Investigation of a Novel Depressant for Flotation Separation of Chalcopyrite and Galena: Experiments and Adsorption Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Methods
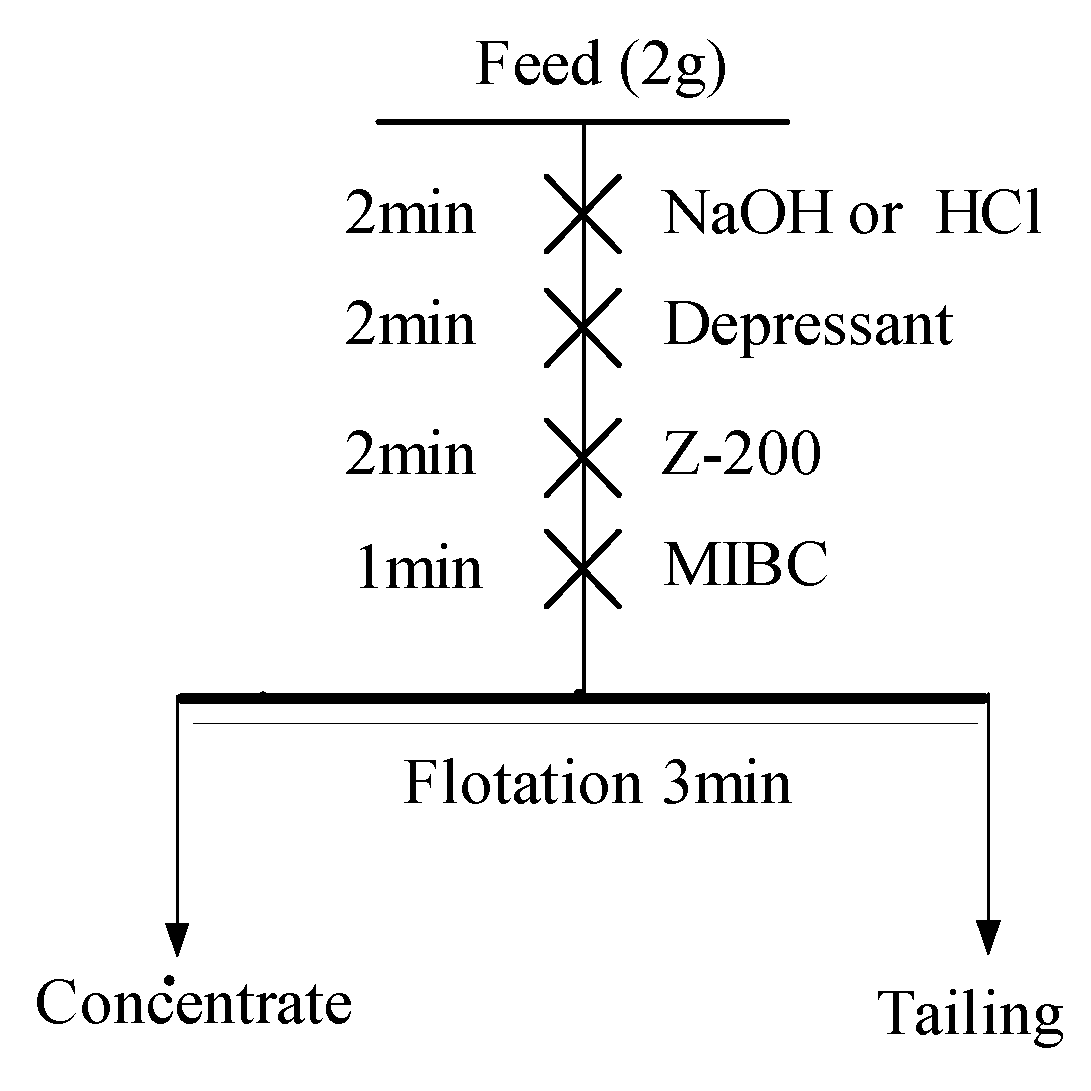
2.2.1. Flotation Experiments
2.2.2. FTIR Measurements
2.2.3. XPS Measurements
2.2.4. Computation Approach
3. Results
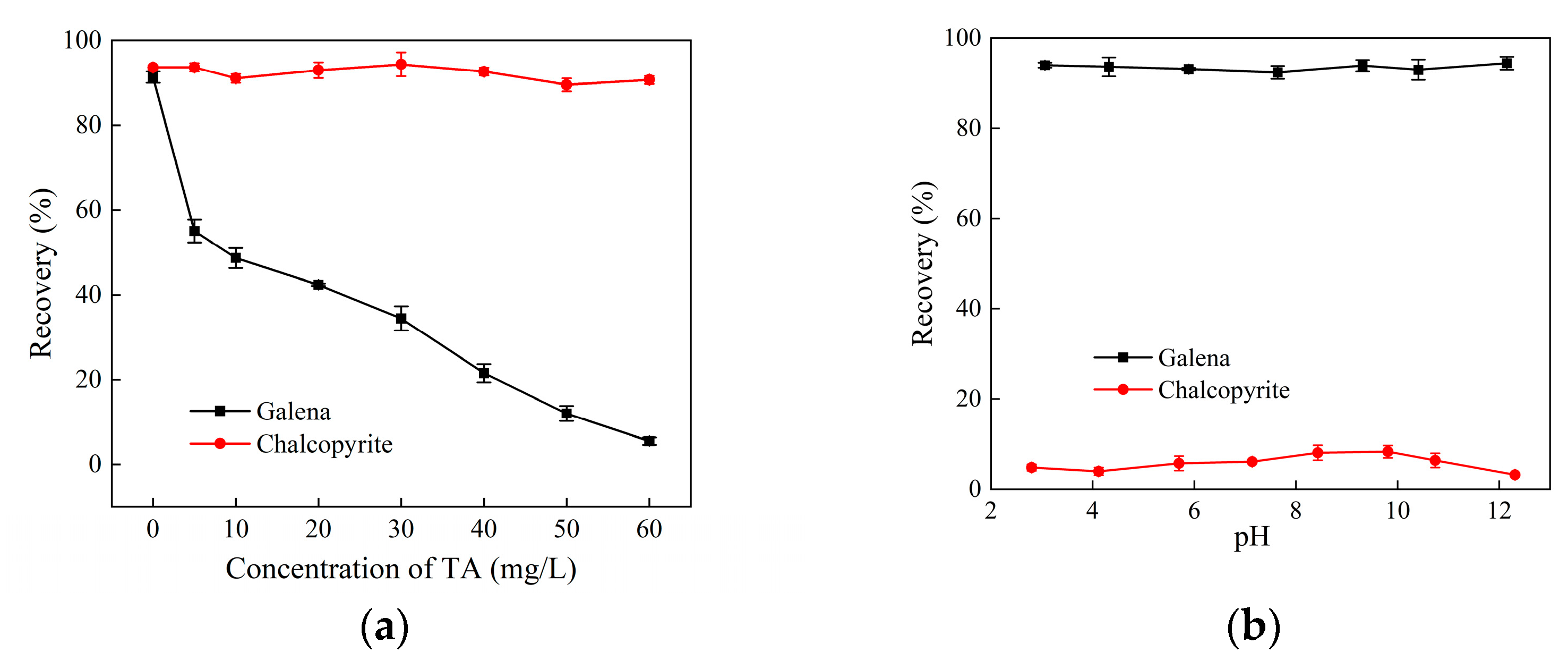
3.1. Micro-Flotation Experiments
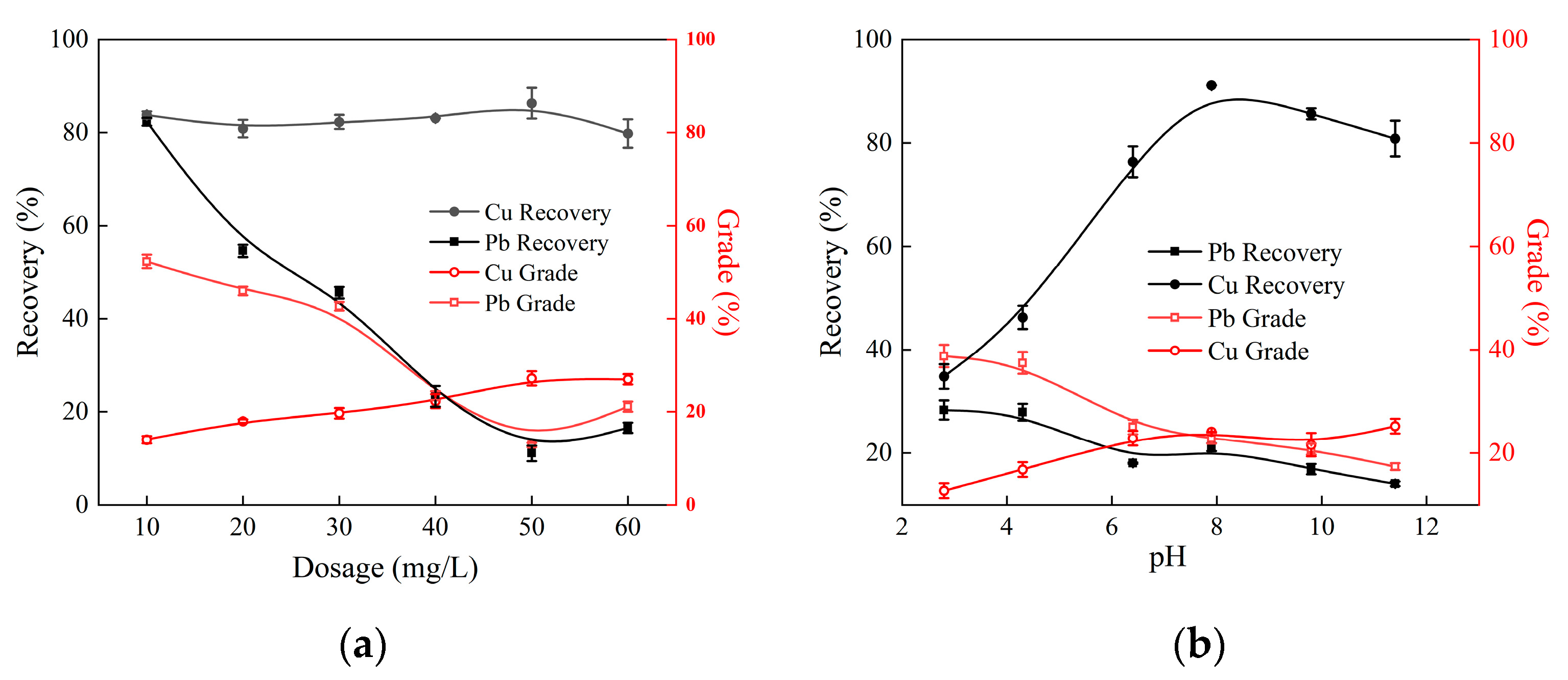
3.2. Artificially Mixed Minerals Experiments
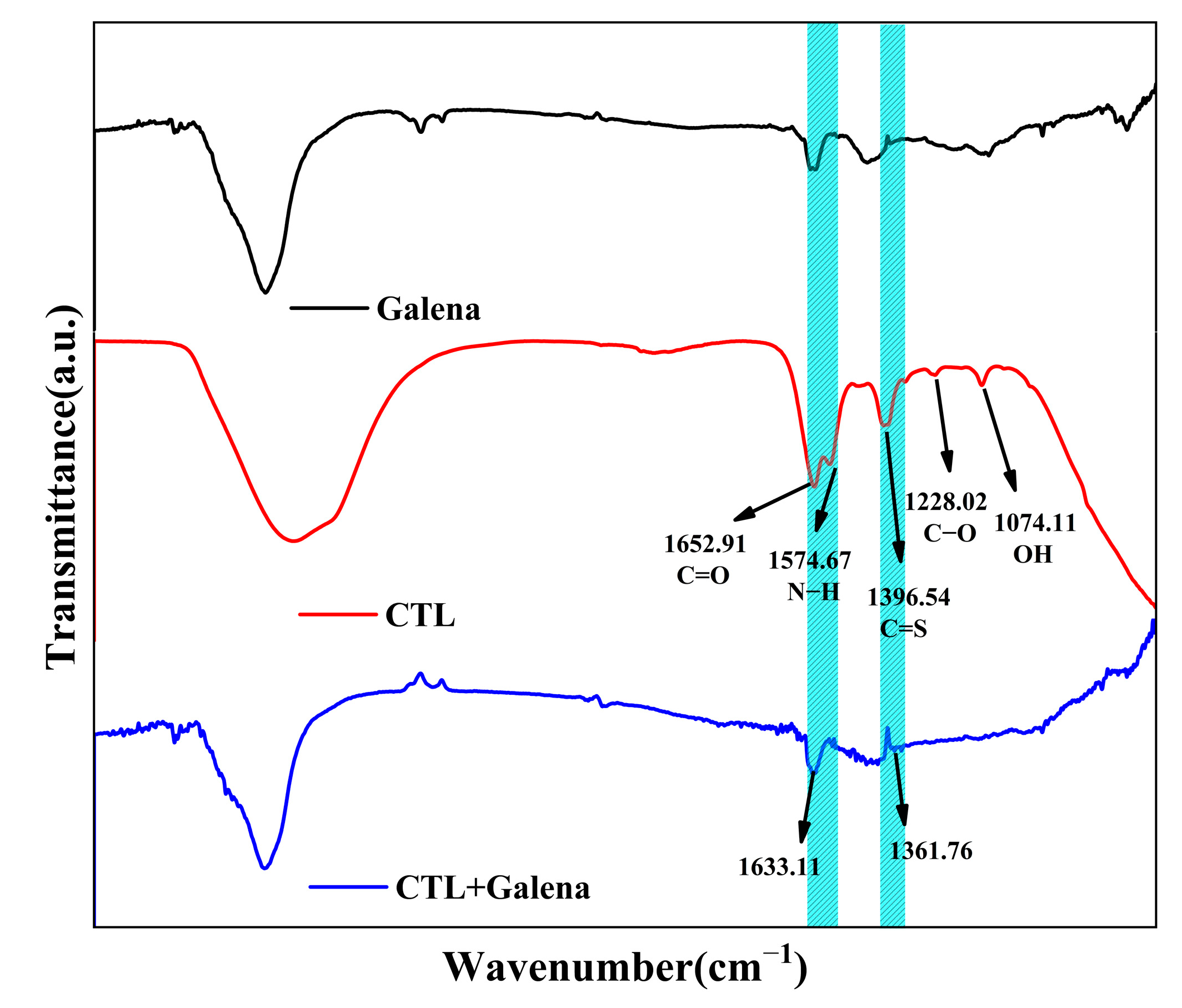
3.3. FTIR Experiments
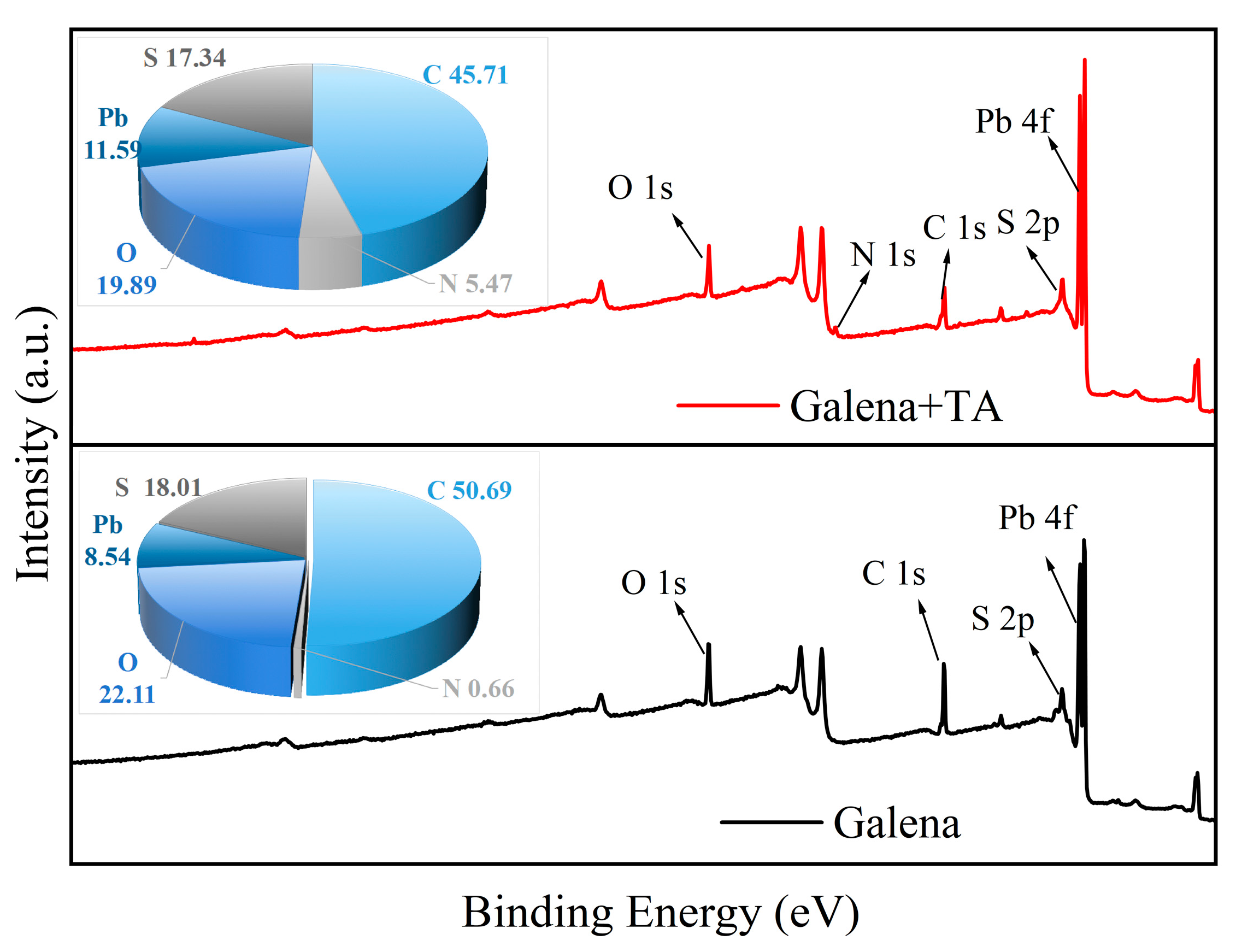
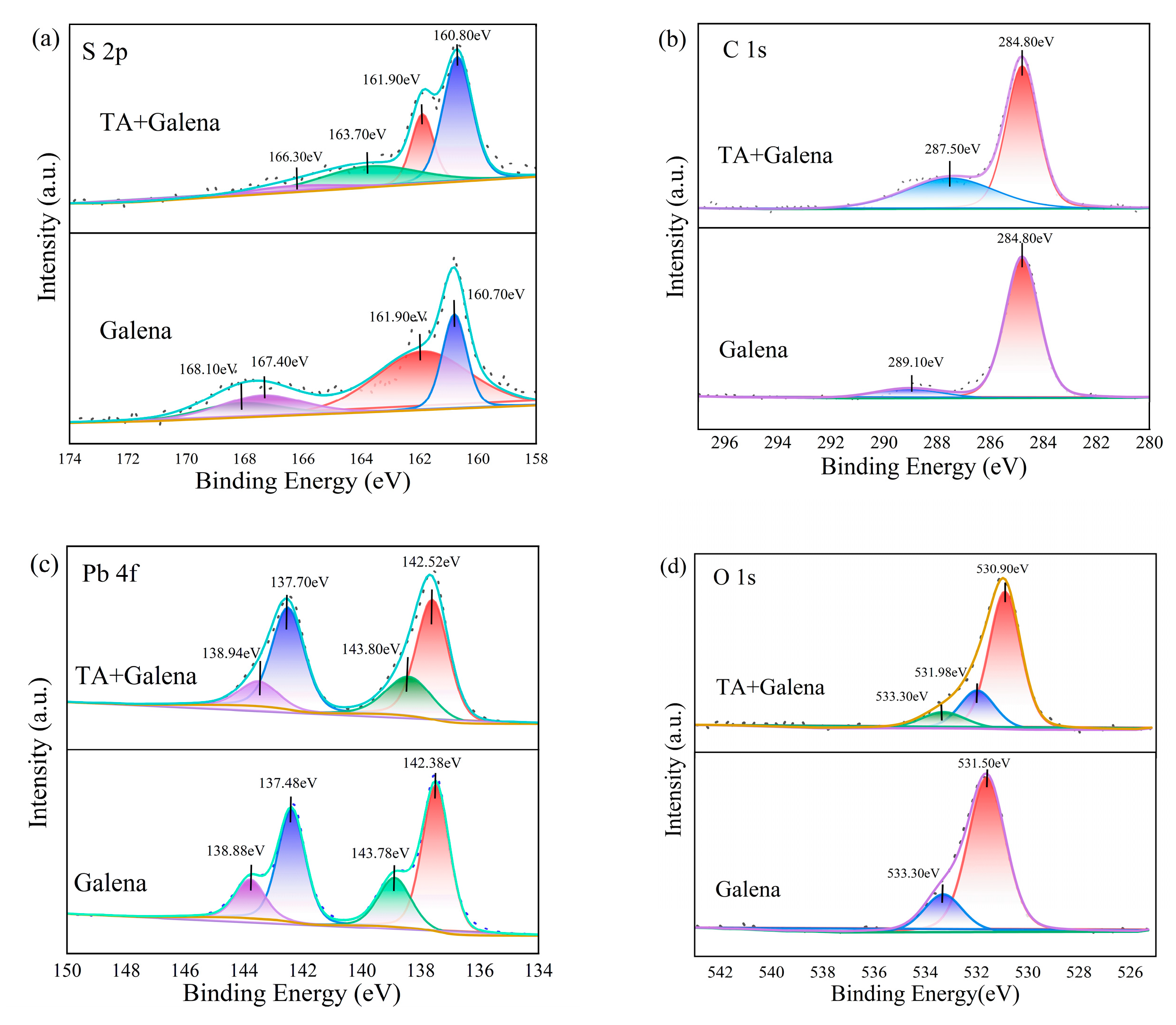
3.4. XPS Experiments
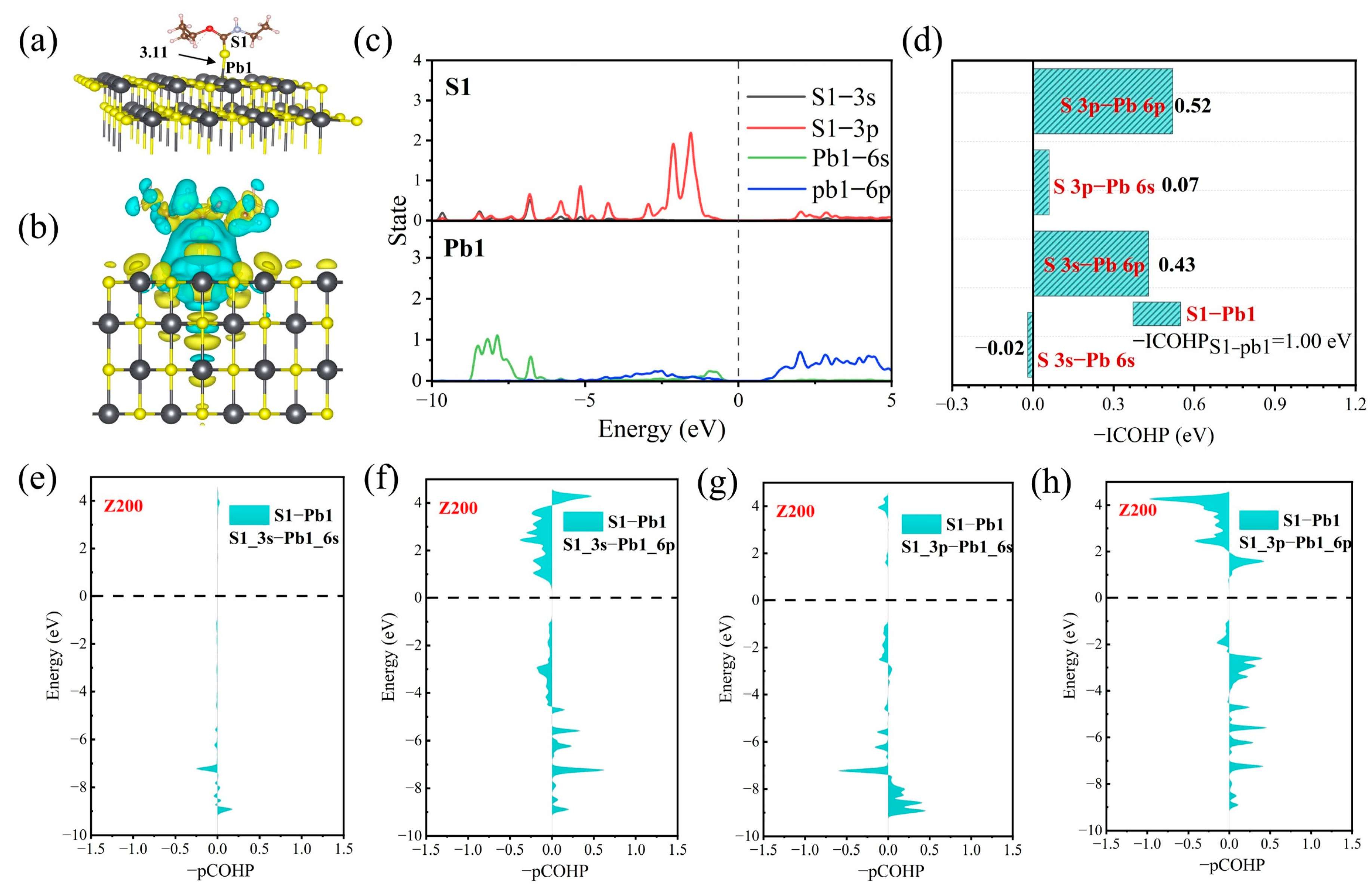
3.5. Adsorption Mechanisms from DFT Calculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Jiao, F.; Qin, W.; Wang, C.; Li, X. Flotation separation of sphalerite from galena using eco-friendly and efficient depressant pullulan. Sep. Purif. Technol. 2022, 295, 121013. [Google Scholar] [CrossRef]
- Su, C.; Cai, J.; Yu, X.; Peng, R.; Zheng, Q.; Ma, Y.; Liu, D. Effect of pre-oxidation on galena in the flotation separation of chalcopyrite from galena with calcium lignosulfonate as depressant. Miner. Eng. 2022, 182, 107520. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Q.; Deng, J.; Wen, S.; Liu, Z. Surface chemistry study of Cu-Pb sulfide ore using ToF-SIMS and multivariate analysis. Appl. Surf. Sci. 2020, 518, 146270. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Q.; Deng, J.; Wen, S. Using ToF-SIMS to study metal ions transfer between chalcopyrite and galena during grinding. Adv. Powder Technol. 2020, 31, 2650–2657. [Google Scholar] [CrossRef]
- Cai, J.; Jia, X.; Ma, Y.; Ibrahim, A.M.; Su, C.; Yu, X.; Liu, D. Effect of Pre-Oxidation on Copper-Lead Bulk Concentrate Flotation Separation with Sodium Polyacrylate as Galena Depressant. Sep. Purif. Technol. 2022, 302, 122276. [Google Scholar] [CrossRef]
- Yang, X.; Lai, H.; Wei, X.; Shen, P.; Wang, Y.; Li, M.; Cai, J.; Liu, D. Adsorption Mechanism of Na2S2O3 and FeSO4 as a Combined Depressant for Galena in Chalcopyrite-Galena Flotation Separation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135799. [Google Scholar] [CrossRef]
- Wills, B. The Separation by Flotation of Copper–Lead–Zinc Sulphides. Min. Mag. 1984, 150, 36–41. [Google Scholar]
- Ralston, J. The chemistry of galena flotation: Principles & practice. Miner. Eng. 1994, 7, 715–735. [Google Scholar]
- Okada, S.; Majima, H. Depressive action of chromate and dichromate salts on galena. Can. Metall. Q. 1971, 10, 189–195. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Zeng, H.; Hu, Z.; Zhu, Y.; Han, L. A macromolecular depressant for galena and its flotation behavior in the separation from molybdenite. Miner. Eng. 2020, 157, 106576. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Z.; Zheng, G.; Zhu, Y.; Wu, W. The design of a macromolecular depressant for galena based on DFT studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Pugh, R. Macromolecular organic depressants in sulphide flotation—A review, 1. Principles, types and applications. Int. J. Miner. Process. 1989, 25, 101–130. [Google Scholar] [CrossRef]
- Cao, J.; Liao, R.; Wu, D.; Zuo, Q.; Liu, J.; Wen, S. Utilizing Phosphonyl Carboxylic Acid Copolymer as an Efficient Depressant for Flotation Separation of Chalcopyrite from Galena: Experimental and DFT Study. Sep. Purif. Technol. 2024, 348, 127725. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Chen, Y. Investigations on the selective depression of fenugreek gum towards galena and its role in chalcopyrite-galena flotation separation. Miner. Eng. 2021, 166, 106886. [Google Scholar] [CrossRef]
- Miao, Y.; Wen, S.; Shen, Z.; Feng, Q.; Zhang, Q. Flotation separation of chalcopyrite from galena using locust bean gum as a selective and eco-friendly depressant. Sep. Purif. Technol. 2022, 283, 120173. [Google Scholar] [CrossRef]
- Qin, W.; Wei, Q.; Jiao, F.; Li, N.; Wang, P.; Ke, L. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena. Int. J. Min. Sci. Technol. 2012, 22, 345–349. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Using chitosan as a selective depressant in the differential flotation of Cu–Pb sulfides. Int. J. Miner. Process. 2012, 106, 8–15. [Google Scholar] [CrossRef]
- Pugh, R. Macromolecular organic depressants in sulphide flotation—A review, 2. Theoretical analysis of the forces involved in the depressant action. Int. J. Miner. Process. 1989, 25, 131–146. [Google Scholar] [CrossRef]
- Pan, Z.; Cai, C.; Liang, G.; Jiao, F.; Yang, C.; Wei, Q.; Qin, W. Pyrolysis Liquid of Pine Wood: A Novel Efficient Depressant of Pyrite in Galena Flotation. Miner. Eng. 2024, 210, 108642. [Google Scholar] [CrossRef]
- Wang, Z.; Han, G.; Feng, Q. Selective Flotation of Galena and Sphalerite Using N-(Phosphonomethyl) Iminodiacetic Acid as an Eco-Friendly Depressant. Green Smart Min. Eng. 2024, 1, 96–103. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Q.; Yan, Y.; Zou, H.; Huang, X.; Liu, D. Flotation Separation of Galena and Chalcopyrite by Using Hydroxyl Radicals from an Fe2+/NaClO System as Depressants. Appl. Surf. Sci. 2025, 686, 162128. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Lu, L.; Zhu, H.; Xiong, W.; Zhu, Y.; Yang, B. Investigation on a Novel Galena Depressant in the Flotation Separation from Molybdenite. Minerals 2021, 11, 12. [Google Scholar] [CrossRef]
- Liu, C.; Wu, G.; Yanhong, M.; Lu, T.; Liu, H.; Zhao, Z.; Chen, L. Application and Quantum Chemical Analysis of Novel Sulfur-Containing Heterocyclic Inhibitors in Separation of Molybdenite and Galena. Conserv. Util. Miner. Resour. 2022, 42, 75–81. [Google Scholar]
- Zhu, H.; Yang, B.; Martin, R.; Zhang, H.; He, D.; Luo, H. Flotation Separation of Galena from Sphalerite Using Hyaluronic Acid (HA) as an Environmental-Friendly Sphalerite Depressant. Miner. Eng. 2022, 187, 107771. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, W.; Liu, R.; Xie, Z.; Cao, Z.; Sun, W. Green Separation of Galena from Molybdenite by Flotation Using DL-Dithiothreitol as a Depressant. Sep. Purif. Technol. 2024, 347, 127676. [Google Scholar] [CrossRef]
- Pearse, M.J. Historical Use and Future Development of Chemicals for Solid–Liquid Separation in the Mineral Processing Industry. Miner. Eng. 2003, 16, 103–108. [Google Scholar] [CrossRef]
- Moudgil, B.M. Effect of Polyacrylamide and Polyethylene Oxide Polymers on Coal Flotation. Colloids Surf. 1983, 8, 225–228. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Selective Depression of Pyrite with Polyacrylamide Polymers. Int. J. Miner. Process. 2001, 61, 13–22. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Gillan, M.J.; Alfe, D.; Michaelides, A. Perspective: How Good Is DFT for Water? J. Chem. Phys. 2016, 144, 130901. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Revealing the Nature of Intermolecular Interaction and Configurational Preference of the Nonpolar Molecular Dimers (H2)2, (N2)2, and (H2)(N2). J. Mol. Model. 2013, 19, 5387–5395. [Google Scholar] [CrossRef]
- Schimka, L.; Harl, J.; Stroppa, A.; Grüneis, A.; Marsman, M.; Mittendorfer, F.; Kresse, G. Accurate Surface and Adsorption Energies from Many-Body Perturbation Theory. Nat. Mater. 2010, 9, 741–744. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Noda, Y.; Masumoto, K.; Ohba, S.; Saito, Y.; Toriumi, K.; Iwata, Y.; Shibuya, I. Temperature Dependence of Atomic Thermal Parameters of Lead Chalcogenides, PbS, PbSe and PbTe. Cryst. Struct. Commun. 1987, 43, 1443–1445. [Google Scholar] [CrossRef]
- Naumov, P.; Makreski, P.; Petruševski, G.; Runčevski, T.; Jovanovski, G. Visualization of a Discrete Solid-State Process with Steady-State X-Ray Diffraction: Observation of Hopping of Sulfur Atoms in Single Crystals of Realgar. J. Am. Chem. Soc. 2010, 132, 11398–11401. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Li, Y.; Chen, J.; Zhao, C.; Chen, Y. DFT Study on the Galvanic Interaction between Pyrite (100) and Galena (100) Surfaces. Appl. Surf. Sci. 2016, 367, 270–276. [Google Scholar] [CrossRef]
- Ke, B.; Chen, J.; Cheng, W. Galvanic Interaction between Different Grinding Media and Galena (1 0 0) Surface and Its Influence on Galena Flotation Behavior: A DFT Study. Appl. Surf. Sci. 2021, 570, 151379. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Wang, T.; Luo, G.; Wang, H.; He, G. The flotation separation of galena and pyrite using serpentine as depressant. Powder Technol. 2019, 342, 486–490. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Karacharov, A.A.; Likhatski, M.N. Effect of adsorption of butyl xanthate on galena, PbS, and HOPG surfaces as studied by atomic force microscopy and spectroscopy and XPS. Int. J. Miner. Process. 2015, 144, 81–89. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, Z.; Sun, C.; Zhang, Y.; Liu, C.; Lu, T.; Hu, J. Novel Depressant Based on Hybridization Theory for the Separation of Galena and Realgar: Experimental Study and Adsorption Mechanism. Minerals 2025, 15, 200. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, C.; Lu, T.; Zhao, Z.; Wu, G.; Zhu, Y. Understanding the Adsorption Mechanism of BTPA, DEPA, and DPPA in the Separation of Malachite from Calcite and Quartz: DFT and Experimental Studies. Minerals 2024, 14, 692. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, C.; Gao, Z.; Lu, T.; Zhao, Z.; Zhu, Y.; Wu, G. First Principle Study of the Relationship between Electronic Properties and Adsorption Energy: Xanthate Adsorption on Pyrite and Arsenopyrite. Minerals 2024, 14, 749. [Google Scholar] [CrossRef]
- Miao, Y.; He, J.; Zhu, X.; Zhu, G.; Cao, S.; Fan, G.; Li, G.; Cao, Y. Hardness of Surface Hydroxyls and Its Pivotal Role in the Flotation of Cassiterite from Quartz via Lead Ions Activation. Sep. Purif. Technol. 2024, 347, 127565. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.; Xu, H.; Cheng, D. Rational Design of Heteroatom-Doped Fe–N–C Single-Atom Catalysts for Oxygen Reduction Reaction via Simple Descriptor. ACS Catal. 2024, 14, 6952–6964. [Google Scholar] [CrossRef]

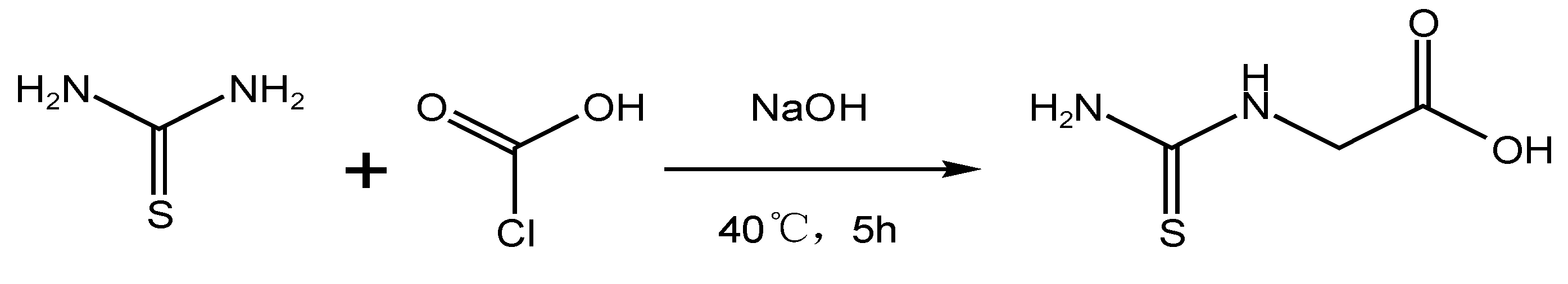



| Inorganic Reagents | Organic Macromolecule | Small Organic Molecule |
|---|---|---|
| Potassium dichromate | Polyacrylic acids | 5-amino-1,3,4-thiadiazole-2-thiol |
| Sodium sulfide | Polyacrylamide | 1,3-oxathiolane derivatives |
| Fe2+/NaClO | Modified lignosulfonates | N-(phosphonomethyl) iminodiacetic acid |
| Sodium periodate | Phosphonyl carboxylic acid copolymer | DL-dithiothreitol |
| Pinewood pyrolysis liquid | ||
| Dextrin | ||
| Tannic acid |
| Sample | P | Zn | Pb | SiO2 | Al2O3 | CaO | MgO | Cu | Tfe | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Chalcopyrite | 0.13 | 0.075 | 0.016 | <0.01 | 0.040 | 0.11 | 0.078 | 33.95 | 30.84 | 34.54 |
| Galena | 0.0021 | <0.01 | 84.99 | <0.01 | 0.047 | 0.032 | 0.013 | 0.031 | 0.067 | 12.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Liu, C.; Lu, T.; Gao, Z.; Zhu, Y.; Sun, C.; Zhao, Z.; Wu, G.; Li, R.; Hu, J. Investigation of a Novel Depressant for Flotation Separation of Chalcopyrite and Galena: Experiments and Adsorption Mechanisms. Minerals 2025, 15, 454. https://doi.org/10.3390/min15050454
Zeng H, Liu C, Lu T, Gao Z, Zhu Y, Sun C, Zhao Z, Wu G, Li R, Hu J. Investigation of a Novel Depressant for Flotation Separation of Chalcopyrite and Galena: Experiments and Adsorption Mechanisms. Minerals. 2025; 15(5):454. https://doi.org/10.3390/min15050454
Chicago/Turabian StyleZeng, Hong, Chongjun Liu, Tong Lu, Zehui Gao, Yangge Zhu, Chuanyao Sun, Zhiqiang Zhao, Guiye Wu, Ruidong Li, and Jun Hu. 2025. "Investigation of a Novel Depressant for Flotation Separation of Chalcopyrite and Galena: Experiments and Adsorption Mechanisms" Minerals 15, no. 5: 454. https://doi.org/10.3390/min15050454
APA StyleZeng, H., Liu, C., Lu, T., Gao, Z., Zhu, Y., Sun, C., Zhao, Z., Wu, G., Li, R., & Hu, J. (2025). Investigation of a Novel Depressant for Flotation Separation of Chalcopyrite and Galena: Experiments and Adsorption Mechanisms. Minerals, 15(5), 454. https://doi.org/10.3390/min15050454






