Study on the Oxidation Inhibition of Pyrite by 2-Mercaptobenzimidazole in the Presence of Acidithiobacillus ferrooxidans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Strains
2.2. Oxidation Resistance Test
2.2.1. Biological Oxidation Resistance Test
2.2.2. Electrochemical Test
2.3. Antimicrobial Activity Test of MBI
2.4. Pyrite Passivation Treatment Steps
2.5. Analysis Method
3. Results and Discussion
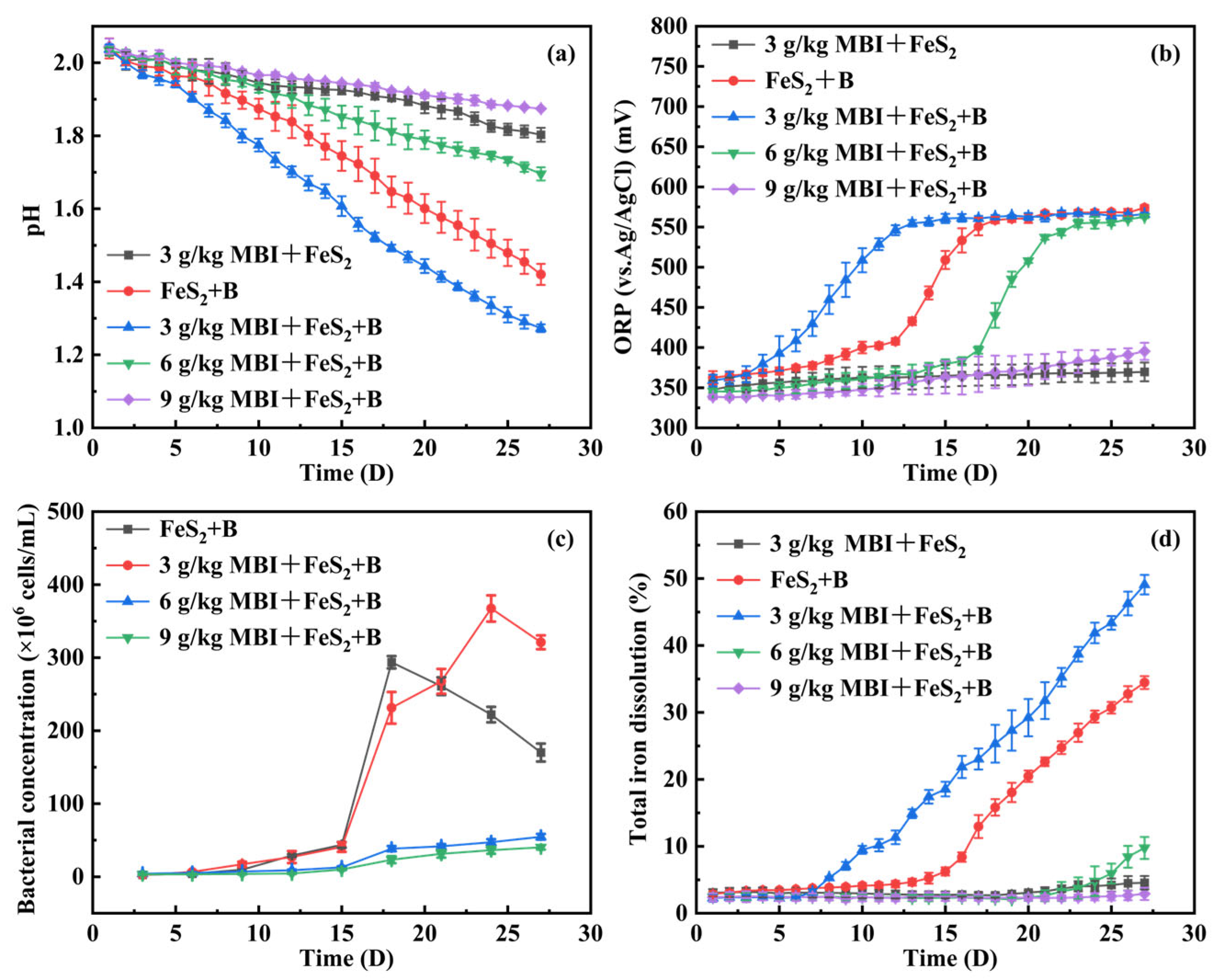
3.1. Effect of MBI on Pyrite Oxidation Under the Action of At. ferrooxidans
3.2. Effect of MBI on the Growth of At. ferrooxidans
3.3. Electrochemical Measurement
3.4. Mechanism of Inhibiting Pyrite Oxidation by MBI Passivation Layer
3.4.1. SEM Analysis
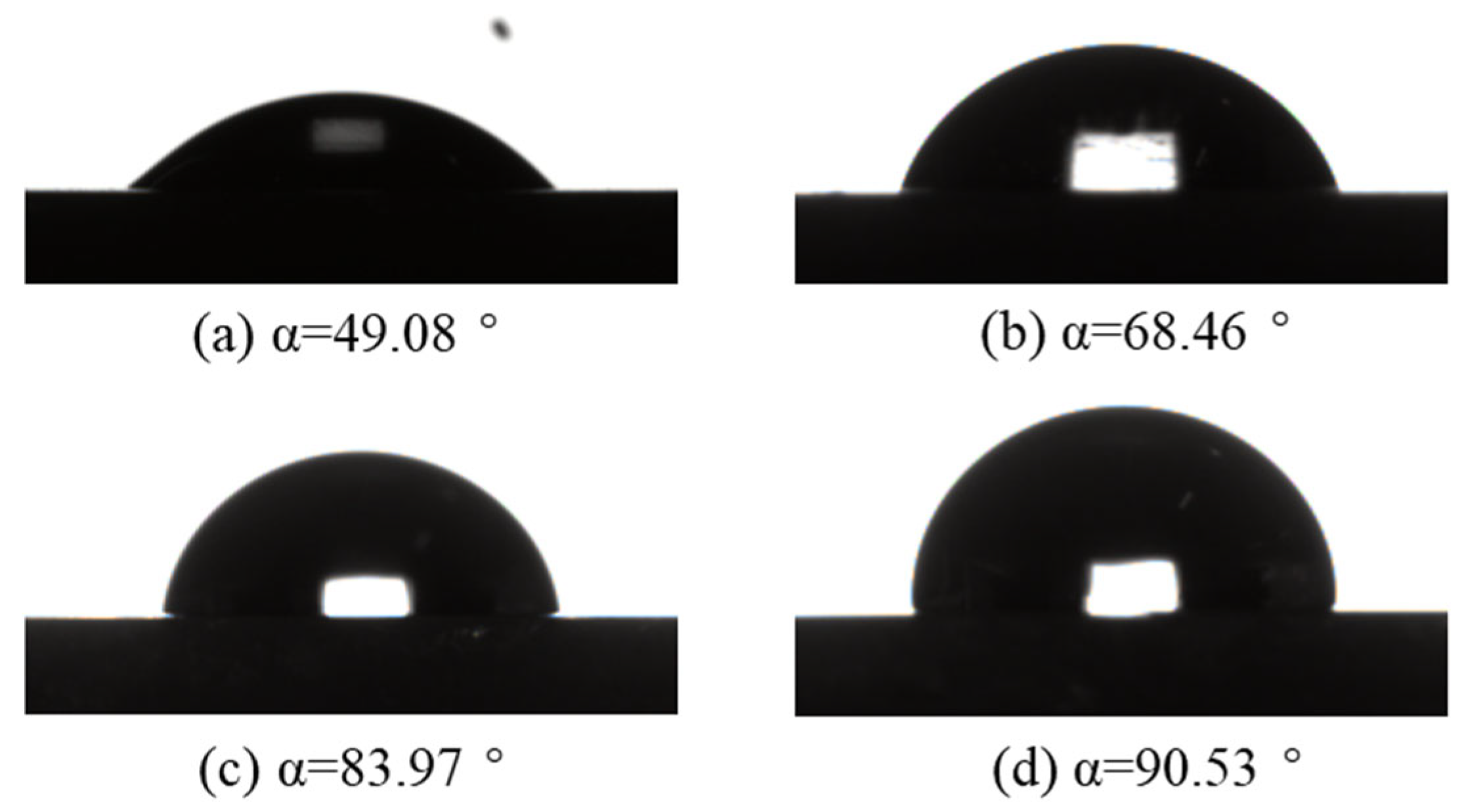
3.4.2. Contact Angle Test
3.4.3. FTIR Analysis
3.4.4. XPS Analysis
3.4.5. Mechanism of MBI Inhibiting Pyrite Oxidation
4. Conclusions
- (1)
- In the presence of At. ferrooxidans, 3 g/kg MBI cannot effectively inhibit pyrite oxidation; on the contrary, it can promote pyrite oxidation. When the dosage is more than or equal to 6 g/kg, MBI can effectively inhibit the oxidation of pyrite, and 9 g/kg MBI has the best inhibition effect, with the inhibition rate reaching 97.1%.
- (2)
- MBI can inhibit the growth of At. ferrooxidans. With the increase in MBI dosage, the growth of At. ferrooxidans was temporarily inhibited, and then returned to normal in a short time. Therefore, the bacteriostatic effect of MBI may be one of the reasons for the inhibition of pyrite oxidation.
- (3)
- When the dosage of MBI is greater than or equal to 16.8 mg, the pyrite electrode will show oxidation resistance, and its oxidation resistance will be enhanced with the increase in MBI dosage.
- (4)
- The contact angle test shows that the adhesion of MBI to the pyrite surface will enhance the hydrophobicity of the pyrite surface, and its hydrophobicity will also increase with the increase in MBI dosage.
- (5)
- The -C=N and -SH groups in MBI can chelate with Fe2+ and Fe3+ on the surface of pyrite, so a large amount of MBI is adsorbed on pyrite, which on the one hand strengthens the hydrophobicity of pyrite, and on the other hand leads to the reduction in oxidation sites on pyrite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hughes, T.A.; Gray, N.F. Co-treatment of acid mine drainage with municipal wastewater: Performance evaluation. Environ. Sci. Pollut. Res. 2013, 20, 7863–7877. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Fan, R.; Yang, S.; Li, C. Development and Status of the Treatment Technology for Acid Mine Drainage. Min. Metall. Explor. 2021, 38, 315–327. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liu, Y.; Zhu, R.; Ge, F.; Xu, T.; Luo, Z.; Liang, L. Pyrite oxidation inhibition by organosilane coatings for acid mine drainage control. Miner. Eng. 2015, 72, 57–64. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Elamraoui, L.; Elghali, A.; Fashae, O.A.; Benzaazoua, M. Harnessing phosphate limestone waste as a cost-effective solution for acid mine drainage treatment. Sci. Total Environ. 2024, 949, 175188. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Li, S. Pyrite oxidation inhibition by hydrophobic films for acid mine drainage control at the source. Physicochem. Probl. Miner. Process. 2019, 55, 1132–1140. [Google Scholar]
- Mosai, A.K.; Ndlovu, G.; Tutu, H. Improving acid mine drainage treatment by combining treatment technologies: A review. Sci. Total Environ. 2024, 919, 170806. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Menzel, K.; Barros, L.; García, A.; Ruby-Figueroa, R.; Estay, H. Metal sulfide precipitation coupled with membrane filtration process for recovering copper from acid mine drainage. Sep. Purif. Technol. 2021, 270, 118721. [Google Scholar] [CrossRef]
- Silva, G.C.; Bertoli, A.C.; Duarte, H.A.; Ladeira, A.C.Q. Recovery of rare earth elements from sulfate-rich acid mine water: Looking through the keyhole the exchange reaction for cationic resin. J. Environ. Chem. Eng. 2022, 10, 108715. [Google Scholar] [CrossRef]
- León-Venegas, E.; Vilches-Arenas, L.F.; Fernández-Baco, C.; Arroyo-Torralvo, F. Potential for water and metal recovery from acid mine drainage by combining hybrid membrane processes with selective metal precipitation. Resour. Conserv. Recycl. 2023, 188, 106629. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; Dos Santos, K.B.; Lautert-Dutra, W.; de Souza Teodoro, L.; de Almeida, V.O.; Weiler, J.; Bogo, M.R. Acid mine drainage (AMD) treatment by neutralization: Evaluation of physical-chemical performance and ecotoxicological effects on zebrafish (Danio rerio) development. Chemosphere 2020, 253, 126665. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Xue, J.; Lv, Q.; Yang, J.; Han, X. Performance and microbial response in a multi-stage constructed wetland microcosm co-treating acid mine drainage and domestic wastewater. J. Environ. Chem. Eng. 2021, 9, 106786. [Google Scholar] [CrossRef]
- Woulds, C.; Ngwenya, B.T. Geochemical processes governing the performance of a constructed wetland treating acid mine drainage, Central Scotland. Appl. Geochem. 2004, 19, 1773–1783. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Equeenuddin, S.M.; Powell, M.A. Current approaches for mitigating acid mine drainage. Rev. Environ. Contam. Toxicol. Vol. 2013, 226, 1–32. [Google Scholar]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Rubinos, D.A.; Jerez, Ó.; Forghani, G.; Edraki, M.; Kelm, U. Geochemical stability of potentially toxic elements in porphyry copper-mine tailings from Chile as linked to ecological and human health risks assessment. Environ. Sci. Pollut. Res. 2021, 28, 57499–57529. [Google Scholar] [CrossRef]
- Peppas, A.; Komnitsas, K.A.; Halikia, I. Use of organic covers for acid mine drainage control. Miner. Eng. 2000, 13, 563–574. [Google Scholar] [CrossRef]
- Sephton, M.G.; Webb, J.A.; McKnight, S. Applications of Portland cement blended with fly ash and acid mine drainage treatment sludge to control acid mine drainage generation from waste rocks. Appl. Geochem. 2019, 103, 1–14. [Google Scholar] [CrossRef]
- Jia, Y.; Maurice, C.; Öhlander, B. Effect of the alkaline industrial residues fly ash, green liquor dregs, and lime mud on mine tailings oxidation when used as covering material. Environ. Earth Sci. 2014, 72, 319–334. [Google Scholar] [CrossRef]
- Georgopoulou, Z.J.; Fytas, K.; Soto, H.; Evangelou, B. Feasibility and cost of creating an iron-phosphate coating on pyrrhotite to prevent oxidation. Environ. Geol. 1996, 28, 61–69. [Google Scholar] [CrossRef]
- Zheng, K.; Li, H.; Xu, L.; Li, S.; Wang, L.; Wen, X.; Liu, Q. The influence of humic acids on the weathering of pyrite: Electrochemical mechanism and environmental implications. Environ. Pollut. 2019, 251, 738–745. [Google Scholar] [CrossRef]
- Leathen, W.W.; Braley Sr, S.A.; McIntyre, L.D. The Role of Bacteria in the Formation of Acid from Certain Sulfuritic Constituents Associated with Bituminous Coal: I. Thiobacillus thiooxidans. Appl. Microbiol. 1953, 1, 61–64. [Google Scholar] [CrossRef]
- Frattini, C.J.; Leduc, L.G.; Ferroni, G.D. Strain variability and the effects of organic compounds on the growth of the chemolithotrophic bacterium Thiobacillus ferrooxidans. Antonie Van Leeuwenhoek 2000, 77, 57–64. [Google Scholar] [CrossRef]
- Elsetinow, A.R.; Schoonen, M.A.; Strongin, D.R. Aqueous geochemical and surface science investigation of the effect of phosphate on pyrite oxidation. Environ. Sci. Technol. 2001, 35, 2252–2257. [Google Scholar] [CrossRef]
- Nyavor, K.; Egiebor, N.O. Control of pyrite oxidation by phosphate coating. Sci. Total Environ. 1995, 162, 225–237. [Google Scholar] [CrossRef]
- Fan, R.; Short, M.D.; Zeng, S.J.; Qian, G.; Li, J.; Schumann, R.C. The formation of silicate-stabilized passivating layers on pyrite for reduced acid rock drainage. Environ. Sci. Technol. 2017, 51, 11317–11325. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 2. Rate control by oxide coatings. Geochim. Et Cosmochim. Acta 1990, 54, 395–402. [Google Scholar] [CrossRef]
- Gao, B.; Han, Z.; Cheng, H.; Zhou, H.; Wang, Y.; Chen, Z. Treating waste with waste: Lignin acting as both an effective bactericide and passivator to prevent acid mine drainage formation at the source. Sci. Total Environ. 2024, 927, 172162. [Google Scholar] [CrossRef] [PubMed]
- Ačai, P.; Sorrenti, E.; Gorner, T.; Polakovič, M.; Kongolo, M.; de Donato, P. Pyrite passivation by humic acid investigated by inverse liquid chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2009, 337, 39–46. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, X.H.; Parekh, B.K. Effect of sodium oleate on inhibiting pyrite oxidation. Int. J. Miner. Process. 2000, 58, 305–318. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Cremer, P.S. Investigations of water structure at the solid/liquid interface in the presence of supported lipid bilayers by vibrational sum frequency spectroscopy. Langmuir 2001, 17, 7255–7260. [Google Scholar] [CrossRef]
- Pierre Louis, A.M.; Yu, H.; Shumlas, S.L.; Van Aken, B.; Schoonen, M.A.; Strongin, D.R. Effect of phospholipid on pyrite oxidation and microbial communities under simulated acid mine drainage (AMD) conditions. Environ. Sci. Technol. 2015, 49, 7701–7708. [Google Scholar] [CrossRef]
- Zhang, X.; Borda, M.J.; Schoonen, M.A.; Strongin, D.R. Adsorption of phospholipids on pyrite and their effect on surface oxidation. Langmuir 2003, 19, 8787–8792. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Xu, Y.; Xu, T. Pyrite passivation by triethylenetetramine: An electrochemical study. J. Anal. Methods Chem. 2013, 2013, 387124. [Google Scholar] [CrossRef]
- Cai, M.F.; Dang, Z.; Chen, Y.W.; Belzile, N. The passivation of pyrrhotite by surface coating. Chemosphere 2005, 61, 659–667. [Google Scholar] [CrossRef]
- Satur, J.; Hiroyoshi, N.; Tsunekawa, M.; Ito, M.; Okamoto, H. Carrier-microencapsulation for preventing pyrite oxidation. Int. J. Miner. Process. 2007, 83, 116–124. [Google Scholar] [CrossRef]
- Thakur Jha, R.K.; Satur, J.; Hiroyoshi, N.; Ito, M.; Tsunekawa, M. Suppression of pyrite oxidation by carrier microencapsulation using silicon and catechol. Miner. Process. Extr. Metall. Rev. 2012, 33, 89–98. [Google Scholar] [CrossRef]
- Khummalai, N.; Boonamnuayvitaya, V. Suppression of arsenopyrite surface oxidation by sol-gel coatings. J. Biosci. Bioeng. 2005, 99, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, Z.; Liu, W.; Lin, H. A new organosilane passivation agent prepared at ambient temperatures to inhibit pyrite oxidation for acid mine drainage control. J. Environ. Manag. 2022, 320, 115835. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, M.; Ashhari, S. Corrosion inhibition performance of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole compounds for protection of mild steel in hydrochloric acid solution. Electrochim. Acta 2010, 55, 1720–1724. [Google Scholar] [CrossRef]
- Wu, X.; Wiame, F.; Maurice, V.; Marcus, P. 2-Mercaptobenzimidazole films formed at ultra-low pressure on copper: Adsorption, thermal stability and corrosion inhibition performance. Appl. Surf. Sci. 2020, 527, 146814. [Google Scholar] [CrossRef]
- Chai, W.; Huang, Y.; Peng, W.; Han, G.; Cao, Y.; Liu, J. Enhanced separation of pyrite from high-sulfur bauxite using 2-mercaptobenzimidazole as chelate collector: Flotation optimization and interaction mechanisms. Miner. Eng. 2018, 129, 93–101. [Google Scholar] [CrossRef]
- Zheng, M.; Peng, T.; Wang, Y.; Luo, L.; Shao, W.; Gao, J.; Lu, Q. Flotability of galena and pyrite using 2-mercaptobenzimidazole as a chelating agent: Adsorption characteristics and flotation mechanisms. Powder Technol. 2021, 393, 449–460. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Recovery of phosphorus from source separated urine by Acidithiobacillus ferrooxidans culture supernatant. Ecol. Eng. 2016, 92, 90–96. [Google Scholar] [CrossRef]
- Caldwell, D.H.; Adams, R.B. Colorimetric determination of iron in water with o-phenanthroline. J. Am. Water Work. Assoc. 1946, 38, 727–730. [Google Scholar] [CrossRef]
- Ogbughalu, O.T.; Vasileiadis, S.; Schumann, R.C.; Gerson, A.R.; Li, J.; Smart, R.S.C.; Short, M.D. Role of microbial diversity for sustainable pyrite oxidation control in acid and metalliferous drainage prevention. J. Hazard. Mater. 2020, 393, 122338. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, Z.; Zhao, X.; Li, J. Review of the recent advances in the prevention, treatment, and resource recovery of acid mine wastewater discharged in coal mines. J. Water Process Eng. 2023, 52, 103555. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Zhang, P.; Nabi, M.; Tao, X.; Zhang, H.; Liu, Y. L-cysteine addition enhances microbial surface oxidation of coal inorganic sulfur: Complexation of cysteine and pyrite, inhibition of jarosite formation, environmental effects. Environ. Res. 2020, 187, 109705. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sayem, A.K.; Perez, J.P.; Hornback, S.; Owusu-Fordjour, E.Y.; Yang, X. Mechanism investigation of food waste compost as a source of passivation agents for inhibiting pyrite oxidation. J. Environ. Chem. Eng. 2024, 12, 113465. [Google Scholar] [CrossRef]
- Meruane, G.; Vargas, T. Bacterial oxidation of ferrous iron by Acidithiobacillus ferrooxidans in the pH range 2.5–7.0. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Rojas-Chapana, J.A.; Tributsch, H. Biochemistry of sulfur extraction in bio-corrosion of pyrite by Thiobacillus ferrooxidans. Hydrometallurgy 2001, 59, 291–300. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D.J.M.E. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Piervandi, Z.; Darban, A.K.; Mousavi, S.M.; Abdollahy, M.; Asadollahfardi, G.; Funari, V. Effect of biogenic jarosite on the bio-immobilization of toxic elements from sulfide tailings. Chemosphere 2020, 258, 127288. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Taheri Shoja, S.; Heydari Zebardast, B.; Mohamadian Samim, P. An electrochemical impedance spectroscopic study of the passive state on AISI 304 stainless steel. Int. J. Electrochem. 2011, 2011, 152143. [Google Scholar] [CrossRef]
- Wu, S.; Ning, Y.; Xie, H.; Tian, H.; Lv, J.; Liu, B. Study on the galvanic corrosion behavior of copper-nickel/titanium alloys under simulated seawater environment. J. Solid State Electrochem. 2024, 28, 2315–2329. [Google Scholar] [CrossRef]
- Walter, G.W. A review of impedance plot methods used for corrosion performance analysis of painted metals. Corros. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Meng, X.; Chen, X.; Wu, M.; Zhao, Y.; Fan, Y. Effect of Hydrostatic Pressure on Electrochemical Behavior of X100 Steel in NaHCO3+NaCl Solution. J. Chin. Soc. Corros. Prot. 2016, 36, 219–224. [Google Scholar]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434. [Google Scholar] [CrossRef]
- Graziano, G. Hydrophobicity of benzene. Biophys. Chem. 1999, 82, 69–79. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Escudey, M.; Vyhmeister, E.; Higueras, P.; Godoy-Faúndez, A.; Salazar, J.L. Adsorption of biosolids and their main components on chalcopyrite, molybdenite and pyrite: Zeta potential and FTIR spectroscopy studies. Miner. Eng. 2015, 78, 128–135. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Xu, Y. PropS-SH/SiO2 nanocomposite coatings for pyrite oxidation inhibition to control acid mine drainage at the source. J. Hazard. Mater. 2017, 338, 313–322. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Liu, Y.; Chen, X.; Wu, W.; Li, F. Tannic acid as an eco-friendly natural passivator for the inhibition of pyrite oxidation to prevent acid mine drainage at the source. Appl. Surf. Sci. 2022, 591, 153172. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar]
- Mu, Y.; Li, L.; Peng, Y. Surface properties of fractured and polished pyrite in relation to flotation. Miner. Eng. 2017, 101, 10–19. [Google Scholar] [CrossRef]
- Hong, M.; Wang, J.; Yang, B.; Liu, Y.; Sun, X.; Li, L. Inhibition of pyrite oxidation through forming biogenic K-jarosite coatings to prevent acid mine drainage production. Water Res. 2024, 252, 121221. [Google Scholar] [CrossRef] [PubMed]
- Schaufuß, A.G.; Nesbitt, H.W.; Kartio, I.; Laajalehto, K.; Bancroft, G.M.; Szargan, R. Incipient oxidation of fractured pyrite surfaces in air. J. Electron Spectrosc. Relat. Phenom. 1998, 96, 69–82. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, S.; Liao, P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Et Cosmochim. Acta 2016, 172, 444–457. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Pratt, A.R.; Scaini, M.J. Sulfur and iron surface states on fractured pyrite surfaces. Am. Mineral. 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Zhang, G.; Ye, G.; Liu, D. Adsorption study of fenugreek gum onto pyrite surface: Implications for chalcopyrite-pyrite flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132404. [Google Scholar] [CrossRef]

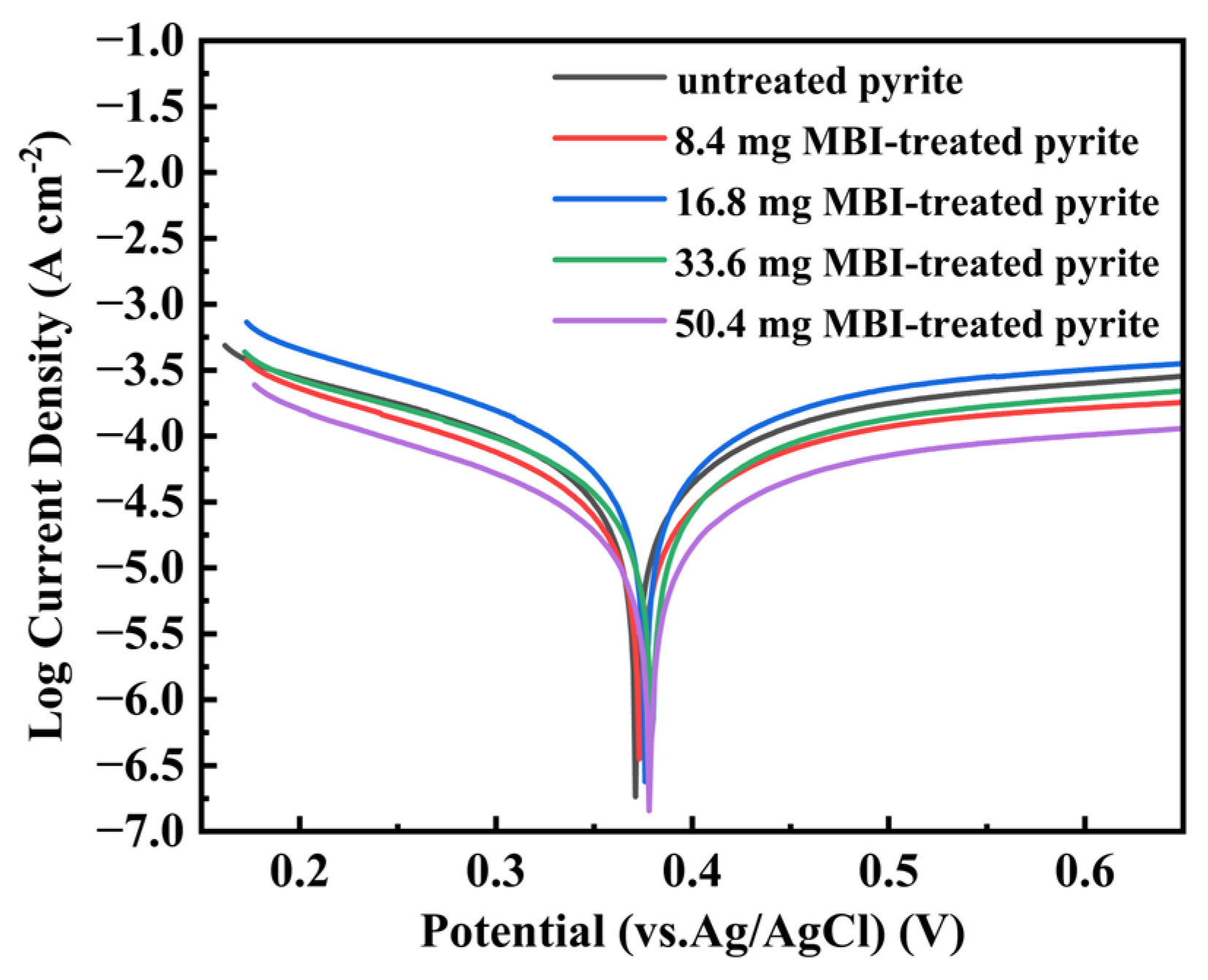

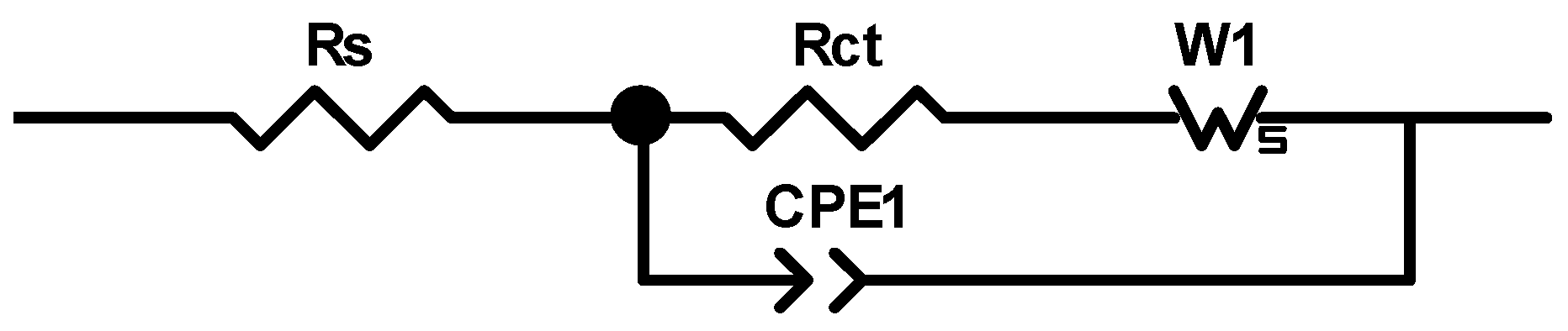

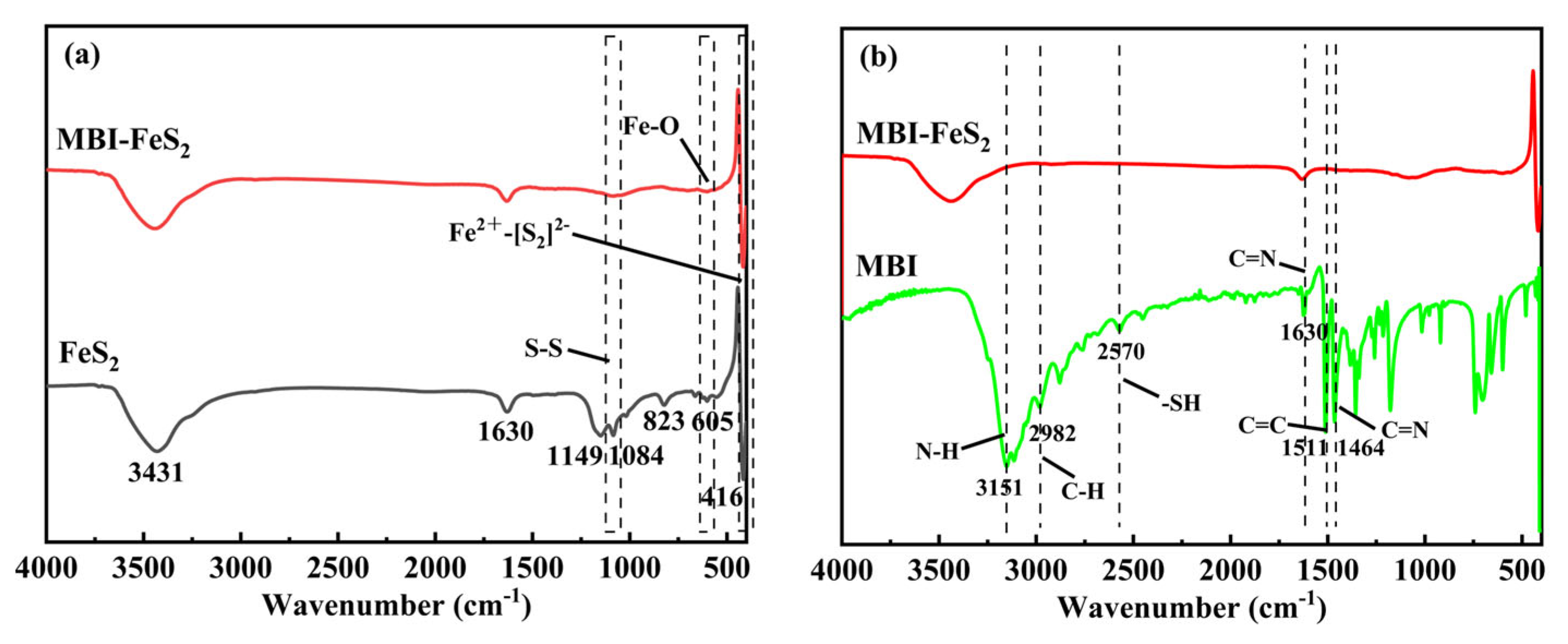
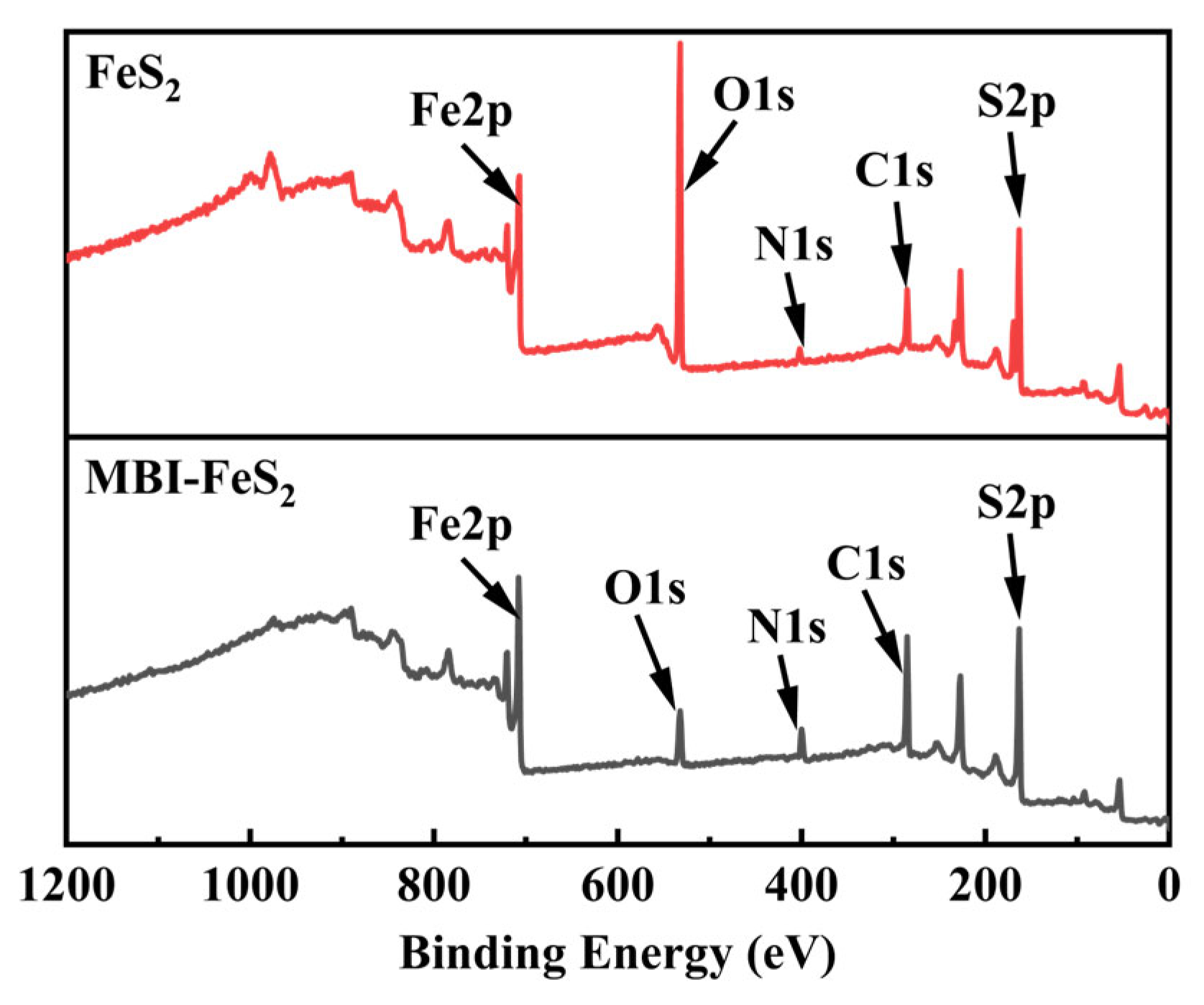
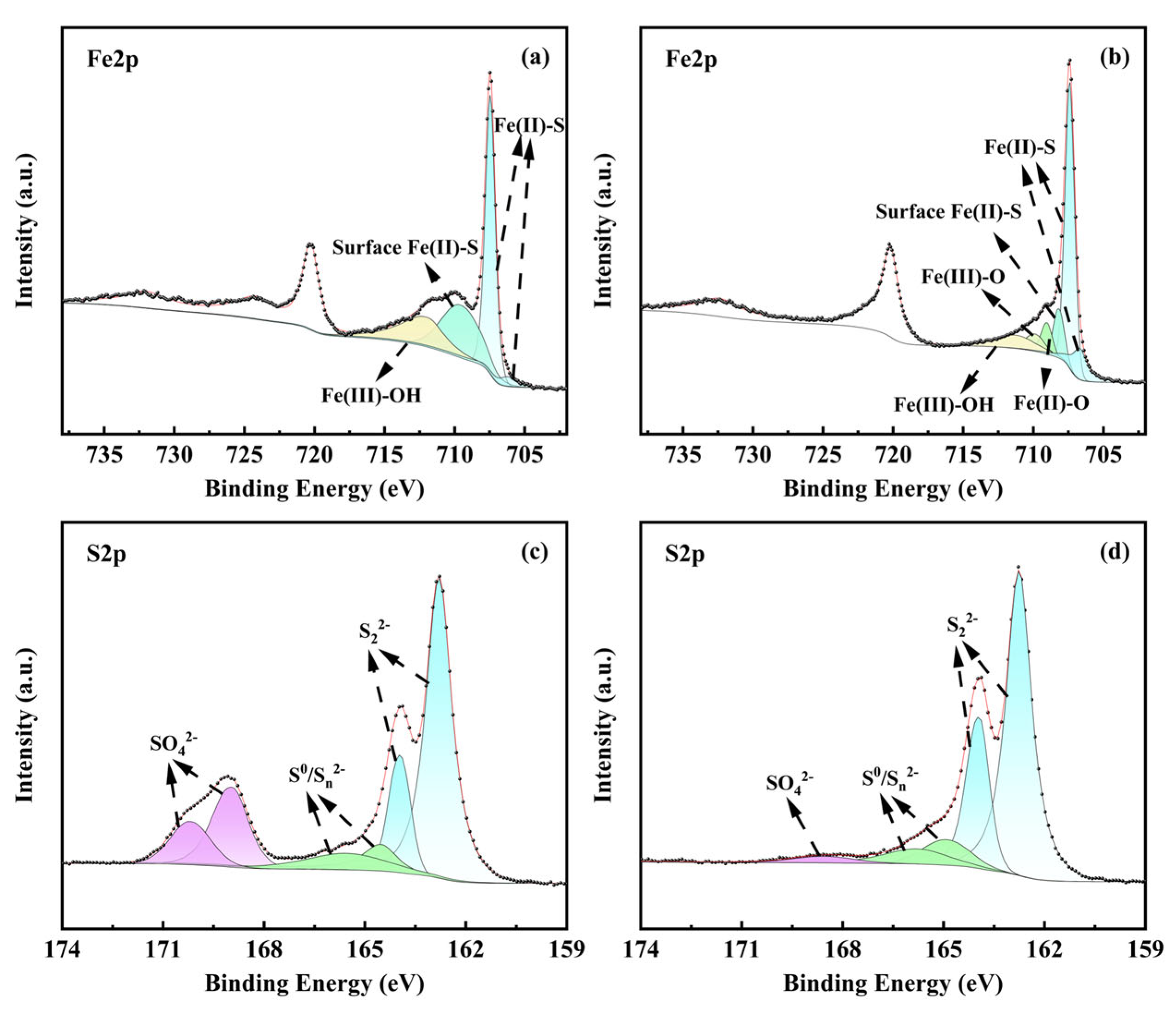

| Electrode | Ecorr (vs. Ag/AgCl) (mV) | Icorr (μA cm−2) |
|---|---|---|
| Untreated pyrite | 371 | 6.803 |
| 8.4 mg MBI-treated pyrite | 373 | 9.358 |
| 16.8 mg MBI-treated pyrite | 376 | 5.708 |
| 33.6 mg MBI-treated pyrite | 379 | 4.872 |
| 50.4 mg MBI-treated pyrite | 378 | 2.952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, X.; Yang, J.; Wang, X.; Zhou, Y.; Liu, B.; Zhang, Y. Study on the Oxidation Inhibition of Pyrite by 2-Mercaptobenzimidazole in the Presence of Acidithiobacillus ferrooxidans. Minerals 2025, 15, 487. https://doi.org/10.3390/min15050487
Huang J, Li X, Yang J, Wang X, Zhou Y, Liu B, Zhang Y. Study on the Oxidation Inhibition of Pyrite by 2-Mercaptobenzimidazole in the Presence of Acidithiobacillus ferrooxidans. Minerals. 2025; 15(5):487. https://doi.org/10.3390/min15050487
Chicago/Turabian StyleHuang, Junjie, Xiang Li, Jingxu Yang, Xiaolong Wang, Yeyang Zhou, Bing Liu, and Yansheng Zhang. 2025. "Study on the Oxidation Inhibition of Pyrite by 2-Mercaptobenzimidazole in the Presence of Acidithiobacillus ferrooxidans" Minerals 15, no. 5: 487. https://doi.org/10.3390/min15050487
APA StyleHuang, J., Li, X., Yang, J., Wang, X., Zhou, Y., Liu, B., & Zhang, Y. (2025). Study on the Oxidation Inhibition of Pyrite by 2-Mercaptobenzimidazole in the Presence of Acidithiobacillus ferrooxidans. Minerals, 15(5), 487. https://doi.org/10.3390/min15050487





