The Recovery of the Strategic Metals from the Nitrate Solutions of Zn-Pb Tailings Using a Solvent Extraction Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Measuring Equipment for Samples
2.3. Characterization of the Zn-Pb Tailings
TXRF Spectrometry
- (a)
- The Suspension Method
- (b)
- The Extraction Method
2.4. ICP-MS Analysis During Solvent Extraction
2.4.1. Validation of the ICP-MS Method
2.4.2. Leaching and Liquid–Liquid Extraction of Transition Metals
3. Results and Discussion
3.1. Analysis of the Samples Using the TXRF Technique
3.2. Analysis of the Aqueous Phase Using ICP-MS
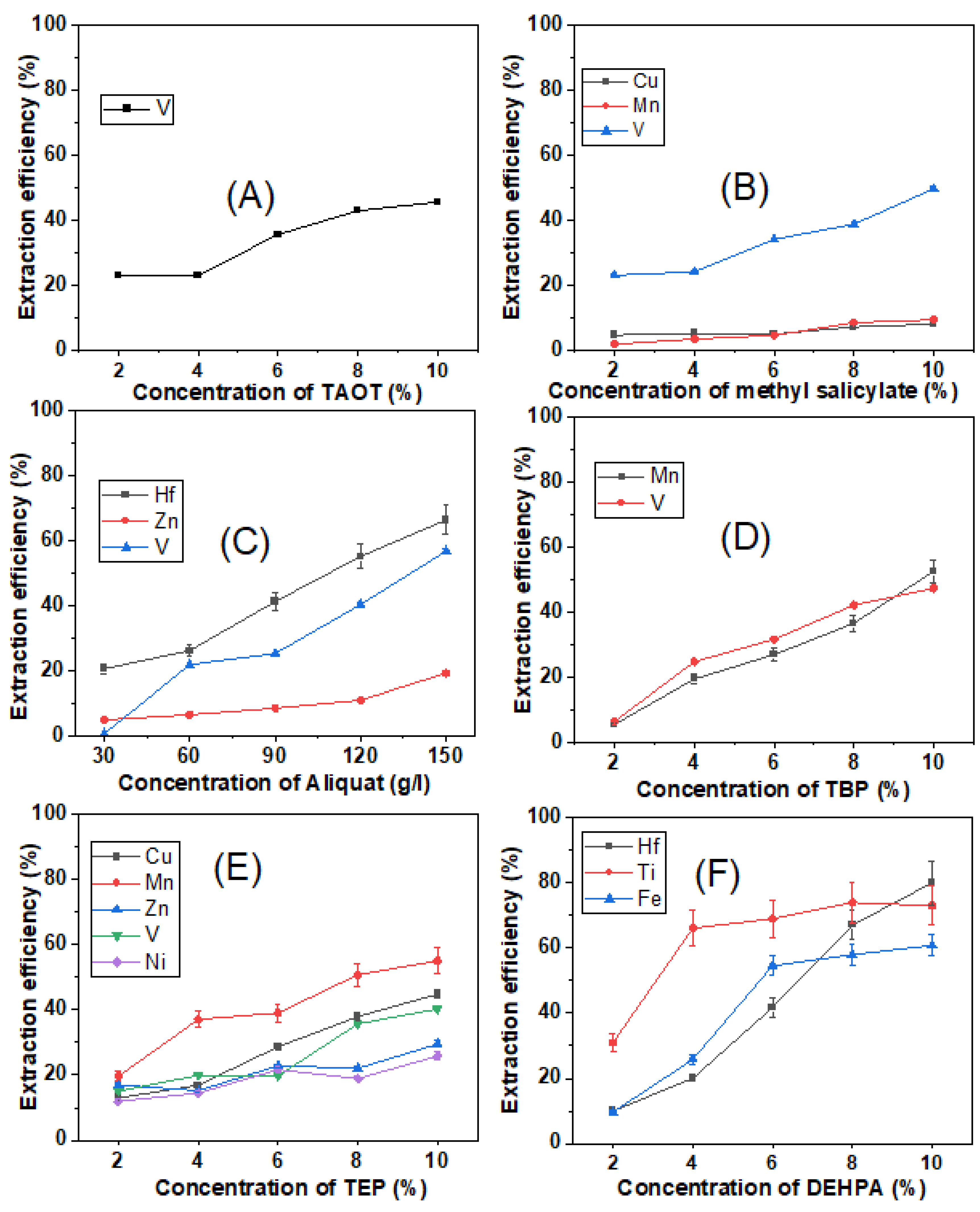
3.3. The Impact of Ligand Concentration on Transition Metals
3.4. The Effect of pH Changes on Transition Metal Extraction
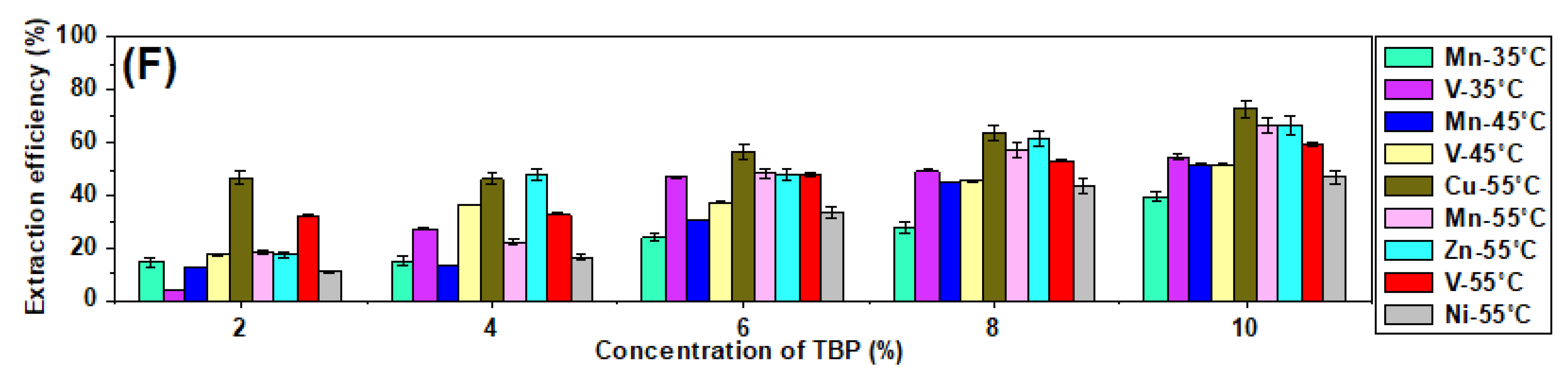
3.5. Evaluation of the Effect of Temperature on the Extraction of Transition Metals
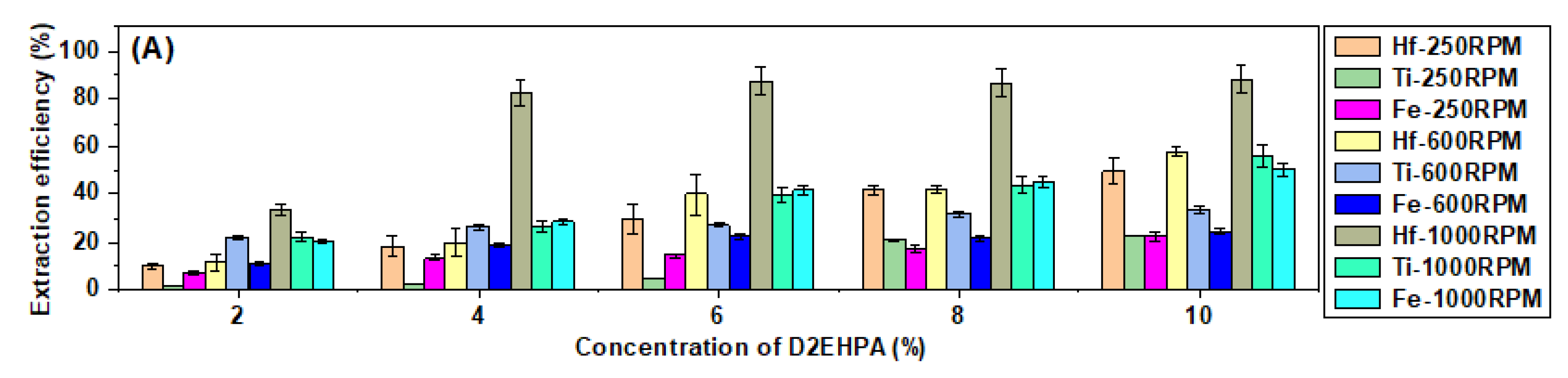
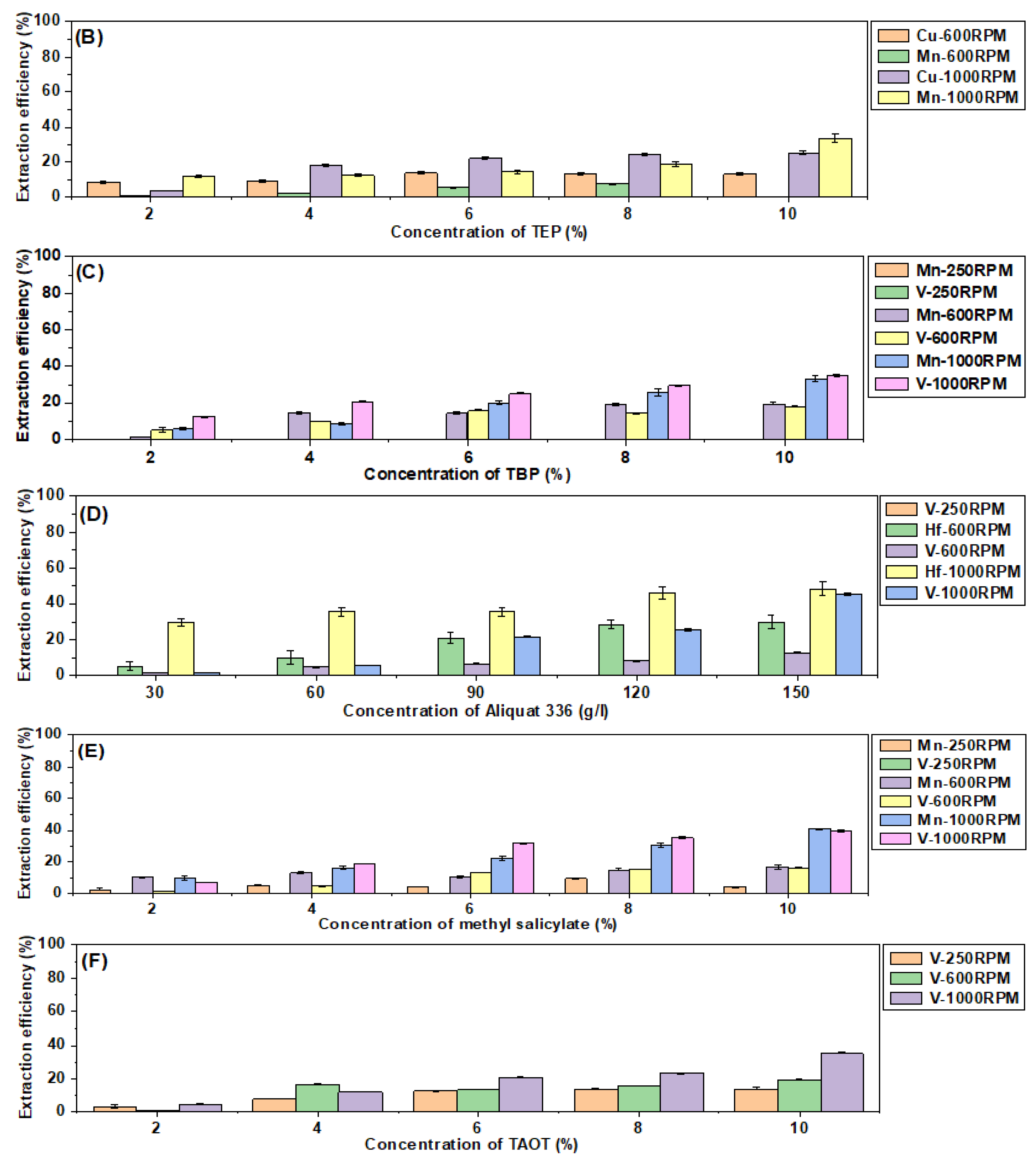
3.6. The Analysis of the Recoveries of Transition Metals at Different Mixing Rates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end-of-life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- Kiprono, N.R.; Smolinski, T.R.; Rogowski, M.; Chmielewski, A.G. The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations 2023, 10, 112. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Wawszczak, D.; Brykała, M. Possibility of uranium and rare metal recovery in the Polish copper mining industry. Hydrometallurgy 2016, 159, 12–18. [Google Scholar] [CrossRef]
- Tarnawczyk, M.; Uzarowicz, Ł.; Perkowska-Pióro, K.; Pędziwiatr, A.; Kwasowski, W. Effect of land reclamation on soil properties, mineralogy and trace-element distribution and availability: The example of technosols developed on the tailing disposal site of an abandoned zn and pb mine. Minerals 2021, 11, 559. [Google Scholar] [CrossRef]
- Duczmal-Czernikiewicz, A.; Baibatsha, A.; Bekbotayeva, A.; Omarova, G.; Baisalova, A. Ore minerals and metal distribution in tailings of sediment-hosted stratiform copper deposits from poland and kazakhstan. Minerals 2021, 11, 752. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Raiguel, S.; Binnemans, K. Separation of transition metals from rare earths by non-aqueous solvent extraction from ethylene glycol solutions using Aliquat 336. Sep. Purif. Technol. 2018, 201, 318–326. [Google Scholar] [CrossRef]
- Lommelen, R.; Vander Hoogerstraete, T.; Onghena, B.; Billard, I.; Binnemans, K. Model for Metal Extraction from Chloride Media with Basic Extractants: A Coordination Chemistry Approach. Inorg. Chem. 2019, 58, 12289–12301. [Google Scholar] [CrossRef]
- Facon, S.; Adekola, F.; Cote, G. Stripping of copper from CYANEX® 301 extract with thiourea-hydrazine-sodium hydroxide solution. Hydrometallurgy 2007, 89, 297–304. [Google Scholar] [CrossRef]
- Lee, L.Y.; Morad, N.; Ismail, N.; Talebi, A.; Rafatullah, M. Optimization for liquid-liquid extraction of Cd (II) over Cu (II) ions from aqueous solutions using ionic liquid Aliquat 336 with tributyl phosphate. Int. J. Mol. Sci. 2020, 21, 6860. [Google Scholar] [CrossRef]
- Pianowska, K.; Leszczyńska-Sejda, K.; Kluczka, J.; Benke, G.; Goc, K.; Malarz, J.; Ochmanski, M.; Leszczyńska-Sejda, K. Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals. Materials 2023, 16, 4681. [Google Scholar] [CrossRef] [PubMed]
- El-Nadi, Y.A. Solvent Extraction and Its Applications on Ore Processing and Recovery of Metals: Classical Approach. Sep. Purif. Rev. 2016, 46, 195–215. [Google Scholar] [CrossRef]
- Rotich, N.K.; Herdzik-Koniecko., I.; Smolinski, T.; Kalbarczyk, P.; Sudlitz, M.; Rogowski, M.; Stosnach, H.; Chmielewski, A.G. Recovery of Metals from Titanium Ore Using Solvent Extraction Process: Part 1—Transition Metals. Minerals 2024, 14, 1212. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Jena, P.K. Studies on the dissolution of metal values in manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1988, 21, 23–39. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar] [CrossRef]
- Gorzin, H.; Ghaemi, A.; Hemmati, A.; Maleki, A. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: Multiple response optimizations by desirability function. J. Mol. Liq. 2021, 324, 115123. [Google Scholar] [CrossRef]
- Cinosi, A.; Siviero, G.; McCaffrey, Z. Elemental analysis of valuable byproducts by TXRF spectrometry: Coal fly ash and activated carbon. Spectrochim. Acta Part B At. Spectrosc. 2024, 220, 107017. [Google Scholar] [CrossRef]
- Lerum, H.V.; Andersen, N.H.; Eriksen, D.Ø.; Hansen, E.W.; Petersen, D.; Wibetoe, G.; Omtvedt, J.P. Study of Cadmium Extraction with Aliquat 336 from Highly Saline Solutions. J. Solut. Chem. 2018, 47, 1395–1417. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y.; Peng, J.; Liu, Q. Separation of Fe(III) and Al via Tributyl Phosphate and 2-Octanol in the Comprehensive Recovery Process of Metals from Hydrochloric Acid Leaching Solution of Fly Ash. Solvent Extr. Res. Dev. Jpn. 2021, 28, 59–68. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Lv, G.; Zhang, W.; Sui, Q. Extraction Separation of Ti (IV) and Fe(II) Using D2EHPA from the Raffinate Obtained After Extraction of Scandium from Titanium Dioxide Waste Acid. JOM 2022, 74, 1061–1069. [Google Scholar] [CrossRef]
- Fedorova, M.I.; Levina, A.V. Application of ionic liquid based on Aliquat 336 and D2EHPA in the extraction of transition metals. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1212, 012021. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Zhang, H.; Sun, G.; Cui, Y. Preferential extraction of hafnium over zirconium with D2EHPA through selective complexation of organic acids. J. Radioanal. Nucl. Chem. 2019, 321, 333–339. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Lee, M.S. Improvement of metal separation process from synthetic hydrochloric acid leaching solution of spent lithium ion batteries by solvent extraction and ion exchange. Physicochem. Probl. Miner. Process. 2021, 57, 1–17. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Nguyen, V.N.H.; Liu, Y.; Lee, M.S. Analysis of the interaction in the mixture of organophosphorus acids and Aliquat 336 through the measurement of dielectric constant and viscosity. J. Mol. Liq. 2020, 315, 113738. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2013, 43, 123–134. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S. Extraction of Heavy Metal Ions Using Ionic Liquids. In Encyclopedia of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2022; pp. 403–410. [Google Scholar] [CrossRef]
- Soeezi, A. Solvent Extraction of Ni and Cu from Synthetic Solution Containing Ni, Cu, Fe and Zn by Using D2EHPA. Mater. Sci. Metall. Eng. 2019, 6, 1–5. [Google Scholar]
- Shulin, S.S.; Galieva, G.N.; Chighevskaya, S.V.; Pletuhina, J.V.; Saveliev, N.S. Extraction Separation of Rare Earth Elements of the Medium Group with Isomolar Mixtures of Aliquat®336–TBP and Cyanex®572–TBP from Nitric Solutions. Inorg. Mater. 2018, 54, 515–519. [Google Scholar] [CrossRef]



| Metal | Suspension Method | Extraction Method |
|---|---|---|
| Sc | - | 100 ± 0.1 |
| Mn | 76 ± 10 | 5 ± 1 |
| Fe | 6137 ± 1010 | 23,505 ± 189 |
| Cu | 2 ± 1 | 3 ± 1 |
| Zn | 1224 ± 182 | 8 ± 1 |
| Ti | 24 ± 3 | 6075 ± 84 |
| As | 58 ± 8 | - |
| Rb | 1 ±1 | 1 ± 0.1 |
| V | - | 144 ± 4 |
| Cr | 4 ± 0.1 | 93 ± 2 |
| Co | 1 ± 0.01 | - |
| Ni | 1 ± 0.01 | 15 ± 1 |
| La | 18 ± 4 | - |
| Pb | 499 ± 39 | 3 ± 1 |
| Sr | 6 ± 1 | - |
| Cd | 9 ± 3 | - |
| Y | 5 ± 1 | - |
| Nd | - | 29 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotich, N.K.; Herdzik-Koniecko, I.; Smolinski, T.; Rogowski, M.; Stosnach, H.; Chmielewski, A.G. The Recovery of the Strategic Metals from the Nitrate Solutions of Zn-Pb Tailings Using a Solvent Extraction Process. Minerals 2025, 15, 357. https://doi.org/10.3390/min15040357
Rotich NK, Herdzik-Koniecko I, Smolinski T, Rogowski M, Stosnach H, Chmielewski AG. The Recovery of the Strategic Metals from the Nitrate Solutions of Zn-Pb Tailings Using a Solvent Extraction Process. Minerals. 2025; 15(4):357. https://doi.org/10.3390/min15040357
Chicago/Turabian StyleRotich, Nelson Kiprono, Irena Herdzik-Koniecko, Tomasz Smolinski, Marcin Rogowski, Hagen Stosnach, and Andrzej G. Chmielewski. 2025. "The Recovery of the Strategic Metals from the Nitrate Solutions of Zn-Pb Tailings Using a Solvent Extraction Process" Minerals 15, no. 4: 357. https://doi.org/10.3390/min15040357
APA StyleRotich, N. K., Herdzik-Koniecko, I., Smolinski, T., Rogowski, M., Stosnach, H., & Chmielewski, A. G. (2025). The Recovery of the Strategic Metals from the Nitrate Solutions of Zn-Pb Tailings Using a Solvent Extraction Process. Minerals, 15(4), 357. https://doi.org/10.3390/min15040357






