Abstract
The Carajás Mineral Province hosts one of the world’s most extensive sulfide-bearing copper belts. These deposits are typically covered by thick regolith, including gossans, laterites, colluviums, and soil, which can be used as important exploration indicators. In some cases, these covers can be mined alongside the parent hypogene ore. Therefore, accurate identification of copper-bearing minerals is essential for selecting the most appropriate metallurgical techniques. This study investigated the saprolite horizon overlying the Alvo 118 deposit, where the parent rocks are chloritites hosting copper-bearing hypogene sulfides, partially altered to an immature gossan. Saprolite formation was primarily controlled by the weathering of chlorite, mostly converted into kaolinite, with smectite and vermiculite serving as intermediates, forming a typical lower saprolite association. During weathering, iron released from chlorite and indirectly by vermiculite and smectite contributed to the formation of ferrihydrite, goethite, and hematite. Magnetite octahedrons, relics of the hypogene ore, pseudomorphic phases, are embedded in the clay mineral matrix. While FTIR analysis of kaolinite showed no evidence of copper retention, Mössbauer spectroscopy enabled the quantification of iron-bearing minerals, revealing a strong correlation between CuO contents and goethite and ferrihydrite. These results suggest that goethite and ferrihydrite may be the main copper carriers in the deposit, consistent with findings from similar deposits. Weak acid leaching is proposed as the most effective technique for copper extraction from this mineralization type.
1. Introduction
In mineral provinces subjected to tropical or paleotropical conditions, the dissolution of ore minerals releases ions or ionic compounds, which may subsequently be retained by secondary minerals derived from the weathering of the host rocks [1,2,3]. This process results in the formation of geochemical dispersion halos, which hold significant economic importance [4,5]. Understanding the mechanisms underlying their formation is essential for mineral exploration, particularly in areas characterized by dense vegetation and limited outcrop exposure.
The Carajás Mineral Province (CMP), Brazil, hosts numerous IOCG copper deposits within metavolcanic–sedimentary sequences, discovered through exploration programs conducted over the past five decades [3,6]. These deposits, typically comprising copper sulfides, underwent gossan formation and were subsequently affected by lateritization events that began approximately 70 Ma [1,7]. Deep hypogene orebodies, their associated gossans, and host rocks remained unaffected by lateritization. However, shallower hypogene orebodies and their host rocks evolved to form saprolites, partially exhumed over the past 10 Ma during the development of the Velhas/Itacaiúnas geomorphic surface [8,9]. These saprolites are distributed throughout the denuded areas surrounding the Carajás Plateaus, reaching thicknesses of up to 100 m and forming dispersion halos that also possess significant economic potential [4,5].
The Alvo 118 deposit is situated in a denuded area south of the Carajás Mountains and was first identified during exploration programs conducted by Docegeo in the 1990s, with further studies resumed by Vale S.A. in 2018. The deposit comprises massive and disseminated copper sulfide orebodies that have undergone two distinct supergene alteration stages. In the first stage, dominated by oxidoreduction processes, primary mineralization was partially transformed into an immature gossan profile at depth [10]. During the second stage, near-surface weathering of the host rocks led to the formation of a thick saprolite cover, preserving high copper content [11,12]. The primary sulfide reserves are estimated at approximately 170 Mt with 1% Cu and 3 ppm Au, while the supergene mineralization totals 55 Mt at 0.92% Cu and 0.3 ppm Au, comprising 30% gossan and 70% saprolite [13].
The high copper content within the saprolite overlying hypogene mineralization contrasts with the apparent absence of copper-bearing minerals, raising questions about the phases responsible for copper retention, an important consideration for metallurgical processes. Previous studies have highlighted the role of phyllosilicates, as well as iron and manganese oxyhydroxides, as copper-bearing phases in CMP saprolite [4,5,14,15]. Copper retention by iron oxyhydroxides can occur through isomorphic substitution or adsorption, while some clay minerals (smectites and vermiculites) can retain metals through ionic substitution within layers or adsorption in interlayer spaces [16,17,18,19]. Precise identification of gangue minerals is also critical for metallurgical processing, as these phases may consume chemical reagents depending on their dissolution kinetics and solubility [20,21].
This study examines the mineralogy and geochemistry of the weathering profile at Alvo 118, focusing on the behavior of copper and other metals within the saprolite horizons. By analyzing their associations with various secondary phases, the findings may support mineral exploration and other metallurgical processes.
2. Geological and Geomorphological Setting
The most prominent geomorphic feature in the Carajás Mineral Province (CMP) is the Carajás Plateaus, a series of plateaus with elevations ranging from 500 to 750 m and occasionally reaching up to 900 m [3,22]. Extensive erosion by the current drainage system has transformed these mountains into flat to undulating lowlands, with elevations ranging from 250 to 350 m. The plateaus are typically capped by ferruginous duricrusts and latosols [23,24], whereas in the surrounding lowlands, the erosion of these surficial materials has exposed the underlying saprolite [22,25].
The Alvo 118 area is composed of mafic to intermediate metavolcanic sequences with mylonitic foliation and intense chloritic alteration characteristic of the Grão Pará Group (Itacaiúnas Supergroup) (Figure 1A). These sequences are intruded by granodioritic, tonalitic, and gabbroic bodies, as well as rhyolitic–dacitic and diabasic dikes [26,27]. These lithotypes host hypogene mineralization and have undergone multiple hydrothermal alteration events, including sodic alteration, marked by albite and scapolite; potassic alteration, characterized by biotite and alkali feldspar; chloritic alteration; and quartz–sericite alteration [27].
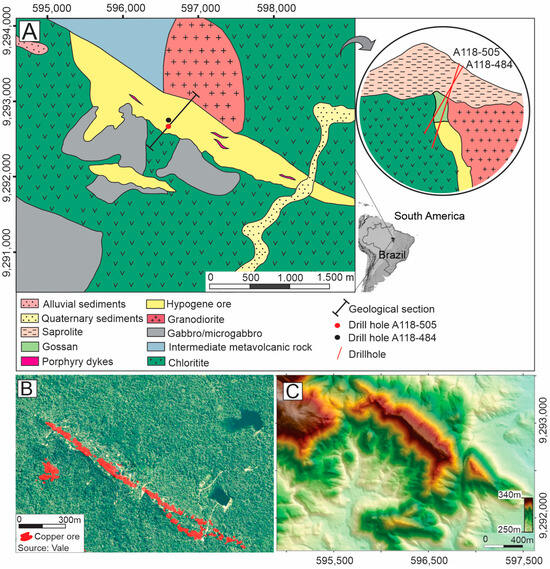
Figure 1.
(A) Simplified geological map of the Alvo 118 deposit area, illustrating the spatial distribution of hypogene mineralization—data compiled and modified from [10] and a simplified geological section including gossan and saprolite, both absent in the geological map. (B) Satellite image of the deposit area, obtained from Google Earth Pro (2018), showing the dense vegetation covering the deposit (source: Vale S.A.). (C) Digital elevation model of the study area, highlighting the relief features [28].
Hypogene mineralization consists of sub-vertical, tabular orebodies trending NW-SE. These orebodies include (1) massive sulfide veins containing chalcopyrite, bornite, magnetite, hematite, and minor amounts of Au-Ag tellurides, galena, and cassiterite, and (2) stockwork veins composed of quartz and calcite with disseminated chalcopyrite [6,27]. The hypogene mineralization transitions upward into an immature gossan. Around the gossan, the host rocks are preserved from weathering but converted into a saprolite closer to the surface [10,12].
The Alvo 118 deposit is located in a hill lowland area approximately 5 km northwest of the Sossego copper mine, both in proximity to a relic plateau of the Carajás Mountains (Figure 1B,C). The weathering profile in this area has an average thickness of 60 m, with localized zones reaching up to 100 m. The soil exhibits colluvial characteristics, including nodules and pebbles embedded in a reddish clayey matrix primarily composed of kaolinite and iron oxyhydroxides. Quartz and magnetite are the most common residual minerals [4].
3. Materials and Methods
Petrographic descriptions were conducted on outcrops at the Alvo 118 pilot pit and four drill holes, followed by the collection of 152 samples, including host rock, primary mineralization, gossan, and saprolite. Color classification was based on the Munsell Soil Color Chart [29]. Mineralogical and microtextural analyses were performed on polished thin sections using a LEICA (DM 2700 P optical microscope equipped with a LEICA MC 170 HD camera), from Wetzlar, Germany. These observations were complemented by scanning electron microscopy (SEM) in secondary electron mode. SEM images were acquired using Zeiss SIGMAVP equipment, from Oberkochen, Germany, with an electron beam current of 80 μA, a constant acceleration voltage of 10 kV, and a working distance of 8.5 mm. Chemical analyses were performed using an energy dispersive system (EDS) on a IXRF Sedona-SD equipment, from Austin, TX, USA, with an electron beam current of 80 μA, a constant acceleration voltage of 20 kV, a working distance of 8.5 mm, and an elemental analysis counting time of 30 s. Optical microscopy and MEV/EDS imaging were performed at the Laboratory of Mineralogy, Geochemistry, and Applications (LAMIGA) and the Microanalysis Laboratory, respectively, both located at the Federal University of Pará.
X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) analyses were conducted on clay fractions separated through sieving and centrifugation. For this purpose, 150 g aliquots were deflocculated in an ultrasonic cleaner, wet sieved at 63 μm, and centrifuged at 1000 rpm for 2 min. The resulting suspension was decanted, centrifuged at 1800 rpm for 10 min, decanted again, and dried at 40 °C for 48 h. XRD analyses were performed on oriented mounts with conventional treatments, including air dry, solvation with ethylene glycol for 48 h, and heating at 550 °C for 2 h. A Bruker D2 PHASER diffractometer equipped with a Cu anode and a Lynxeye detector (1D mode) was used over an angular range of 4° to 75° 2θ, with a 0.02° 2θ increment, a 0.2 s step time, and a 0.1 mm slit, operating at 300 W (30 kV and 10 mA). FTIR spectra were obtained using a Bruker Vertex 70 spectrometer, from Ettlingen, Germany, with a deuterated L-alanine-doped triglycine sulfate detector, operating at room temperature (RT-DLaTGS). Analyses were controlled using Opus 7 software in the spectral range of 400 to 4000 cm−1, in transmission mode, with a spectral resolution of 4 cm−1 and a mirror movement speed of 10 kHz. All XRD and FTIR analyses were conducted at LAMIGA.
Iron oxyhydroxides were identified and quantified using 57Fe Mössbauer spectroscopy on raw samples at room temperature (298 K). Measurements were performed on a conventional spectrometer with transmission geometry and a 57Co source under constant acceleration. Spectra were collected over a velocity range of ±10 mm/s and calibrated with an α-Fe standard at room temperature. The spectra were refined using WinNormos-for-Igor software, version 3.0 (May 2009). These analyses were performed at the Brazilian Center for Research in Physics (Rio de Janeiro).
Measurements of pH and Eh were conducted on twelve samples representing hypogene mineralization, gossan, and saprolite. For each sample, 1 g of powdered material was mixed with 10 mL of distilled water in Falcon tubes, following the procedure outlined by [30]. Five measurements were taken per sample using an Orion Star A211 pH meter (Thermo Scientific, from Waltham, MA, USA.
A total of 146 samples (44 from hypogene mineralization, 62 from gossan, and 40 from saprolite), each weighing approximately 10 kg, were analyzed for whole-rock chemical composition at ALS laboratories Ltd. The elements analyzed included Ag, Al, As, Ba, Be, Bi, Cd, Ce, Cu, Co, Cr, Cs, Fe, Ga, Ge, Hf, In, K, La, Li, Mg, Mn, Mo, Na, Nb, Ni, P, Pb, Rb, Re, S, Sb, Sc, Se, Sn, Sr, Ta, Te, Th, Ti, Tl, U, V, W, Y, Zn, and Zr. Aliquots of 0.25 g were digested using a mixture of perchloric, nitric, hydrofluoric, and hydrochloric acids. After digestion, the resulting solution was brought to volume with dilute hydrochloric acid prior to analysis through inductively coupled plasma–atomic emission spectrometry (ICP-AES). Gold was determined via fire assay, with final measurement through ICP-AES.
Additionally, the Fe2O3 and CuO contents of six selected samples were analyzed at ALS laboratories Ltd. to support Mössbauer spectroscopy investigations. For Fe2O3 determination, 0.1 g aliquots were mixed with lithium borate and fused at 1025 °C. The resulting melt was cooled, dissolved in a mixture of nitric, hydrochloric, and hydrofluoric acids, and analyzed using an inductively coupled plasma mass spectrometer (ICP-MS). For copper, 0.4 g aliquots were digested in aqua regia, with the resulting solution also analyzed through ICP-MS.
4. Results
4.1. Bedrock
The bedrocks of the investigated boreholes consist of foliated chloritites, found between depths of 35 to 43 m and 70 to 150 m. These chloritites are composed of approximately 80% chlorite, 15% plagioclase, and 5% quartz, with accessory magnetite. The chloritites are crosscut by stockwork veins made up of quartz and chalcopyrite, with accessory calcite, apatite, and fluorite (Figure 2A). They are also intersected by massive sulfide veins, which feature a chalcopyrite matrix, embedded in chlorite (Figure 2B,C), along with quartz, calcite, fluorite, and apatite. The 43 to 70 m interval is dominated by granodiorites, which contain about 40% quartz, 35% oligoclase, 15% orthoclase, and accessory biotite and hornblende (Figure 2D). The granodiorites exhibit extensive chloritization along a network of chlorite veinlets, with disseminated chalcopyrite (Figure 2E).
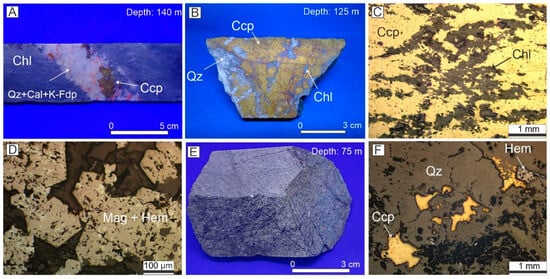
Figure 2.
(A) Chloritite crosscut by quartz veins with minor chalcopyrite, indicative of disseminated mineralization. (B) Massive mineralization consisting of a chalcopyrite matrix with chloritite clasts. (C) Chlorite surrounded by chalcopyrite, observed under optical microscopy (OM) with reflected light (RL). (D) Magnetite partially hydrothermally altered to hematite, observed under OM/RL. (E) Granodiorite crosscut by chlorite veinlets. (F) Chalcopyrite disseminations in granodiorite, observed under OM/RL. Abbreviations: Chl (chlorite), Mag (magnetite), Hem (hematite), Qz (quartz), Ccp (chalcopyrite), Cal (calcite), K-Fdp (alkali feldspar), optical microscopy (OM), reflected light (RL).
The presence of sulfide mineralization has led to the development of an immature gossan, which is interspersed with both chloritites and granodiorites (Figure 3A). In the chloritites, the gossan hosts goethite and malachite zones, while the granodiorites contain cuprite and libethenite zones. The goethite zone is characterized by goethite micromass coating dissolution cavities (cavity-filling facies), filling the chloritite fracture system (fracture-filling facies), or forming a matrix around quartz grains (breccia facies). In this zone, goethite is associated with chalcocite, native copper, malachite, and pseudomalachite. The malachite zone consists of a malachite micromass, with chrysocolla and ramsbeckite, surrounding cuprite and tenorite nodules. The cuprite zone is defined by cuprite micromass with relics of goethite and chalcocite, associated with millimeter-sized quartz grains. Finally, the libethenite zone features a micromass of libethenite, along with malachite and pseudomalachite, distributed along fractures in the granodiorite (Figure 3A).
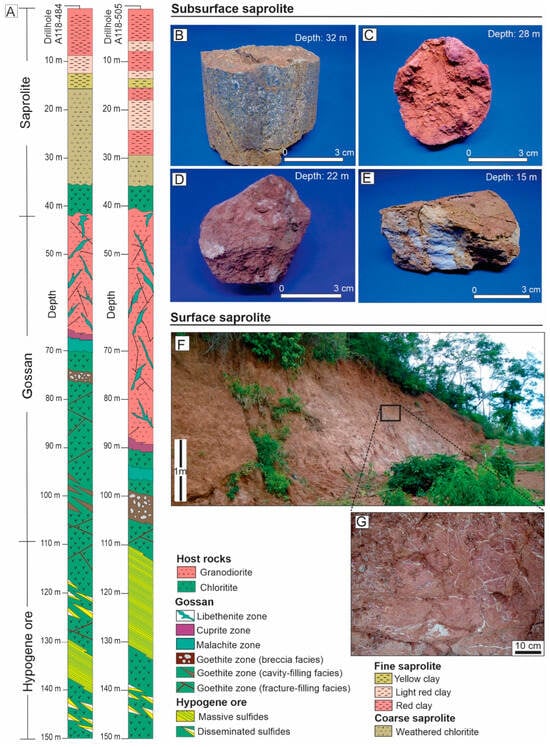
Figure 3.
Vertical distribution of parent rocks and saprolite in the Alvo 118 deposit, modified from [10,12] (A). Weathered chlorite characteristic of the coarse saprolite (B). Typical fine saprolite samples consisting of a clay–sandy matrix with iron oxyhydroxides, showing red (C), light red (D), and yellow (E) coloration. Exposure of fine saprolite on the hillside (F). Kaolinite veinlets crosscutting the clay–sandy matrix in the fine saprolite (G).
4.2. Structure of the Weathering Profile
The host rocks of the hypogene mineralization and gossans (chloritite and granodiorite) transition towards the surface to form a saprolite (Figure 3A), comprising a coarse and a fine saprolite. The coarse saprolite extends from 15 m to 35 m in drill hole A118-484 and from 29 m to 35 m in drill hole A118-484. This zone retains the typical foliation inherited from the chloritites, where chlorite remains predominant, although it is now associated with a secondary plasma of kaolinite and goethite. These secondary minerals impart a friable texture and a yellow color (10YR 6/6) to the saprolite (Figure 3B).
The fine saprolite extends from 15 m to the surface in drill hole A118-484 or from 29 m to the surface in drill hole A118-484, consisting of a friable clayey–sandy matrix primarily composed of kaolinite, with minor smectite and vermiculite, in addition to quartz. These minerals are coated with iron oxyhydroxide films, which, depending on their distribution, form distinct red (10R 5/8), light red (10R 7/8), and yellow (2.5Y 7/8) zones, with occasional white (10R 8/1) patches of kaolinite (Figure 3C–E). The light red portion of the fine saprolite outcrops on the hillsides as a sandy–clayey matrix containing kaolinite veinlets (Figure 3F,G).
Microscopic analyses show that chlorite is preserved in the coarse saprolite, maintaining the original foliation (Figure 4A). As the profile transitions to fine saprolite, the presence of goethite becomes more prominent, occurring in a botryoidal patter, typically along the chlorite cleavage planes (Figure 4B). In the fine saprolite, kaolinite crystallites are the dominant phase, often forming booklets (Figure 4C). EDS data indicate that kaolinite is encased by goethite films, which lack the botryoidal structure seen earlier. The kaolinite matrix surrounds numerous magnetite octahedra, either isolated or cemented together (Figure 4D,E). These magnetite crystals are relics of the parent rocks, suggesting that the saprolite was derived from mineralized protoliths. Hematite nodules are also present within the clayey matrix (Figure 4F).
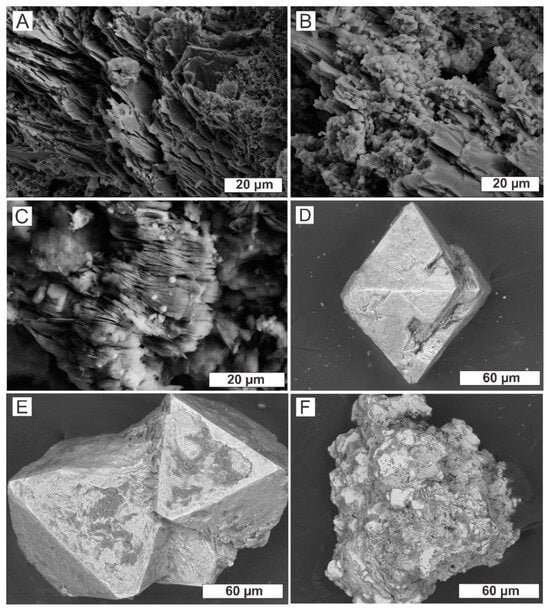
Figure 4.
Micromorphology of the coarse saprolite showing unweathered chlorite plates that preserve relic foliation (A). Botryoidal goethite distributed along chlorite cleavage planes (B). Typical micromorphology of fine saprolite, with kaolinite crystallites arranged in booklets (C). Octahedral magnetite crystals in the fine saprolite, occurring as isolated crystals (D) or in aggregates (E). Hematite nodules characteristic of the saprolite (F).
4.3. Multi-Elemental Chemical Composition of the Studied Saprolite
The Fe2O3 and MgO contents are naturally elevated in the chloritites and hypogene mineralization, both exceeding the values of the Upper Continental Crust (UCC), reflecting the presence of chlorite and, to a lesser extent, chalcopyrite in the case of Fe2O3. In contrast, the Fe2O3 and MgO contents in the granodiorites are lower than in the UCC, with higher concentrations of CaO, K2O, and Na2O. TiO2 content in the chloritites is slightly higher compared to in the granodiorites (Table 1). These lithotypes host an immature gossan, whose distribution is limited and contributes minimally to the formation of saprolite.

Table 1.
Chemical composition of selected samples from drill hole A118-505, expressed in wt.% (major elements) and ppm (trace elements). Not detected (n.d.); Chloritite (Chlo.); Granodiorite (Gran.); hypogene mineralization (Hypo.).
In the saprolite, the contents of Al2O3 (15.38 to 23.59%) and Fe2O3 (8.33 to 16.64%) are variable. The concentrations of CaO, K2O, and Na2O are lower than those in the bedrock, while TiO2 contents are slightly higher. MgO contents are lower than in the chloritites but higher than in the granodiorites. The CuO content in a selected sample from the massive hypogene mineralization is 25.24%. However, because the mineralized zones are interspersed with chloritites, the average Cu content of the hypogene deposit is approximately 1%. This average is slightly higher than the CuO content in the saprolite, which ranges from 0.51% to 1.37%, with an average of 0.92%.
Concentrations of Au, Ag, Be, Co, Ga, La, Mn, Mo, Nb, P, Sc, Sn, U, V, W, Y, and Zr are consistently higher than the UCC values and exhibit significant variability, reflecting the typical spatial distribution pattern in the drill holes investigated, with non-mineralized zones alternating with massive and disseminated mineralization.
The contents of Al2O3 and Fe2O3 exhibit oscillations and show opposite vertical distributions throughout most of the profile, reflecting the variable abundance of clay minerals and iron oxyhydroxides, respectively (Figure 5A). CuO contents follow a trend similar to that of MgO, both displaying a distribution pattern comparable to Al2O3 but not Fe2O3 (Figure 5A). TiO2 contents show minimal vertical variation, remaining around 1%, consistent with the intermediate composition of the underlying granodiorites and chloritites. P2O5 behaves similarly to CuO, suggesting the presence of trace amounts of pseudomalachite and libethenite in the saprolite. K2O contents are low in the lower portion of the profile (22 to 40 m), with a significant increase towards the top (above 22 m). Au contents exhibit strong fluctuations, peaking at up to 3% at a depth of 8 m (Figure 5B).
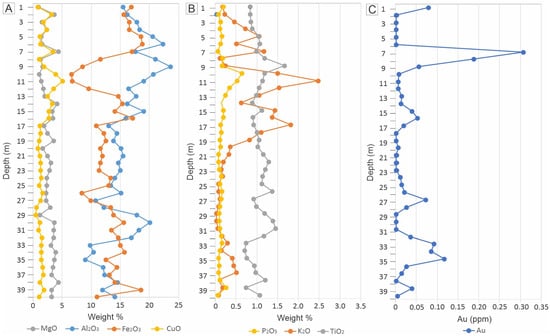
Figure 5.
Vertical distribution of selected major elements in the saprolite of drill hole A118-505: (A) MgO, Al2O3, Fe2O3, CuO; (B) P2O5, K2O, TiO2; Au (C).
4.4. Clay Minerals
Clay mineral identification followed the criteria outlined by [31,32]. In the coarse saprolite, the predominant clay mineral is chlorite, while kaolinite becomes progressively more abundant towards the top (fine saprolite), often in association with vermiculite, smectite, quartz, and goethite.
Kaolinite was identified by its major peak (001) between 7.10 and 7.12 Å in natural samples, which remains unchanged after treatment with ethylene glycol and disappears after heating at 550 °C (Figure 6A–D). Chlorite was identified by its strongest peak at 14.04 Å in natural samples, which remains stable after ethylene glycol treatment and heating at 550°C (Figure 6A).
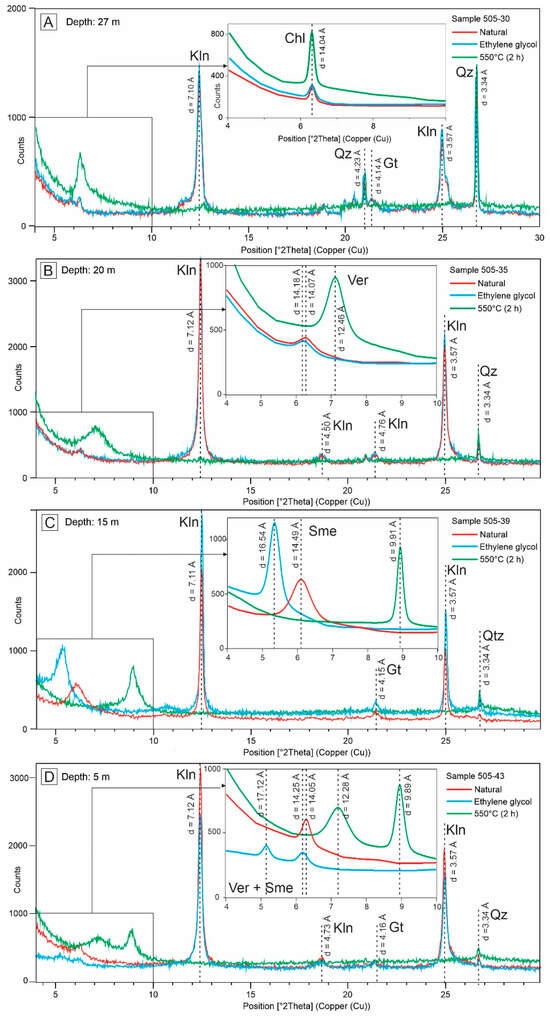
Figure 6.
X-ray diffractograms of selected fine saprolite samples (drill hole A118-505) collected at different depths: 27 m (A), 20 m (B), 15 m (C), and 5 m (D). Air-dried (red), ethylene glycol solvated (blue), and heated at 550°C (green) oriented clay mounts. Abbreviations: Kln (kaolinite), Chl (chlorite), Ver (vermiculite), Sme (smectite), Gt (goethite), Qz (quartz).
The presence of vermiculite was indicated by peaks at 14.18 Å in natural samples, which shifted to 14.07 Å after ethylene glycol solvation and collapsed to 12.46 Å after heating at 550°C (Figure 6B). Smectite showed a peak at 14.49 Å in the natural sample, expanding to 16.54 Å after ethylene glycol treatment and collapsing to 9.91 Å upon heating (Figure 6C). In some samples, smectite and vermiculite coexist (Figure 6D).
The mid-infrared transmittance spectra (Figure 7) exhibit a typical kaolinite pattern across all analyzed samples, with absorbance values consistent with those reported by [33,34]. The absorption bands at 431, 468, and 537 cm−1 are attributed to the Si-O, Si-O-Si, and Al-O-Si deformation vibrations, respectively. The bands at 695, 753, and 788 cm−1 correspond to the Si-O bond, with the first two bands being of the perpendicular type. The band at 912 cm−1 represents the deformation of internal hydroxyl groups. The Si-O stretching vibrations are observed at 1030 cm−1 and 1112 cm−1, parallel and perpendicular to the bond, respectively. The band at 1635 cm−1 indicates the OH deformation vibration of water. The bands at 3619 and 3695 cm−1 are related to the stretching vibrations of internal hydroxyl groups. No absorption bands typically associated with smectites, such as the 3420 cm−1 band, indicative of water molecules, were detected. Therefore, the FTIR spectrum confirms the dominance of kaolinite among clay minerals.
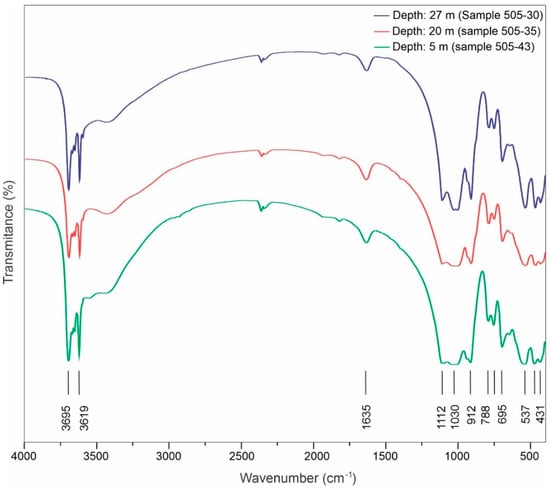
Figure 7.
FTIR spectra of selected fine saprolite samples (drill hole A118-505), representing different depths considered most characteristic of the profile.
4.5. Iron-Bearing Minerals
The room temperature Mössbauer spectra of the saprolite samples revealed the presence of various iron-bearing phases, which were identified based on their hyperfine parameters. The spectra generally consist of four subspectra: three doublets and one sextet (Figure 8A–C,E). However, samples 505-35 and 505-43 exhibited more complex spectra, featuring an additional sextet (Figure 8D,F). The hyperfine parameters derived from these spectra are summarized in Table 2. Iron-bearing phases were identified by comparing the hyperfine parameters with those reported in the literature [35,36,37]. This comparison considered the influence of room temperature on the results obtained. For example, Mössbauer spectra of ferrihydrite measured at 4 K exhibit well-developed sextets, indicative of magnetic ordering [38,39], while at room temperature it displays a broad paramagnetic doublet, with a specific range of isomer shift and quadrupole splitting values, which we used for subspectral fitting [40,41,42,43].
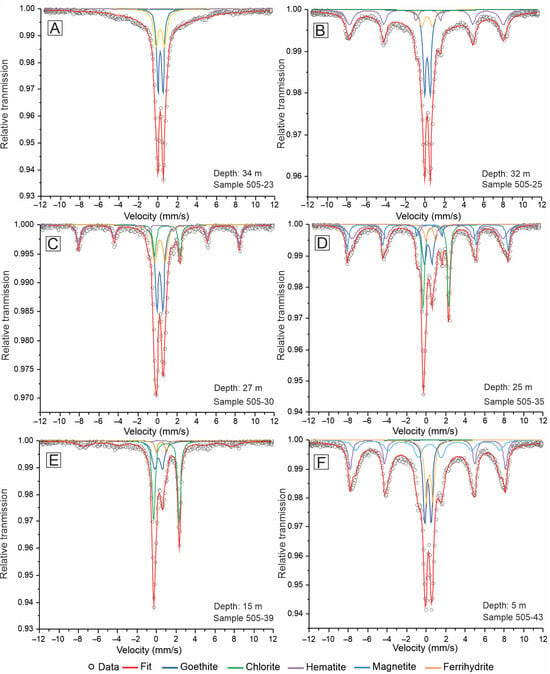
Figure 8.
Mössbauer spectra of selected samples from the coarse saprolite (A,B) and fine saprolite (C–F) taken at room temperature (drill hole A118-505). Solid colored lines are the best fits, and black dots indicate the experimental data.

Table 2.
Hyperfine parameters derived from Mössbauer spectra refinement at room temperature for selected saprolite samples (drill hole A118-505). Bhf (hyperfine field intensity); δ/Fe (isomeric shift relative to α-Fe); QS (quadrupole splitting); RA (relative subspectral area).
Two main groups of iron-bearing phases were identified. The first group includes goethite, ferrihydrite, chlorite, and hematite, which were present in samples 505-23, 505-25, 505-30, and 505-39. The second group, present in samples 505-35 and 505-43, contains the same phases as the first group but also includes magnetite. The relative areas of the subspectra suggest that chlorite is the most abundant iron-bearing phase in the coarse saprolite, while goethite and hematite are more dominant in the fine saprolite. Despite this, chlorite remains present in significant amounts in the fine saprolite, as well.
5. Discussion
5.1. Mineralogical Transformations and Iron Oxyhydroxide Formation
The mineral assemblage comprising chlorite, vermiculite, smectite, kaolinite, goethite, hematite, ferrihydrite, and quartz is commonly reported in saprolite profiles, often associated with chlorite-bearing protoliths [44,45,46]. The vermiculites identified in this study are relics from the initial stage of chlorite weathering, typically involving the oxidation of Fe²+ to Fe³+ and the removal of Fe and Mg [47,48,49].
As weathering progressed, the vermiculites were largely converted into a mixture of kaolinite and iron oxyhydroxides [45,50]. This transformation from vermiculite to kaolinite requires substantial chemical alterations, including the substitution of Al for Si in the tetrahedral sheets and the replacement of Mg and Fe by Al in the octahedral sheets. Such changes necessitate dissolution and reprecipitation processes, during which the major components of vermiculite are retained in kaolinite, as both minerals share a similar polymerization pattern [51].
The presence of smectite in samples containing kaolinite and vermiculite, or kaolinite alone, suggests that smectite may have formed as an intermediate phase during the conversion of vermiculite to kaolinite [45]. Alternatively, smectite formation may have occurred independently through the weathering of chlorite or plagioclase in minor amounts [46]. The formation of smectite from chlorite involves the removal of hydroxyl interlayers and a reduction in sheet charge, primarily due to organic compound complexation [52,53]. Mössbauer spectroscopy data confirm that chlorite, inherited from the protolith, is present throughout the saprolite, even if it is not always detectable through XRD [46].
Goethite and hematite were also identified throughout the saprolite, associated with supergene clay minerals (vermiculite, smectite, and kaolinite) and chloritite. Mössbauer spectroscopy further revealed the presence of ferrihydrite, a phase that is difficult to detect through XRD. The identification of the iron-bearing phases was based on a detailed comparison of the hyperfine parameters with those reported in the literature [35,36,37]. The presence of magnetite in samples 505-35 and 505-43 suggests a more complex mineralogical environment, likely influenced by variations in weathering conditions or differences in the parent material composition. The hyperfine parameters of magnetite, particularly the Bhf values of ~49 T and ~51 T, align well with those reported for magnetite in previous studies, confirming its presence in these samples.
The dominance of chlorite in the coarse saprolite indicates its relative resistance to weathering processes compared to goethite and hematite, which are more prevalent in the fine saprolite. This observation suggests that the fine saprolite has undergone more extensive weathering, leading to the breakdown of chlorite and the subsequent formation of goethite and hematite. The isomeric shifts (δ/Fe) and quadrupole splittings (QS) of goethite and hematite in the fine saprolite are consistent with their formation under oxidizing conditions, which is typical of advanced weathering environments. The presence of ferrihydrite in all samples, characterized by its relatively low Bhf values and specific QS and δ/Fe parameters, indicates ongoing weathering processes. Ferrihydrite is often an intermediate phase in the transformation of iron-bearing minerals, and its presence suggests that the weathering process is still active. The hyperfine parameters of ferrihydrite, particularly its QS values ranging from ~0.87 to ~1.07 mm/s, are consistent with its identification as a poorly crystalline iron oxyhydroxide. The differences in mineral composition between the coarse and fine saprolite highlight the varying degrees of weathering and mineral transformation. The coarse saprolite, with its higher chlorite content, likely represents a less weathered material, while the fine saprolite, dominated by goethite and hematite, reflects a more advanced stage of weathering. The presence of magnetite in samples 505-35 and 505-43 adds further complexity to the mineralogical profile, suggesting localized reducing conditions or specific geochemical processes that favor magnetite formation.
Similar studies suggest that iron released during the conversion of chlorite to vermiculite was retained in the profile as ferrihydrite. Over time, ferrihydrite underwent dehydration, ultimately evolving to form goethite and hematite [54,55,56]. Direct precipitation of goethite is rarely observed [56,57]. Conversely, much of the magnesium released from chlorite was leached out due to its high mobility [58,59].
Magnetite, a common iron mineral in IOCG deposits [60,61], is generally resistant to oxidation in the surface environment and tends to accumulate in weathering profiles [62]. However, some magnetite can be partially oxidized to ferrihydrite, maghemite, or hematite [63]. The presence of relic magnetite octahedra supports the interpretation that the saprolite derives from mineralized chloritites, as magnetite is a typical accessory mineral in hypogene mineralization.
This mineral assemblage suggests that the investigated saprolite represents the lower portion of a thicker lateritic profile, with weak to moderate mineral transformations. In this environment, ferromagnesian minerals, such as chlorite, are partially preserved, leading to the retention of high MgO content throughout the profile. Secondary minerals, including smectites and vermiculites, which are less stable than kaolinite, are partially preserved [58,59].
5.2. Elemental Retention
The distributions of Al2O3, Fe2O3, and MgO contents are mainly controlled by chlorite weathering, which is partially preserved in the coarse and fine saprolite due to decreased susceptibility to dissolution caused by iron oxyhydroxides’ generation on its edges and planes [64]. These ferruginous films prevent the diffusion of the more mobile elements [58,59].
The hypogene mineralization presents higher oxidation potential than the hosting chloritites, gossan, and saprolite due to the abundance of chalcopyrite, which faces a rapid and profound change in the S oxidation state [65] (Figure 9). The chalcopyrite dissolution releases H+, resulting in pH values between four and five, in accordance with its typical low acidifying potential [66,67,68]. Under such conditions, mineral inclusions in the chalcopyrite are also dissolved, leading to a redistribution of Cu, Au, Ag, Bi, Pb, Sn, Zn, and U, as will be discussed below.
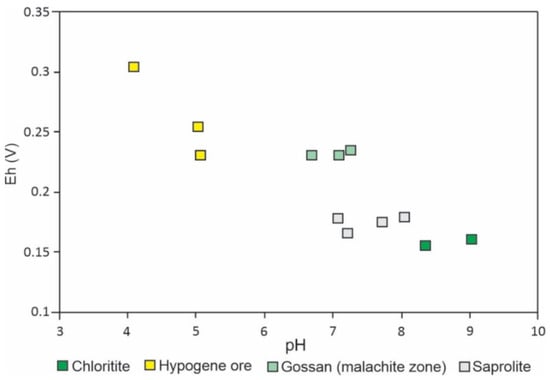
Figure 9.
pH-Eh conditions measured for selected samples of hypogene mineralization, gossan, and saprolite.
Conversely, new formation of gossan minerals and hydrolysis of chlorites from the host rocks results in the consumption of H+, with pH elevation. The malachite predominance in gossan samples accounts for the more alkaline values [69,70,71]. Chlorites from the host rocks and kaolinite from the saprolite tend to consume H+ during hydrolysis reactions, leading to higher pH values [67]. These data demonstrate a minor effect of hypogene mineralization on the pH and Eh conditions of the saprolite.
In addition, selected elements were analyzed to assess the contribution of hypogene mineralization to the saprolite’s geochemistry: Au, Ag, Bi, Pb, Sn, Zn, and U. Except for Zn and Bi, these metals are attributed to chalcopyrite and its mineral inclusions, including petzite, altaite, galena, cassiterite, stannite, sphalerite, and uraninite. In the studied deposit, the gossan is considered a direct product of hypogene mineralization, while the saprolite is derived from the host rocks.
The high chemical variability observed in all analyzed elements is attributed to the alternation between massive and disseminated sulfides, resulting in a gossan with a corresponding spatial distribution (Figure 10). Therefore, the lower concentrations of most elements (except Zn) in the gossan, compared to the hypogene mineralization, are primarily due to the more disseminated distribution of newly formed minerals, rather than intense leaching.
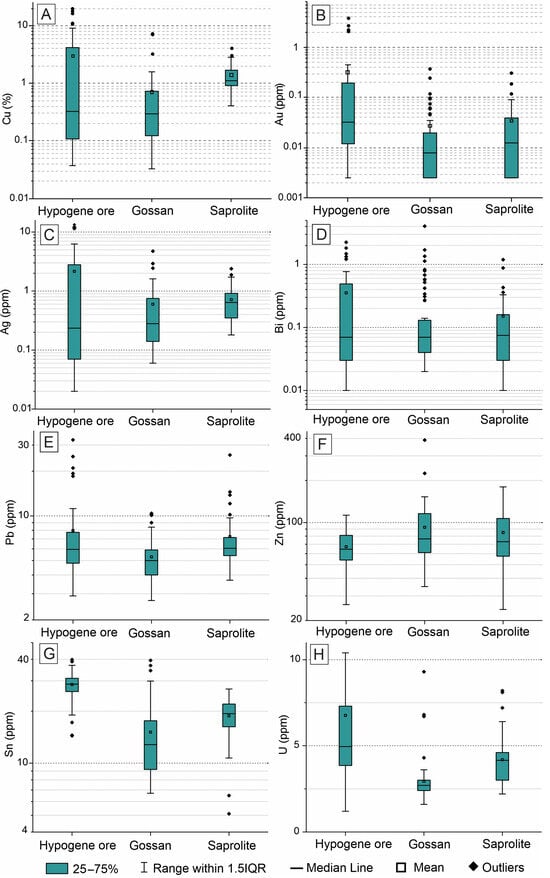
Figure 10.
Geochemical fractionation of selected elements in hypogene mineralization, gossan, and saprolite from drill hole A118-505: Cu (A), Au (B), Ag (C), Bi (D), Pb (E), Zn (F), Sn (G), and U (H). Sample size: hypogene mineralization (n = 44); gossan (n = 62); saprolite (n = 40).
However, the saprolite is not a direct product of hypogene mineralization or gossan but is derived from the silicate host rocks. Consequently, the increase in average concentrations of these elements reflects the saprolite’s capacity for chemical retention. The average CuO content in the saprolite is generally higher or similar to that in the gossan and hypogene mineralization. This same trend is observed for Ag, Bi, Pb, Sn, and U (Figure 10C–G), supporting the strong retention capacity of saprolite minerals for metals beyond Cu. No other minerals containing Ag, Bi, Pb, Sn, or U as major components were identified in the saprolite, except for cassiterite inclusions within magnetite octahedrons. However, iron oxyhydroxides are thought to have contributed significantly to the partial retention of these elements [58].
5.3. Copper Immobilization and Metallurgical Implications
Due to their relatively low abundance in the saprolite, chlorite, vermiculite, and smectite were considered to contribute minimally to the economically relevant copper contents. However, CuO values of up to 2 wt.% have been reported in both natural and synthesized smectites [72,73]. In contrast, kaolinite, as a major component, appears more likely to contribute to copper retention, which is typically attributed to Cu/Al substitution in the octahedral sheets rather than Cu²+ adsorption [74].
FTIR analysis suggests that the presence of CuO in kaolinite would cause a broadening and loss of resolution in the 3619 to 3695 cm−1 bands [74], but such effects were not observed in the Alvo 118 samples. Thus, based on a comparison with the theoretical data of [74], the behavior of the OH stretching bands indicates that the CuO concentration in Alvo 118 kaolinites is below 10 ppm. Consequently, although kaolinite is the predominant mineral in the saprolite profile, it cannot account for the Cu contents of around 1% observed in whole rock analyses.
The estimation of goethite, ferrihydrite, hematite, chlorite, and magnetite contents through Mössbauer spectroscopy enabled the determination of Pearson correlations with CuO contents. This analysis revealed a moderate to strong positive correlation between copper and iron oxyhydroxides. Specifically, the moderate correlation between CuO contents in whole samples and goethite content (r = 0.71) suggests that this mineral plays a significant role in copper retention in the saprolite (Figure 11A). Additionally, the correlation of CuO with ferrihydrite (r = 0.80) suggests that amorphous or low-crystallinity ferruginous phases make a greater contribution to copper retention (Figure 11B). The distribution of hematite and chlorite shows no significant correlation with CuO contents (Figure 11C,D).
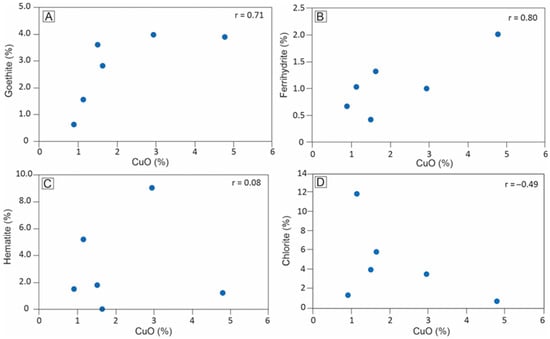
Figure 11.
Distribution of CuO (wt.%) versus potential Cu mineral sink in the Alvo 118 saprolite (drill hole A118-505): CuO × goethite (A); CuO × ferrihydrite (B); CuO × hematite (C); CuO × chlorite (D). CuO contents were determined through ICP-AES analysis of whole samples of the saprolite.
The stronger correlation of CuO with ferrihydrite compared to goethite is consistent with data from selective extraction studies in the Salobo mine (CMP), located 6 km northwest of the Alvo 118 deposit, where iron oxyhydroxides exhibit a wide range of crystallinity, and copper is primarily associated with the lower crystallinity phases [5]. The role of iron oxyhydroxides as carriers of base metals is well-established and generally attributed to their high structural imperfection and nanometer-scale particle size, which enhance surface reactivity [75,76]. The intermediate correlation between CuO and both goethite and ferrihydrite contents contrasts with the weak correlation between Fe2O3 and CuO (r = 0.03) due to the presence of other iron-bearing minerals, such as hematite, chlorite, and magnetite, which show weak correlations with CuO.
For saprolite ores, such as those discussed in this study, the development of metallurgical processes must account for the fact that copper is hosted by at least two distinct phases (goethite and ferrihydrite), each with different strength constraints. Percolation leaching is commonly recommended for the exploitation of this type of mineralization. This process effectively removes copper associated with iron oxyhydroxides under moderately acidic conditions, as iron oxyhydroxides like goethite and ferrihydrite are highly susceptible to breaking down into ferric ions in such environments. This breakdown releases adsorbed metals, including copper [77]. It is important to consider the associated phyllosilicates, as they can act as acid consumers during the process [5,21].
The dominance of kaolinite in the Alvo 118 saprolite, with low levels of swelling clays, is advantageous for percolation leaching, as smectite can trap acid molecules within its structure [20,21]. In contrast, chlorite, which is more abundant in the coarse saprolite, is more prone to acid consumption in strongly acidic environments due to its incongruent dissolution [77,78,79].
Recent advancements in percolation leaching technology have significantly improved operational efficiency and recovery rates [21,80,81]). Similar mineralized saprolite in the nearby Sossego mine has been described but remains unexploited [4,5]. This consideration is crucial for maximizing the profitability of Alvo 118.
6. Conclusions
The saprolite profile overlying the Alvo 118 deposit is primarily derived from the underlying chloritites given the chemical influence of the total decomposition of hypogene mineralization, without significant contribution from the granodiorites. Weathering of chlorite is the primary factor controlling the mineralogical composition of the saprolite, where vermiculite and smectite form as intermediate phases, and kaolinite (the predominant phase) represents the final product. Iron released during the weathering of chlorite is retained in the profile as ferrihydrite, goethite, and hematite.
The parent rock sections hosting hypogene copper ore at depth underwent initial supergene alteration, resulting in the formation of an immature gossan. Closer to the surface, disseminated hypogene mineralization weathered along with the chloritites, forming a saprolite horizon exposed by subsequent erosion. This horizon displays a mineralogical association typical of weak to moderate weathering, with abundant magnetite octahedrons still preserved within the kaolinite–vermiculite–smectite matrix.
Copper released from chalcopyrite formed a supergene dispersion halo within the saprolite, which also inherited Au, Ag, Bi, Pb, Sn, Zn, and U from hypogene mineralization. This study shows that ferrihydrite and goethite played a key role in copper retention across the weathering profile, while no evidence was found for copper retention by clay minerals. Consequently, percolation leaching is considered a suitable copper extraction technique for this mineralization type, especially given the low content of expandable clay minerals.
Author Contributions
Conceptualization, P.H.C.d.S. and M.L.d.C.; methodology, P.H.C.d.S. and R.d.S.S.d.S.; validation, N.S.F. and M.A.C.; formal analysis, P.H.C.d.S., R.d.S.S.d.S. and M.A.C.; investigation, P.H.C.d.S.; resources, M.L.d.C.; writing—original draft preparation, P.H.C.d.S.; writing—review and editing, M.L.d.C., N.S.F. and M.A.C.; supervision, M.L.d.C.; project administration, M.L.d.C.; funding acquisition, M.L.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq) grant numbers 305015/2016-8; 442871/2018-0; 304.967/2022-0. The APC was funded by MDPI.
Data Availability Statement
Data available on request due to legal restrictions.
Acknowledgments
The authors want to thank Vale S.A. for granting access to the exploration areas, providing logistical support during field activities, and granting access to drill cores and exploratory data. Special thanks to geologist Clóvis Maurity for facilitating the partnership with Vale S.A. and to the Geosciences Institute of the Federal University of Pará and the Brazilian Center for Research in Physics for providing the analytical facilities. Finally, we thank the editors and reviewers of Minerals for their thoughtful comments and constructive suggestions, which helped improve the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Costa, M.L.; Angélica, R.S.; Fonseca, L.R. Geochemical exploration for gold in deep weathered lateritized gossans in the Amazon region-Brazil: A case history of the Igarapé Bahia deposit. Geochim. Bras. 1996, 10, 13–26. [Google Scholar]
- Costa, M.L.; Angélica, R.S.; Costa, N.C. The geochemical association Au–As–B–(Cu)–Sn–W in latosol, colluvium, lateritic iron crust and gossan in Carajás, Brazil: Importance for primary ore identification. J. Geochem. Explor. 1999, 67, 33–49. [Google Scholar] [CrossRef]
- Porto, C.G. Geochemical exploration challenges in the regolith dominated Igarapé Bahia gold deposit, Carajás, Brazil. Ore Geol. Rev. 2016, 73, 432–450. [Google Scholar] [CrossRef]
- Toledo-Groke, M.C.; Melfi, A.J.; Parisot, J.C. Comportamento do cobre durante o intemperismo das rochas xistosas cupríferas do Salobo 3A, Serra dos Carajás. Geochim. Bras. 1987, 2, 187–200. [Google Scholar]
- Veiga, M.M.; Schorscher, H.D.; Fyfe, W.S. Relationship of copper with hydrous ferric oxides: Salobo, Carajás, PA, Brazil. Ore Geol. Rev. 1991, 6, 245–255. [Google Scholar] [CrossRef]
- Grainger, C.J.; Groves, D.I.; Tallarico, F.H.B.; Fletcher, I.R. Metallogenesis of the Carajás Mineral Province, Southern Amazon Craton, Brazil: Varying styles of Archean through Paleoproterozoic to Neoproterozoic base- and precious-metal mineralisation. Ore Geol. Rev. 2008, 33, 451–489. [Google Scholar] [CrossRef]
- Vasconcelos, P.M.; Renne, P.R.; Brimhall, G.H.; Becker, T.A. Direct dating of weathering phenomena by 40Ar39Ar and K-Ar analysis of supergene K-Mn oxides. Geochim. Cosmochim. Acta 1994, 58, 1635–1665. [Google Scholar] [CrossRef]
- King, L.C. A Geomorfologia do Brasil Oriental. Rev. Bras. Geogr. 1956, 18, 147–265. [Google Scholar]
- Monteiro, H.S.; Vasconcelos, P.M.P.; Farley, K.A.; Lopes, C.A.M. Age and evolution of diachronous erosion surfaces in the Amazon: Combining (U-Th)/He and cosmogenic 3He records. Geoch Cosmo Acta 2018, 229, 162–183. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Costa, M.L. Mineralogical and textural evolution of the Alvo 118 copper-bearing gossan: Implications for supergene metallogenesis in Carajás Mineral Province, Brazil. J. S. Am. Earth Sci. 2023, 121, 104108. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Andrade, P.J.M.B.; Maurity, C.; Kwitko, R. Geologia e características das mineralizações cupríferas do depósito Alvo 118. In VII Simpósio de Geologia da Amazônia; Província Mineral de Carajás: Pará, Brazil, 2001; pp. 5–8. [Google Scholar]
- Santos, P.H.S.; Costa, M.L.; Roerdink, D.R. Geochemical and Isotopic Fractionation in the Hypogene Ore, Gossan, and Saprolite of the Alvo 118 Deposit: Implications for Copper Exploration in the Regolith of the Carajás Mineral Province. Minerals 2023, 13, 1441. [Google Scholar] [CrossRef]
- Docegeo; Internal Report; Departamento Nacional de Produção Mineral: Rio de Janeiro, Brazil, 1991.
- Mano, E.S.; Caner, L.; Petit, S.; Chaves, A.P. Mineralogical characterization of copper lateritic ore from the Furnas deposit—Carajás, Brazil. Int. Eng. J. 2020, 73, 329–335. [Google Scholar] [CrossRef]
- Mano, E.S.; Caner, L.; Chaves, A.P. Methodology for laterítics Cu-bearing clay minerals characterization. Holos 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Ildefonse, P.; Manceau, A.; Prost, D.; Toledo-Groke, M.C. Hydroxy-Cu vermiculite formed by the weathering of Fe-biotites at Salobo, Carajás, Brazil. Clays Clay Miner. 1986, 34, 338–345. [Google Scholar] [CrossRef]
- Oliveira, S.M.B.; Silva, M.L.M.; Toledo, M.C.M. The role of residual 2, 1 phyllosilicates in lateritic metallogenesis: Ni and Cu deposits in Serra dos Carajás, Brazilian Amazônia. Geochim. Bras. 1995, 9, 161–171. [Google Scholar]
- Oliveira, S.M.B.; Imbernon, R.A.L.; Partiti, C.S.M.; Rechenberg, H.R. Mössbauer spectroscopic study of iron oxides and oxyhydroxides in gossans. Geoderma 1996, 73, 245–256. [Google Scholar] [CrossRef]
- Dube, A.; Zbytniewski, R.; Kowalkowski, T.; Cukrowska, E.; Buszewski, B. Adsorption and migration of heavy metals in soil. Pol. J. Environ. Stud. 2001, 10, 1–10. [Google Scholar]
- Chetty, D. Acid-gangue interactions in heap leach operations: A review of the role of mineralogy for predicting ore behavior. Minerals 2018, 8, 47. [Google Scholar] [CrossRef]
- Thomas, M. Understanding gangue acid consumption in copper sulfide heap leaching: Predicting the impact of carbonates, silicates and secondary precipitates. Miner. Eng. 2021, 171, 107090. [Google Scholar] [CrossRef]
- Silva, E.R.P.; Kotschoubey, B. Alteração supergênica do depósito de cobre-ouro do Salobo, Serra dos Carajás-PA—Ênfase no comportamento do cobre. Rev. Bras. Geociências 2000, 30, 623–630. [Google Scholar] [CrossRef]
- Ker, J.C. Latossolos do Brasil: Uma Revisão. Rev. Geonomos 1997, 5, 17–40. [Google Scholar] [CrossRef]
- Eggleton, R.A. The Regolith Glossary, Surficial Geology, Soils and Landscapes; CRC LEME: Camberra, Australia, 2001; p. 144. [Google Scholar]
- Maurity, C.W.; Kotschoubey, B. Evolução recente da cobertura de alteração no platô N1- Serra dos Carajás-Pa: Degradação, pseudocarstificação, espeleotemas. Bol. Mus. Para Emílio Goeldi 1995, 7, 331–362. [Google Scholar]
- Tallarico, F.H.B.; Figueiredo, B.R.; Groves, D.I.; Kositcin, N.; McNaughton, N.J.; Fletcher, I.R.; Rego, J.L. Geology and SHRIMPU–Pb geochronology of the Igarapé Bahia Deposit, Carajás Copper–Gold Belt, Brazil: An Archean (2.57 Ga) example of iron–oxide Cu–Au–(U–REE) mineralization. Econ. Geol. 2005, 100, 7–28. [Google Scholar] [CrossRef]
- Torresi, I.; Xavier, R.P.; Bortholoto, D.F.A.; Monteiro, L.V.S. Hydrothermal alteration, fluid inclusions and stable isotope systematics of the Alvo 118 iron oxide–copper–gold deposit, Carajás Mineral Province (Brazil): Implications for ore genesis. Miner. Depos. 2011, 47, 299–323. [Google Scholar] [CrossRef]
- USGS. Shuttle Radar Topography Mission (SRTM). 2018. Available online: https://www.usgs.gov/centers/eros/science/usgs-eros-archive-digital-elevation-shuttle-radar-topography-mission-srtm-1 (accessed on 5 May 2025).
- Munsell Color (Firm). Munsell Soil-Color Charts; Munsell Color: Boston, MA, USA, 1992. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Starkey, H.C.; Blackmon, P.D.; Hauff, P.L. The Routine Mineralogical Analysis of Clay-Bearing Samples. 1984. Available online: https://pubs.usgs.gov/bul/1563/report.pdf (accessed on 5 May 2025).
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 400. [Google Scholar]
- Farmer, V.C. Differing effects of particle size and shape in the infrared and Raman spectra of kaolinite. Clay Miner. 1998, 33, 601–604. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Murad, E.; Schwertmann, U. The Möessbauer spectrum of ferrihydrite and its relations to those of other iron oxides. Ame Min. 1980, 65, 1044–1049. [Google Scholar]
- Imbernon, R.A.L.; Blot, A.; Pereira, V.P.; Franco, D.R. Characterization of Zn-bearing chlorite by Mössbauer (ME) and infrared spectroscopy (IR)—Occurrence associated to the Pb-Zn-Ag deposits of Canoas, PR, Brazil. Braz. J. Geol. 2011, 41, 28–236. [Google Scholar] [CrossRef][Green Version]
- Vandenberghe, R.E.; De Grave, E. Application of Mössbauer spectroscopy in earth sciences. In Mössbauer Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–185. [Google Scholar] [CrossRef]
- Bowen, L.H.; De Grave, E.; Vandernberghe, R.E. Mössbauer Effect Studies of Magnetic Soils and Sediments. In Mössbauer Spectroscopy Applied to Magnetism and Materials Science; Long, G.J., Grandjean, F., Eds.; Plenum Press: New York, NY, USA, 1993; Volume 1, pp. 115–159. [Google Scholar]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Smitn, S.C.; Dohnalkova, A.C.; Russell, C.K. Transformation of 2-line ferrihydrite to 6-line ferrihydrite under oxic and anoxic conditions. Miner. Soc. Am. 2003, 88, 11–12. [Google Scholar] [CrossRef]
- Guyodo, Y.; Banerjee, S.K.; Lee Penn, R.; Burleson, D.; Berqui, T.S.; Seda, T.; Solheid, P. Magnetic properties of synthetic six-line ferrihydrite nanoparticles. Phys. Earth Planet. Inter. 2006, 154, 222–233. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Ziganshina, E.E.; Byrne, J.; Gerlach, R.; Struve, E.; Biktagirov AKappler, A. Fe(III) mineral reduction followed by partial dissolution and reactive oxygen species generation during 2,4,6-trinitrotoluene transformation by the aerobic yeast Yarrowia lipolytica. AMB Express 2015, 5, 8. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Koopal, L.K.; Li, W.; Xu, W.; Liu, F.; Zhang, J.; Liu, Q.; Feng, X.; Sparks, D. Effects of crystallite size on the structure and magnetism of ferrihydrite. Envron. Sci. Nano 2016, 3, 190–202. [Google Scholar] [CrossRef]
- Zhao, Z.; Yao, L.; Li, J.; Ma, X.; Han, L.; Lin, Z.; Guan, S. Determination of interactions of ferrihydrite-humic acid-Pb (II) system. Environ. Sci. Pollut. Res. 2022, 29, 21561–21575. [Google Scholar] [CrossRef]
- Airey, P.L. Radionuclide migration around uranium ore bodies in the Alligator Rivers Region of the Northern Territory of Australia–Analogue of radioactive waste repositories—A review. Chem. Geol. 1986, 55, 255–268. [Google Scholar] [CrossRef]
- Murakami, T. Weathering of Chlorite in a Quartz-Chlorite Schist: I. Mineralogical and Chemical Changes. Clays Clay Miner. 1996, 44, 244–256. [Google Scholar] [CrossRef]
- Sirbu-Radasanu, D.S.; Huzum, R.; Dumitraş, D.-G.; Stan, C.O. Mineralogical and Geochemical Implications of Weathering Processes Responsible for Soil Generation in Mănăila Alpine Area (Tulgheş 3 Unit—Eastern Carpathians). Minerals 2022, 12, 1161. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Little, I.P. Weathering of chlorite and some associations of trace elements in Permian phyllites in Southeast Queensland. Geoderma 1972, 7, 233–247. [Google Scholar] [CrossRef]
- Ross, G.J.; Kodama, H. Experimental alteration of a chlorite into a regularly interstratified chlorite vermiculite by chemical oxidation. Clays Clay Miner. 1976, 24, 183–190. [Google Scholar] [CrossRef]
- Schulze, D.G. Clay minerals. In Encyclopedia of Soils in the Environment; Hille, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 246–254. [Google Scholar]
- Aspandiar, M.F.; Eggleton, R.A. weathering of chlorite: I. Reactions and products in microsystems controlled by the primary mineral. Clays Clay Miner. 2002, 50, 685–698. [Google Scholar] [CrossRef]
- Banfield, J.F. Transmission Electron Microscope Study of Biotite Weathering. Clays Clay Miner. 1988, 36, 47–60. [Google Scholar] [CrossRef]
- Carnicelli, S.; Mirabella, A.; Cecchini, G.; Sanesi, G. Weathering of Chlorite to a Low-Charge Expandable Mineral in a Spodosol on the Apennine Mountains, Italy. Clays Clay Miner. 1997, 45, 28–41. [Google Scholar] [CrossRef]
- Castaldini, M.; Mirabella, A.; Sartori, G.; Fabiani, A.; Santomassimo, F.; Miclaus, N. Soil development and microbial community along an altitudinal transect in trentino mountains. Dev. Soil Sci. 2002, 28, 217–228. [Google Scholar] [CrossRef]
- Nickel, E.H.; Daniels, J.L. Gossans. In Handbook of Strata-Bound and Stratiform Ore Deposits; Wolf, K.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 261–390. [Google Scholar]
- Spier, C.A.; Levett, A.; Rosière, C.A. Geochemistry of canga (ferricrete) and evolution of the weathering profile developed on itabirite and iron ore in the Quadrilátero Ferrífero, Minas Gerais, Brazil. Miner. Depos. 2018, 54, 983–1010. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Moore, F.; Asadi, S. Leached caps mineralogy and geochemistry as supergene enrichment fertility indicators, Meiduk and Parkam porphyry copper deposits, SW Iran. J. Geochem. Explor. 2018, 194, 198–209. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. Effect of pH on the Formation of Goethite and Hematite from Ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Butt, C.R.M.; Zeegers, H. Regolith Exploration Geochemistry in Tropical and Subtropical Terrains. In Handbook of Exploration Geochemistry, 4; Elsevier: Amsterdam, The Netherlands, 1992; p. 607. [Google Scholar]
- Butt, C.R.M.; Lintern, M.J.; Anand, R.R. Evolution of regoliths and landscapes in deeply weathered terrain—Implications for geochemical exploration. Ore Geol. Rev. 2000, 16, 167–183. [Google Scholar] [CrossRef]
- Barley, M.E.; Pickard, A.L.; Hagemann, S.G.; Folkert, S.L. Hydrothermal origin for the 2-billion-year-old Mount Tom Price giant iron ore deposit, Hamersley Province, Western Australia. Miner. Depos. 1999, 34, 784–789. [Google Scholar] [CrossRef]
- Montreuil, J.-F.; Potter, E.G.; Corriveau, L.; Davis, W.J. Element mobility patterns in magnetite-group IOCG systems: The Fab IOCG system, Northwest Territories, Canada. Ore Geol. Rev. 2016, 72, 562–584. [Google Scholar] [CrossRef]
- Graham, R.C. Weathering of Iron-Bearing Minerals in Soils and Saprolite on the North Carolina Blue Ridge Front: I. Sand-Size Primary Minerals. Clays Clay Miner. 1989, 37, 19–28. [Google Scholar] [CrossRef]
- Habteselassie, M.M.; Mathison, C.I.; Gilkes, R.J. Vanadium in magnetite gabbros and its behaviour during lateritic weathering, Windimurra Complex, Western Australia. Aust. J. Earth Sci. 1996, 43, 555–566. [Google Scholar] [CrossRef]
- Acker, J.G.; Bricker, O.P. The influence of pH on biotite dissolution and alteration kinetics at low temperature. Geochim. Cosmochim. Acta 1992, 56, 3073–3092. [Google Scholar] [CrossRef]
- Thornber, M.R.; Taylor, G.F. The mechanisms of sulphide oxidation and gossan formation. Handb. Explor. Geochem. 1992, 4, 119–138. [Google Scholar] [CrossRef]
- Nickel, E.H. The mineralogy and geochemistry of the weathering profile of the Teutonic Bore Cu-Pb-Zn-Ag sulphide deposit. J. Geochem. Explor. 1984, 22, 239–263. [Google Scholar] [CrossRef]
- Chávez, W. Supergene oxidation of copper deposits: Zoning and distribution of copper oxide minerals. Soc. Econ. Geol. News 2000, 41, 10–21. [Google Scholar] [CrossRef]
- Atapour, H.; Aftabi, A. The geochemistry of gossans associated with Sarcheshmeh porphyry copper deposit, Rafsanjan, Kerman, Iran: Implications for exploration and the environment. J. Geochem. Explor. 2007, 93, 47–65. [Google Scholar] [CrossRef]
- Brown, A.C. Refinements for footwall red-bed diagenesis in the sediment-hosted stratiform copper deposits model. Econ. Geol. 2005, 100, 765–771. [Google Scholar] [CrossRef]
- Putter, T.; Mees, F.; Decrée, S.; Dewaele, S. Malachite, an indicator of major Pliocene Cu remobilization in karstic environment (Katanga, Democratic Republic of Congo). Ore Geol. Rev. 2010, 38, 90–100. [Google Scholar] [CrossRef]
- Papineau, D. Chemically oscillating reactions in the formation of botryoidal malachite. Ame Miner. 2020, 105, 447–454. [Google Scholar] [CrossRef]
- Mosser, C.; Mestdagh, M.; D’Ecarreau Herbillon, A.J. Spectroscopic (ESR, EXAFS) evidence of Cu for (Al-Mg) substitution in octahedral sheets of smectites. Clay Miner. 1990, 25, 271–282. [Google Scholar] [CrossRef]
- Mosser, C.; Mosser, A.; Romeo, M.; Petit, S.; Decarreau, A. Natural and synthetic copper phyllosilicates studied by XPS. Clays Clay Miner. 1992, 40, 593–599. [Google Scholar] [CrossRef]
- Petit, S.; Decarreau, A.; Mosser, C.; Ehret, G.; Grauby, O. Hydrothermal Synthesis (250 °C) of Copper-Substituted Kaolinites. Clays Clay Miner. 1995, 43, 482–494. [Google Scholar] [CrossRef]
- Strauss, R.; Brummer, G.W.; Barrow, N.J. Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Frost, R.L. An overview of the role of goethite surfaces in the environment. Chemosphere 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Taylor, A. Overview of Gangue Mineralogy Issues in Oxide Copper Heap Leaching. International Project Development Services Pty Limited. 2003. Available online: https://emrlibrary.gov.yk.ca/minerals/MajorMines/carmackscopper/overview_of_gangue_mineralogy_issues.pdf (accessed on 5 May 2025).
- Snäll, S.; Liljefors, T. Leachability of major elements from minerals in strong acids. J. Geochem. Explor. 2000, 71, 1–12. [Google Scholar] [CrossRef]
- Tan, H.; Skinner, W.; Addai-Mensah, J. Leaching behaviour of low and high Fe substituted chlorite clay minerals at low pH. Hydrometallurgy 2012, 125, 100–108. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Min. Proc. Ext. Met. Rev. 2015, 37, 73–119. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Alvear Flores, G.R.F. Hydrometallurgical copper extraction. In Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2022; pp. 361–406. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).