Trace Metal and Phosphorus Enrichments in Cyanobacteria Cells and Cyanobacterial Precipitated Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Sampling the Experiments
2.3. Sample Digestion and Dilution for ICP-MS Measurements
2.4. Sample Digestion and Dilution for ICP-AES Measurements
2.5. SEM, EDS, and EPMA Sample Preparation and Methods
2.6. Using PHREEQC to Calculate Mineral Saturation States
3. Results
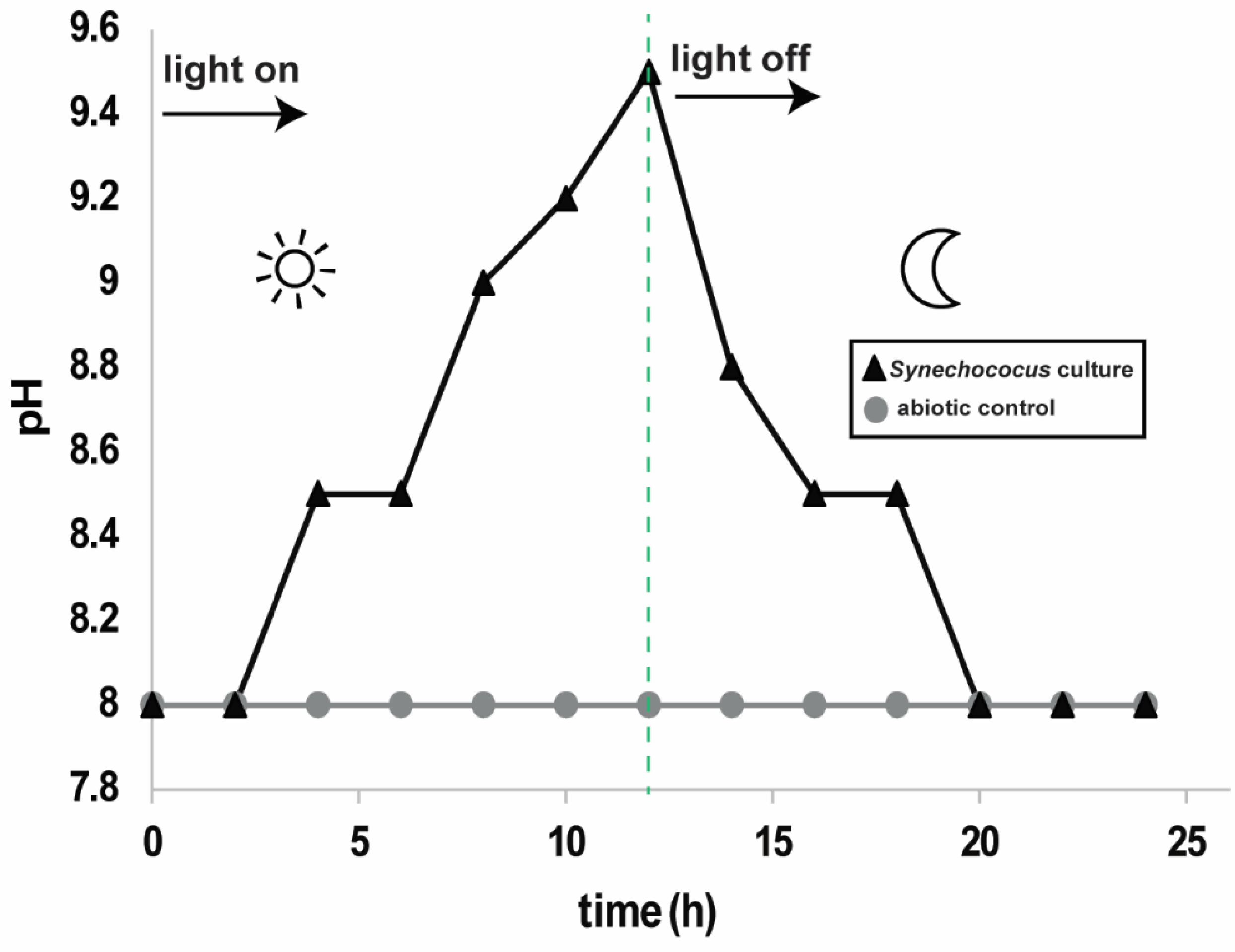
3.1. pH and Carbonate Mineral Saturation
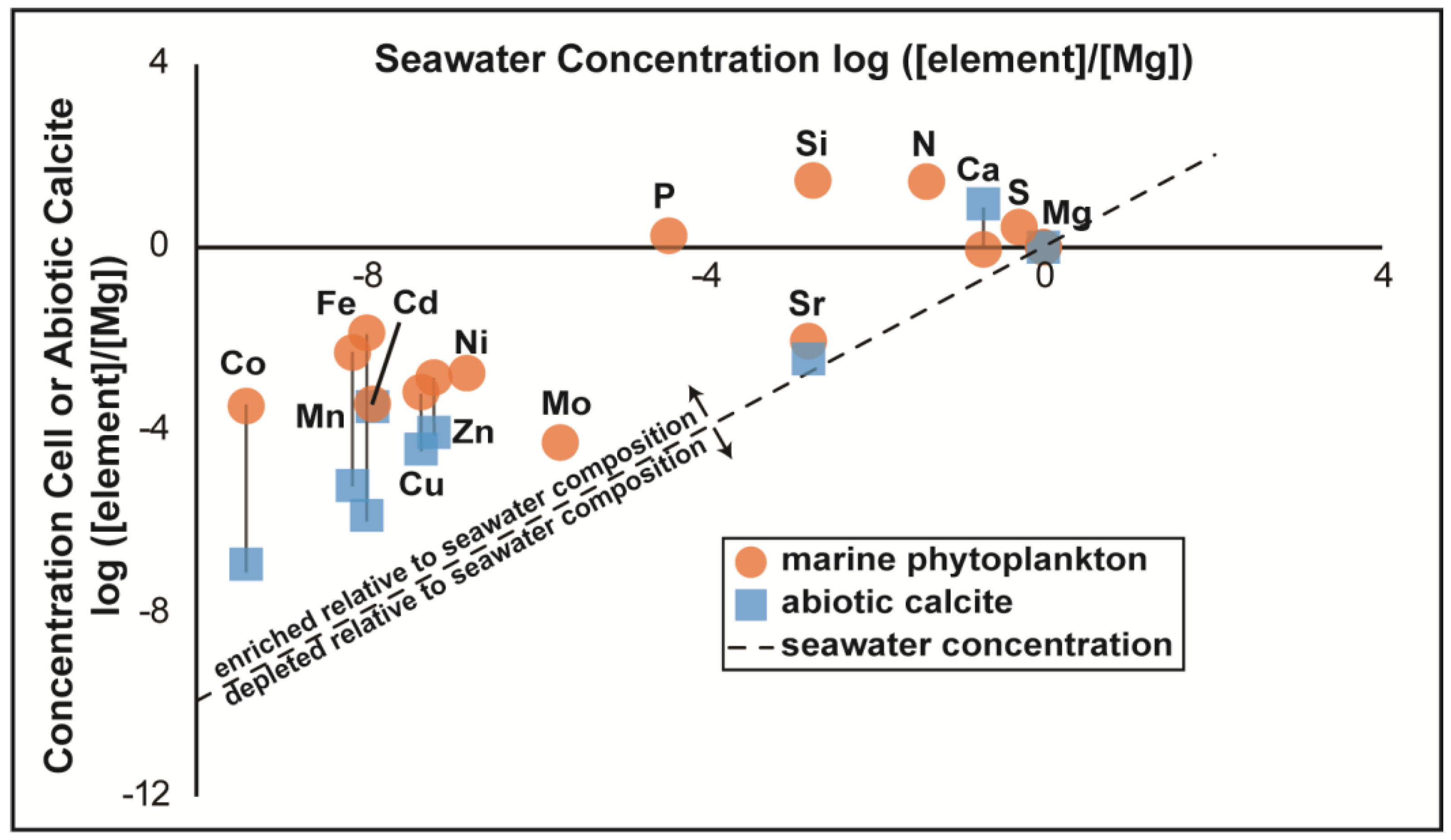
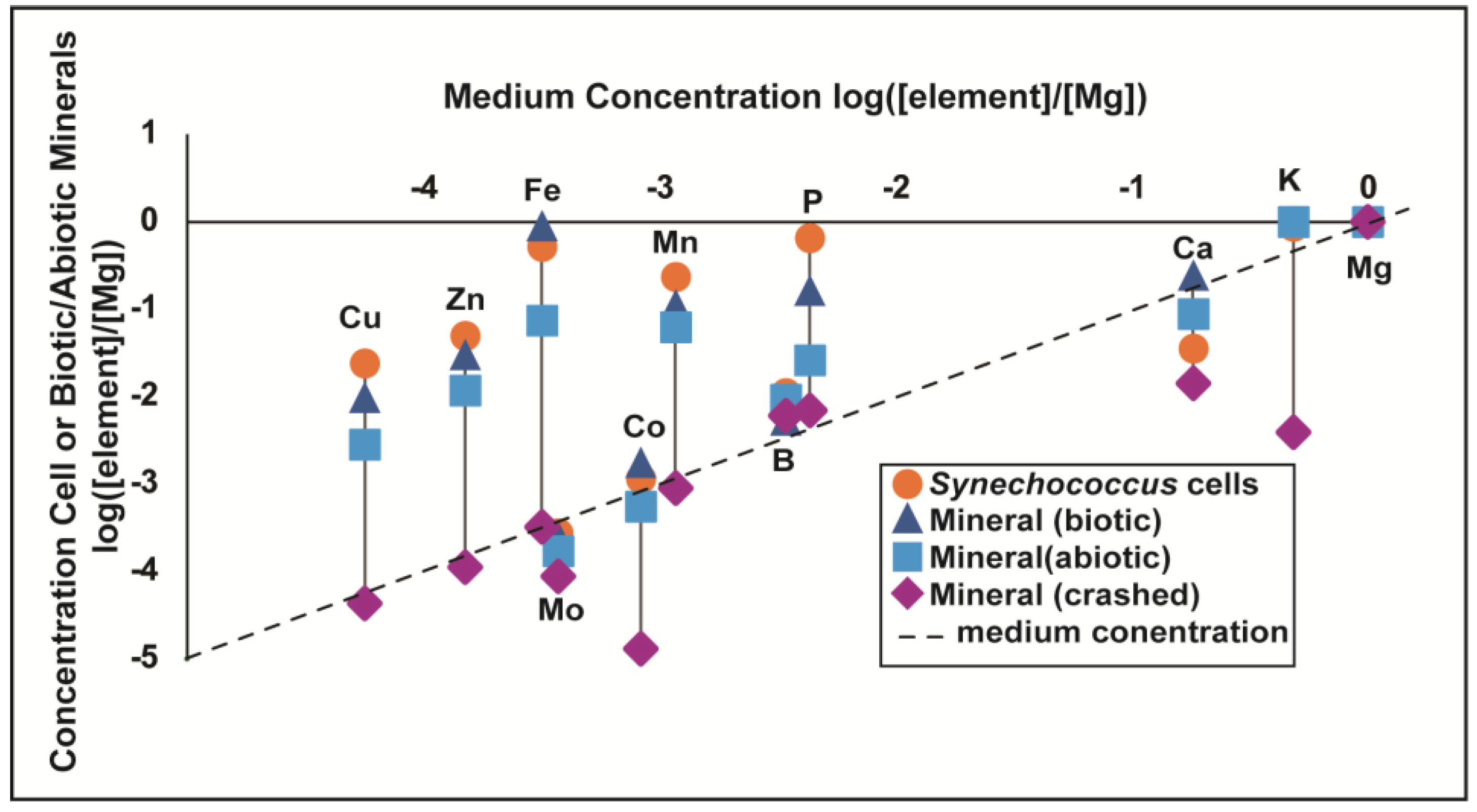
3.2. Trace Element Enrichment Factors from ICP-MS Trace Element Measurements
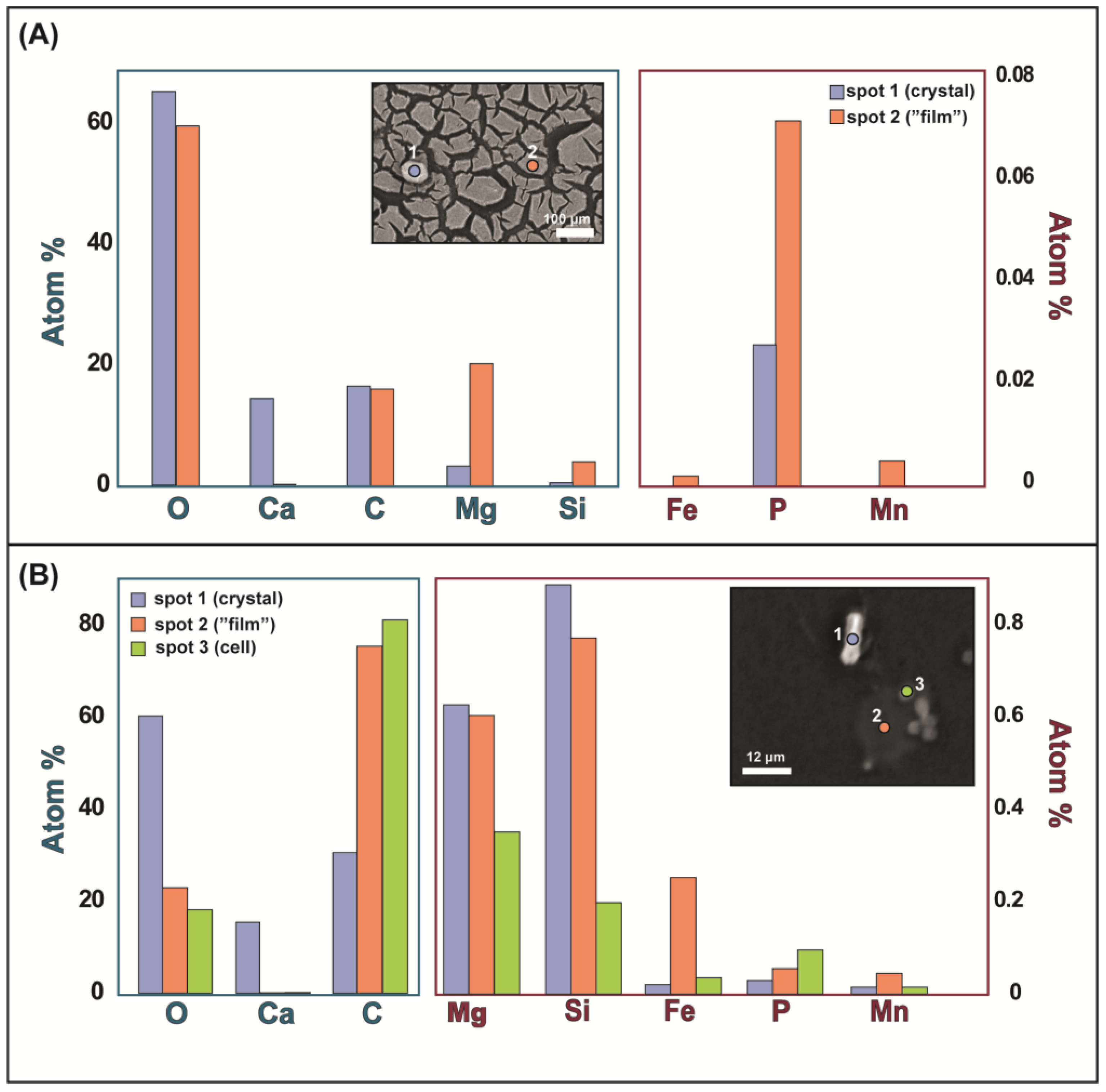
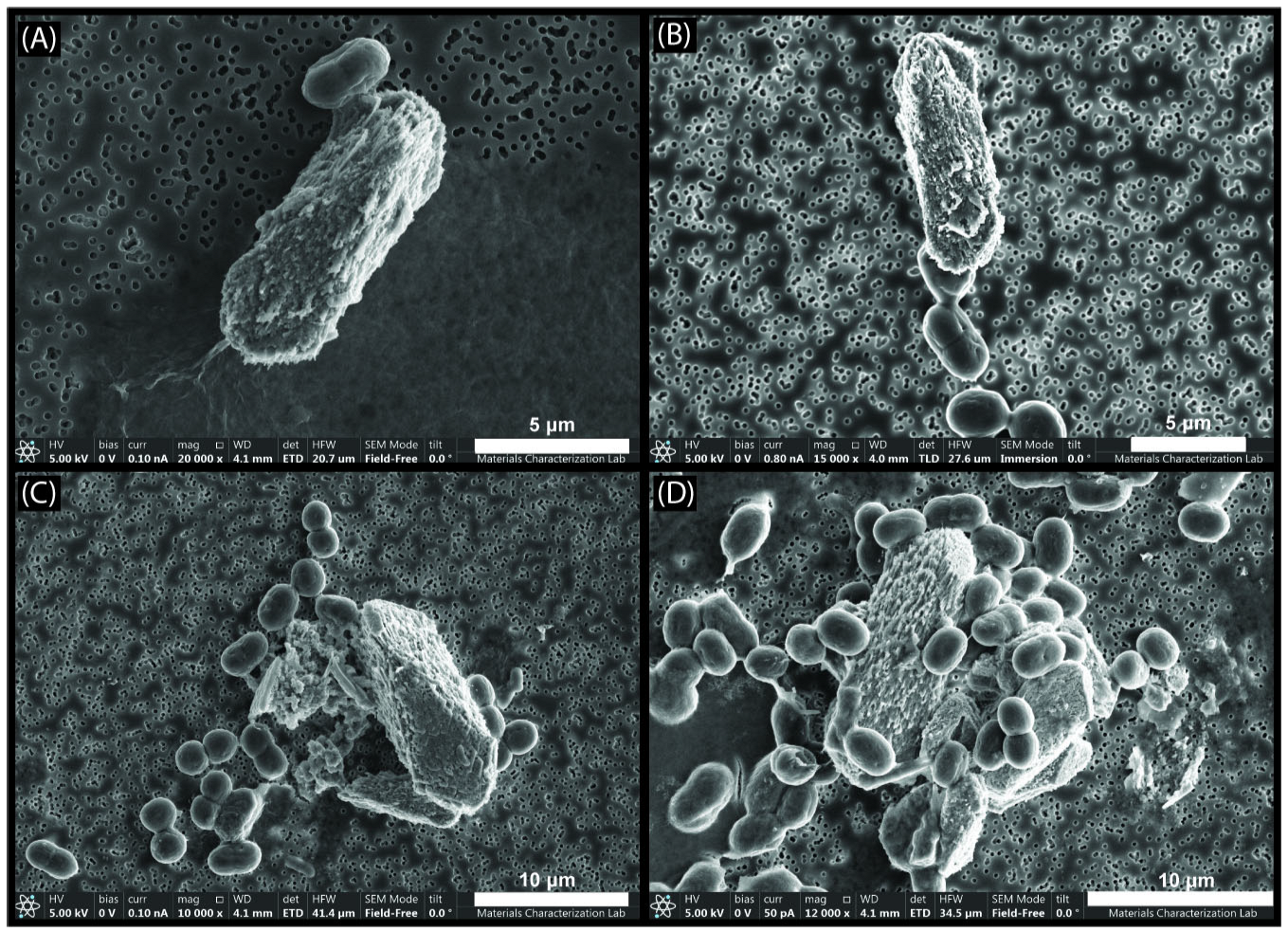
3.3. Scanning Electron Microscopy and Electron Probe Micro-Analysis
4. Discussion
4.1. Enrichments of Trace Elements in Cells and Biotic Calcite
4.1.1. Fe
4.1.2. Cu, Zn, and Mn
4.1.3. P
4.1.4. Enrichment in Abiotic Minerals—Slow vs. Rapid Precipitation
4.2. Caveats Due to Mg-O-C-Si Mineral Phase
4.3. Implications for Biosignature Use and Microbial Carbonates in the Rock Record
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The MSR Campaign Science Group (MCSG); Czaja, A.D.; Zorzano, M.; Kminek, G.; Meyer, M.A.; Beaty, D.W.; Sefton-Nash, E.; Carrier, B.L.; Thiessen, F.; Haltigin, T.; et al. Report of the Science Community Workshop on the proposed First Sample Depot for the Mars Sample Return Campaign. Meteorit. Planet. Sci. 2023, 58, 885–896. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Redfield, A.C. The Biological Control of Chemical Factors in the Environment. Am. Sci. 1958, 46, 230A-221. [Google Scholar]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Huertas, M.J.; López-Maury, L.; Giner-Lamia, J.; Sánchez-Riego, A.M.; Florencio, F.J. Metals in Cyanobacteria: Analysis of the Copper, Nickel, Cobalt and Arsenic Homeostasis Mechanisms. Life 2014, 4, 865–886. [Google Scholar] [CrossRef]
- Bergmann, K.D.; Grotzinger, J.P.; Fischer, W.W. Biological Influences on Seafloor Carbonate Precipitation. Palaios 2013, 28, 99–115. [Google Scholar]
- de Brito, M.M.; Bundeleva, I.; Marin, F.; Vennin, E.; Wilmotte, A.; Plasseraud, L.; Visscher, P.T. Properties of exopolymeric substances (EPSs) produced during cyanobacterial growth: Potential role in whiting events. Biogeosciences 2023, 20, 3165–3183. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Beveridge, T.J.; Des Marais, D.J. Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton. Limnol. Oceanogr. 1997, 42, 133–141. [Google Scholar] [CrossRef]
- Stanton, C.; Barnes, B.D.; Kump, L.R.; Cosmidis, J. A re-examination of the mechanism of whiting events: A new role for diatoms in Fayetteville Green Lake (New York, USA). Geobiology 2022, 21, 210–228. [Google Scholar] [CrossRef]
- Thompson, J.B. Microbial Whitings. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 250–260. [Google Scholar] [CrossRef]
- Obst, M.; Wehrli, B.; Dittrich, M. CaCO3 nucleation by cyanobacteria: Laboratory evidence for a passive, surface-induced mechanism. Geobiology 2009, 7, 324–347. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Harauz, G.; Beveridge, T.J. Participation of a cyanobacterial S layer in fine-grain mineral formation. J. Bacteriol. 1992, 174, 7971–7981. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lam, S.; Beveridge, T.J. Physicochemical characteristics of the mineral-forming S-layer from the cyanobacterium Synechococcus strain GL24. Can. J. Microbiol. 1994, 40, 216–223. [Google Scholar] [CrossRef]
- Webb, G.E.; Kamber, B.S. Trace Element Geochemistry as a Tool for Interpreting Microbialites. In Earliest Life on Earth: Habitats, Environments and Methods of Detection; Golding, S.D., Glikson, M., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 127–170. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Kamber, B.S.; Webb, G.E. Transition metal abundances in microbial carbonate: A pilot study based on in situ LA-ICP-MS analysis. Geobiology 2007, 5, 375–389. [Google Scholar] [CrossRef]
- Sforna, M.C.; Daye, M.; Philippot, P.; Somogyi, A.; van Zuilen, M.A.; Medjoubi, K.; Gérard, E.; Jamme, F.; Dupraz, C.; Braissant, O.; et al. Patterns of metal distribution in hypersaline microbialites during early diagenesis: Implications for the fossil record. Geobiology 2017, 15, 259–279. [Google Scholar] [CrossRef]
- Warke, M.R.; Edwards, N.P.; Wogelius, R.A.; Manning, P.L.; Bergmann, U.; Egerton, V.M.; Kimball, K.C.; Garwood, R.J.; Beukes, N.J.; Schröder, S. Decimeter-scale mapping of carbonate-controlled trace element distribution in Neoarchean cuspate stromatolites. Geochim. Cosmochim. Acta 2019, 261, 56–75. [Google Scholar] [CrossRef]
- Lorens, R.B. Sr, Cd, Mn and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 1981, 45, 553–561. [Google Scholar] [CrossRef]
- Rimstidt, J.; Balog, A.; Webb, J. Distribution of trace elements between carbonate minerals and aqueous solutions. Geochim. Cosmochim. Acta 1998, 62, 1851–1863. [Google Scholar] [CrossRef]
- Lee, B.D.; Apel, W.A.; Walton, M.R. Calcium carbonate formation by Synechococcus sp. strain PCC 8806 and Synechococcus sp. strain PCC 8807. Bioresour. Technol. 2006, 97, 2427–2434. [Google Scholar] [CrossRef]
- Yamamura, H.; Hayakawa, M.; Iimura, Y. Application of sucrose-gradient centrifugation for selective isolation of Nocardia spp. from soil. J. Appl. Microbiol. 2003, 95, 677–685. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Basic expression of ‘PAP’ computation for quantitative EPMA. In Proceedings of the 11th International Congress on X-ray Optics and Microanalysis (ICXOM), London, ON, Canada, 4–8 August 1986; pp. 249–253. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2013; p. 6-A43. [Google Scholar] [CrossRef]
- Plummer, L.N.; Parkhurst, D.L.; Fleming, G.W.; Dunkle, S.A. A Computer Program Incorporating Pitzer’s Equations for Calculation of Geochemical Reactions in Brines; Water-Resources Investigations Report; Department of the Interior, US Geological Survey: Reston, VA, USA, 1988; Volume 88. [CrossRef]
- Pitzer, K.S. Ion Interaction Approach: Theory and Data Correlation. In Activity Coefficients in Electrolyte Solutions, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauß, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Kurisu, G.; Zhang, H.; Smith, J.L.; Cramer, W.A. Structure of the Cytochrome b6f Complex of Oxygenic Photosynthesis: Tuning the Cavity. Science 2003, 302, 1009–1014. [Google Scholar] [CrossRef]
- Stroebel, D.; Choquet, Y.; Popot, J.-L.; Picot, D. An atypical haem in the cytochrome b6f complex. Nature 2003, 426, 413–418. [Google Scholar] [CrossRef]
- Ferreira, F.; Straus, N.A. Iron deprivation in cyanobacteria. J. Appl. Phycol. 1994, 6, 199–210. [Google Scholar] [CrossRef]
- Morrissey, J.; Bowler, C. Iron Utilization in Marine Cyanobacteria and Eukaryotic Algae. Front. Microbiol. 2012, 3, 43. [Google Scholar] [CrossRef]
- Qiu, G.-W.; Koedooder, C.; Qiu, B.-S.; Shaked, Y.; Keren, N. Iron transport in cyanobacteria-from molecules to communities. Trends Microbiol. 2022, 30, 229–240. [Google Scholar] [CrossRef]
- Dromgoole, E.L.; Walter, L.M. Iron and manganese incorporation into calcite: Effects of growth kinetics, temperature and solution chemistry. Chem. Geol. 1990, 81, 311–336. [Google Scholar] [CrossRef]
- Paquette, J.; Reeder, R.J. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite. Geochim. Cosmochim. Acta 1995, 59, 735–749. [Google Scholar] [CrossRef]
- Demirel, S.; Ustun, B.; Aslim, B.; Suludere, Z. Toxicity and uptake of iron ions by Synechocystis sp. E35 isolated from Kucukcekmece Lagoon, Istanbul. J. Hazard. Mater. 2009, 171, 710–716. [Google Scholar] [CrossRef]
- Swanner, E.D.; Wu, W.; Hao, L.; Wüstner, M.L.; Obst, M.; Moran, D.M.; McIlvin, M.R.; Saito, M.A.; Kappler, A. Physiology, Fe(II) oxidation, and Fe mineral formation by a marine planktonic cyanobacterium grown under ferruginous conditions. Front. Earth Sci. 2015, 3, 60. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Shaked, Y.; Keren, N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 2011, 13, 2990–2999. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Finkel, O.M.; Schmetterer, G.; Shaked, Y.; Keren, N. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J. 2014, 8, 409–417. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Osman, D.; Cavet, J.S. Chapter 8-Copper Homeostasis in Bacteria. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2008; Volume 65, pp. 217–247. [Google Scholar] [CrossRef]
- Tottey, S.; Rich, P.R.; Rondet, S.A.; Robinson, N.J. Two Menkes-type ATPases Supply Copper for Photosynthesis in Synechocystis PCC 6803*. J. Biol. Chem. 2001, 276, 19999–20004. [Google Scholar] [CrossRef]
- Barnett, J.P.; Millard, A.; Ksibe, A.Z.; Scanlan, D.J.; Schmid, R.; Blindauer, C.A. Mining Genomes of Marine Cyanobacteria for Elements of Zinc Homeostasis. Front. Microbiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Bishop, B.A.; Flynn, S.L.; Warchola, T.J.; Alam, S.; Robbins, L.J.; Liu, Y.; Owttrim, G.W.; Alessi, D.S.; Konhauser, K.O. Adsorption of biologically critical trace elements to the marine cyanobacterium Synechococcus sp. PCC 7002: Implications for marine trace metal cycling. Chem. Geol. 2019, 525, 28–36. [Google Scholar] [CrossRef]
- Dyhrman, S.T. Nutrients and Their Acquisition: Phosphorus Physiology in Microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 155–183. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic Polyphosphate: Essential for Growth and Survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Ingalls, M.; Blättler, C.L.; Higgins, J.A.; Magyar, J.S.; Eiler, J.M.; Fischer, W.W. P/Ca in Carbonates as a Proxy for Alkalinity and Phosphate Levels. Geophys. Res. Lett. 2020, 47, e2020GL088804. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochim. Cosmochim. Acta 2011, 75, 2911–2923. [Google Scholar] [CrossRef]
- Ma, X.; Ma, H.; Jiang, X.; Jiang, Z. Preparation of magnesium hydroxide nanoflowers from boron mud via anti-drop precipitation method. Mater. Res. Bull. 2014, 56, 113–118. [Google Scholar] [CrossRef]
- Butt, D.P.; Lackner, K.S.; Wendt, C.H.; Conzone, S.D.; Kung, H.; Lu, Y.; Bremser, J.K. Kinetics of Thermal Dehydroxylation and Carbonation of Magnesium Hydroxide. J. Am. Ceram. Soc. 1996, 79, 1892–1898. [Google Scholar] [CrossRef]
- Dung, N.; Unluer, C. Carbonated MgO concrete with improved performance: The influence of temperature and hydration agent on hydration, carbonation and strength gain. Cem. Concr. Compos. 2017, 82, 152–164. [Google Scholar] [CrossRef]
- Nguyen, H.; Santos, H.; Sreenivasan, H.; Kunther, W.; Carvelli, V.; Illikainen, M.; Kinnunen, P. On the carbonation of brucite: Effects of Mg-acetate on the precipitation of hydrated magnesium carbonates in aqueous environment. Cem. Concr. Res. 2022, 153, 106696. [Google Scholar] [CrossRef]
- Sforna, M.C.; Philippot, P.; Somogyi, A.; van Zuilen, M.A.; Medjoubi, K.; Schoepp-Cothenet, B.; Nitschke, W.; Visscher, P.T. Evidence for arsenic metabolism and cycling by microorganisms 2.7 billion years ago. Nat. Geosci. 2014, 7, 811–815. [Google Scholar] [CrossRef]
- Swart, P.K. The geochemistry of carbonate diagenesis: The past, present and future. Sedimentology 2015, 62, 1233–1304. [Google Scholar] [CrossRef]
- Dodd, M.S.; Zhang, Z.; Li, C.; Algeo, T.J.; Lyons, T.W.; Hardisty, D.S.; Loyd, S.J.; Meyer, D.L.; Gill, B.C.; Shi, W.; et al. Development of carbonate-associated phosphate (CAP) as a proxy for reconstructing ancient ocean phosphate levels. Geochim. Cosmochim. Acta 2021, 301, 48–69. [Google Scholar] [CrossRef]
- Ingalls, M.; Grotzinger, J.P.; Present, T.; Rasmussen, B.; Fischer, W.W. Carbonate-Associated Phosphate (CAP) Indicates Elevated Phosphate Availability in Neoarchean Shallow Marine Environments. Geophys. Res. Lett. 2022, 49, e2022GL098100. [Google Scholar] [CrossRef]
- Allwood, A.C.; Kamber, B.S.; Walter, M.R.; Burch, I.W.; Kanik, I. Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem. Geol. 2010, 270, 148–163. [Google Scholar] [CrossRef]
- Banner, J.L. Application of the trace element and isotope geochemistry of strontium to studies of carbonate diagenesis. Sedimentology 1995, 42, 805–824. [Google Scholar] [CrossRef]
- Wacey, D.; Gleeson, D.; Kilburn, M.R. Microbialite taphonomy and biogenicity: New insights from NanoSIMS. Geobiology 2010, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]

| Ωcalcite | Ωaragonite | Ωdolomite | Ωmagnesite | Ωbrucite | |
|---|---|---|---|---|---|
| pH = 8 | 1.03 | 0.69 | 17.74 | 2.76 | 2.77 × 10−3 |
| pH = 9.4 | 14.13 | 9.39 | 3266.30 | 37.14 | 0.17 |
| B | Mn | Co | Cu | Zn | Mo | Ca | Fe | K | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Synechococcus cells | 3.21 | 199.98 | 1.39 | 424.05 | 329.38 | 0.73 | 0.20 | 1622.22 | 1.81 | 149.27 |
| Minerals (biotic) | 1.56 | 94.56 | 2.10 | 171.46 | 197.71 | 0.66 | 1.33 | 2800.50 | b.d. * | 37.10 |
| Minerals (abiotic) | 2.68 | 52.88 | 0.66 | 50.25 | 78.38 | 0.46 | 0.48 | 231.43 | b.d. * | 5.99 |
| Minerals (crashed) | 1.76 | 0.76 | 0.02 | 0.76 | 0.74 | 0.24 | 0.08 | 1.01 | 0.01 | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leapaldt, H.; Ingalls, M.; Soares, G.; House, C.H. Trace Metal and Phosphorus Enrichments in Cyanobacteria Cells and Cyanobacterial Precipitated Minerals. Minerals 2025, 15, 378. https://doi.org/10.3390/min15040378
Leapaldt H, Ingalls M, Soares G, House CH. Trace Metal and Phosphorus Enrichments in Cyanobacteria Cells and Cyanobacterial Precipitated Minerals. Minerals. 2025; 15(4):378. https://doi.org/10.3390/min15040378
Chicago/Turabian StyleLeapaldt, Hanna, Miquela Ingalls, Georgia Soares, and Christopher H. House. 2025. "Trace Metal and Phosphorus Enrichments in Cyanobacteria Cells and Cyanobacterial Precipitated Minerals" Minerals 15, no. 4: 378. https://doi.org/10.3390/min15040378
APA StyleLeapaldt, H., Ingalls, M., Soares, G., & House, C. H. (2025). Trace Metal and Phosphorus Enrichments in Cyanobacteria Cells and Cyanobacterial Precipitated Minerals. Minerals, 15(4), 378. https://doi.org/10.3390/min15040378









