Advances in Glendonite Understanding and Its Potential for Carbon Capture
Abstract
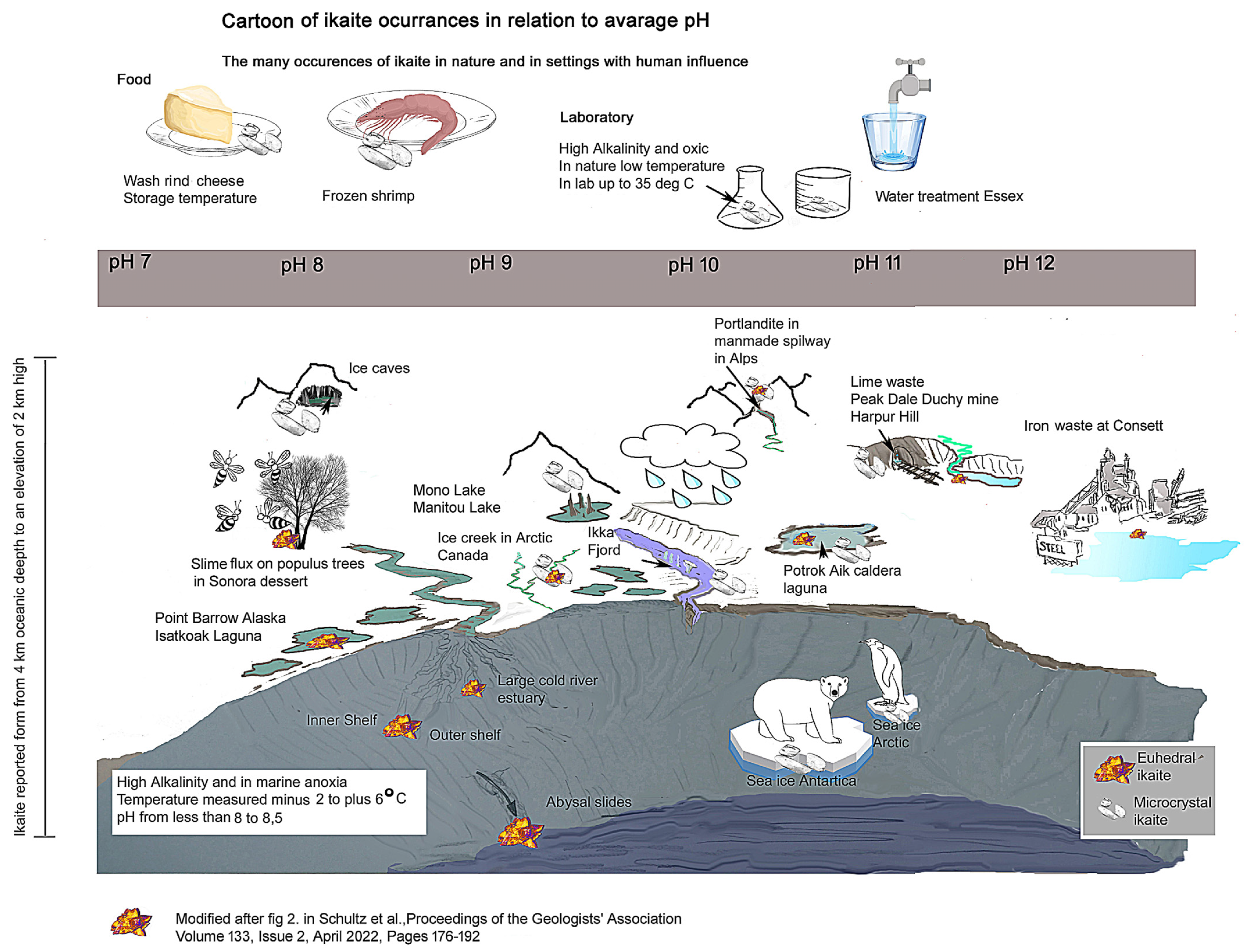
1. Introduction
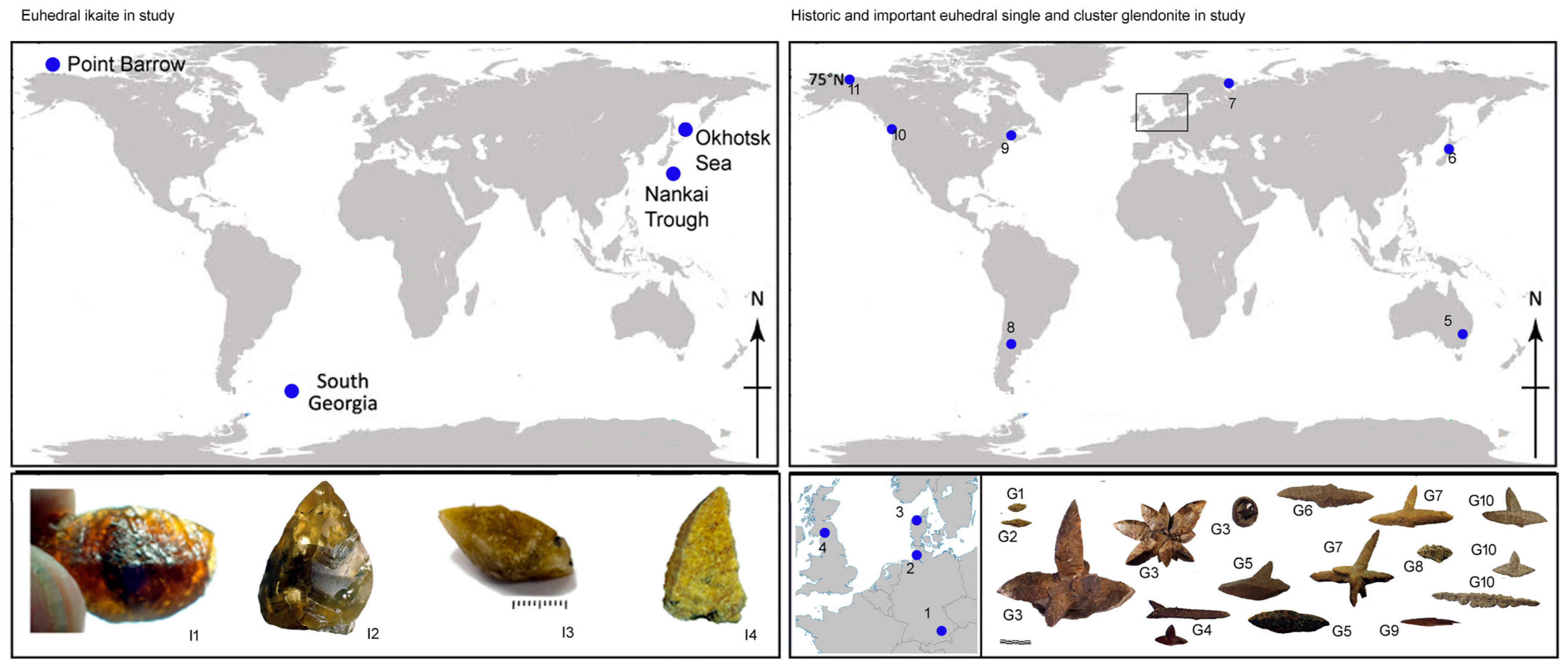
2. Study Sites
3. Methods
4. Linking Ikaite to Glendonite
Identification of Glendonite
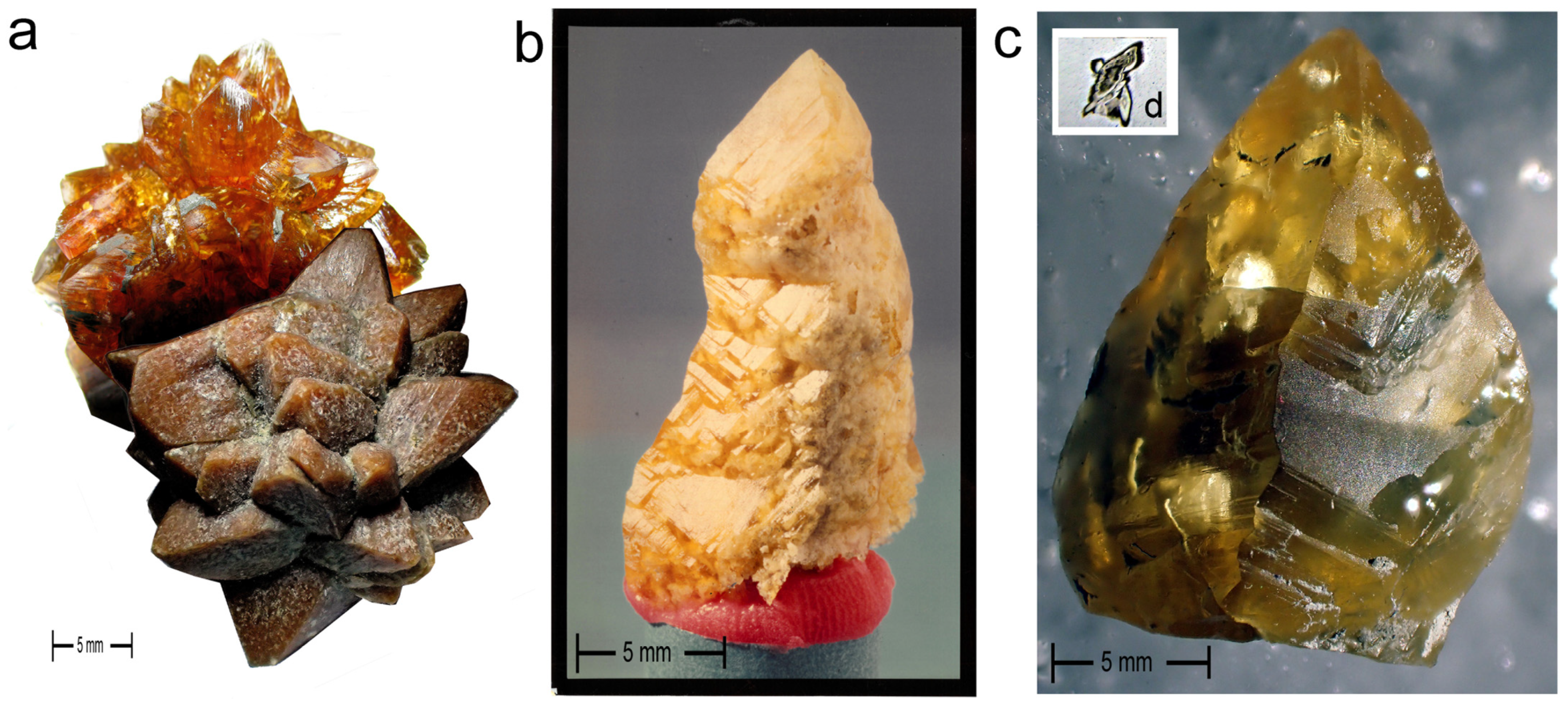
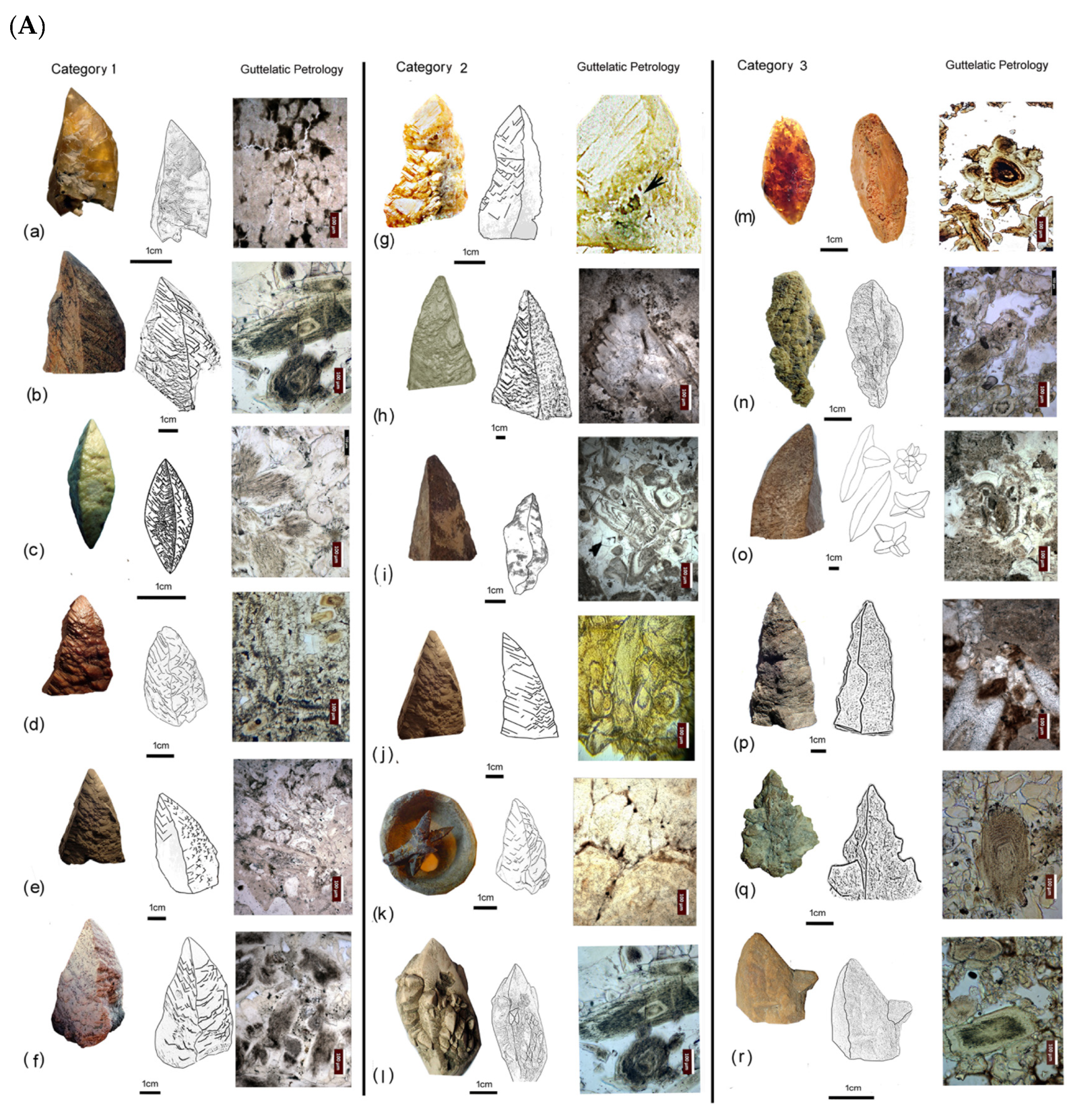
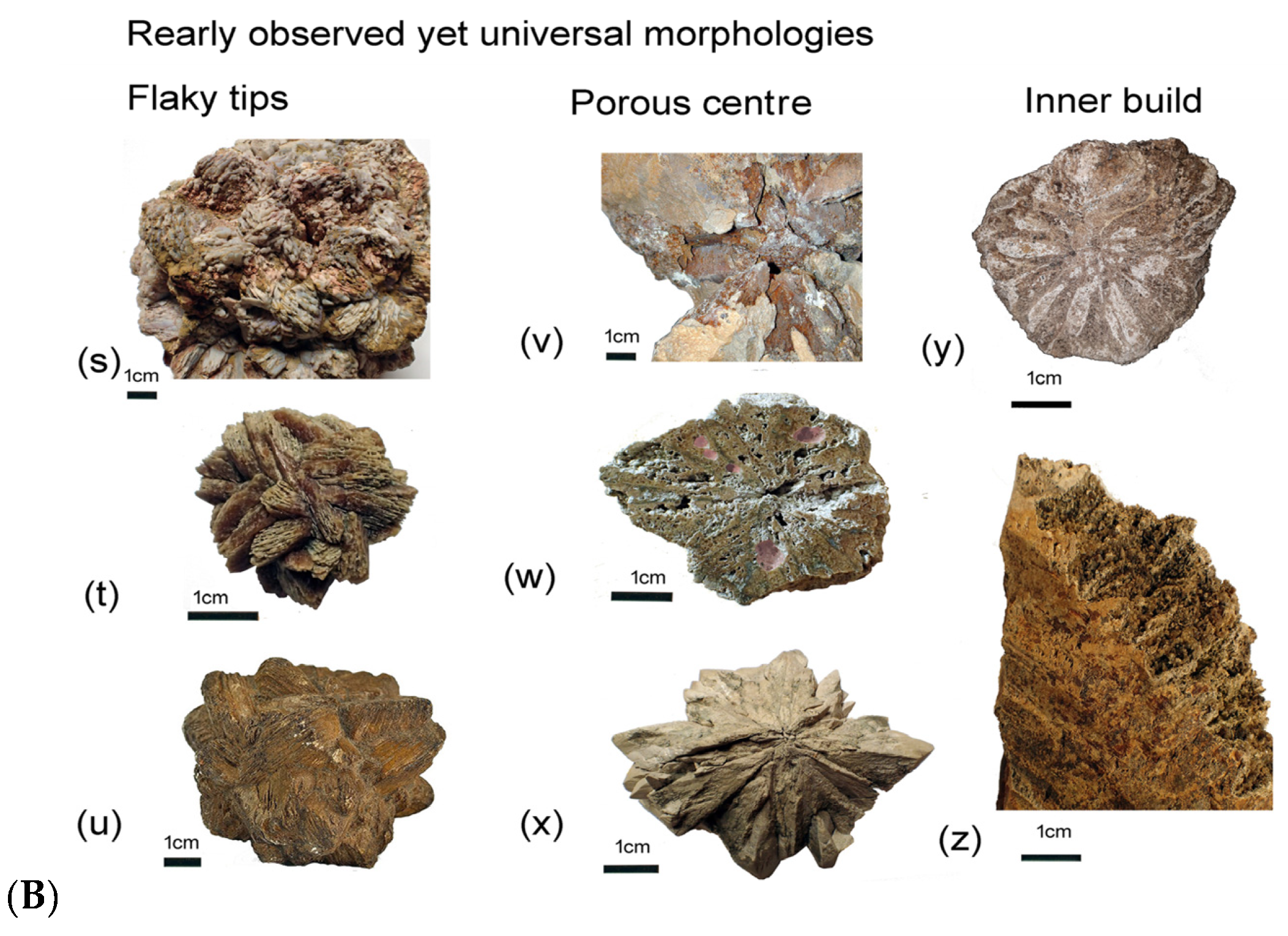
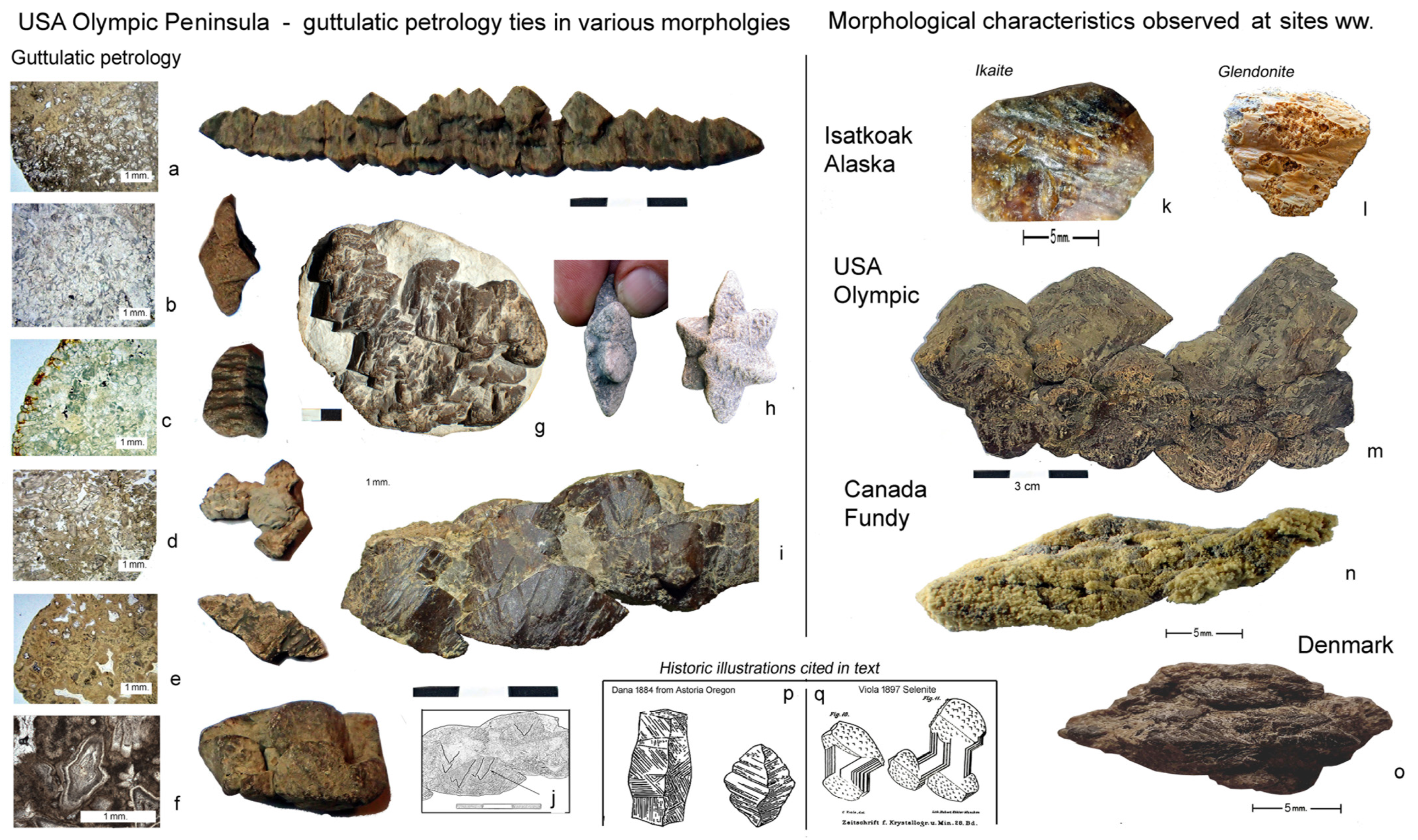
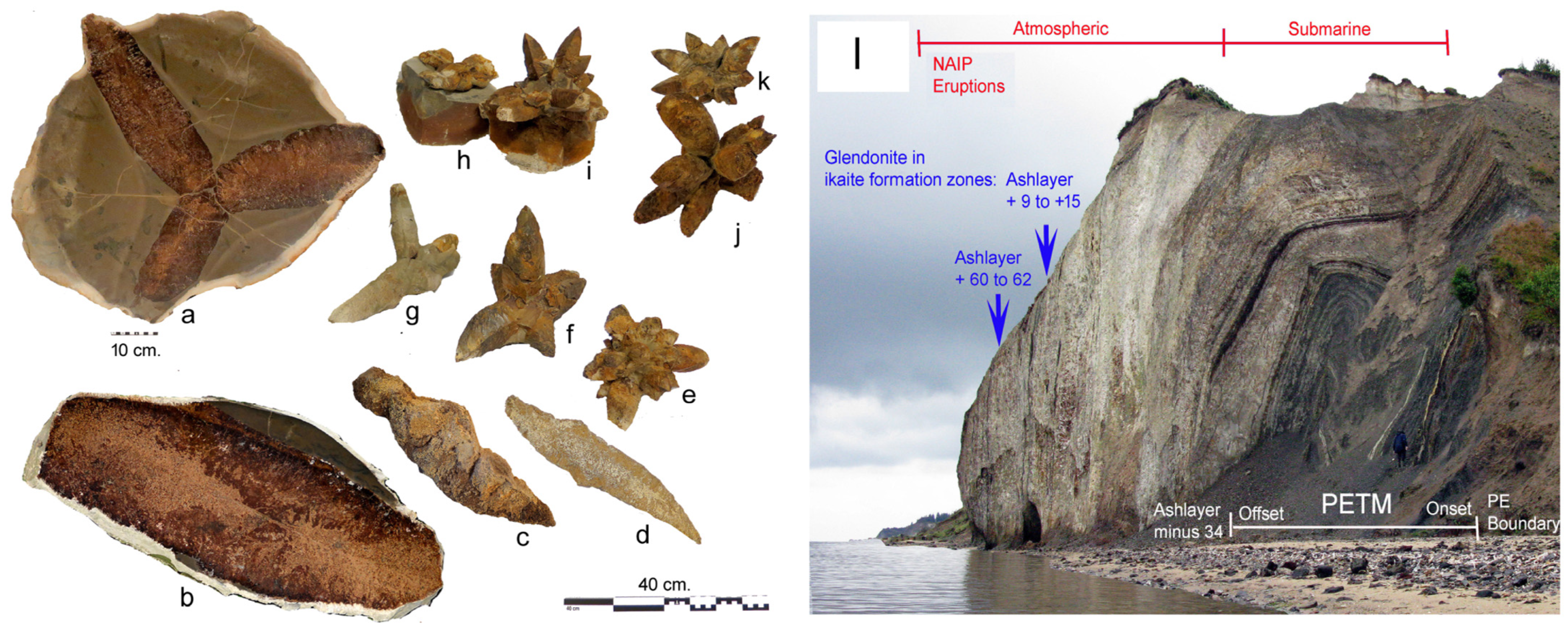
5. Spectacular Morphologies from Historic Sites United by Guttulatic Petrography
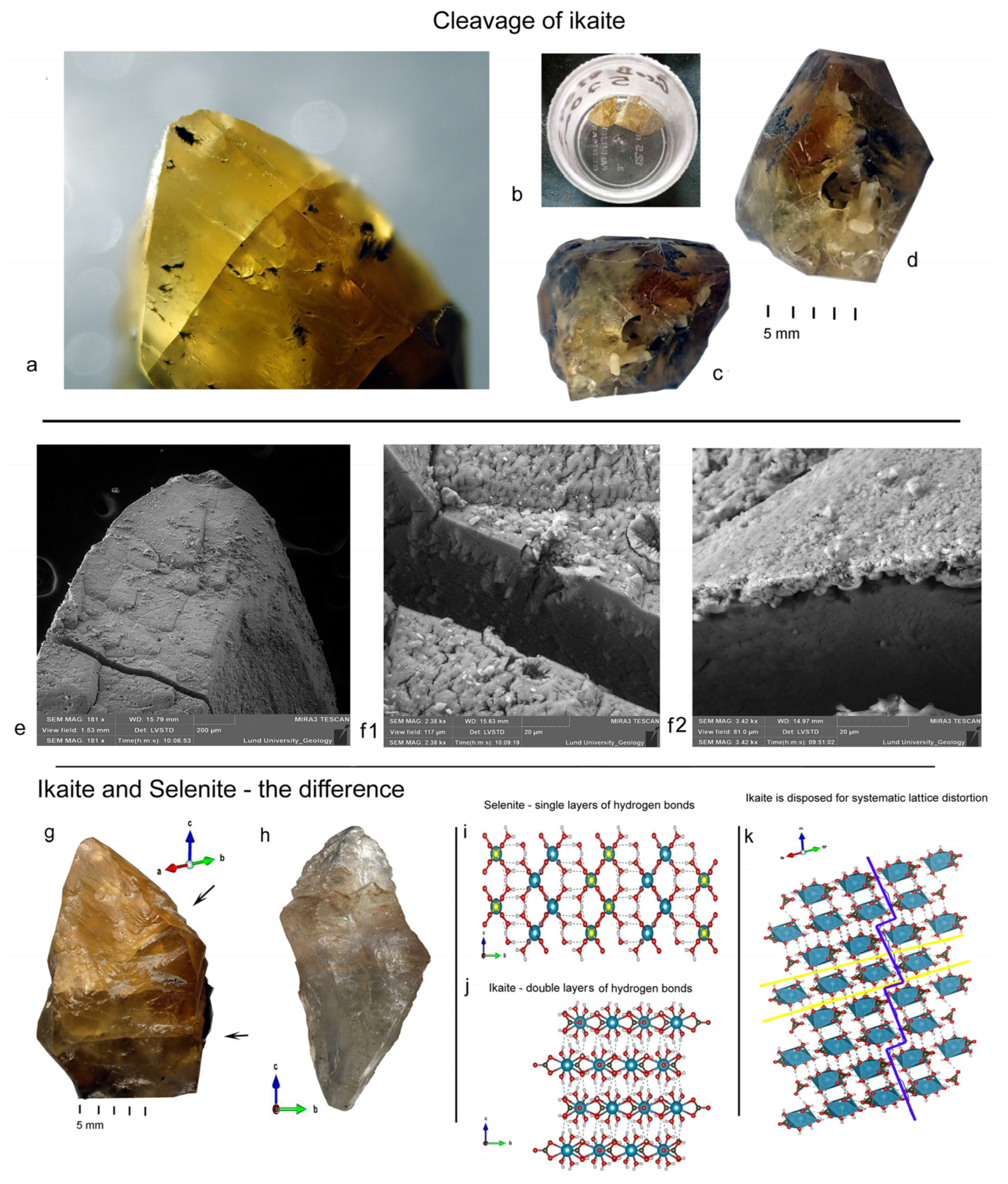
5.1. Preliminary Observations of Ikaite Cleavage Suggest Symmetry Alignment
- Gypsum’s prismatic faces exhibit perfect mirroring, whereas glendonite prismatic faces do not [3].
- Gypsum typically has pointed terminations, whereas glendonite has tabular prismatic tips.
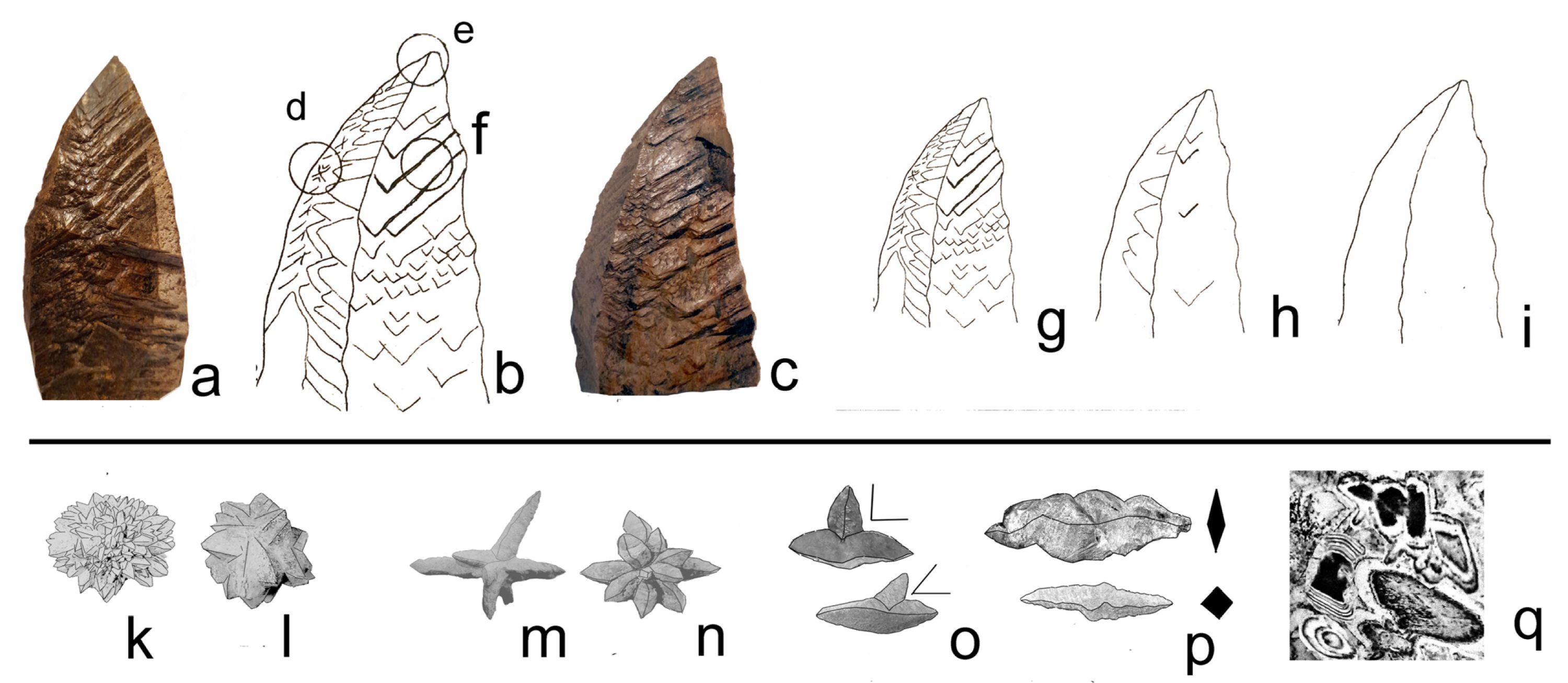
5.2. Frequently Shared Petrographic Structures
- Type 1: Zoned primary calcite grains
- Type 2: Spherulitic calcite
- Type 3: Later infill spar
- Guttulatic texture (Greek for “little drop”), characterised by a zoned internal structure.
- Non-zoned texture, lacking apparent internal differentiation.
5.3. Glendonites from the Limfjord of Denmark
6. Potential for Carbon Capture and Storage
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultz, B.P.; Thibault, N.; Huggett, J.M. The minerals ikaite and its pseudomorph glendonite: Historical perspective and legacies of Douglas Shearman and Alec, K. Smith. Proc. Geol. Assoc. 2022, 133, 176–192. [Google Scholar] [CrossRef]
- Rogov, M.; Ershova, V.; Gaina, C.; Vereshchagin, O.; Vasileva, K.; Mikhailova, K.; Krylov, A. Glendonites throughout the Phanerozoic. Earth-Sci. Rev. 2023, 241, 104430. [Google Scholar] [CrossRef]
- Schultz, B.P.; Vickers, M.L.; Huggett, J.; Madsen, H.; Heilmann-Clausen, C.; Friis, H.; Suess, E. Palaeogene glendonites from Denmark. Palaeogene glendonites from Denmark. Bull. Geol. Soc. Den. 2020, 68, 23–35. [Google Scholar] [CrossRef]
- Schultz, B.P.; Huggett, J.M.; Kennedy, G.L.; Burger, P.; Jensen, A.M.; Kanstrup, M.; Bernasconi, S.M.; Thibault, N.; Ullmann, C.V.; Vickers, M.L.; et al. Petrography and geochemical analysis of Arctic ikaite pseudomorphs from Utqiagvik (Barrow), Alaska. Nor. J. Geol. 2023, 103, 202303. [Google Scholar] [CrossRef]
- Schultz, B.P.; Huggett, J.; Ullmann, C.V.; Kassens, H.; Kölling, M. Links between Ikaite Morphology, Recrystallised Ikaite Petrography and Glendonite Pseudomorphs Determined from Polar and Deep-Sea Ikaite. Minerals 2023, 13, 841. [Google Scholar] [CrossRef]
- Schultz, B.P.; Huggett, J.; Schootbrugge, B.V.D.; Ullmann, C.V.; Broch, M.C. Transgression Related Holocene Coastal Glendonites from Historic Sites. Minerals 2023, 13, 1159. [Google Scholar] [CrossRef]
- Scheller, E.L.; Grotzinger, J.; Ingalls, M. Guttulatic calcite: A carbonate microtexture that reveals frigid formation conditions. Geology 2022, 50, 48–53. [Google Scholar] [CrossRef]
- Frank, T.D.; Thomas, S.G.; Fielding, C.R. On using carbon and oxygen isotope data from glendonites as palaeoenvironmental proxies: A case study from the Permian system of eastern Australia. J. Sediment. Res. 2008, 78, 713–723. [Google Scholar] [CrossRef]
- Swainson, I.P.; Hammond, R.P. Ikaite, CaCO3·6H2O: Cold comfort for glendonites as paleothermometers. Am. Mineral. 2001, 86, 1530–1533. [Google Scholar] [CrossRef]
- Vickers, M.L.; Vickers, M.; Rickaby, R.E.; Wu, H.; Bernasconi, S.M.; Ullmann, C.V.; Bohrmann, G.; Spielhagen, R.F.; Kassens, H.; Schultz, B.P.; et al. The ikaite to calcite transformation: Implications for palaeoclimate studies. Geochim. Cosmochim. Acta 2022, 334, 201–216. [Google Scholar] [CrossRef]
- Vickers, M.L.; Jones, M.T.; Longman, J.; Evans, D.; Ullmann, C.V.; Stokke, E.W.; Vickers, M.; Frieling, J.; Harper, D.T.; Clementi, V.J. IODP Expedition 396 Scientists: Paleocene-Eocene age glendonites from the Mid-Norwegian Margin—Indicators of cold snaps in the hothouse? Clim. Past 2024, 20, 1–23. [Google Scholar] [CrossRef]
- Krylov, A.A.; Logvina, L.A.; Metveeva, T.M.; Prasolov, E.M.; Sapega, V.F.; Demedova, A.L.; Radchenko, M.S. Ikaite CaCO3·6H2O in bottom sediments of the Laptev Sea and the role of anaerobic methane oxidation in this mineral-forming process. Proc. Russ. Mineral. Soc. 2015, 4, 61–76. [Google Scholar]
- Whiticar, M.J.; Suess, E.; Wefer, G.; Müller, P.J. Calcium Carbonate Hexahydrate (Ikaite): History of Mineral Formation as Recorded by Stable Isotopes. Minerals 2022, 12, 1627. [Google Scholar] [CrossRef]
- Bohrmann, G.; Aromokeye, A.D.; Bihler, V.; Dehning, K.; Dohrmann, I.; Gentz, T.; Grahs, M.; Hogg, O.; Hüttich, D.; Kasten, S.; et al. R/V METEOR Cruise Report M134, Emissions of Free Gas from Cross-shelf Troughs of South Georgia: Distribution, Quantification, and Sources for Methane Ebullition Sites in Sub-Antarctic Waters, Port Stanley (Falkland Islands)-Punta Arenas (Chile), 16 January–18 February 2017; Berichte, MARUM—Zentrum für Marine Umweltwissenschaften, Fachbereich Geowissenschaften, Universität Bremen: Bremen, Germany, 2017; Volume 317, pp. 1–220. Available online: https://nbn-resolving.de/urn:nbn:de:gbv:46-00106081-12 (accessed on 23 February 2025).
- Vickers, M.L.; Lengger, S.; Bernasconi, S.M.; Thibault, N.; Schultz, B.P.; Fernandez, A.; Ullmann, C.V.; McCormack, P.; Bjerrum, C.J.; Rasmussen, J.A.; et al. Cold spells in a Greenhouse world? The early Eocene glendonite paradox. Nat. Commun. 2020, 11, 4713. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.L.; Hopkins, D.M.; Pickthorn, W.J. Ikaite, the glendonite precursor, in estuarine sediments at Barrow, Arctic Alaska, Abstracts with Programs. Geol. Soc. Am. 1987, 19, 725. [Google Scholar]
- Wefer, G.; Strasser, M.; Besuden, E.; Büttner, H.; Diekamp, V.; Dinten, D.; dos Santos Ferreira, C.; Fink, H.; Franke, P.; Fujiwara, T.; et al. Report and Preliminary Results of R/V SONNE Cruise SO219A, Tohoku-Oki-Earthquake-Japan Trench, Yokohama-Yokohama, 8 March–6 April 2012; Berichte, MARUM—Zentrum für Marine Umweltwissenschaften, Fachbereich Geowissenschaften, Universität Bremen: Bremen, Germany, 2012; pp. 1–83. Available online: http://publications.marum.de/id/eprint/2362 (accessed on 20 February 2025).
- Greinert, J.; Derkachev, A. Glendonites and methane-derived Mg-calcites in the Sea of Okhotsk, Eastern Siberia: Implications of a venting-related ikaite/glendonite formation. Mar. Geol. 2004, 204, 129–144. [Google Scholar] [CrossRef]
- Muramiya, Y.; Yoshida, H. A review of the occurrence and the origin of glendonite and glendonite concretion. J. Geol. Soc. Jpn. 2022, 128, 395–409. [Google Scholar] [CrossRef]
- Geptner, A.R.; Vetoshkina, O.S.; Petrova, V.V. New data on the composition of stable isotopes in glendonites of the White Sea and their genesis. Lithol. Miner. Resour. 2014, 49, 473–490. [Google Scholar] [CrossRef]
- Vasileva, K.; Zaretskaya, N.; Ershova, V.; Rogov, M.; Stockli, L.D.; Stockli, D.; Khaitov, V.; Maximov, F.; Chernyshova, I.; Soloshenko, N.; et al. New model for seasonal ikaite precipitation: Evidence from White Sea glendonites. Mar. Geol. 2022, 449, 106820. [Google Scholar] [CrossRef]
- Gonzalez, C.R. Sobre la presencia de “glendonita” en el Paleozoico Superior de Patagonia. Rev. Asoc. Geol. Argent. 1980, 35, 417–420. [Google Scholar]
- Steacy, H.R.; Grant, D.R. Tidal muds reveal mineral curiosity. Can. Geogr. J. 1974, 88, 36–38. [Google Scholar]
- Qu, Q.; Teichert, B.M.A.; Birgel, D.; Goedert, J.L.; Peckmann, J. The prominent role of bacterial sulfate reduction in the formation of glendonite: A case study from Paleogene marine strata of western Washington State. Facies 2017, 63, 10. [Google Scholar] [CrossRef]
- Freisleben, J.K. Uber einige interessante Vorkommnisse im Schlotztenleimen (Alluvialthon) bey Obersdorf, ohnweit Sangerhausen. Isis Von Oken 1827, 20, 334–337. [Google Scholar]
- Counts, J.W.; Vickers, M.L.; Stokes, M.R.; Spivey, W.; Gardner, K.F.; Self-Trail, J.M.; Gooley, J.T.; McAleer, R.J.; Jubb, A.M.; Houseknecht, D.W.; et al. Insights into glendonite formation from the upper Oligocene Sagavanirktok Formation, North Slope, Alaska, USA. J. Sediment. Res. 2024, 94, 179–206. [Google Scholar] [CrossRef]
- Suess, E.; Balzer, W.; Hesse, K.F.; Müller, P.J.; Ungerer, C.T.; Wefer, G. Calcium carbonate hexahydrate from organic-rich sediments of the Antarctic shelf: Precursors of glendonites. Science 1982, 216, 1128–1131. [Google Scholar] [CrossRef]
- Brooks, R.; Clark, L.M.; Thurston, E.F. Calcium carbonate and its hydrates. Philos. Trans. R. Soc. Lond. 1950, 243, 145–167. [Google Scholar]
- Buchardt, B.; Seaman, P.; Stockmann, G.; Vous, M.; Wilken, U.; Düwel, L.; Kristiansen, A.; Jenner, C.; Whiticar, M.J.; Kristensen, R.M.; et al. Submarine columns of ikaite tufa. Nature 1997, 390, 129–130. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Z.; Rickaby, R.E.M.; Domack, E.W.; Wellner, J.S.; Kennedy, H.A. Ikaite abundance controlled by porewater phosphorus level: Potential links to dust and productivity. J. Geol. 2015, 123, 269–281. [Google Scholar] [CrossRef]
- Purgstaller, B.; Dietzel, M.; Baldermann, A.; Mavromatis, V. Control of temperature and aqueous Mg2+/Ca2+ ratio on the (trans-) formation of ikaite. Geochim. Cosmochim. Acta 2017, 217, 128–143. [Google Scholar] [CrossRef]
- Stockmann, G.; Tollefsen, E.; Skelton, A.; Brüchert, V.; Balic-Zunic, T.; Langhof, J.; Skogby, H.; Karlsson, A. Control of a Calcite inhibitor (phosphate) and Temperature on Ikaite precipitation in Ikka Fjord, Southwest Greenland. Appl. Geochem. 2018, 89, 11–22. [Google Scholar] [CrossRef]
- Stockmann, G.J.; Seaman, P.; Balic-Zunic, T.; Peternell, M.; Sturkell, E.; Liljebladh, B.; Gyllencreutz, R. Mineral Changes to the Tufa Columns of Ikka Fjord, SW Greenland. Minerals 2022, 12, 1430. [Google Scholar] [CrossRef]
- Tollefsen, E.; Balic-Zunic, T.; Morth, C.-M.; Bruchert, V.; Lee, C.C.; Skelton, A. Ikaite nucleation at 35 degrees C challenges the use of glendonite as a paleotemperature indicator. Sci. Rep. 2020, 10, 8141. [Google Scholar] [CrossRef]
- Huggett, J.M.; Schultz, B.P.; Shearman, D.J.; Smith, A.J. The petrology of ikaite pseudomorphs and their diagenesis. Proc. Geol. Assoc. 2005, 116, 207–220. [Google Scholar] [CrossRef]
- Stein, C.L.; Smith, A.J. Authigenic Carbonate Nodules in the Nankai Trough, Site 583. Initial Reports of the Deep Sea Drilling Project, 77; U.S. Government Printing Office: Washington, DC, USA, 1985; p. 668.
- Bell, J.B.; Aquilina, A.; Woulds, C.; Glover, A.G.; Little, C.T.S.; Reid, W.D.K.; Hepburn, L.E.; Newton, J.; Mills, R.A. Geochemistry, faunal composition and trophic structure at an area of weak methane seepage on the southwest South Georgia margin. R. Soc. Open Sci. 2016, 3, 160284. [Google Scholar] [CrossRef]
- Dana, E.S. A crystallographic study of the thinolite of Lake Lahontan. United States Geol. Surv. Bull. 1884, 12, 429–455. [Google Scholar]
- Van Calker, F.J.P. XXIX. Beitrag zur Kenntniss des Pseudogaylussite und uber dessenVorkommen in Holland. P.Groth. Z. Kryst. Mineral. 1897, 28, 556–571. [Google Scholar]
- MacNair, P. On pseudogaylussite dredged from the Clyde at Cardross and other recent additions to the Mineral Collections in the Kelvingrove Museum. Proc. R. Philos. Soc. Glasg. 1904, 35, 250–262. [Google Scholar]
- Port, R.B. Paleoclimate of the Late Oligocene Arctic Ocean: Molluscan Isotopic and Biotoc Evidence; Florida Atlantic University: Boca Raton, FL, USA, 2008; pp. 1–214. [Google Scholar]
- Selleck, B.; Carr, P.F.; Jones, B.G. A review and synthesis of glendonites (pseudomorphs after ikaite) with new data: Assessing applicability as recorders of ancient cold water conditions. J. Sediment. Res. 2007, 77, 980–991. [Google Scholar] [CrossRef]
- Nesbitte, E.A.; Martin, R.A.; Campbell, K.A. New records of Oligocene diffuse hydrocarbon seeps, northern Cascadia margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 390, 116–129. [Google Scholar] [CrossRef]
- Boggs, S. Petrography and geochemistry of rhombic calcite pseudomorphs from mid-Tertiary mudstone of the Pacific Northwest, USA. Sedimentology 1972, 19, 219–235. [Google Scholar] [CrossRef]
- Brookes, I.A.; McAndrews, J.H.; von Bitter, P.H. Quaternary interglacial and associated deposits in southwest Newfoundland. Can. J. Earth Sci. 1982, 19, 410–423. [Google Scholar] [CrossRef]
- David, T.W.E.; Taylor, T.G. Occurrence of pseudomorph glendonite in New South Wales, part 2. Rec. New South Wales Geol. Surv. 1905, 8, 162–179. [Google Scholar]
- De Lurio, J.L.; Frakes, L.A. Glendonites as a paleoenvironmental tool: Implications for early Cretaceous high latitude climates in Australia. Geochim. Cosmochim. Acta 1999, 633, 1039–1048. [Google Scholar] [CrossRef]
- Carr, P.; Southwood, M.; Jones, B.; Dowton, G. Opal Pineapples from White Cliffs New South Wales, Australia, Rocks and Minerals 2023, 98, 404–417. Rocks Miner. 2023, 98, 404–417. [Google Scholar] [CrossRef]
- Carr, P.F.; Jones, B.G.; Middleton, R.G. Precursor and formation of glendonites in the Sydney Basin. Aust. Mineral. 1989, 4, 980–991. [Google Scholar]
- Kaplan, M.E. Calcite pseudomorphs (pseudogaylussite, Jarrowite, Thinolite, Glendonite, Gennoishi, White Sea Hornlets) in Sedimentary Rocks. Origins of the pseudomorphs. Litol. I Polezn. Iskop. 1979, 14, 125–141. (In Russian) [Google Scholar]
- Kemper, E. Das Klima der Kreide-Zeit. Geols. Jahr. 1987, 96, 185. [Google Scholar]
- Grasby, S.; McCune, G.; Beauchamp, B.; Galloway, J. Lower Cretaceous cold snaps led to widespread glendonite occurrences in the Sverdrup Basin, Canadian High Arctic. Geol. Soc. Am. Bull. 2017, 129, 771–787. [Google Scholar] [CrossRef]
- Mikhailova, K.; Rogov, M.; Ershova, V.; Vereshchagina, O.; Shurekovab, O.; Feodorovabc, A.; Zakharovb, V. Middle Jurassic–Lower Cretaceous glendonites from the eastern Barents Shelf as a tool for paleoenvironmental and paleoclimatic reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 579, 110600. [Google Scholar] [CrossRef]
- Spielhagen, R.F.; Tripati, A. Evidence from Svalbard for near-freezing temperatures and climate oscillations in the Arctic during the Paleocene and Eocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 278, 48–56. [Google Scholar] [CrossRef]
- Vickers, M.; Watkinson, M.; Price, G.D.; Jerrett, R. An improved model for the ikaite glendonite transformation: Evidence from the Lower Cretaceous of Spitsbergen, Svalbard. Nor. J. Geol. 2018, 98, 1–15. [Google Scholar] [CrossRef]
- Vasileva, K.; Rogov, M.; Ershova, V.; Mikhailova, K.; Vereshchagin, O.; Pokrovsky, B. Ikaite versus seep-related carbonate precipitation in the Late Jurassic–Early Cretaceous of West Spitsbergen: Evidence for cold versus warm climates? Int. J. Earth Sci. 2024, 113, 417–439. [Google Scholar] [CrossRef]
- Vickers, M.L.; Jelby, M.E.; Blok, C.N.; Price, G.D.; Jerrett, R.M.; Jensen, M.A.; Jones, M.T. Early Cretaceous giant glendonites: A record of (sub-)millennial-scale cooling? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2025, 661, 112739. [Google Scholar] [CrossRef]
- Teichert, B.M.A.; Luppold, F.W. Glendonites from an Early Jurassic methane seep—Climate or methane indicators? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 390, 81–93. [Google Scholar] [CrossRef]
- Rogov, M.; Ershova, V.; Vereshchagin, O.; Vasileva, K.; Mikhailova, K.; Krylov, A. Database of global glendonite and ikaite records throughout the Phanerozoic. Earth Syst. Sci. Data 2021, 13, 343–356. [Google Scholar] [CrossRef]
- Kennedy, G.L. Glendonites: Enigmatic mineral pseudomorphs and their ephemeral precursor. Rocks Min. 2022, 97, 496–508. [Google Scholar] [CrossRef]
- Viola, C. XXX. Über Aetzfiguren am Gyps. Z. Kryst. Mineral. 1897, 28, 573–578. [Google Scholar]
- Goldschmidt, V. Atlas der Krystallformen; Winter: Heidelberg, Germany, 1913. [Google Scholar]
- Goldschmidt, V.M. Entwicklung d. Krystallformen II, Taf VIII & IX. Z. Kryst. Mineral. 1923, 28, 414–451. [Google Scholar]
- Hintze, C. Nitrate, Jodate, Karbonate, Selenite, Tellurite, Manganite, Plumbate. In Handbuch der Mineralogie, Veit & Comp; Der Verlag Walter de Gruyter & Co.: Berlin, Germany, 1915; pp. 2790–2802+3650. [Google Scholar]
- Blum, J.R. Pseudomorphosen; dritter Nachtrag, p. 13.; 1863; vierter Nachtrag p. 8, l879.
- Ito, T. Ikaite from cold spring water at Shiowakka, Japan. J. Econ. Mineral. Petrol. 1996, 91, 209–219. [Google Scholar] [CrossRef]
- Friedel, G. Leçons de Cristallographie Proféssés à la Faculté des Sciences de Strasbourg; Berger-Levrault: Paris, France, 1926; 602p. [Google Scholar]
- Shearman, D.J.; Smith, A.J. Ikaite, the parent mineral of jarrowite-type pseudomorphs. Proc. Geol. Assoc. 1985, 96, 305–314. [Google Scholar] [CrossRef]
- Mitterer, R.M.; Cunningham, R., Jr. The interaction of natural organic matter with grain surfaces: Implications for calcium carbonate precipitation. Soc. Econ. Paleontol. Mineral. 1985, 36. [Google Scholar] [CrossRef]
- Raven, M.J.; Dickson, J.A.D. Fir-tree zoning: An indicator of pulsed crystallization in calcite cement crystals. Sediment. Geol. 1989, 65, 249–259. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Surlyk, F. The Fur Formation, a late Paleocene ash-bearing diatomite from northern Denmark. Bull. Geol. Soc. Den. 1983, 32, 43–65. [Google Scholar] [CrossRef]
- Schoon, P.L.; Heilmann-Clausen, C.; Schultz, B.P.; Sluijs, A.; Sinninghe Damsté, J.S.; Schouten, S. Recognition of Early Eocene global carbon isotope excursions using lipids of marine Thaumarchaeota. Earth Planet. Sci. Lett. 2013, 373, 60–168. [Google Scholar] [CrossRef]
- Stokke, E.W.; Jones, M.T.; Tierney, J.E.; Svensen, H.H.; Whiteside, J.H. Temperature changes across the Paleocene-Eocene Thermal Maximum—A new high-resolution TEX86 temperature record from the Eastern North Sea Basin. Earth Planet. Sci. Lett. 2020, 544, 116388. [Google Scholar] [CrossRef]
- Stokke, E.W.; Jones, M.T.; Riber, L.; Haflidason, H.; Midtkandal, I.; Schultz, B.P.; Svensen, H.H. Rapid and sustained environmental responses to global warming: The Paleocene–Eocene Thermal Maximum in the eastern North Sea. Clim. Past 2021, 17, 1989–2013. [Google Scholar] [CrossRef]
- Jones, M.T.; Percival, L.M.E.; Stokke, E.W.; Frieling, J.; Mather, T.A.; Riber, L.; Schubert, B.A.; Schultz, B.; Tegner, C.; Planke, S.; et al. Mercury anomalies across the Palaeocene-Eocene Thermal Maximum. Clim. Past 2019, 15, 217–236. [Google Scholar] [CrossRef]
- Jones, M.T.; Stokke, E.W.; Rooney, A.D.; Frieling, J.; Pogge von Strandmann, P.A.E.; Wilson, D.J.; Svensen, H.H.; Planke, S.; Adatte, T.; Thibault, N.; et al. Tracing North Atlantic volcanism and seaway connectivity across the Paleocene–Eocene Thermal Maximum (PETM). Clim. Past 2023, 19, 1623–1652. [Google Scholar] [CrossRef]
- Turner, S.K. Constraints on the onset duration of the paleocene–eocene thermal maximum. Philosophical Transactions of the Royal Society. Math. Phys. Eng. Sci. 2018, 376, 20170082. [Google Scholar] [CrossRef]
- Baumann, N.B.; Regelous, M.; Adatte, T.; Thibault, N.R.; Regelous, A.; Schultz, B.P.; Fantasia, A.; Madsen, H.; Haase, K.M. Linking the PETM and North Atlantic volcanism using tellurium in sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 656, 112575. [Google Scholar] [CrossRef]
- Heingård, M.; Sjövall, P.; Sylvestersen, R.L.; Schultz, B.P.; Lindgren, J. Crypsis in the pelagic realm: Evidence from exceptionally preserved fossil fish larvae from the Eocene Stolleklint Clay of Denmark. Palaeontology 2021, 64, 805–815. [Google Scholar] [CrossRef]
- Heingård, M.; Sjövall, P.; Schultz, B.P.; Sylvestersen, R.L.; Lindgren, J. Preservation and Taphonomy of Fossil Insects from the Earliest Eocene of Denmark. Biology 2022, 11, 395. [Google Scholar] [CrossRef]
- Lindgren, J.; Uvdal, P.; Sjövall, P.; Nilsson, D.E.; Schultz, B.P.; Theil, V. Molecular preservation of the pigment melamin in fossil melanosomes. Nat. Commun. 2012, 3, 824. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Uvdal, P.; Gren, J.A.; Dyke, G.; Schultz, B.P.; Shawkey, M.D.; Barnes, K.R.; Polcyn, M.J. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 2014, 506, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.; Moyer, A.; Higby Schweitzer, M.; Sjövall, P.; Uvdal, P.; Nilsson, D.-E.; Heimdal, J.; Engdahl, A.; Gren, J.; Schultz, B.P.; et al. Interpreting melanin-based coloration through deep time: A critical review. Royal Society of London. Proc. B Biol. Sci. 2015, 282, 20150614. [Google Scholar] [CrossRef]
- Lindgren, J.; Nilsson, D.; Sjövall, P.; Jarenmark, M.; Ito, S.; Wakamatsu, K.; Kear, B.P.; Schultz, B.P.; Sylvestersen, R.L.; Madsen, H.; et al. Fossil insect eyes shed light on trilobite optics and the arthropod pigment screen. Nature 2019, 573, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, T.J.; Sylvestersen, R.L.; Olsen, K.; Rasmussen, J.A. Apachyus madseni (Dermaptera: Apachyidae) sp. nov. from the Ypresian Fur Formation of Denmark: The first fossil record of the enigmatic earwig family Apachyidae. Palaeoentomology 2024, 7, 638–644. [Google Scholar] [CrossRef]
- Schroeder, A.; Carnevale, G. The argentiniform Surlykus longigracilis gen. et sp. nov.; the most abundant fish from the Eocene Fur Formation of Denmark. Bull. Geol. Soc. Den. 2024, 72, 1–18. [Google Scholar] [CrossRef]
- Nenning, F. Mega-Glendonites in the Early Eocene Fur Formation: Unraveling Paleoenvironmental Conditions in the Danish Basin and Their Influence on Glendonite Formation. 2017. Available online: https://nbn-resolving.de/urn:nbn:de:hbz:6-51229483616 (accessed on 20 February 2025).
- Larsen, L.M.; Fitton, J.G.; Pedersen, A.K. Paleogene volcanic ash layers in the Danish Basin: Compositions and source areas in the North Atlantic Igneous Province. Lithos 2003, 71, 47–80. [Google Scholar] [CrossRef]
- Heilmann-Oausen, C.; Nielsen, O.B.; Gersner, P. Lithostratigraphy and depositional environments in the Upper Paleocene and Eocene of Denmark. Bull. Geol. Soc. Den. 1985, 33, 287–323. [Google Scholar] [CrossRef]
- Rasmussen, J.A.; Madsen, H.; Schultz, B.P.; Sylvestersen, R.L.; Bonde, N. The lowermost Eocene deposits and biota of the western Limfjord region, Denmark—Field Trip Guidebook. In Proceedings of the 2nd International Mo-Clay Meeting, Nykøbing Mors, Denmark, 2–4 November 2016; p. 35. [Google Scholar]
- Pedersen, G.K.; Pedersen, S.A.S.; Bonde, N.; Heilman-Clausen, C.; Larsen, L.M.; Lindow, B.; Madsen, H.; Pedersen, A.K.; Rust, J.; Schultz, B.P.; et al. Molerområdets geologi—Sedimenter, fossiler, askelag og glacialtektonik. In Geologisk Tidsskrift; Dansk Geologisk Forening: Copenhagen, Denmark, 2011; pp. 41–135. Available online: https://2dgf.dk/wp-content/uploads/2016/05/gt2011-41-135.pdf (accessed on 23 February 2025).
- Pedersen, G.K.; Buchardt, B. The calcareous concretions (cementsten) in the Fur Formation (Paleogene, Denmark). Isotopic evidence of early diagenetic growth. Bull. Geol. Soc. Den. 1996, 43, 78–86. [Google Scholar] [CrossRef]
- McNamara, M.E.; Briggs, D.E.G.; Orr, P.J.; Field, D.J.; Wang, Z. Experimental maturation of feathers: Implications for reconstructions of fossil feather colour. Biol. Lett. 2013, 9, 20130184. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, G.J.; Ranta, E.; Trampe, E.; Sturkell, E.; Seaman, P. Carbon mineral storage in seawater: Ikaite (CaCO3·6H2O) columns in Greenland. Energy Procedia 2018, 146, 59–67. [Google Scholar] [CrossRef]
- Carmiggelt, J.; King, H.; Wolthers, M.; Schulz, B.P.; Van de Schootbrugge, B. Promotion of ikaite precipitation by bentonite fertilisation in the Eocene Fur formation, northern Denmark. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Andersen, A.B.; Engelsen, S.B.; Hansen, H.C.B.; Larsen, O.; Skibsted, L.H. Presence and dehydration of ikaite, calcium carbonate hexahydrate, in frozen shrimp shell. J. Agric. Food Chem. 1999, 47, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.; Jones, B.G.; Selleck, B. The Sydney Basin and Frozen Prawns—The Cool Mineral Connection. 2006. Available online: https://ro.uow.edu.au/scipapers/4837 (accessed on 20 February 2025).
- Tansman, G.; Kindstedt, P.; Hughes, J. Minerals in Food: Crystal Structures of Ikaite and Struvite from Bacterial Smears on Washed-Rind Cheese. Can. Mineral. 2017, 55, 89–100. [Google Scholar] [CrossRef]
- Garvie, L.A.J. Seasonal formation of ikaite in slime flux jelly on an infected tree (Populus fremontii) wound from the Sonoran Desert. Sci Nat. 2022, 109, 48. [Google Scholar] [CrossRef]
- Milodowski, A.; Shaw, R.; Stewart, D. The Harpur Hill Site: Its Geology, Evolutionary History and a Catalogue of Materials Present. CR/13/104; British Geological Survey: Nottingham, UK, 2013; 43p. [Google Scholar]
- Shaikh, A.M. A new crystal growth form for vaterite, CaCO3. J. Appl. Crystallogr. 1990, 23, 263–265. [Google Scholar] [CrossRef]
- Zabel, M.; Schulz, H.D. Importance of submarine landslides for non-steady state conditions in pore water systems-lower Zaire (Congo) deep-sea fan. Mar. Geol. 2001, 176, 87–99. [Google Scholar] [CrossRef]
- Nielsen, T.; Laier, T.; Kuijpers, A.; Rasmussen, T.; Mikkelsen, N.; Nørgaard-Pedersen, N. Fluid flow and methane occurrences in the Disko Bugt area offshore West Greenland: Indications for gas hydrates? Geo-Mar. Lett. 2014, 34, 511–523. [Google Scholar] [CrossRef][Green Version]
- Kodina, L.A.; Tokarev, V.G.; Vasova, L.N.; Korobeinik, G.S. Contribution of biogenic methane to ikaite formation in the Kara Sea: Evidence from the Stable Carbon Isotope Geochemistry. In Siberian River Run-Off in the Kara Sea: Characterisation, Quantification, Variability, and Environmental Significance; Stein, R., Fahl, K., Fütterer, D.K., Galimov, E.M., Stepanets, O.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 349–375. [Google Scholar]
- Bornhold, B.D.; Firth, J.V.; Adamson, L.M.; Baldauf, J.G.; Blais, A.P.; Elvert, M.; Fox, P.J.; Hebda, R.; Kemp, A.E.S.; Mora, K.; et al. Sites 1033 and 1034. Proc. Ocean. Drill. Program Initial. Rep. 1998, 27, 11–61. Available online: http://www-odp.tamu.edu/publications/169S_IR/PRELIM.PDF (accessed on 20 February 2025).
- Kassen, H.; Dmitrenko, I. The TRANSDRIFT II Expedition to the Laptev Sea Reports on Polar Research, Laptev Sea Systems Expedition Beretten. Polarforsch 1994, 182, 93. [Google Scholar]
- Lu, Z.; Rickaby, R.E.M.; Kennedy, H.; Kennedy, P.; Pancost, R.D.; Shaw, S.; Lennie, A.; Wellner, J.; Anderson, J.B. An ikaite record of late Holocene climate at the Antartic Peninsula. Earth Planet. Sci. Lett. 2012, 325, 108–115. [Google Scholar] [CrossRef]
- Rysgaard, S.; Glud, R.N.; Lennert, K.; Cooper, M.; Halden, N.; Leakey, R.J.G.; Hawthorne, F.C.; Barber, D. Ikaite crystals in melting sea ice—Implications for pCO2 and pH levels in Arctic surface waters. Cryosphere 2012, 6, 901–908. [Google Scholar] [CrossRef]
- Dieckmann, G.S.; Nehrke, G.; Papadimitriou, S.; Gottlicher, J.; Steininger, R.; Kennedy, H.; Wolf-Gladrow, D.; Thomas, D.N. Calcium carbonate as ikaite crystals in Antarctic sea ice. Geophys. Res. Lett. 2008, 35, 1–3. [Google Scholar] [CrossRef]
- Pauly, H. Ikaite, a New Mineral from Greenland. Arctic 1963, 16, 213–292. [Google Scholar] [CrossRef]
- Bischoff, J.L.; Stine, S.; Rosenbauer, R.J.; Fitzpatrick, J.A.; Stafford, T.W. Ikaite precipitation by mixing of shoreline springs and lake water, Mono Lake, California, USA. Geochim. Cosmochim. Acta 1993, 57, 3855–3866. [Google Scholar] [CrossRef]
- Last, F.M.; Last, W.M.; Fayek, M.; Halden, N. Occurrence and significance of a coldwater carbonate pseudomorph in microbialites from a saline lake. J. Paleolimnol. 2013, 50, 505–517. [Google Scholar] [CrossRef]
- Pollard, W.; Omelon, C.; Andersen, D.; McKay, C. Perennial spring occurrence in the Expedition Fiord area of western Axel Heiberg Island, Canadian High Arctic. Can. J. Earth Sci. 1999, 36, 105–120. [Google Scholar] [CrossRef]
- Ohlendorf, C.; Fey, M.; Massaferro, J.; Haberzettl, T.; Laprida, C.; Lücke, A.; Maidana, N.; Mayr, C.; Oehlerich, M.; Ramón, M.J.; et al. Late Holocene hydrology inferred from lacustrine sediments of Laguna Cháltel (southeastern Argentina). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 411, 229–248. [Google Scholar] [CrossRef]
- Nèmeth, P.; Toechterle, P.; Dublyansky, Y.; Stalder, R.; Molnár, Z.; Klébert, S.; Spötl, C. Tracing structural relicts of the ikaite-to-calcite transformation in cryogenic cave glendonite. Am. Mineral. 2022, 10, 1960–1967. [Google Scholar] [CrossRef]
- Marland, G.H. The stability of CaCO3·6H2O (ikaite). Geochim. Cosmochim. Acta 1975, 39, 83–91. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Wolf-Gladrow, D.A.; Dieckmann, G.S.; Völker, C.; Nehrke, G. A laboratory study of ikaite (CaCO3·6H2O) precipitation as a function of pH, salinity, temperature and phosphate concentration. Mar. Chem. 2014, 162, 10–18. [Google Scholar] [CrossRef]
- Lázár, A.; Molnár, Z.; Demény, A.; Kótai, L.; Trif, L.; Béres, K.; Bódis, E.; Bortel, G.; Aradi, L.; Karlik, M.; et al. Insights into the amorphous calcium carbonate (ACC) → ikaite → calcite transformations. Cryst. Eng. Comm. 2022, 25, 738–750. [Google Scholar] [CrossRef]
- Tollefsen, E.; Stockmann, G.; Skelton, A.; Mörth, C.M.; Dupraz, C.; Sturkell, E. Chemical controls on ikaite formation. Mineral. Mag. 2018, 82, 1–22. [Google Scholar] [CrossRef]
- Slack, J.G. Calcium carbonate hexahydrate: Its properties and formation in lime-soda softening. Water Res. 1980, 14, 799–804. [Google Scholar] [CrossRef]
- Boch, R.; Dietzel, M.; Reichl, P.; Leis, A.; Baldermann, A.; Mittermayr, F.; Poelt, P. Rapid ikaite (CaCO3·6H2O) crystallization in a man-made river bed: Hydrogeochemical monitoring of a rarely documented mineral formation. Appl. Geochem. 2015, 63, 366–379. [Google Scholar] [CrossRef]
- Field, L.P.; Milodowski, A.E.; Shaw, R.P.; Stevens, L.A.; Hall, M.R.; Kilpatrick, A.; Gunn, J.; Kemp, S.J.; Ellis, M.A. Unusual morphologies and the occurrence of pseudomorphs after ikaite (CaCO3·6H2O) in fast growing, hyperalkaline speleothems. Mineral. Mag. 2017, 81, 565–589. [Google Scholar] [CrossRef]
- Bastianini, L.; Rogerson, M.; Brasier, A.; Prior, T.J.; Hardman, K.; Dempsey, E.; Bird, A.; Mayes, W.M. Ikaite formation in streams affected by steel waste leachate: First report and potential impact on contaminant dynamics. Chem. Geol. 2023, 644, 121842. [Google Scholar] [CrossRef]
- Németh, P. Diffraction Features from (101¯4) Calcite Twins Mimicking Crystallographic Ordering. Minerals 2021, 11, 720. [Google Scholar] [CrossRef]
- Vasileva, K.; Ershova, V.; Rogov, M.; Gritsenko, Y.; Maximov, F.; Ovsepyan, Y.; Okuneva, T.; Rybakova, A.; Kiseleva, D.; Vereshchagin, O. Mineralogical composition, isotopic and geochemical characteristics of Pleistocene glendonites from the outcrops of Bol’shaya Balakhnya River, Eastern Taimyr, Russia. J. Sediment. Res. 2024, 94, 355–366. [Google Scholar] [CrossRef]
- Oenema, S. Pseudogaylussiet in Het Nederlandse en Duitse Kustgebied; Grondboor en Hamer: München, Germany, 1990; p. 163. [Google Scholar]
- Trechmann, C.O. Uber einen Fund von ausgezeichneten Pseudogaylussite (Thinolite Jarrowite -) Krystallen. Z. Kryst. 1901, 25, 283–285. [Google Scholar]

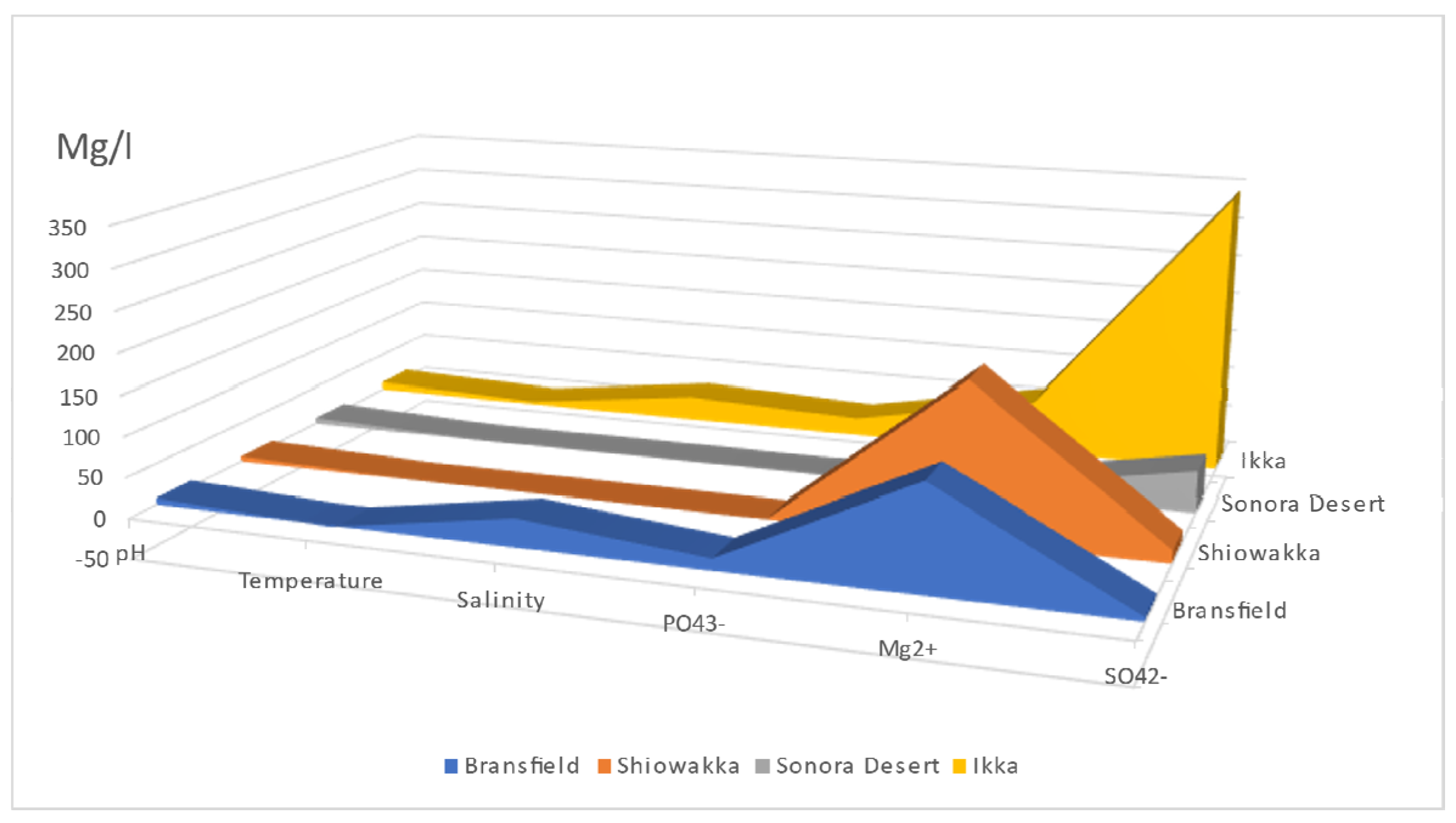
| pH | Temperature | Salinity | PO43− | Mg2+ | SO42− | |
|---|---|---|---|---|---|---|
| °C | g/L | mg/L | mg/L | mg/L | ||
| Bransfield [13] | 7.7 | −1.5 | 34.5 | 14.4 | 127 | 5 |
| Shiowakka [66] | 6.7 | <1 | 0.1 | 0.18 | 190 | 15.6 |
| Sonora Desert [99] | 8.2 | <5 | 0.8 | 0.21 | 11.12 | 51.8 |
| Ikka [29,32] | 10.2 | 3.5 | 31 | 23 | 65.9 | 345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, B.P.; Huggett, J. Advances in Glendonite Understanding and Its Potential for Carbon Capture. Minerals 2025, 15, 410. https://doi.org/10.3390/min15040410
Schultz BP, Huggett J. Advances in Glendonite Understanding and Its Potential for Carbon Capture. Minerals. 2025; 15(4):410. https://doi.org/10.3390/min15040410
Chicago/Turabian StyleSchultz, Bo Pagh, and Jennifer Huggett. 2025. "Advances in Glendonite Understanding and Its Potential for Carbon Capture" Minerals 15, no. 4: 410. https://doi.org/10.3390/min15040410
APA StyleSchultz, B. P., & Huggett, J. (2025). Advances in Glendonite Understanding and Its Potential for Carbon Capture. Minerals, 15(4), 410. https://doi.org/10.3390/min15040410







