Mineralogy of Petrified Wood from Costa Rica
Abstract
1. Introduction
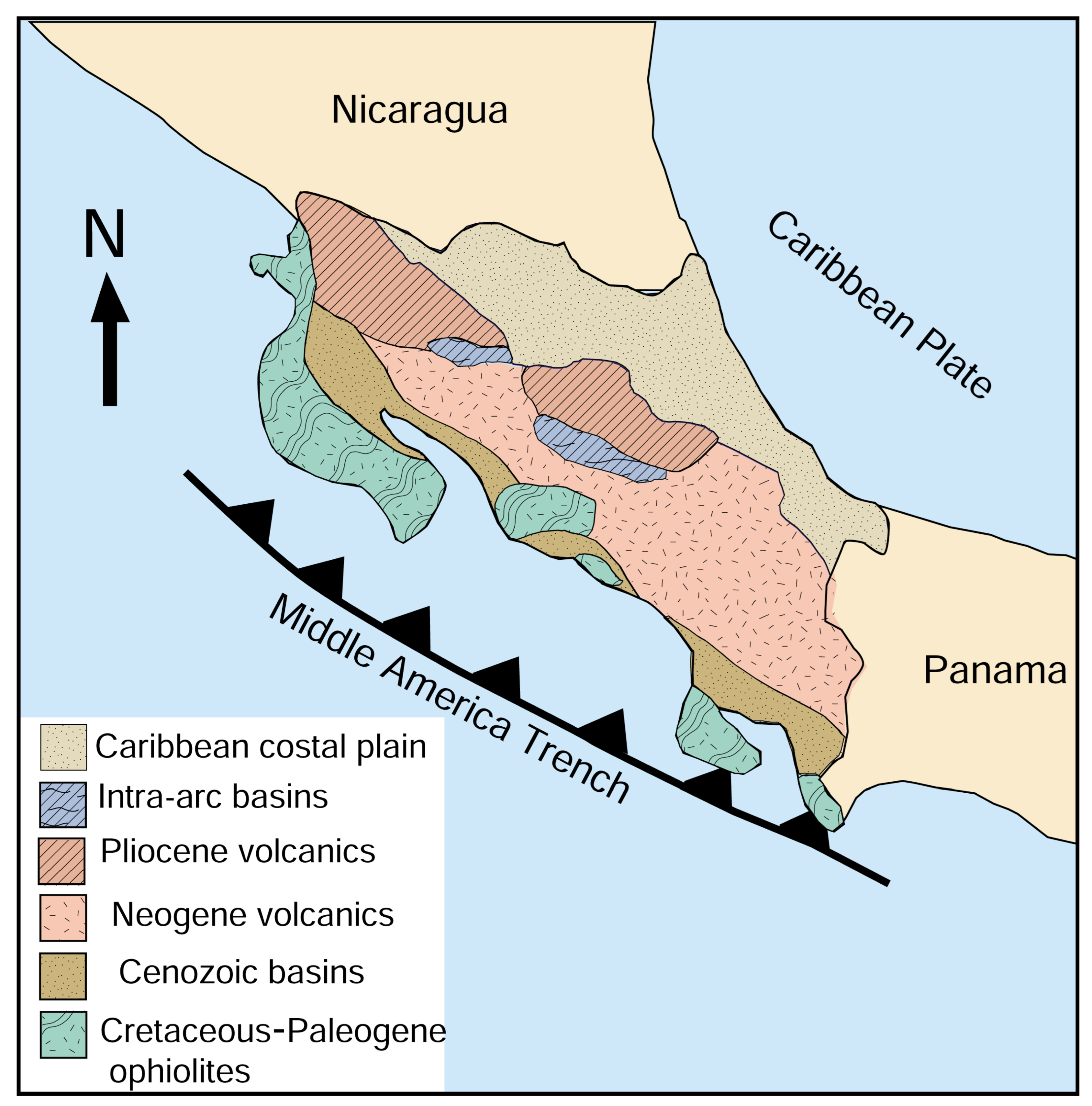
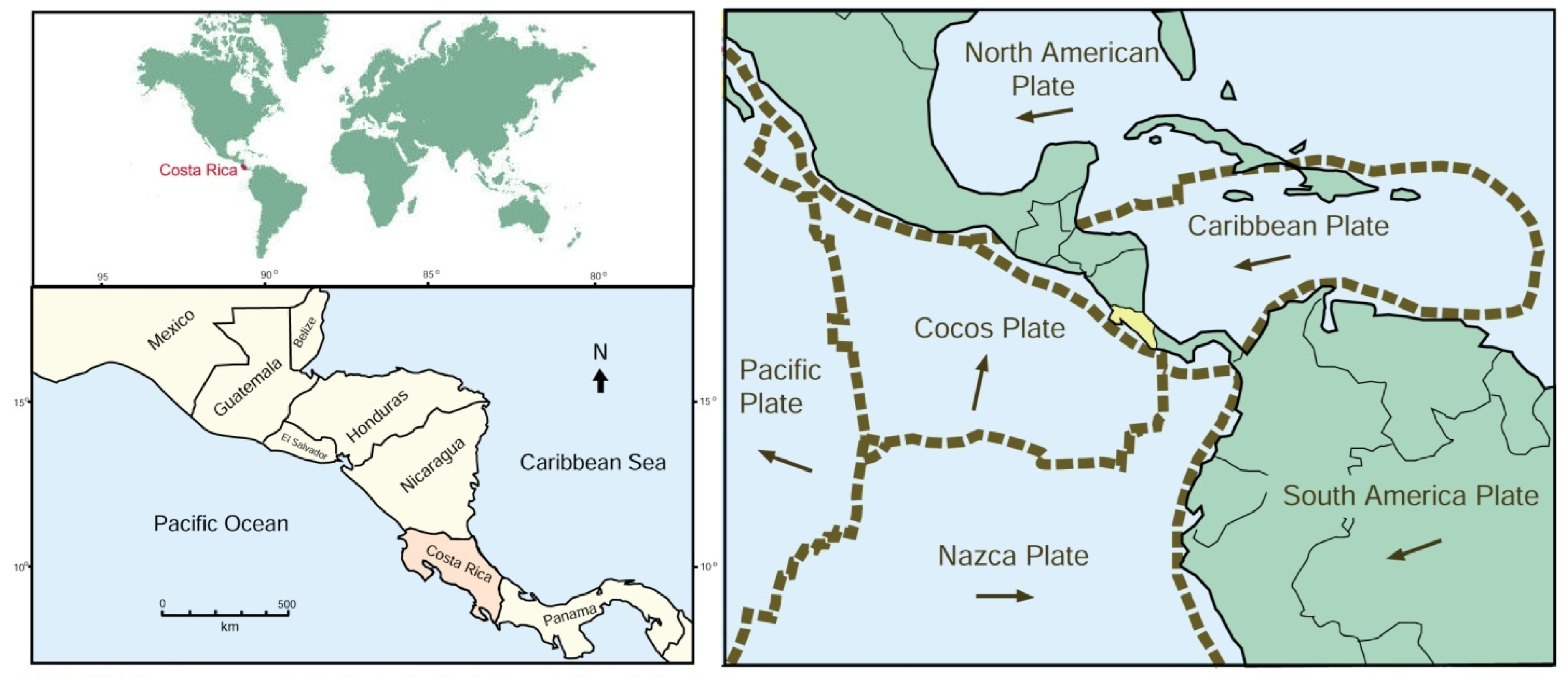
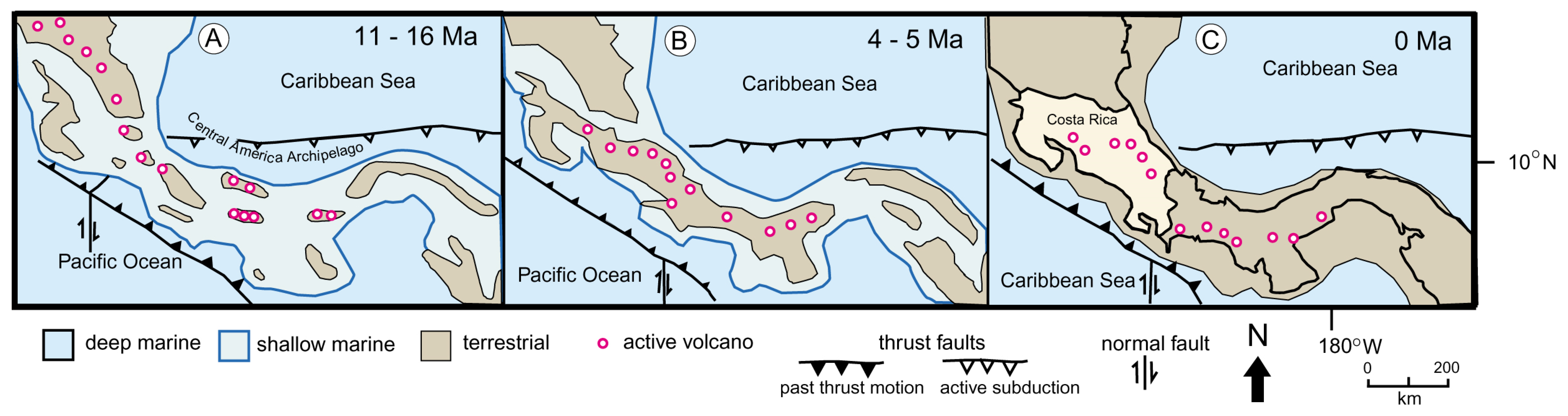
1.1. Geologic Setting
1.2. Paleobiology of Central America
1.3. History of Paleobotany Research in Central America
2. Methods and Materials
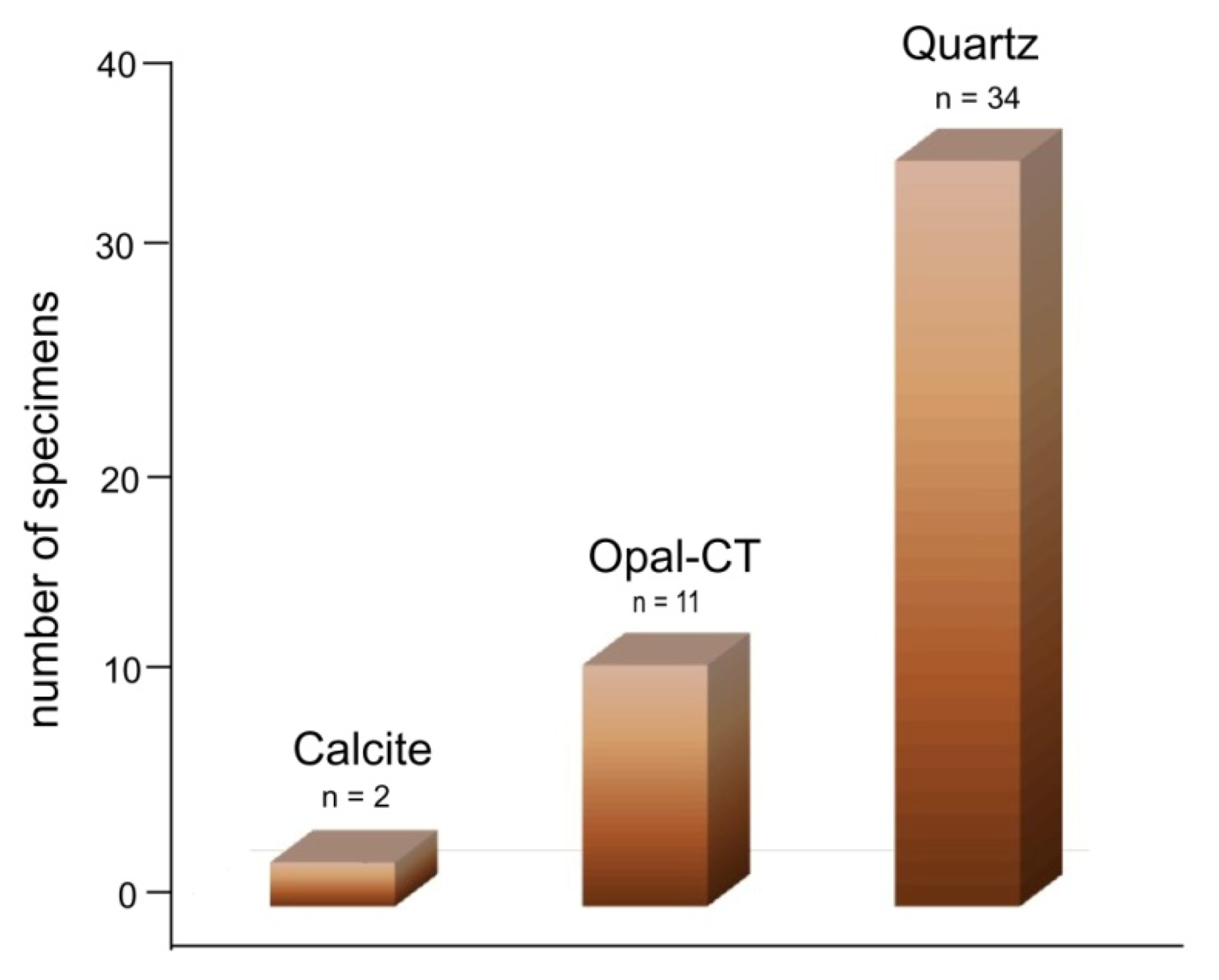
3. Results
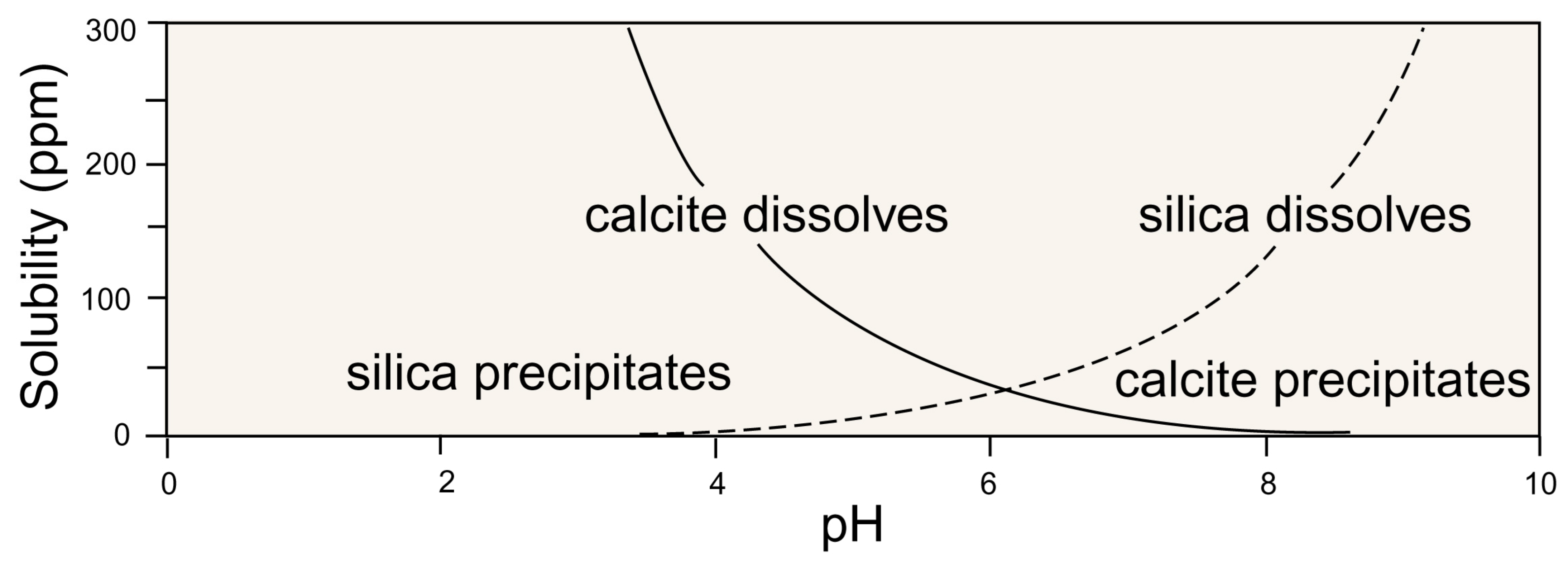
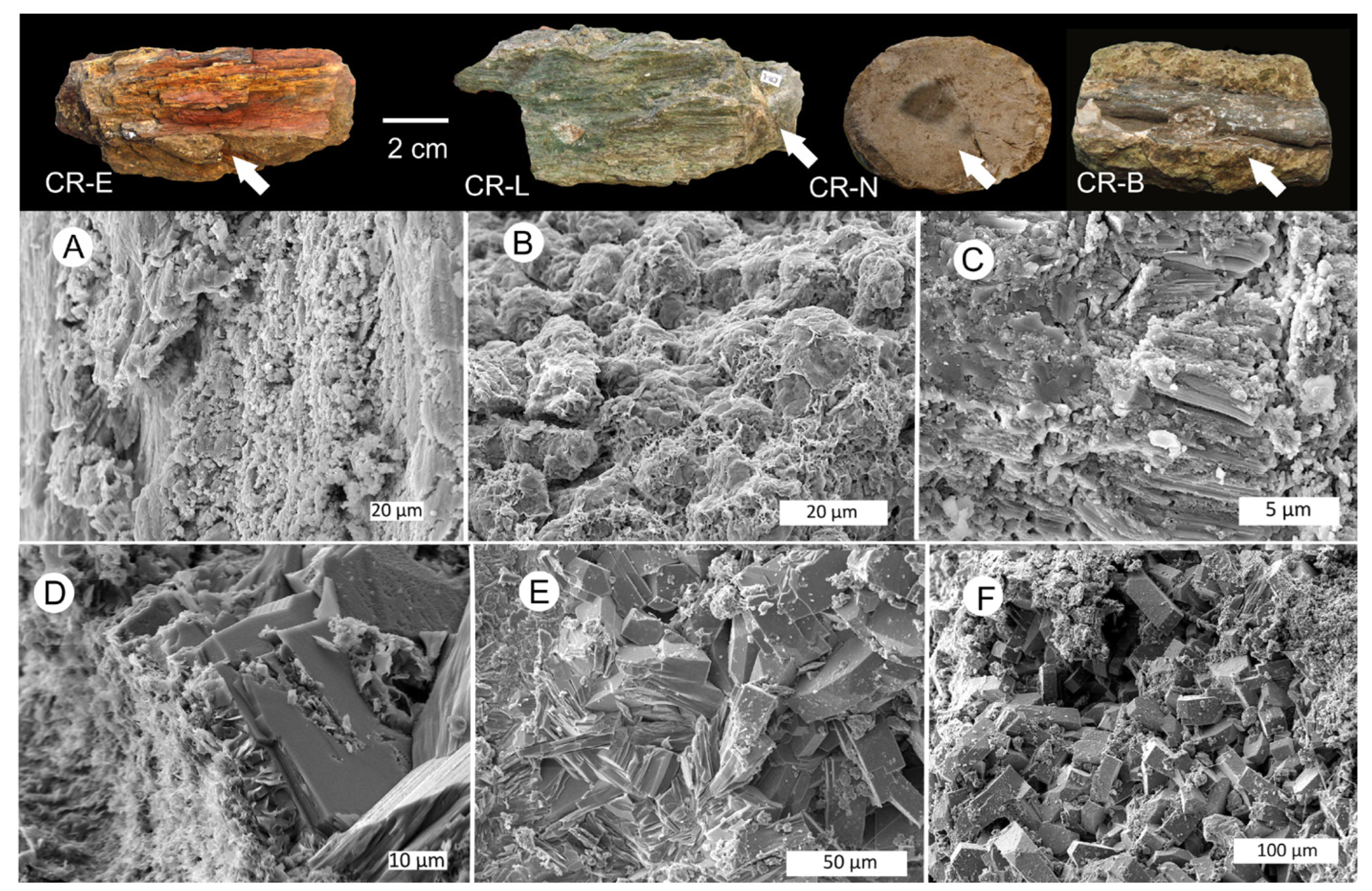
3.1. Wood Fossilization Processes
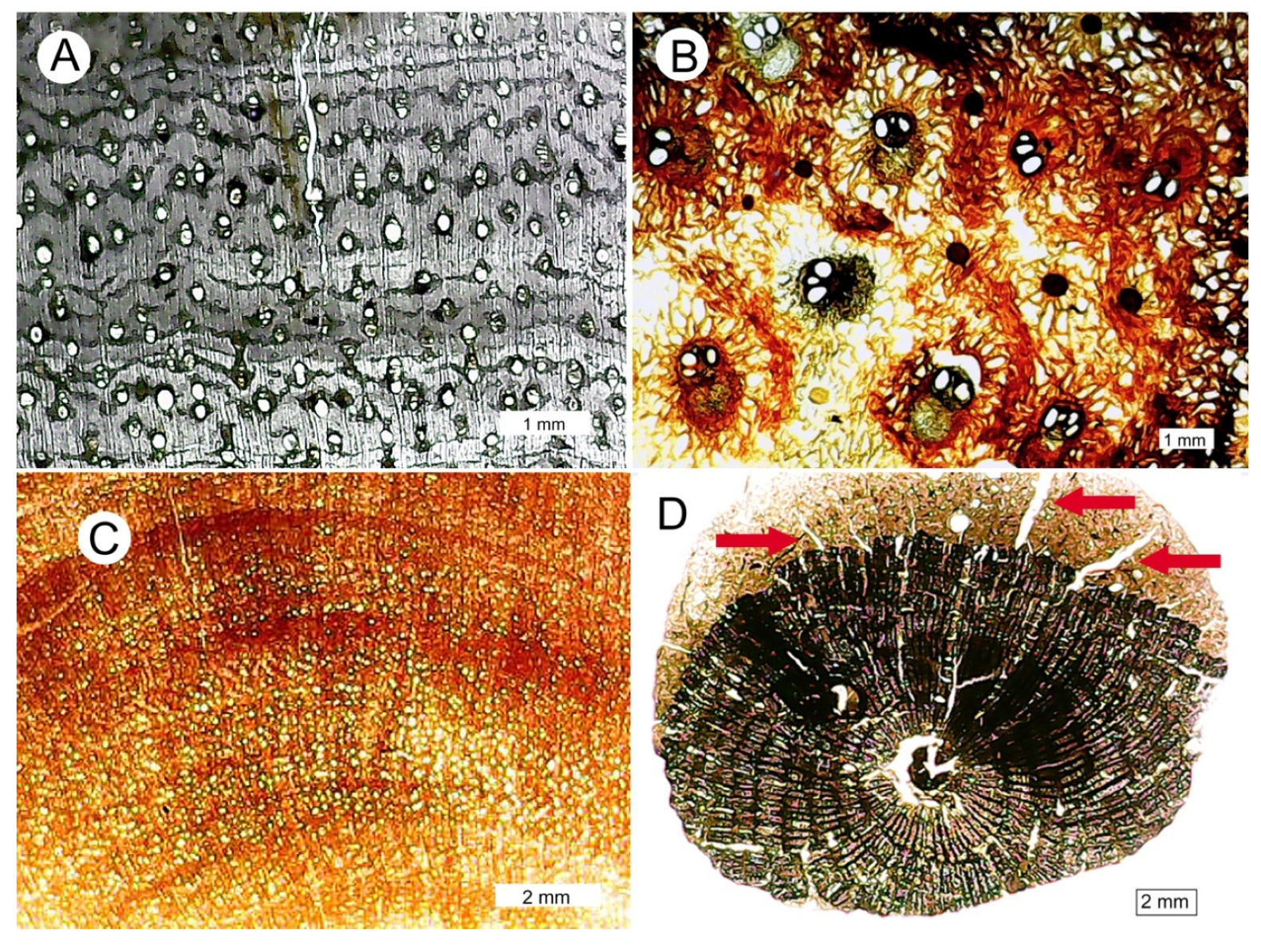
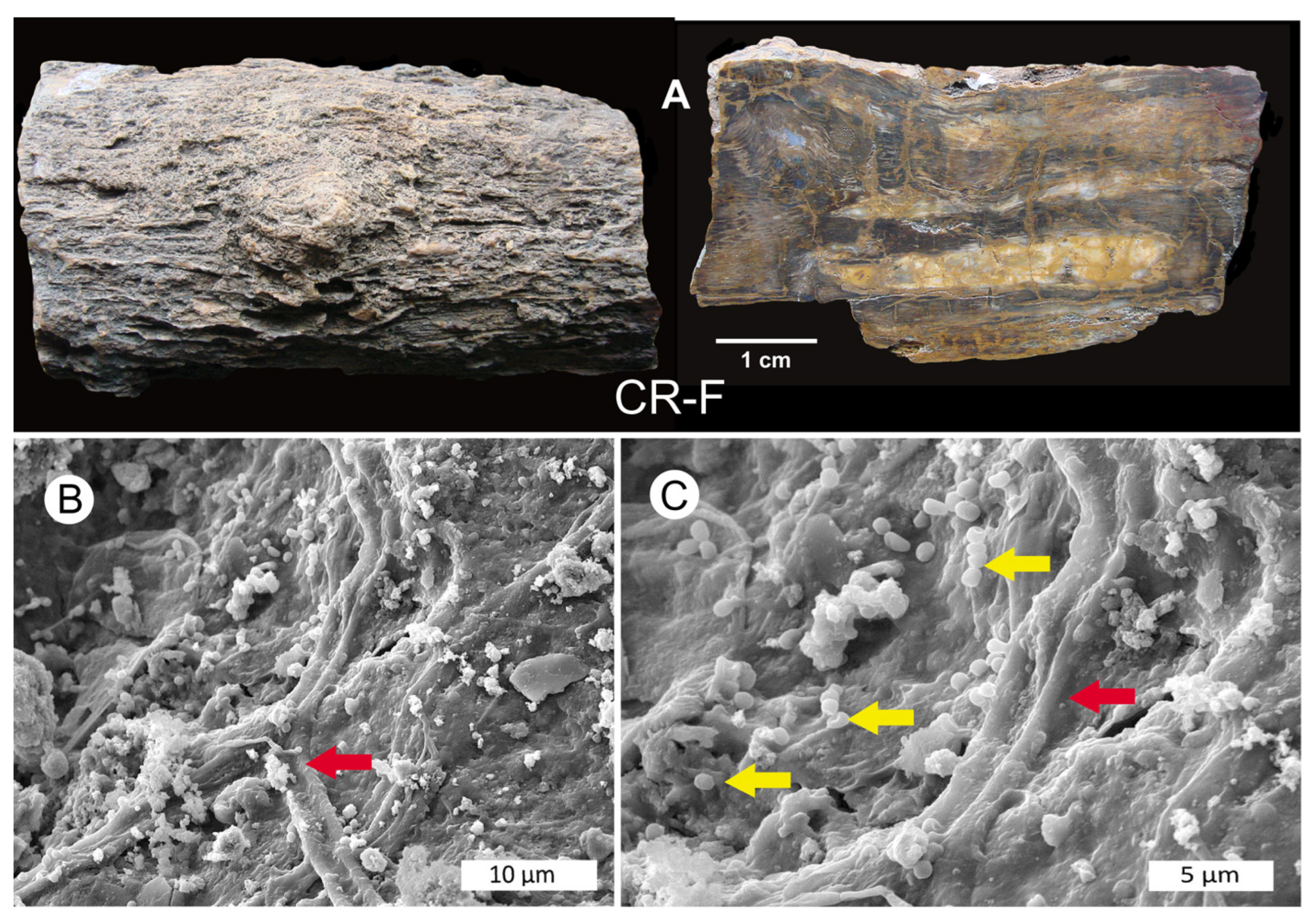
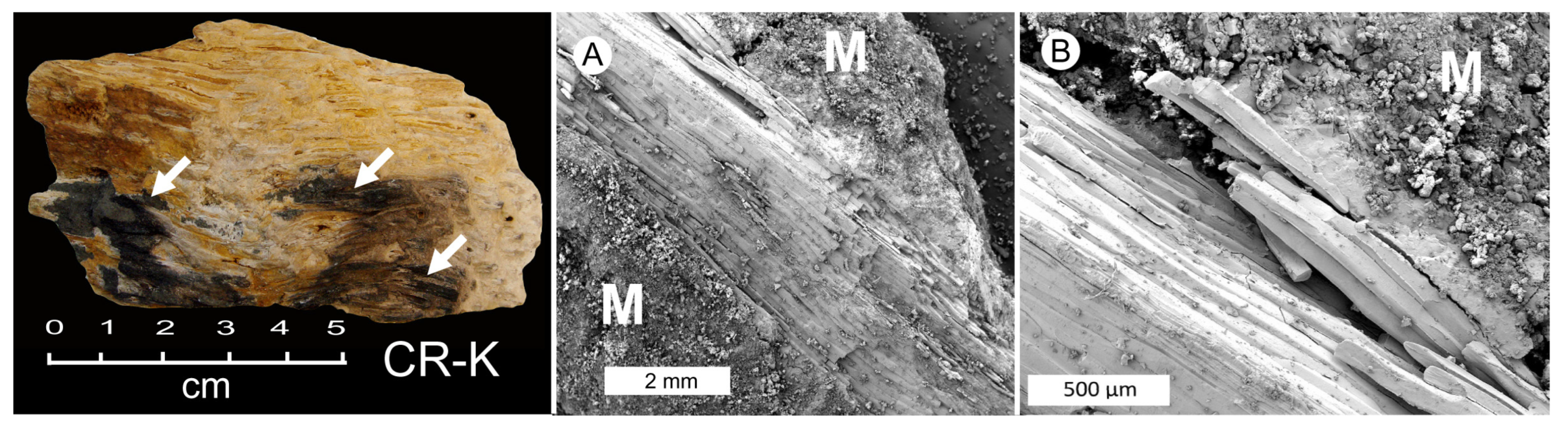
3.2. Evidence of Decay
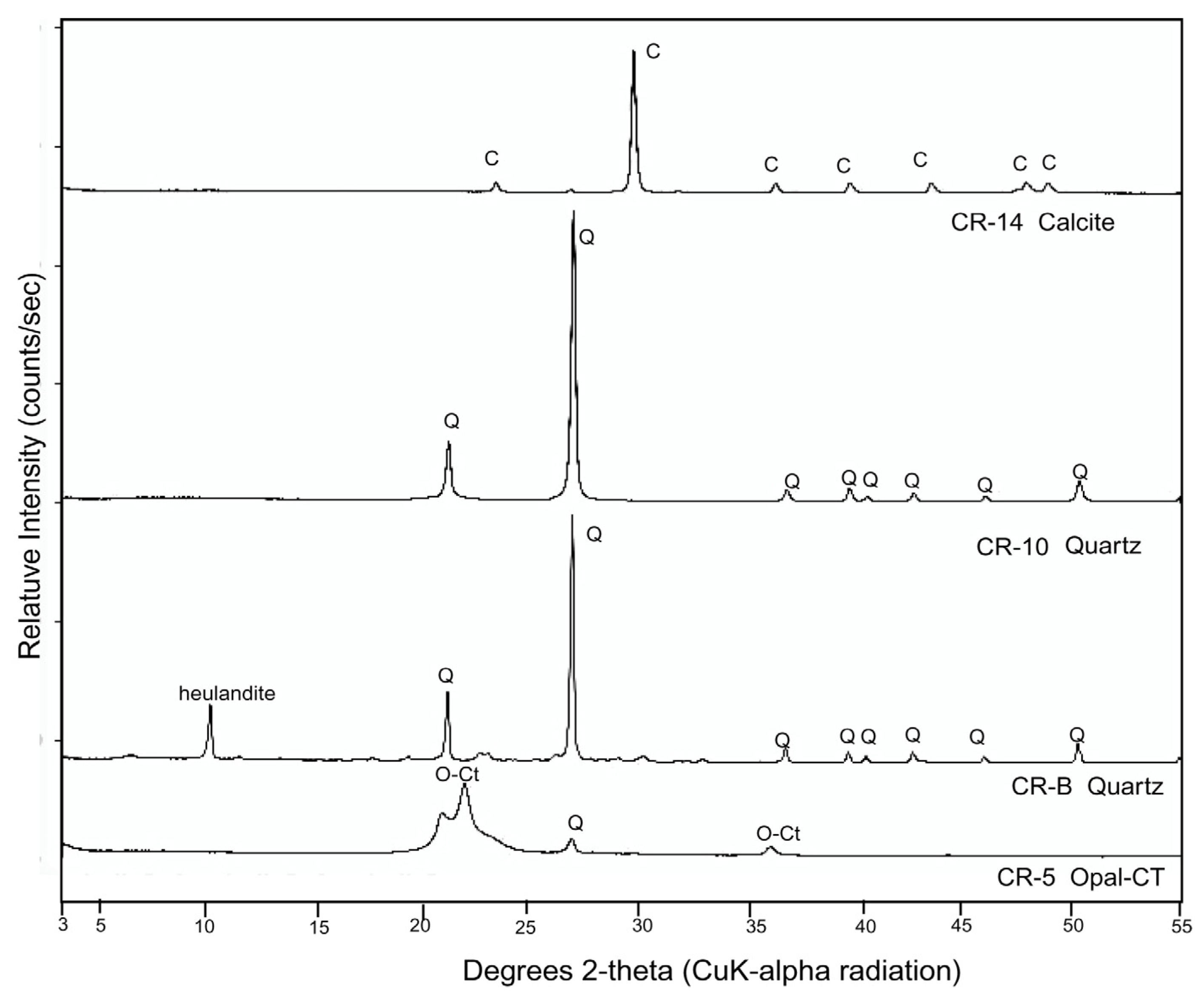
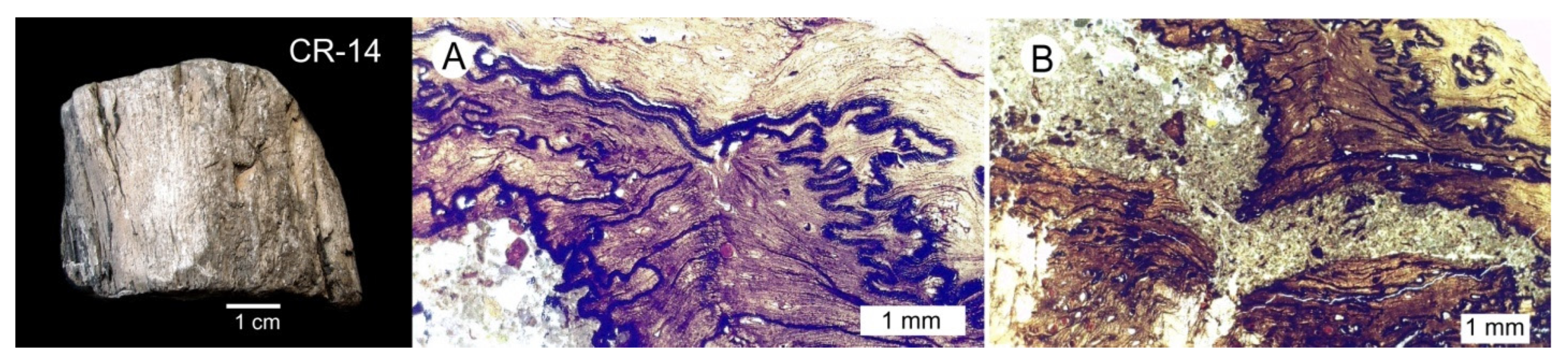
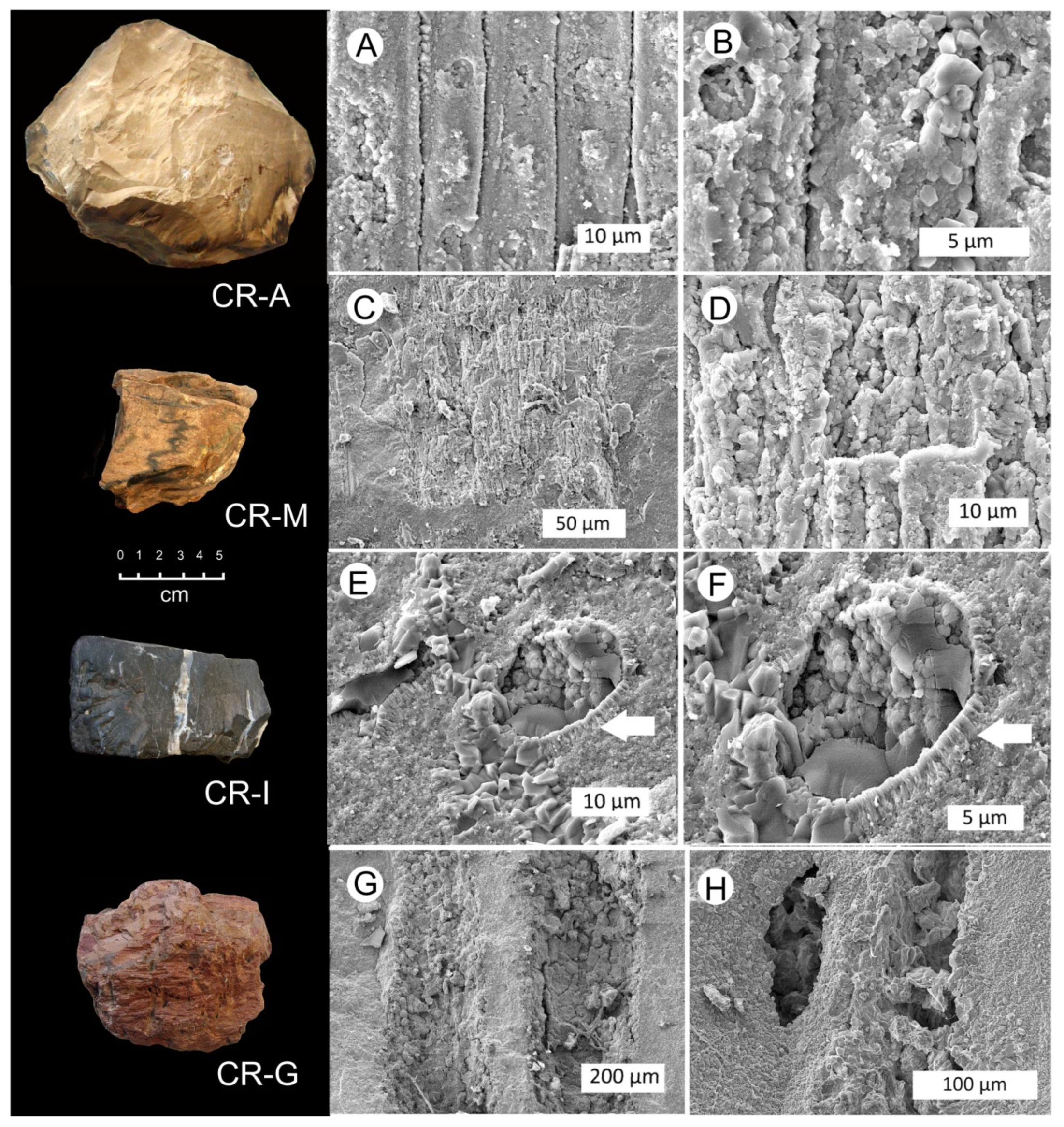
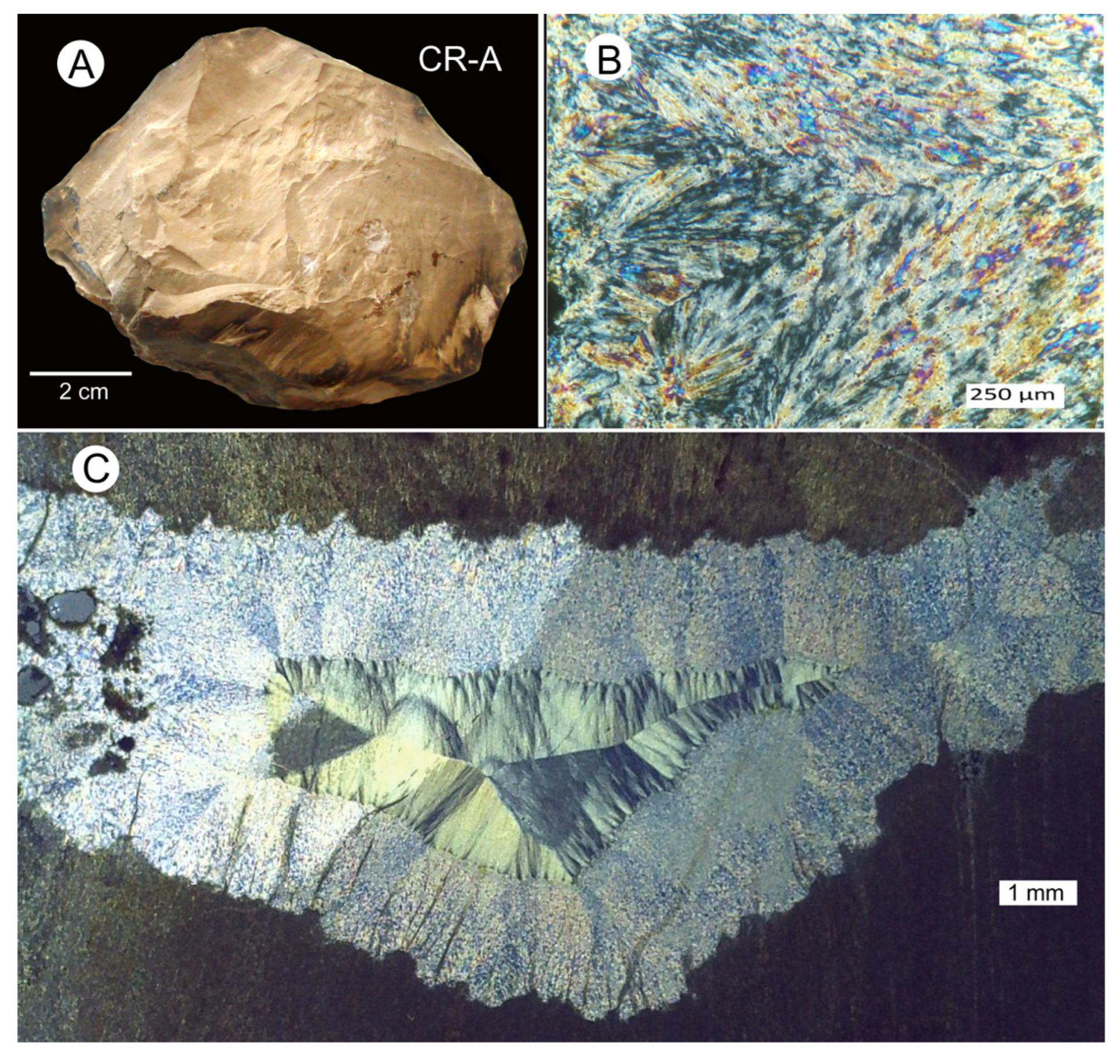
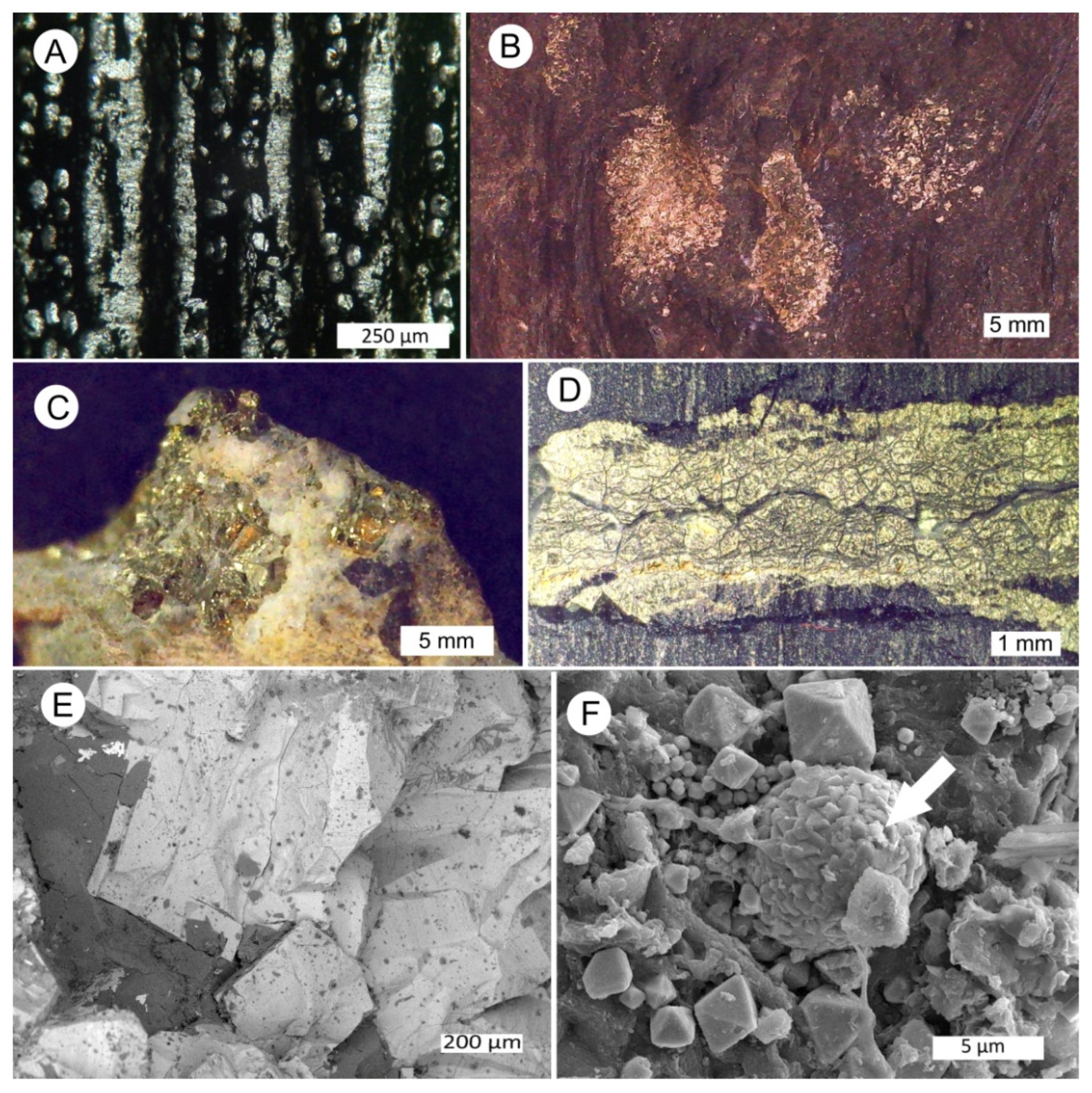
3.3. Silicified Woods: Microcrystalline Quartz
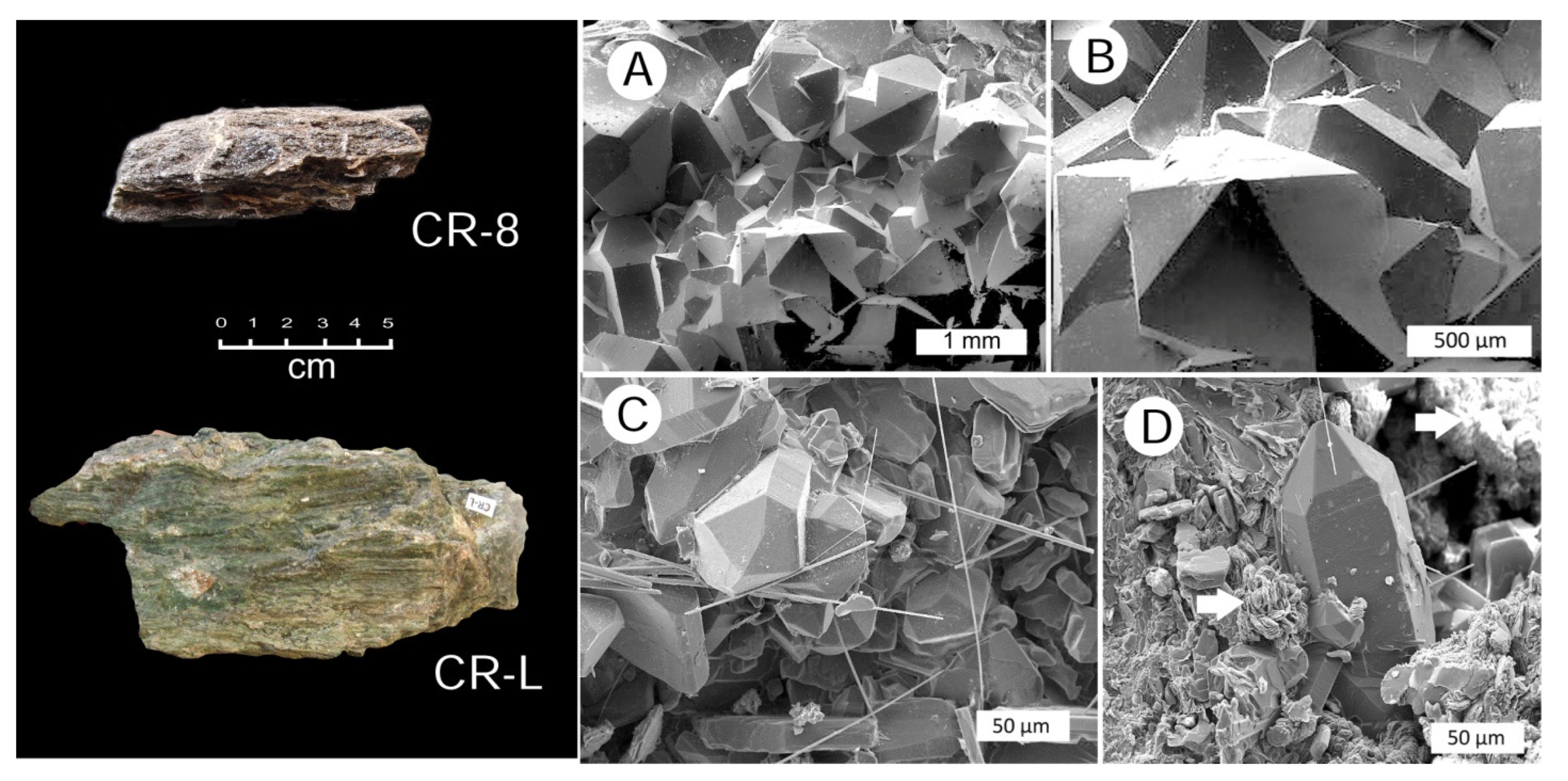
3.4. Silicified Wood: Megacrystalline Quartz
3.5. Chalcedony Mineralization
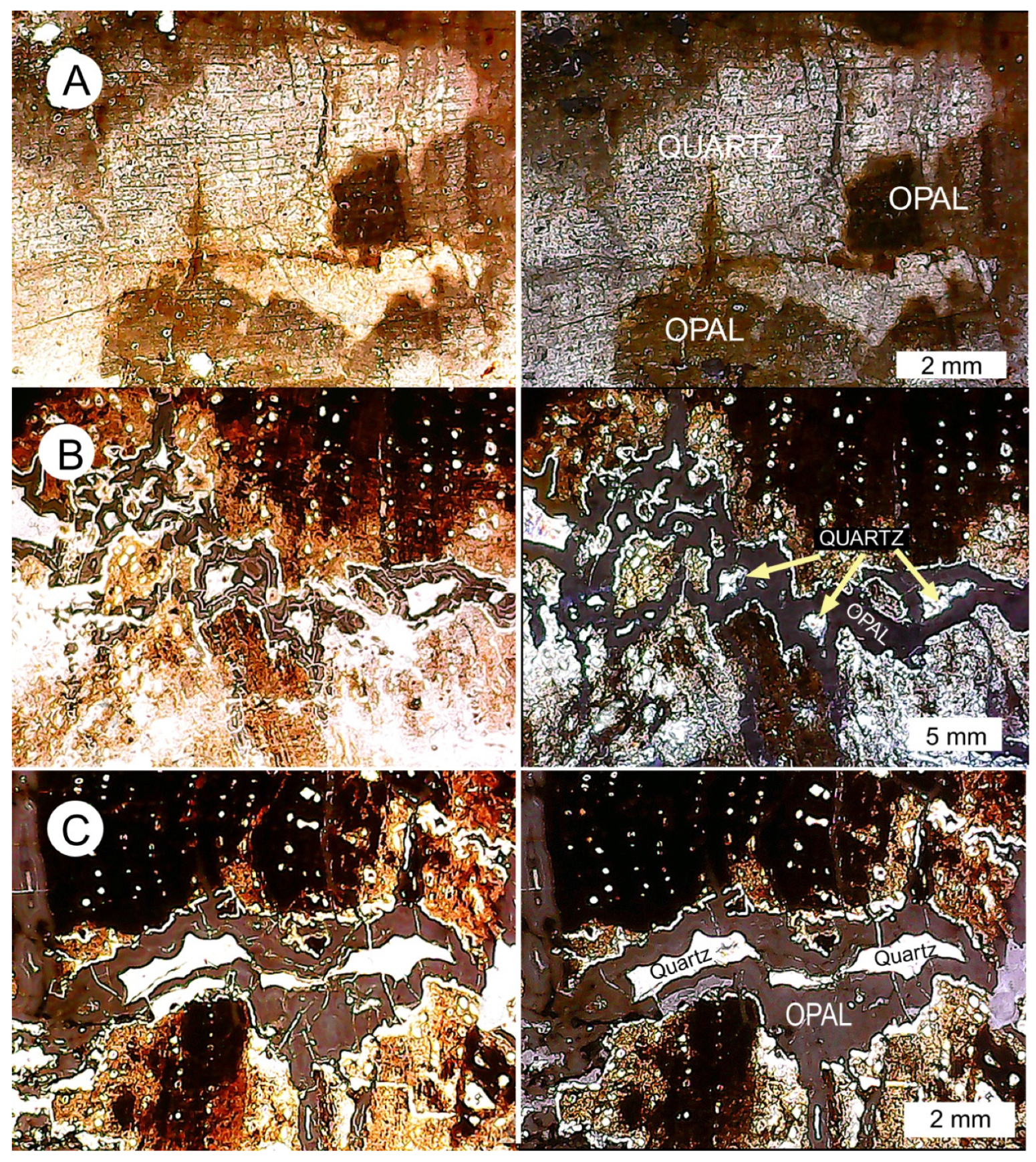
3.6. Silicified Wood: Opal-CT
3.7. Opal and Quartz
3.8. Calcite Wood
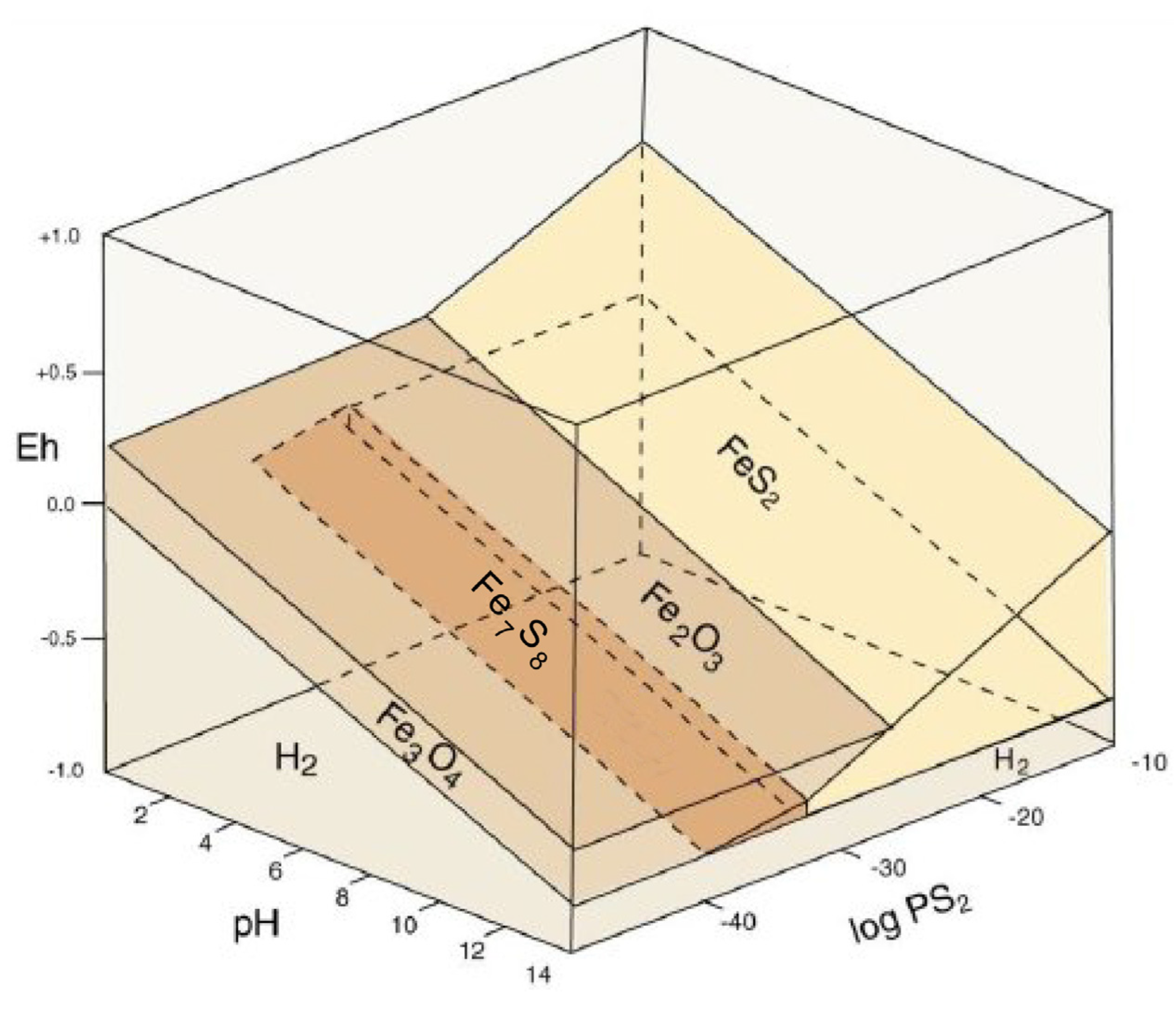
3.9. Pyrite as an Accessory Mineral in Fossil Wood
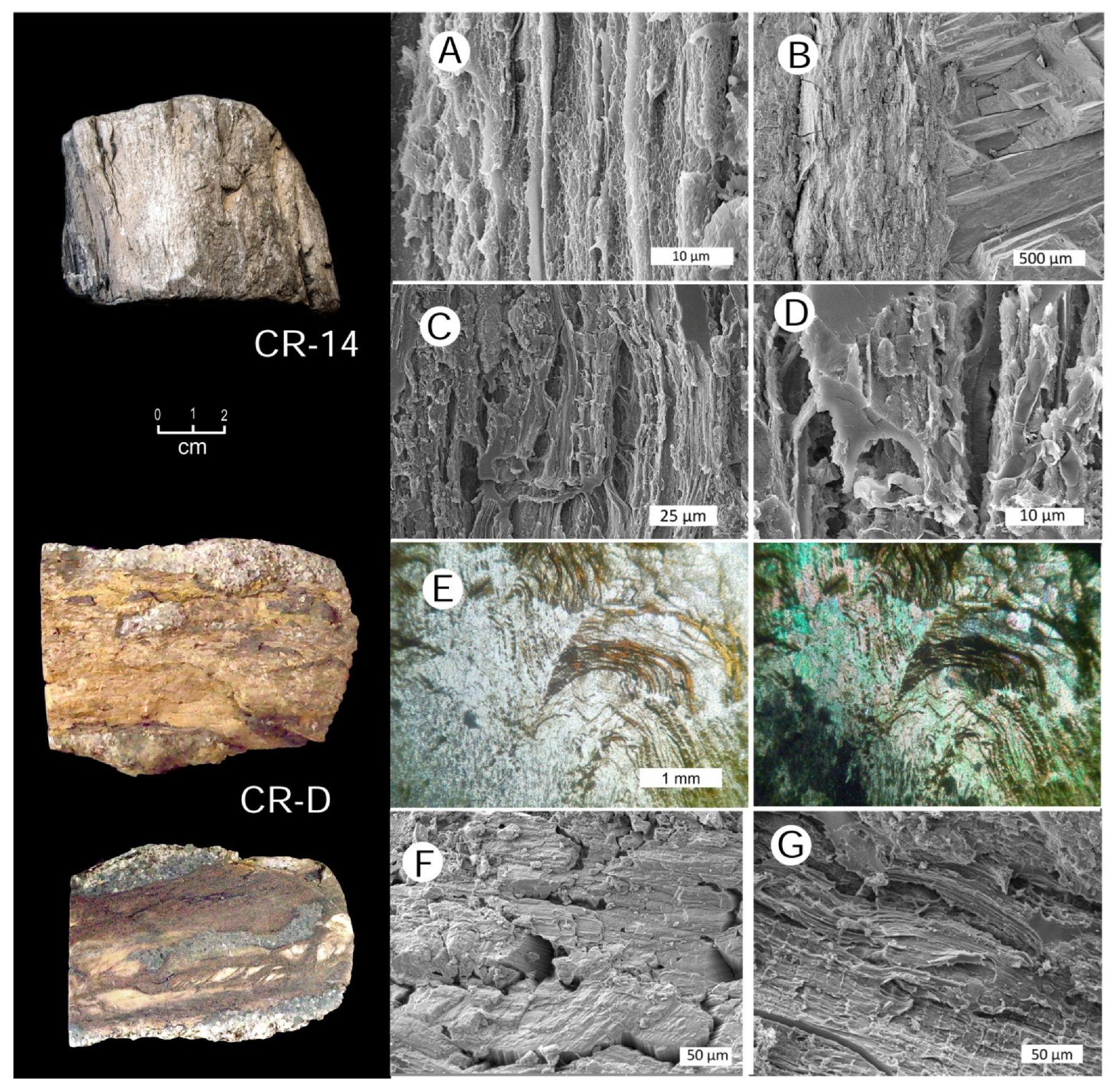
3.10. Preservation of Relict Tissue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaro, A. Comprobaciones geológicas. Boletín Del Fom. 1911, 1, 123–131. [Google Scholar]
- Alfaro, A. Rocas sedimentarias de Costa Rica. Boletín Del Fom. 1913, 3, 853–861. [Google Scholar]
- Madrigal, R. Geología del Mapa Básico “Barranca”, Costa Rica, Informes Técnicos y Notas Geológicas; Ciudad Univeritaria “Rodorigo Facio”: San José, Costa Rica, 1970; 55p. [Google Scholar]
- Alfaro, A. Caracterización petrográfica y geoquímica de las rocas ígneas en el sector Pacífico de la cordillera de Talamanca, Costa Rica. Licentiure Thesis, Universidad de Costa Rica, San José, Costa Rica, 2017. [Google Scholar]
- Alvarado, G.E.; Bonilla-Mata, A.; Cárdenes-Sandí, G. A Synopsis of the Geology, Geotectonic, and Geological History of Costa Rica. In Landscapes and Landforms of Costa Rica; Quesada-Román, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 3–34. [Google Scholar]
- Denyer, P.; Alvarado, G.E. Desarrollo y evolución de la geología. In Geología de Costa Rica; Denyer, P., Kussmaul, S., Eds.; Editorial Tecnólógica de Costa Rica: Cartago, Costa Rica, 2000; pp. 185–218. [Google Scholar]
- Weyl, R. Geology of Central America, 2nd ed.; Gebruder-Borntraeger: Berlin, Germany, 1980. [Google Scholar]
- Laurito, C.A. Sinópsis de la estratigrafía de la cuenca Barranca-Herradura y el promontorio Herradura. Provincia de Puntarenas, Costa Rica. Brenesia 1988, 29, 21–32. [Google Scholar]
- Denyer, P.; Aguilar, T.; Alvarado, G.E. Geología y estratigrafía de la hoja Barranca, Costa Rica. Rev. Geol. Amér. Central 2003, 29, 105–125. [Google Scholar] [CrossRef][Green Version]
- Alvarado, G.E.; Dengo, C.; Martens, U.; Bundschuh, J.; Aguilar, T.; Bonis, S.B. Stratigraphy and geologic history. In Central America: Geology, Resources and Hazards; Bundschuh, J., Alvarado, G.E., Eds.; Taylor and Francis: London, UK, 2007; pp. 345–394. [Google Scholar]
- Denyer, P.; Alvarado, G.E. Mapa Geológico de Costa Rica 2007(1: 400 000); Librería Francesa S.A: San Jóse, Costa Rica, 2007. [Google Scholar]
- Denyer, P.; Aguilar, T.; Alvarado, G.E.; Chavarría, M.M.; Herrera, P. Mapa geológico de la hoja Bahía Salinas. Escala 1:50 000. In Perspectiva Geológica del Noroeste de Costa Rica: Historia, Evolución y Cartografía; Denyer, P., Ed.; EUCR-CICG: San Jóse, Costa Rica, 2019. [Google Scholar]
- Alvarado, G.E.; Cárdenes, G. Geology, tectonics, and geomorphology of Costa Rica: A natural history approach. In Costa Rica Ecosystems; Kappelle, M., Ed.; University Chicago Press: Chicago, IL, USA, 2017; pp. 30–61. [Google Scholar]
- Murata, K.J. Volcanic ash as a source of silica for the silicification of wood. Am. J. Sci. 1940, 238, 586–596. [Google Scholar] [CrossRef]
- Matysová, P.; Rössler, R.; Götze, J.; Leichmann, J.; Forbes, G.; Taylor, E.I.; Dakala, J.; Grygar, T. Alluvial and volcanic pathways to silicified plant stems (Upper Carboniferous-Triassic) and their taphonomic and paleoenvironmental meaning. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 292, 127–143. [Google Scholar] [CrossRef]
- O’Dea, O.; Lessios, H.A.; Coates, A.G.; Eytan, R.J.; Resrepo-Moreno, S.A.; Cine, A.L.; Collins, L.S.; de Queiroz, A.; Farris, D.W.; Norris, R.D.; et al. Formation of the Isthmus of Panama. Sci. Adv. 2016, 2, e1600883. [Google Scholar] [CrossRef]
- Lucas, S.G. Vertebrate paleontology in Central America 30 years of progress. Rev. Geológica De América 2014, 30, 139–155. [Google Scholar]
- Lucas, S.G.; Alvarado, G.E. Vertebrate paleoentology in Central America: A narrative and analytical history. In History of Geoscience Celebrating 50 Years of INHIGEO; Mayer, W., Clary, R.M., Azuela, L.F., Mota, T.S., Wolkowicz, S., Eds.; Geological Society of London: London, UK, 2017; pp. 155–169. [Google Scholar]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 502–528. [Google Scholar] [CrossRef]
- Janzen, D.H.; Martin, P.S. The fruit the gompotheres ate. Science 1982, 215, 19–27. [Google Scholar] [CrossRef]
- Herrera, C.M. Vertebrate frugivores and their interaction with invertebrate fruit predators: Evidence from the Costa Rican dry forest. Oikos 1989, 54, 185–288. [Google Scholar] [CrossRef]
- Berry, E.W. Fossil plants in the Panama Canal Zone. Science 1914, 39, 357. [Google Scholar] [CrossRef]
- Berry, E.W. The fossil higher plants from the Canal Zone. U.S. Natl. Mus. Bull. 1919, 33, 15–44. [Google Scholar]
- Berry, E.W. A palm fruit from the Miocene of western Panama. J. Wash. Acad. Sci. 1928, 18, 455–457. [Google Scholar]
- Berry, E.W. Tertiary fossil plants from Costa Rica. Proc. United States Natl. Mus. 1921, 59, 169–185. [Google Scholar] [CrossRef]
- Berry, E.W. Mesozoic and Cenozoic plants of South America, Central America, and the Antilles. In Proceedings of the Eighth American Scientific Conference, Washington, DC, USA, 10–18 May 1940; 1942; Volume 4, p. 3650373. [Google Scholar]
- Stern, W.L.; Eyde, R.H. Fossil forests of Ocú Panama. Science 1963, 140, 2014. [Google Scholar] [CrossRef]
- Reyes, O.R.; Falcon-Lang, H.; Gasson, P.; Collinson, M.; Jaramillo, C. Fossil woods (Malvaceae) from the lower Miocene (early to mid-Burdigalian) part of the Cucaracha Formation of Panama (Central America) and their biogeographic implications. Rev. Palaeobot. Palynol. 2014, 209, 11–14. [Google Scholar] [CrossRef]
- Judd, N.; Nelson, C.W. A liana from the lower Miocene of Panama and the fossil record of Connaraceae. Am. J. Bot. 2017, 104, 1–9. [Google Scholar] [CrossRef]
- Judd, N.; Dunham, J.I. Fossil woods from the Cenozoic of Panama (Azuero Peninsula) reveal an ancient neotropical rainforest. IAWA J. 2017, 38, 366–411. [Google Scholar] [CrossRef]
- McFadden, B.J.; Jones, D.S.; Judd, N.A.; Moreno-Bernai, J.W.; Morgan, G.S.; Poretell, R.W.; Perez, V.J.; Moran, S.M.; Wood, A.R. Integrated chronology flora and faunas and paleoecology of the Alajuela Formation, Late Miocene of Panama. PLoS ONE 2017, 104, 1–9. [Google Scholar] [CrossRef]
- Rodriquez-Reyes, O.; Gasson, P.; Thornton, C.; Falcon-Lang, H.; Judd, N.A. Panascleroticoxylon crytstllosa gen. et. sp. nov.: A new Miocene malpghialean tree from Panama. IAWA J. 2017, 38, 437–455. [Google Scholar] [CrossRef]
- Mustoe, G.E.; Eberl, M. New discovery of Neogene fossil forests in Guatemala. Geosciences 2020, 10, 49. [Google Scholar] [CrossRef]
- Laurito, C. Los proboscídeos fósiles de Costa Rica y sucontexto en la América Central. Vínculos 1988, 14, 29–58. [Google Scholar]
- Amann, H. Randmarine und terrestrische Ablagerungsräume des neogenen Inselbogensystems in Costa Rica (Mittelamerika). Profil 1993, 4, 1–161. [Google Scholar]
- Rojas, M. Geología de la hoja Monterrey—3247 I escala 1:50 000; Depart. Investigación, Dirección de Geología y Minas: Minae, San Jóse, Costa Rica, 2019. [Google Scholar]
- Hooghiemstra, H.; Cleef, A.M.; Noldus, G.; Kappelle, M. Upper Quaternary vegetation dynamics and palaeoclimatology of the La Chonta bog area (Cordillera de Talmanca, Costa Rica). J. Quat. Sci. 1992, 7, 205–225. [Google Scholar] [CrossRef]
- Islebe, G.A.; Hooghiemstra, H. Vegetation and climate history of montane Costa Rica since the Last Glacial. Quat. Sci. 1997, 16, 589–604. [Google Scholar] [CrossRef]
- Islebe, G.A.; Hooghiemstra, H.; van’t Veer, R. Historia holocénica de la vegetación y del niveldeagua endos tuberas de la cordillera de Talmanca, Costa Rica. In Páramos d Costa Rica; Kapelle, M., Horn, S., Eds.; Instituto Nacional de Biodiveridad, INBio, Santo Domingo de Heredia: Santo Domingo, Costa Rica, 2005; pp. 337–352. [Google Scholar]
- Kappelle, M.; Haberyan, K.A.; Horn, S.P. Algas fósiles y recientes de los páramos de Costa Rica. In Páramos de Costa Rica; Kapelle, M., Horn, S., Eds.; Instituto Nacional de Biodiveridad, INBio: Santo Domingo de Herdia, Costa Rica, 2005; pp. 331–341. [Google Scholar]
- Horn, S.P. Late Quaternary lake and swamp sediments: Rocorders of climate and environment. In Central America: Geology, Resources and Hazards; Bundschuh, J., Alvarado, G.E., Eds.; Taylor & Francis: London, UK, 2007; pp. 423–441. [Google Scholar]
- Kesel, R.H. Quaternary history of the Rio General valley, Costa Rica. Natl. Geogr. Soc. Res. Rep. 1983, 15, 339–358. [Google Scholar]
- Pérez, E.A. Comparación de la flora fósil del pleistoceno, con la flora actual en la localidad de la Palmera de San Carlos, Provincia del Alajuela. Escuela la de Ciencia Exactas y Naturales. Programa de licenciatura en Manejo de Recursos Naturals, Universidad Estatal a Distancia, San José, Costa Rica, 2001; 126p. [Google Scholar]
- Pérez, E.A.; Laurito, C.A. Quercus corrugata Hooker (Fagaceae) como un indicatorpaleoclimático del Pleistoceno del Costa Rica. Rev. Geológica De América Central 2003, 28, 83–90. [Google Scholar]
- Pérez, E.A. Presencia de Juglans olanchana Standley & L.O. Williams (Juglandaceae) en el territorio costrarricenes durante el Pleistoceno. Rev. Geológica De América Central 2003, 28, 77–81. [Google Scholar]
- Gómez, L.D. A first report of a fossil fern-like Pteropsida from Costa Rica. Rev. Biol. Trop. 1970, 16, 255–258. [Google Scholar]
- Gómez, L.D. Palmacitesberry anum, a new palm fossil from the Costa Rican Tertiary. Rev. Biol. Trop. 1971, 19, 121–132. [Google Scholar]
- Gómez, L.D. Keratophyllum bromeliodes (Bromeliaceae), nov. gen. et sp., del Terciario Medio de Costa Rica. Rev. Biol. Trop. 1972, 20, 221–229. [Google Scholar]
- Gómez, L.D. Criptonemiales calcáreas fossils en las terciarias de Patarrá. Rev. Biol. Trop. 1973, 21, 107–110. [Google Scholar]
- Gómez, L.D. Ficus padifolia H.B.K. en la diatomita pliocena/pleistocena de la Formación Bagaces, Guanacaste, Costa Rica. Veröff. Uberseemuseum Brem. Reihe A 1974, 4, 141–148. [Google Scholar]
- Gómez, L.D. Preliminary note on a fossil Equisetum from Costa Rica. Fern. Gaz. 1978, 11, 401–404. [Google Scholar]
- Cevallos-Ferriz, S.R.S.; Alvarado, G.E.; Huerta-Vergara, A.R.; Cárdenes, G.; Ramírez, T.D.; Bonilla, N.W.; Gusmán, E. Fossil woods from the Miocene of Costa Rica and Nicaragua: Insights into the Neotropical floral puzzle. J. South Am. Earth Sci. 2024, 144, 1–14. [Google Scholar] [CrossRef]
- Fournier, L.A.; Jiménez, A.; Salas, S.; Marin, F. Las familias y géneros de árboles y arbustos de Costa Rica. Rev. Biol. Trop. 1966, 14, 317–328. [Google Scholar]
- Gómez-Laurito, J.; Fournier, L.A. Las Familias y Los Generos de Plantas Leñosas de Costa Rica. Available online: https://tropicalstudies.org/rbt/attachments/volumes/vol14-2/11-Fournier-Arboles.pdf (accessed on 20 April 2025).
- Alvarado, G.E.; Gans, P.B. Síntesis geocronológica del magmatismo, metamorfismo y metalogenia de Costa Rica, América Central. Rev. Geol. Amér. Central 2012, 46, 7–122. [Google Scholar] [CrossRef]
- Denyer, P.; Montero, W.; Aguilar, T.; Herrera, P.; Alvarado, G.E.; Barrantes, M.; y Chavarría, M.M. Mapa Geológico de la Hoja Carrillo Norte (Escala 1:50 000); Editorial Universidad de Costa Rica: San José, Costa Rica, 2019. [Google Scholar]
- Tournon, J.; Alvarado, G.E. Mapa Geológico de Costa Rica. Folleto Explicativo, 1: 500,000; Tecnológica de Costa Rica: Cartago, Costa Rica, 1997. [Google Scholar]
- Lucas, S.G.; Sequeira, C.; Chesnel, V.; Rodríguez, D.; Alvarado, G.E.; Méndez, J.C.; Vargas, A.; Vargas, C.; Ruiz, G. Boid snake fossils from the Neogene of Costa Rica. Rev. Geológica De América Cent. 2025, 72, 1–11. [Google Scholar]
- Mustoe, G.E. Density and loss on ignition as indicators of the fossilization of silicified wood. IAWA J. 2016, 37, 98–111. [Google Scholar] [CrossRef]
- Mustoe, G.E.; Acosta, M. Origin of petrified wood color. Geosciences 2016, 6, 25. [Google Scholar] [CrossRef]
- The Quartz Page. Available online: www.quartzpage.de (accessed on 7 January 2025).
- Mustoe, G.E. Silicification of wood: An overview. Minerals 2023, 13, 206. [Google Scholar] [CrossRef]
- Rasbury, E.T.; Hatton, K.; Cox, S.E.; Sousa, F.J.; Hemming, S.R.; Steponaitis, E.; Saslaw, M.; Rowan, C.M.; Boyd, M.D.; Gaiku, M.; et al. Carbonate mineralized wood from Oligocene-Miocene Deposits in the Turkana Basin. Turkana Miocene Project Publication #6. 2025; in press. [Google Scholar]
- Mustoe, G.E.; Beard, G. Calcite-mineralized fossil wood from Vancouver Island, British Columbia, Canada. Geosciences 2021, 11, 38. [Google Scholar] [CrossRef]
- Mustoe, G.E. Mineralogy of non-silicified wood. Geosciences 2018, 8, 85. [Google Scholar] [CrossRef]
- Mustoe, G.E. Wood petrifaction: A new view of permineralization and replacement. Geosciences 2017, 7, 119. [Google Scholar] [CrossRef]
- Utami, W.S.; Herdianita, N.R.; Atmaja, R.W. The effect of temperature and pH on the formation of silica scaling at Dieng Geothermal Field, central Java, Indonesia. In Proceedings of the Thirty-Ninth Workshop on Geothermal Field Engineering Stanford University, Stanford, CA, USA, 24–26 February 2014. [Google Scholar]
- Nicholson, K. Geothermal Fluids: Chemistry and Exploration Techniques; Springer: Berlin, Germany, 1968; 263p. [Google Scholar]
- Garcia-Guinea, J.; Martinez-Frias, A.; Harffy, M. Cell-hosted pyrite framboids in fossil woods. Naturwissenschaft 1988, 85, 78–81. [Google Scholar] [CrossRef]
- Tibbs, S.L.; Briggs, D.E.G.; Prossl, K.F. Pyritization of plant fossils from the Devonian Hunsrücck Slate of Germany. Palaeontol. Z. 2003, 77, 241–246. [Google Scholar] [CrossRef]
- Kendrick, P.; Edwards, D. Anatomy of the Lower Devonian Gosslingia breconenis Herad based on pyritized axes with some comments on the permineralizaton process. Bot. J. Linnean Soc. 1988, 97, 95–123. [Google Scholar] [CrossRef]
- Grimes, S.T.; Davies, K.L.; Butler, I.B.; Brock, F.; Edwards, D.; Rickard, D.; Briggs, D.E.G.; Parkes, R.J. Understanding fossilization: Experimental pyritization of plants. Geology 2001, 29, 123–126. [Google Scholar] [CrossRef]
- Camacho-Umaña, M.E.; Quesada-Román, A.; Villatro-Sánchez, M.; Alemán-Montes, B.; Mata, R.; Henríquez-Henríquez, C.; Céspedes-Rivera, J.; Alvarado, A. Highly weathered landscapes of Costa Rica. In Landscapes and Landforms of Costa Rica; Quesada-Román, A., Ed.; Springer: Cham, Switzerland, 2024; pp. 431–451. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria; Harper and Row: New York, NY, USA; John Weatherhill, Inc.: Tokyo, Japan, 1965. [Google Scholar]
- Cody, S.; Richardson, J.E.; Pennington, R.T. The great American biotic exchange revisited. Ecography 2010, 33, 326–332. Available online: https://www.nature.com/articles/s41598-020-58575-6 (accessed on 3 May 2024). [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustoe, G.E.; Alvarado, G.E.; Palacios, A.J. Mineralogy of Petrified Wood from Costa Rica. Minerals 2025, 15, 497. https://doi.org/10.3390/min15050497
Mustoe GE, Alvarado GE, Palacios AJ. Mineralogy of Petrified Wood from Costa Rica. Minerals. 2025; 15(5):497. https://doi.org/10.3390/min15050497
Chicago/Turabian StyleMustoe, George E., Guillermo E. Alvarado, and Armando J. Palacios. 2025. "Mineralogy of Petrified Wood from Costa Rica" Minerals 15, no. 5: 497. https://doi.org/10.3390/min15050497
APA StyleMustoe, G. E., Alvarado, G. E., & Palacios, A. J. (2025). Mineralogy of Petrified Wood from Costa Rica. Minerals, 15(5), 497. https://doi.org/10.3390/min15050497