stable pH value. Without aging, the resulting solution was filtrated to obtain REC, which was washed 3 times with pure water and dried for characterization.
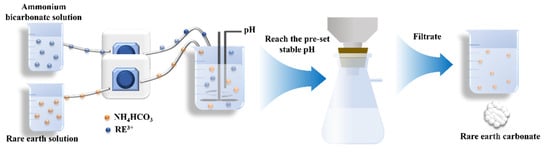
Figure 1.
Experimental flow chart.
2.3. Characterization
The EDTA titration method was used to determine the concentration of total REEs in the rare earth leaching solution before and after the precipitation process [26]. Before analyzing the amount of non-REE impurities and REEs in the REC, the REC was dissolved completely by HCl. The concentration of non-REE impurities in the REC was detected by atomic absorption spectroscopy (Agilent 240Z AA, Agilent Technologies, Santa Clara, CA, USA), and the concentration of REEs was determined by the EDTA titration method. The amount of SO42− in the REC was analyzed by infrared absorption spectroscopy (USCS-844). A PHS-3C pH meter (Mettler-Toledo Technology (China), Shanghai, China) was used to measure the pH values of the rare earth leaching solution. The REC was characterized by X-ray diffraction (Rigaku D/max2550VB 18 Kw, Rigaku Corporation, Tokyo, Japan), scanning electron microscopy (SEM) (MLA 650F, FEI Company, Hillsboro, OR, USA), and infrared spectroscopy (Magna IR 750, Thermo Nicolet Corporation, Madison, WI, USA). The particle size of the REC was tested using a laser particle size analyzer (Mastersizer3000, Malvern Panalytical, Malvern, UK).
3. Results and Discussion
3.1. Species Distribution of La, Nd, Y, and Mg in SO42−–CO32−–H2O Solution System
In the precipitation of REEs by ammonium bicarbonate, the pH is a significant factor influencing the existence states of the REEs and Mg in the solution, thereby affecting the content of impurities in the REC and the precipitation rate of the REC. To provide a theoretical basis for the subsequent efficient precipitation and purification of REC, the software Visual MINTEQ 3.1 was used to calculate the species distribution of the main REEs, represented by La, Nd, Y, and Mg, in the SO42−–CO32−–H2O solution system. The concentrations of La, Nd, Y, Mg, SO42−, and CO32− were set according to those in the rare earth leaching solution. The results are shown in Figure 2.
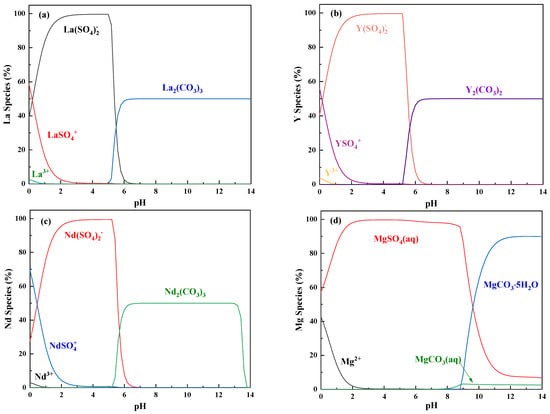
Figure 2.
Species distribution of La in the SO42−–CO32−–H2O solution system. (a) La: 0.1 mol/L; (b) Y: 0.15 mol/L; (c) Nd: 0.1 mol/L; and (d) Mg: 0.5 mol/L; SO42−: 1.75 mol/L; CO32−: 0.6 mol/L.
As shown in Figure 2a–c, the La, Nd, and Y in the solution behaved similarly, existing in four main forms: RESO4+, RE(SO4)2−, RE3+, and RE2(CO3)3. When the pH was less than 5, RE3+ could combine with SO42− to form RESO4+ and RE(SO4)2−. When the pH was increased from 0 to 3, the content of RESO4+ dramatically decreased, while that of RE(SO4)2− significantly increased at the same time. A low amount of free RE3+ existed when the pH was below 1, and RE(SO4)2− was the predominant species in the pH range of 3 and 5. When the pH was greater than 5, the REEs started to precipitate in the form of RE2(CO3)3, which became the predominant species when the pH was above 6. For the impurity Mg, as seen in Figure 2d, when the pH was less than 3, its main forms were Mg2+ and aqueous MgSO4. When the pH was increased from 0 to 3, the amount of Mg2+ decreased, while the amount of aqueous MgSO4 increased. When the pH was in the range of 3 and 8, aqueous MgSO4 accounted for 100% of the Mg species. As the pH reached and exceeded 8, aqueous MgCO3 started to appear as a small amount less than 5% of all the Mg species. Meanwhile, MgCO35H2O precipitated and became the predominant species. Above all, it can be concluded that changing the pH in the range of 6 and 8 can realize selective precipitation of REEs from aqueous MgSO4 thermodynamically.
3.2. Determination of the Terminal pH of the Precipitation Reaction
According to the above research on the precipitation thermodynamics, a pH in the range of 6 and 8 was the optimal condition to precipitate REC. To determine the actual reaction pH, the initial precipitation pH was set to 6 in the experiment. The conditions included a reaction temperature of 35 °C, a feeding rate of 1.5 mL/min, and an ammonium bicarbonate concentration of 1 mol/L. The results are shown in Figure 3.
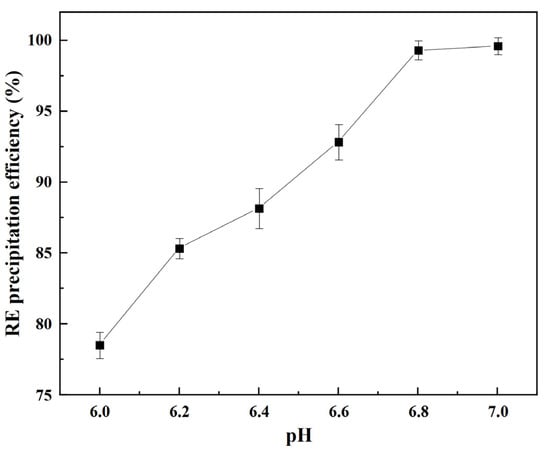
Figure 3.
Rare earth precipitation efficiency under different pH conditions.
As shown in Figure 3, the precipitation efficiency of the REEs continuously increased with an increasing pH. When the pH was 6, the precipitation efficiency of the REEs was 78.54%. With the continuous addition of ammonium bicarbonate to the solution, the pH of the system continued to rise, and when the pH increased to 6.8, the precipitation efficiency of the REEs reached 99.63%. Under this condition, the REEs in the solution were basically completely precipitated. The precipitation of REC can be expressed by Formula (1) [27]. During the precipitation process, HCO3− entered the solution system and dissociated CO32− and H+, rare earth ions combined with CO32− to form REC, and H+ easily reacted with HCO3− to release CO2. When the pH was high, the complete precipitation of REC could be ensured. Therefore, to ensure a high precipitation efficiency of REC and avoid an excessive dosage of ammonium bicarbonate, a precipitation pH of 6.8 was the best choice.
3.3. Effect of Factors on the Impurity Content and Particle Size of REC
3.3.1. Effect of Feeding Rate Under Parallel Flow
During the precipitation process, different feeding rates of ammonium bicarbonate solution and rare earth leaching solution affect the supersaturation of the precipitation system, thus affecting the nucleation, growth rate, and impurity content of REC [21]. Therefore, the changes in the content of Mg and SO42− in the REC and the particle size of the REC were investigated at different concurrent feeding rates. The experiments were carried out with a terminal precipitation pH of 6.8, a reaction temperature of 35 °C, and an ammonium bicarbonate concentration of 1 mol/L. Here, the feeding rate of the ammonium bicarbonate solution changed with changes in the pH of the reaction solution, because ammonium bicarbonate solution was seen as a pH regulator to make sure that the pH of the reaction solution reached the pre-set stable value. Thus, the feeding rate of the rare earth solution was seen as the variable. The results are shown in Figure 4 and Figure 5.
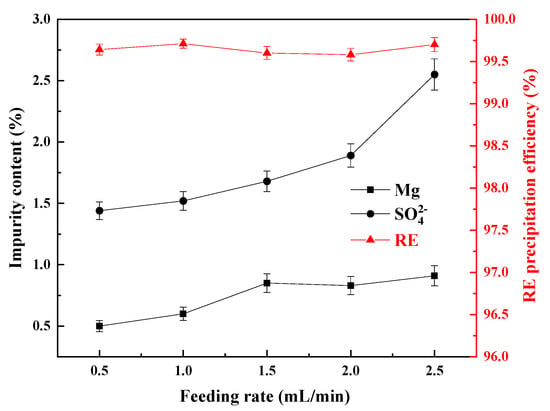
Figure 4.
Influence of the feeding rate on the impurity content of REC and the RE precipitation efficiency.
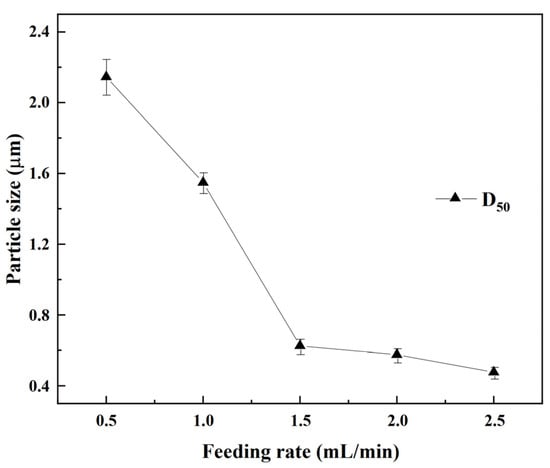
Figure 5.
Particle size of REC at different feeding rates.
From Figure 4, the rare earth (RE) precipitation efficiencies could reach more than 99.50% at different feeding rates, and the content of Mg in REC gradually increased when increasing the feeding rate. When the feeding rate increased from 0.5 mL/min to 1.5 mL/min, the content of Mg increased from 0.44% to 0.85%; with a further increase of the feeding rate, the content of Mg in the REC exhibited little change. The content of SO42− in the REC increased rapidly when increasing the feeding rate. When the feeding rate was 0.5 mL/min, the content of SO42− was 1.44%, and when the feeding rate was further increased to 2.5 mL/min, the content of SO42− was increased to 2.55%. This illustrated that a high feeding rate can cause a high content of Mg and SO42−. It might be attributed to the fact that the continuous increase in the feeding rate will lead to an increase in the supersaturation of the solution system, accelerate nucleation, but shorten the crystal growth time, thus forming fine particles with more surface defects and promoting the adsorption or encapsulation of the impurities Mg and SO42− [28,29]. Therefore, to ensure a low content of impurities, the feeding rate should be reduced as much as possible.
The changes in the median particle size of the REC under different feeding rates are shown in Figure 5. As seen in Figure 5, when increasing the feeding rate, the median particle size of the REC gradually decreased from 2.15 μm with a feeding rate of 0.5 mL/min to 0.48 μm with a feeding rate of 2.5 mL/min. Compared to the small particles, larger particles are easier to filtrate. Therefore, to ensure the low impurity content and large particle size of REC, a feeding rate of 0.5 mL/min should be selected.
3.3.2. Effects of Temperature
Different temperatures affect the supersaturation degree of the solution and the molecular diffusion rate in the solution. When the temperature increased, the supersaturation degree of the solution was easily reduced, while the diffusion rate of the solute molecules was accelerated at the same time. When the temperature decreased, the solute diffusion rate was reduced, while the supersaturation degree of the solution could increase [21]. Therefore, the changes in the content of Mg and SO42− in the REC and the particle size of the REC at different temperatures were investigated. The precipitation endpoint pH was set at 6.8, the feeding rate was 0.5 mL/min, and the concentration of ammonium bicarbonate was 1 mol/L. The results are shown in Figure 6.
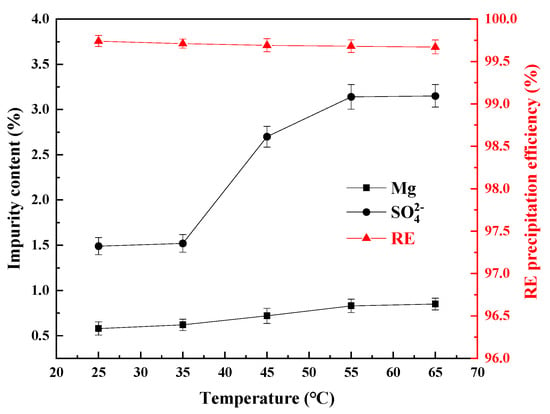
Figure 6.
Influence of the temperature on the impurity content of REC and the RE precipitation efficiency.
From Figure 6, the RE precipitation efficiencies could reach more than 99.50% at all the temperatures and the content of SO42− in the REC continued to rise with an increasing reaction temperature, while the content of Mg changed slightly. When the temperature increased from 25 °C to 65 °C, the content of Mg increased from 0.58% to 0.85%. When the temperature was higher than 35 °C, the content of SO42− in the REC began to rise significantly and reached a maximum value of 3.15% when the temperature was 65 °C. This is because the increase in the temperature will accelerate the reaction rate of ammonium bicarbonate with rare earth ions, which may lead to the fast formation of precipitated particles, forming loose or amorphous structures, thereby entailing more SO42− and Mg. At the same time, the solubility of sulfate may decrease with the increase in temperature, resulting in the co-precipitation of some SO42− and magnesium with the rare earth carbonate [30,31]. Therefore, to reduce the content of Mg and SO42− in the REC, a lower temperature should be chosen.
The changes in the median particle size of the REC under different temperatures are shown in Figure 7. As seen in Figure 7, when increasing the temperature, the median particle size of the REC increased within a limited range so that the D50 of the REC increased from 0.57 μm to 0.61 μm when the temperature increased from 25 °C to 65 °C. This indicated that the temperatures had a little effect on the particle size of the REC. Therefore, when preparing REC, to ensure its purity, 25 °C was selected as the optimal reaction temperature.
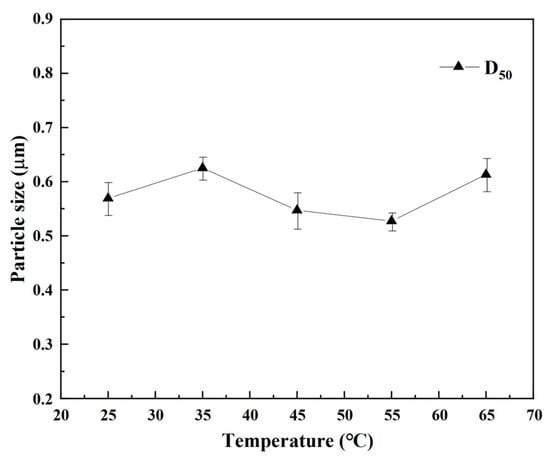
Figure 7.
Particle size of REC at different temperatures.
3.3.3. Effect of Ammonium Bicarbonate Concentration
Different concentrations of ammonium bicarbonate affect the reaction rate of the precipitation process, further affecting the precipitation of impurities and the particle size of REC. Therefore, the changes in the content of Mg and SO42− in the REC and the particle size of the REC with different ammonium bicarbonate concentrations were investigated. During the controlled precipitation process, the final pH was 6.8, the feeding rate was 0.5 mL/min, and the reaction temperature was 25 °C. The results are shown in Figure 8.
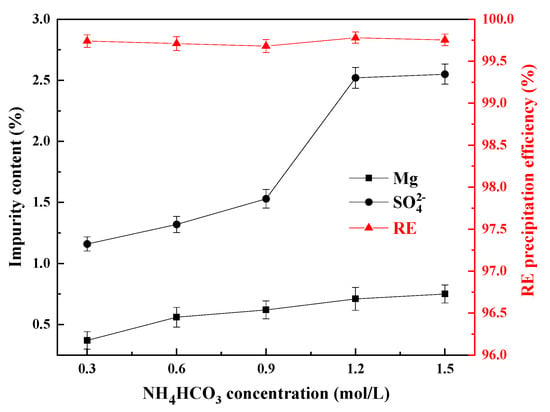
Figure 8.
Influence of the NH3HCO3 concentration on the impurity content of the REC and the RE precipitation efficiency.
From Figure 8, the RE precipitation efficiencies could reach more than 99.50% at all the ammonia bicarbonate concentrations, and when the initial concentration of ammonium bicarbonate was 0.3 mol/L, the contents of Mg and SO42− reached the lowest values of 0.37% and 1.16%, respectively. As the concentration of ammonium bicarbonate continued to increase, the contents of Mg and SO42− continued to increase at the same time. When the concentration of ammonium bicarbonate reached 1.5 mol/L, the contents of Mg and SO42− increased to 0.75% and 2.55%, respectively. This is because as the concentration of ammonium bicarbonate increases, the solution system is prone to local supersaturation, which can accelerate the nucleation of rare earth carbonate and shorten its crystal growth time. During this process, it is easy to encapsulate Mg and SO42−, and the result is consistent with the effect of increasing the feeding rate. Therefore, in order to reduce the content of impurities in REC as much as possible, the concentration of ammonium bicarbonate should be low.
The changes in the median particle size of the REC under different concentrations of ammonium bicarbonate are shown in Figure 9. As seen in Figure 9, with increasing concentrations of ammonium bicarbonate, the particle size of the REC decreased continuously, and the D50 of REC decreased from 3.15 μm to 0.42 μm. To obtain a large particle size of REC, the concentration of ammonium bicarbonate should also be low. However, in the experiment, the low-concentration ammonium bicarbonate solution changed the pH slowly, and when considering shortening the reaction time, 0.6 mol/L was selected.
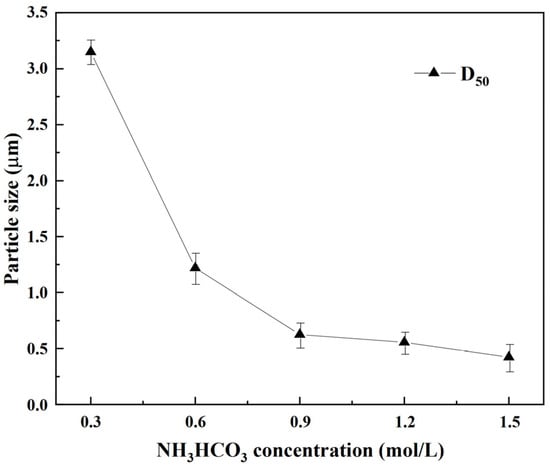
Figure 9.
Particle size of REC at different ammonium bicarbonate concentrations.
3.3.4. Effect of Crystal Seed Dosage
During the precipitation and crystallization process, the addition of crystal seeds can provide the crystal nucleus for crystal growth, helping the crystalline ions directly form directional growth on the crystal seeds to achieve rapid crystallization, thus increasing the particle size of REC [21]. Therefore, the changes in the content of Mg and SO42− in the REC and the particle size of the REC with different amounts of crystal seeds were investigated. In the experiment, the obtained REC from the above optimal conditions was seen as the crystal seed to be added. During the precipitation process, the final pH was 6.8, the feeding rate was 0.5 mL/min, the ammonium bicarbonate concentration was 0.6 mol/L, and the reaction temperature was 25 °C. The results are shown in Figure 10.
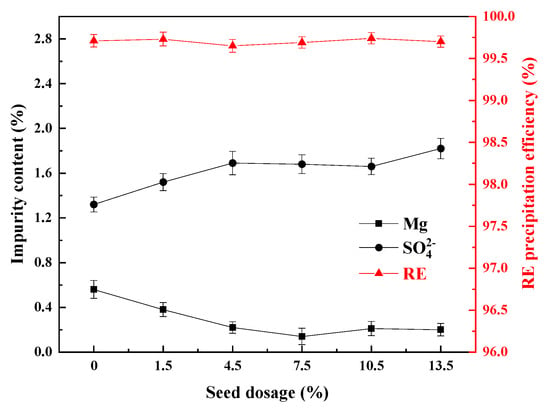
Figure 10.
Influence of the crystal seed dosage on the impurity content of the REC and the RE precipitation efficiency.
From Figure 10, the RE precipitation efficiencies could reach more than 99.50% under the addition amounts of all the crystal seeds, and the content of Mg in the REC gradually decreased when increasing the amount of crystal seeds, from 0.54% without crystal seeds to 0.14% when the dosage of crystal seeds was 7.5%, and after further adding crystal seeds, the content of Mg tended to be stable. By contrast, the content of SO42− in the REC did not change significantly when increasing the amount of crystal seeds. When the amount of crystal seeds was increased to 13.5%, the content of SO42− in the REC reached the maximum value of 1.75%. Nevertheless, compared to the above other factors, the dosage of seeds reduced the amount of the Mg and SO42− in the REC. This is because the seed crystal acts as a nucleation site, reducing the supersaturation of the system and stabilizing the crystal growth rate, forming more complete particles with fewer surface defects, thereby reducing the adsorption sites of Mg and SO42− [32].
The changes in the median particle size of the REC under different amounts of crystal seeds are shown in Figure 11. As seen in Figure 11, the dosage of crystal seeds significantly affected the particle size of the REC. With increasing amounts of crystal seeds, the particle size of the REC gradually increased. The D50 of the REC without the addition of crystal seeds was 1.25 μm, and it was increased to 11.37 μm when the crystal seed dosage was increased to 7.5%. When the crystal seed dosage was further increased to 13.5%, the particle size of the REC reached the highest value of 14.68 μm. This indicated that the addition of crystal seeds could greatly promote the crystallization reaction and increase the crystallization rate. Therefore, to reduce the content of impurities in REC and obtain REC with large particles, the crystal seed dosage of 7.5% should be selected.
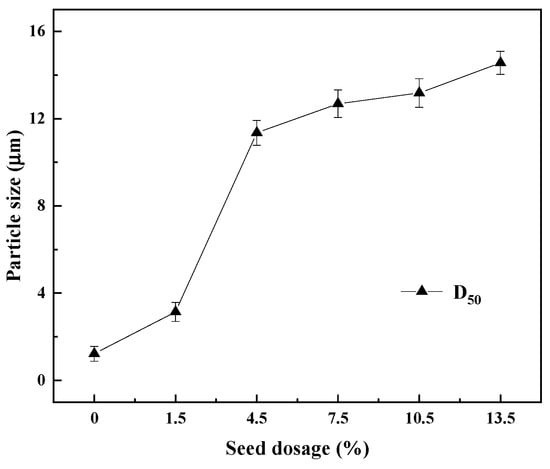
Figure 11.
Particle size of REC with different amounts of crystal seeds.
3.3.5. Effect of Stirring Rate
The stirring rate can affect the supersaturation distribution and ion diffusion in the reaction solution [21]. The changes of the Mg and SO42− in the REC and the median particle size of the REC at different stirring speeds were investigated. During the precipitation process, the final pH was controlled at 6.8, the feeding rate was 0.5 mL/min, the reaction temperature was 25 °C, and the dosage of crystal seed was 7.5%. The results are shown in Figure 12.
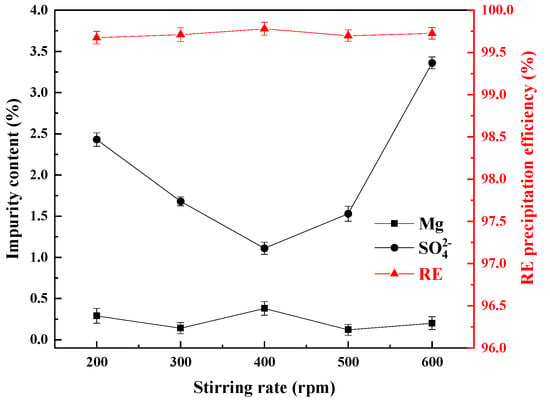
Figure 12.
Influence of different stirring rates on the impurity content of the REC and the RE precipitation efficiency.
As can be seen from Figure 12, the RE precipitation efficiencies could reach more than 99.50% at all the rotational speeds, and with the continuous increase of the stirring speed, the Mg content in the REC had a small variation range, mainly fluctuating between 0.12% and 0.38%, while the SO42− content in the rare earth carbonate showed a trend of first decreasing and then increasing with the increase of the stirring speed. When the initial rotation speed was 200 rpm, the contents of Mg and SO42− were 0.29% and 2.43%, respectively. When the stirring speed was increased to 400 rpm, the content of Mg increased to 0.38%, and the content of SO42− reached the lowest value of 1.11%. When the stirring speed was increased to 500 rpm, the content of Mg decreased to 0.12%. The content of SO42− was 1.53%, and when the stirring speed was increased to 600 rpm, the content of Mg changed little, and the content of SO42− increased to 3.36%. This is because moderate stirring can enhance the mixing uniformity of the reaction system, reduce the local concentration gradients, and avoid sulfate ions being trapped inside the crystal due to local supersaturation. However, excessively high stirring speeds (such as >400 rpm) can cause crystal breakage, resulting in the formation of small particles and new surfaces, increasing the adsorption sites of sulfate ions. In addition, micro-crystals formed by secondary nucleation may encapsulate more SO₄²⁻ [33]. Therefore, to ensure a low impurity content, the stirring speed should be within the range of 300–500 rpm.
The changes in the median particle size of the REC are shown in Figure 13. With the continuous increase of the stirring speed, the particle size of the REC gradually decreased. When the initial stirring speed was 200 rpm, the D50 of the REC was 12.33 μm; when the stirring speed increased to 500 rpm, the D50 of the REC was 9.75 μm; when the stirring speed continued to increase to 600 rpm, the median particle size of the REC was only 8.39 μm. This is because at high speeds, supersaturation is rapidly consumed to generate a large number of small crystal nuclei instead of being used for the growth of existing crystals, resulting in a large number of grains but a small average particle size. Meanwhile, the diffusion rate on the crystal surface is rapidly stirred and dispersed, shortening the crystal growth time and further limiting the increase in grain size [33]. To obtain large particles of REC with a low impurity content, a stirring rate of 300 rpm was selected.
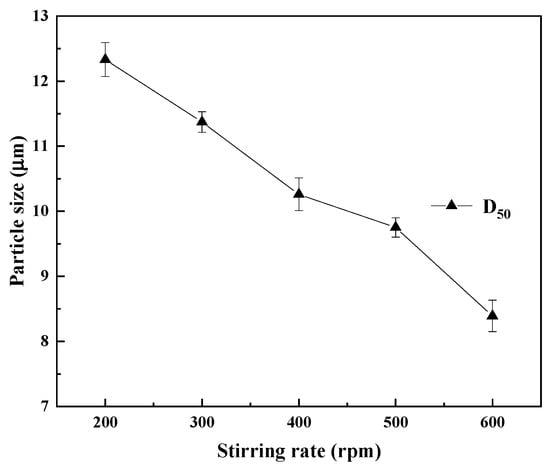
Figure 13.
Particle size of REC at different stirring rates.
3.3.6. One-Way ANOVA for the Effect of Factors on the Impurity Content and the Particle Size of REC
To compare the effect of factors on the Mg and SO42− amounts in the REC and the median particle sizes of the REC, a one-way ANOVA was used to illustrate the influencing differences among the factors. The results are shown in Figure 14.
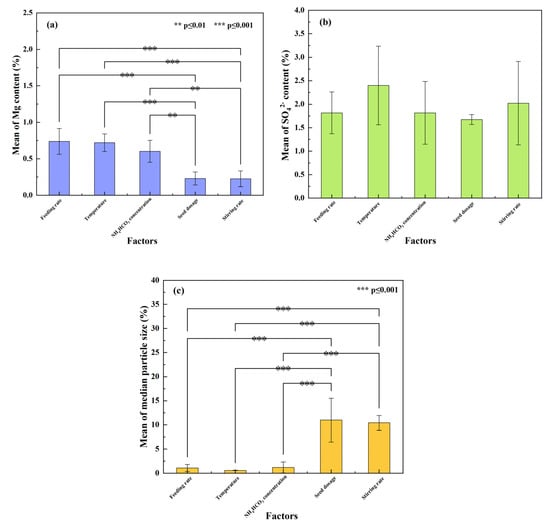
Figure 14.
Results of the one-way ANOVA for the effect of factors on the (a) Mg content of the REC; (b) SO42− content of the REC; and (c) D50 of the REC.
As shown in Figure 14a, the mean values of the Mg content in the REC for the feeding rate, temperature, NH4HCO3 concentration, seed dosage, and stirring rate were 0.74%, 0.72%, 0.60%, 0.23%, and 0.23%. The p values between factors including feeding rate and temperature and factors with seed dosage were below 0.001, and the p values between NH4HCO3 concentration and factors with seed dosage were below 0.01. This indicated a significant difference between the conditions without seed dosage and the conditions with seed dosage. However, for SO42−, in Figure 14b, its mean values were fluctuant among all the factors without significant differences. The changes in the particle size of the REC were similar to the Mg content. As seen in Figure 14c, the conditions with seed dosage dramatically increased the median particle size (D50) of the REC. Accordingly, the p values between factors without seed dosage and factors with seed dosage were below 0.001. It was concluded that the seed dosage was the key factor for decreasing the Mg content and increasing the particle size of REC. To decrease the amount of SO42−, other methods outside the process can be further studied.
3.4. Morphology and Phase Characterization of REC
According to the above optimal experimental conditions, when the feeding rate was controlled at 0.5 mL/min, the temperature was 25 °C, the ammonium bicarbonate concentration was 0.6 mol/L, the crystal seed dosage was 7.5%, and the stirring rate was 300 rpm, REC was obtained. Its chemical content is shown in Table 3. The contents of REO, MgO, and SO42− in the REC were 57.69%, 0.23%, and 1.68%, respectively, superior to the requirements of the current Chinese national standard (GB/T 16479-2020) [34].

Table 3.
Total amount of REO and impurities in the REC obtained under the optimal conditions (wt%).
The morphology and phase characterization results are shown in Figure 15, Figure 16, Figure 17 and Figure 18. In Figure 15, there were almost no intensity peaks to be detected, demonstrating that the generated REC phase was amorphous. As shown in Figure 16, the resulting rare earth carbonate crystals were irregularly flocculent and agglomerated, and the surface was very rough, which was due to the strong shear force generated by stirring destroying the surface morphology of the rare earth carbonate crystal [35,36]. At the same time, these defects provide adsorption sites for tiny grains, promoting the growth and agglomeration of particles [37]. The SEM—EDS result showed that La, Y, and Nd had a good correlation with a high degree of coincidence, and the atomic percentages of La, Y, Nd, C, and O at Point 1 were similar to those of the REC. The result of the infrared spectral analysis is shown in Figure 17. The characteristic peak at 1350~1510 cm−1 corresponded to the stretching vibration of CO32− in the REC, and the characteristic peak at 740~860 cm−1 can be attributed to the bending vibration of CO32−. The characteristic peaks at 3451.61 cm−1 and 3743.66 cm−1 were attributed to the bending and stretching vibrations of OH− groups. The above characteristic peaks demonstrated that the obtained REC was rare earth orthocarbonate. The result of the particle size analysis shown in Figure 18 indicate that the overall particle size distribution was uniform, and the median particle size of the REC (D50) was 11.37 μm. According to Equation (2), the particle size distribution (R) of the REC under the optimal conditions was 1.17.
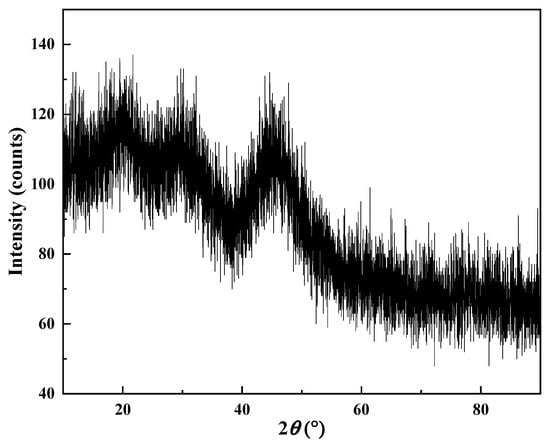
Figure 15.
XRD pattern of REC.
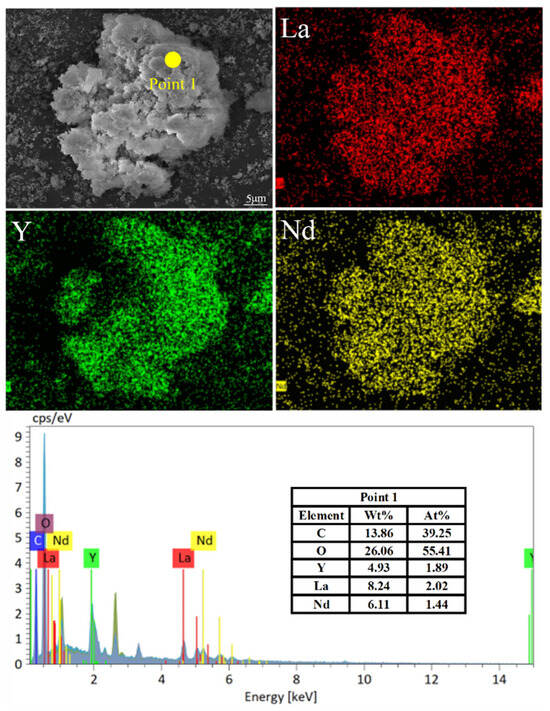
Figure 16.
SEM micrographs of REC with the EDS elemental mapping results.
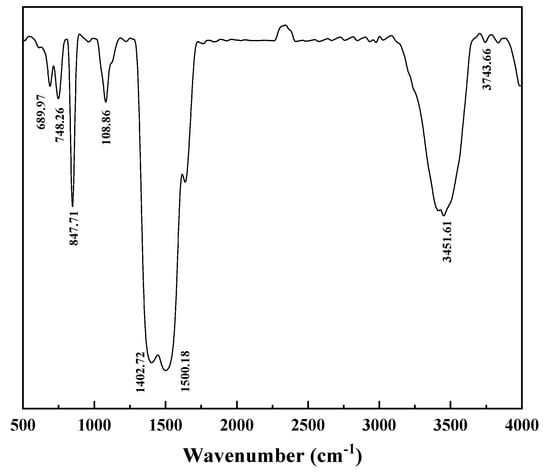
Figure 17.
Infrared spectrum of REC.
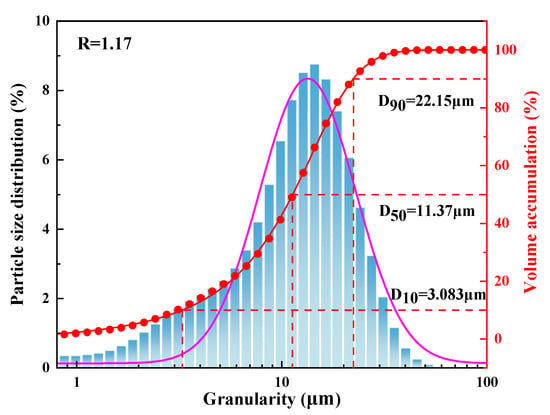
Figure 18.
The particle size distribution of the REC.
4. Conclusions
In this study, the effects of different reaction conditions on the rare earth precipitation efficiency, the impurity content, and the median particle size of REC precipitated from a concentrated ionic rare earth and magnesium sulfate solution by ammonium bicarbonate were investigated. Under the optimal conditions, the obtained REC was superior to the requirements of the current Chinese national standard (GB/T 16479-2020). The rare earth precipitation efficiency was 99.78%. The pH was the main factor found to affect the rare earth precipitation efficiency, such that when the pH was 6.8, the REEs in the solution precipitated nearly completely. The dosage of crystal seeds was the key factor controlling the particle size and Mg content of the REC. The average particle size of the REC was increased from 1.25 μm without crystal seeds to 11.37 μm, and the amount of Mg was decreased from 0.54% without crystal seeds to 0.14% with the crystal seed dosage of 7.5%. Though the seed dosage promoted particle growth and impurity decrease, the obtained REC was amorphous rare earth orthocarbonate. For the impurity SO42−, its amount was fluctuant among all the factors and could be controlled at a relatively low value by the seed dosage and specific stirring rare. To realize the lowest amount of SO42− in REC, other ways outside the process are suggested.
Author Contributions
Conceptualization, T.Q. and H.Y.; writing—original draft, Q.W.; writing—review and editing, Q.W., Y.L. and L.Z.; methodology and investigation, Q.W. and D.S.; formal analysis, L.Z.; visualization, Y.L.; supervision, T.Q., H.Y. and T.L.; project administration and funding acquisition, H.Y.; resources, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Soviet Light talent sponsorship program by Ganzhou Municipal Government [2023(10)]; the National Natural Science Foundation of China (42202345); the Rural Revitalization Project of Chinese Academy of Sciences (KFJ-XCZX-202305); and the Technology and Demonstration Project for Efficient Leaching of Ammonia and Nitrogen in Ionic Rare Earth Goaf (YHD060002).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Authors Qiang Wang and Lue Huang were employed by the company China Rare Earth Group Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, Y.X.; Zhang, L.; Zhou, X.M. Resource and environmental protection mining mode of Ion-type rare earth in South China. Chin. Rare Earths 2010, 31, 6. [Google Scholar]
- Li, Y.X.; Zhou, X.M.; Liu, Y.Z. Advances in efficient extraction and separation of rare earth by ion adsorption. J. Rare Earths 2012, 30, 8. [Google Scholar]
- Wang, D.H.; Zhao, Z.; Yu, Y. Research progress, existing problems and future research directions of ion-adsorbed rare earth resources. Rock Miner. Anal. 2013, 32, 796–802. [Google Scholar]
- Zhang, L.; Wu, K.X.; Chen, L.K. Metallogenic characteristics of ion-adsorbed rare earth deposits in southern Jiangxi Province. J. Rare Earths 2015, 33, 10–12. [Google Scholar]
- Zhang, Z.H. Ion-adsorbed rare earth deposit of weathering crust in South China. Collect. Geol. Prospect. Pap. 1990, 1, 57–58. [Google Scholar]
- Yang, X.J.; Lin, A.; Li, X.L.; Wu, Y.; Zhou, W.; Chen, Z. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xi, Y.L.; Li, D. Rare earth precipitation process in magnesium salt rare earth leaching solution. Non-Ferr. Met. (Smelt. Part) 2022, 30, 45–50. [Google Scholar]
- Xiao, Y.F.; Huan, X.W.; Feng, Z.Y. Research progress of green extraction technology of ion-adsorbed rare earth ore. Chin. Rare Earths 2015, 36, 109–115. [Google Scholar]
- Chen, X.; He, Q.; Chen, J.F. Research progress on green and efficient leach technology and principle of ion-adsorbed rare earth ore. J. Rare Earths 2022, 40, 936–947. [Google Scholar]
- He, Z.H.; Guo, A.; Qiu, X.Y.; Liao, C.S.; Lan, Q.F.; Ge, L.S.; Xiao, L.; Huang, D.S. Extraction technology of rare earth from ionic rare earth magnesium salt concentrates. J. Non-Ferr. Met. (Smelt. Part) 2024, 1, 61–67. [Google Scholar]
- Huan, X.W.; Yu, Y.; Feng, Z.Y. The Invention Relates to a Method for Recovering Rare Earth from Ionic Rare Earth Raw Ore. CN102190325B, 16 July 2014. [Google Scholar]
- Lai, A.; Lai, F.; Huang, L.; Qiu, J.; Zhou, X.; Xiao, Y. Non-ammonia enrichment of rare earth elements from rare earth leaching liquor in a magnesium salt system I: Precipitation by calcium oxide. Hydrometallurgy 2020, 193, 53–58. [Google Scholar] [CrossRef]
- Cai, X.D.; Yang, L.F.; Sun, Y.Y. Organic phase loss during P507-kerosene extraction of rare earth and oil removal from raffinate by aluminum sulfate coagulation. Chin. Rare Earths 2017, 38, 33–40. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Chen, Y.Y.; Feng, Z.Y. Leaching characteristics of magnesium sulfate leaching ion adsorption type rare earth ore. Trans. Nonferrous Met. Soc. China 2015, 25, 38–40. [Google Scholar] [CrossRef]
- Xiao, Y.F. Study on Green and Efficient Leaching Technology of Ion Adsorbed Rare Earth Mineral Magnesium Salt System. Ph.D. Thesis, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- He, S.Z. Study on the Process and Mechanism of pH Regulation-Complexation Precipitation Combined with Impurity Removal in Ionic Rare Earth Magnesium Salt Leaching Solution. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2022. [Google Scholar]
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Oliveira, É.D. Selective precipitation of rare earth from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate. Miner. Eng. 2019, 134, 402–416. [Google Scholar] [CrossRef]
- Zhou, H.C.; Zheng, Z.; Chen, Y.J.; Wan, Y.H. A novel ion exchange-electrodialysis hybrid system to treat rare-earth oxalic precipitation mother liquid: Contamination reduction, efficient Y3+ recovery, and acid separation. Desalination 2023, 565, 116815. [Google Scholar] [CrossRef]
- Feng, J.; Wu, X.Y.; Xue, Y.P. Leaching of rare earth ore by weathering crust leaching based on direct reuse of rare earth mother liquor precipitated by oxalic acid. J. Tianjin Polytech. Univ. 2024, 43, 40–49. [Google Scholar]
- Qiang, Y.J. Study on Precipitation and Crystallization Process of Ammonium Bicarbonate in Low Concentration Rare Earth Solution. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2013. [Google Scholar]
- Rao, G.H.; Yao, H.Q. Study on aluminum inhibition leaching from rare earth ore in South China. Rare Met. Cem. Carbide 2003, 4, 1–3. [Google Scholar]
- Zhao, Z.H.; San, X.Y.; Zhang, W.B. Removal of impurities aluminum and iron from rare earth solution by centrifugal sedimentation. China Rare Earths 2007, 95–97. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Peng, S.S. Study on precipitation of rare earth with ammonium bicarbonate. China Rare Earths 1997, 16–19. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, T.; Liu, Y.; Yu, H.; Zhang, L.; Zhan, W. Sulfuric Acid Leaching of Ionic Rare Earth Magnesium Salt Enrichment and Removing Aluminum by MgO Precipitation. Minerals 2025, 15, 189. [Google Scholar] [CrossRef]
- GB/T 14635-2020; National Technical Committee for Standardization of Rare Earths. Methods for Chemical Analysis of Rare Earth Metals and Their Compounds Determination of Total Rare Earth Amounts; China Standard Press: Beijing, China, 2020.
- Yu, Z.H. Study on the Process of Preparation of RECs by Mg(HCO3)2 Precipitation Method in Sulfuric Acid System. Master’s Thesis, Beijing Nonferrous Metals Research Institute, Beijing, China, 2019. [Google Scholar]
- Wang, X.Y.; Cui, J.G.; Hang, X.C.; Wei, L.X.; Li, X.F.; Xu, M.; Gao, T. A Method for Preparing Low-Sulfate Rare Earth Carbonate Using a Mixed Precipitating Agent. CN119287183A, 10 January 2025. [Google Scholar]
- Cui, J.G.; Wang, X.Y.; Hang, X.C.; Wei, L.X.; Wang, Z.; Zhang, S.W.; Li, N.; Li, J.L. A Method for the Continuous Preparation of Low-Impurity Rare Earth Carbonate Using Mixed Precipitants. CN119287182A, 10 January 2025. [Google Scholar]
- Wuyuan County Runze Rare Earth Limited Liability Company. Production Method of Rare Earth Carbonate Without Ammonia Nitrogen Emission: 201110093638.0[P]; Wuyuan County Runze Rare Earth Limited Liability Company: Jiangxi, China, 2011. [Google Scholar]
- Zhang, L.L.; Deng, X.Y.; Ding, Y.G. Study on the preparation of crystalline mixed rare earth carbonate with ammonium bicarbonate. J. Huangshi Inst. Technol. 2011, 27, 20–24. [Google Scholar] [CrossRef]
- Ding, L. Function and Environment Oriented Precipitation and Crystallization Technologies of Rare Earth Compounds; Nanchang University: Nanchang, China, 2014. [Google Scholar]
- Li, Y.X.; Zhou, L.; Zhou, X.M.; Song, L.S.; Zhou, X.Z.; Liu, Y.Z.; Li, J. A Method for Preparing Rare Earth Carbonate and the Recycling and Utilization of Its Materials. CN103224248B, 1 July 2015. [Google Scholar]
- GB/T 16479-2020; National Technical Committee for Standardization of Rare Earths. Light Rare Earth Carbonate. China Standard Press: Beijing, China, 2020.
- Jiao, X.Y.; Luo, X.M.; Yang, Y.J.; Gu, Z.Y.; Li, Y.X. Study of crystallization process during La and La2(CO3)3pre-cipitation with sodium bicarbonate. Rare Met. Cem. Carbides 2001, 29, 4–8. [Google Scholar]
- Ding, J.W.; Li, Y.X.; Huang, T.; Luo, X.M.; Yang, Y.; He, X.B. Crystalline neodymium carbonate with lanthanide structure: Preparation and promoting action of seeding on crystallization. Chin. J. Inorg. Chem. 2005, 21, 1213–1217. [Google Scholar]
- Mutaftschiev, B. Adsorption and crystal growth. Crit. Rev. Solid State Mater. Sciences 1976, 6, 157–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).