An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
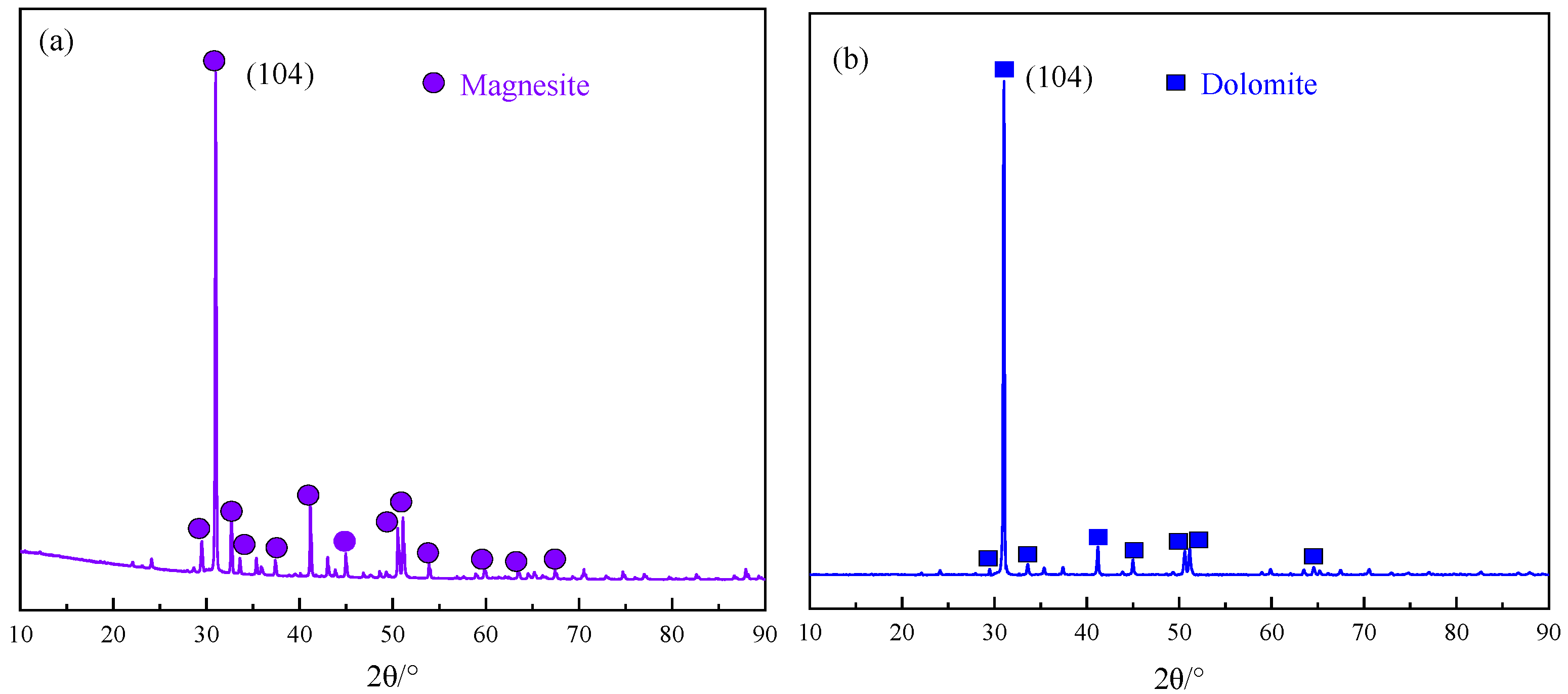
2.1.1. Minerals
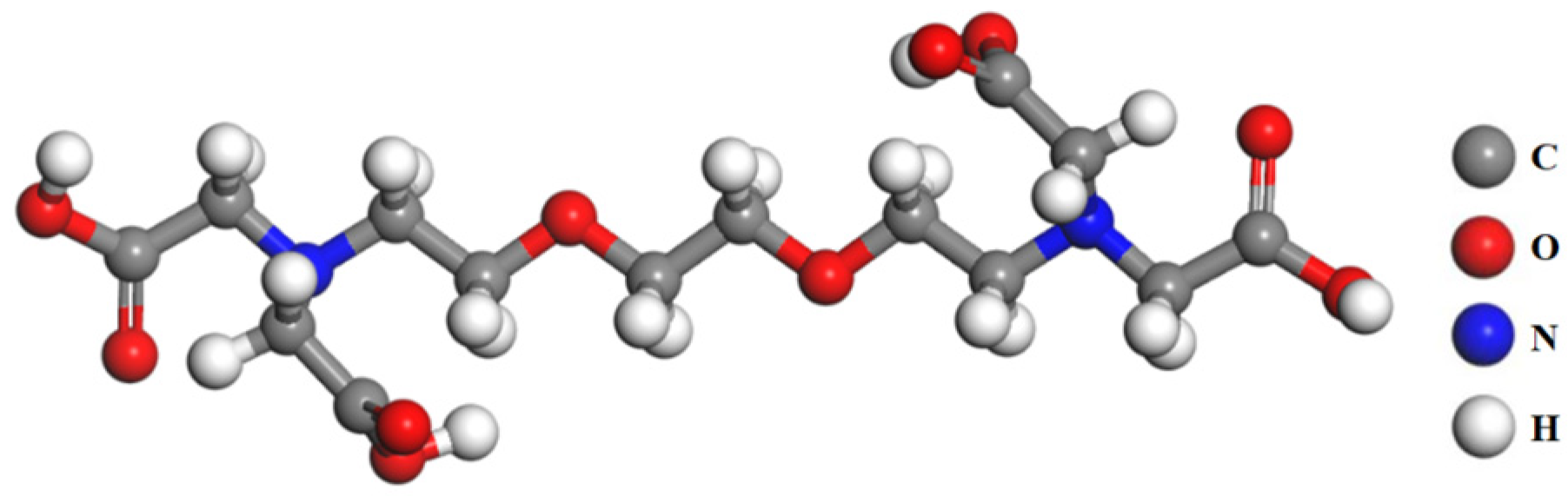
2.1.2. Reagents
2.2. Methods
2.2.1. Flotation Test
2.2.2. Contact Angle Detection
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Detection
2.2.4. Zeta Potential Detection
2.2.5. X-Ray Photoelectron Spectroscopy (XPS) Testing
2.2.6. Inductively Coupled Plasma Emission Spectrometer (ICP-OES) Detection
3. Results and Discussion
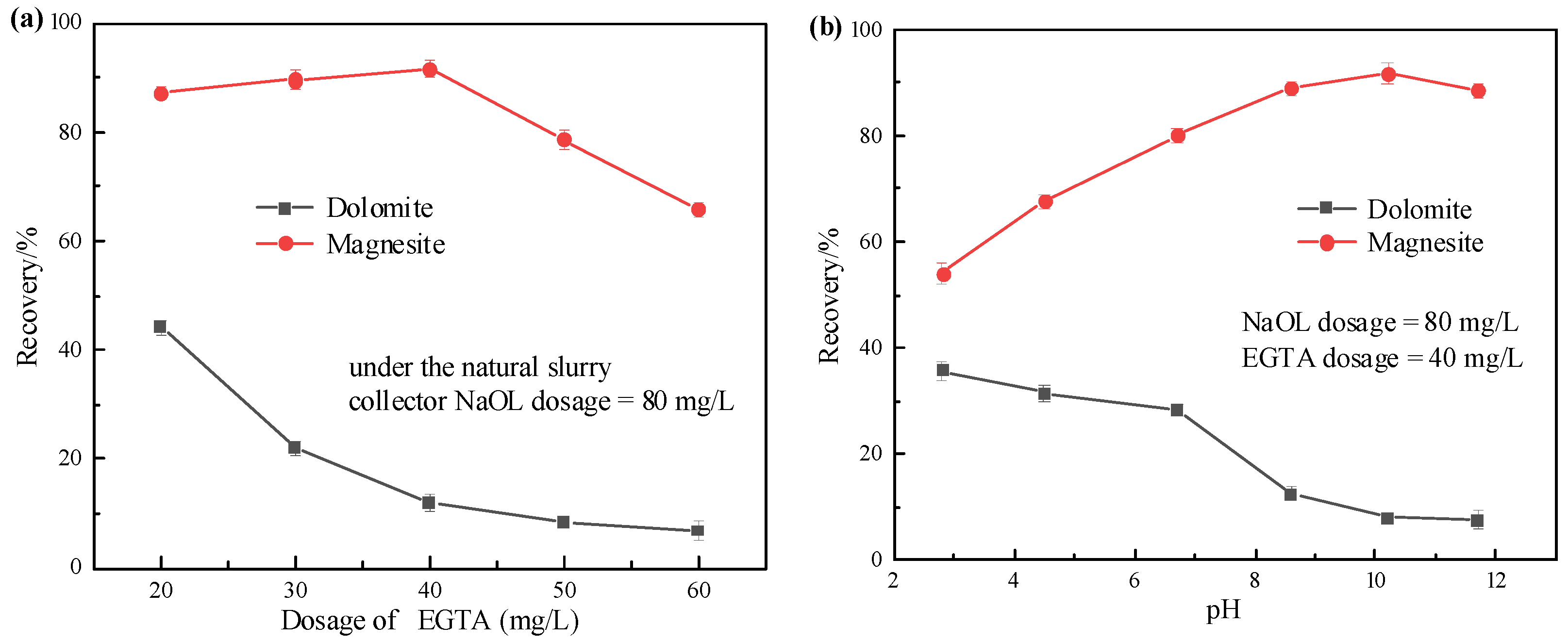
3.1. Flotation Test
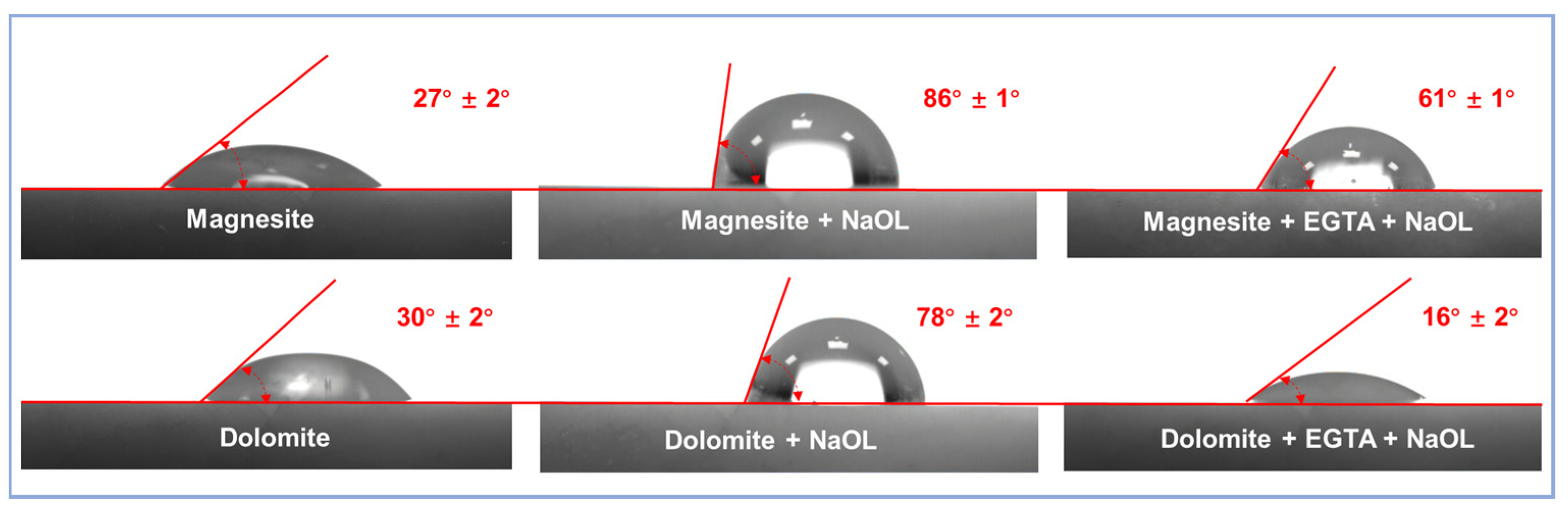
3.2. Contact Angle Detection
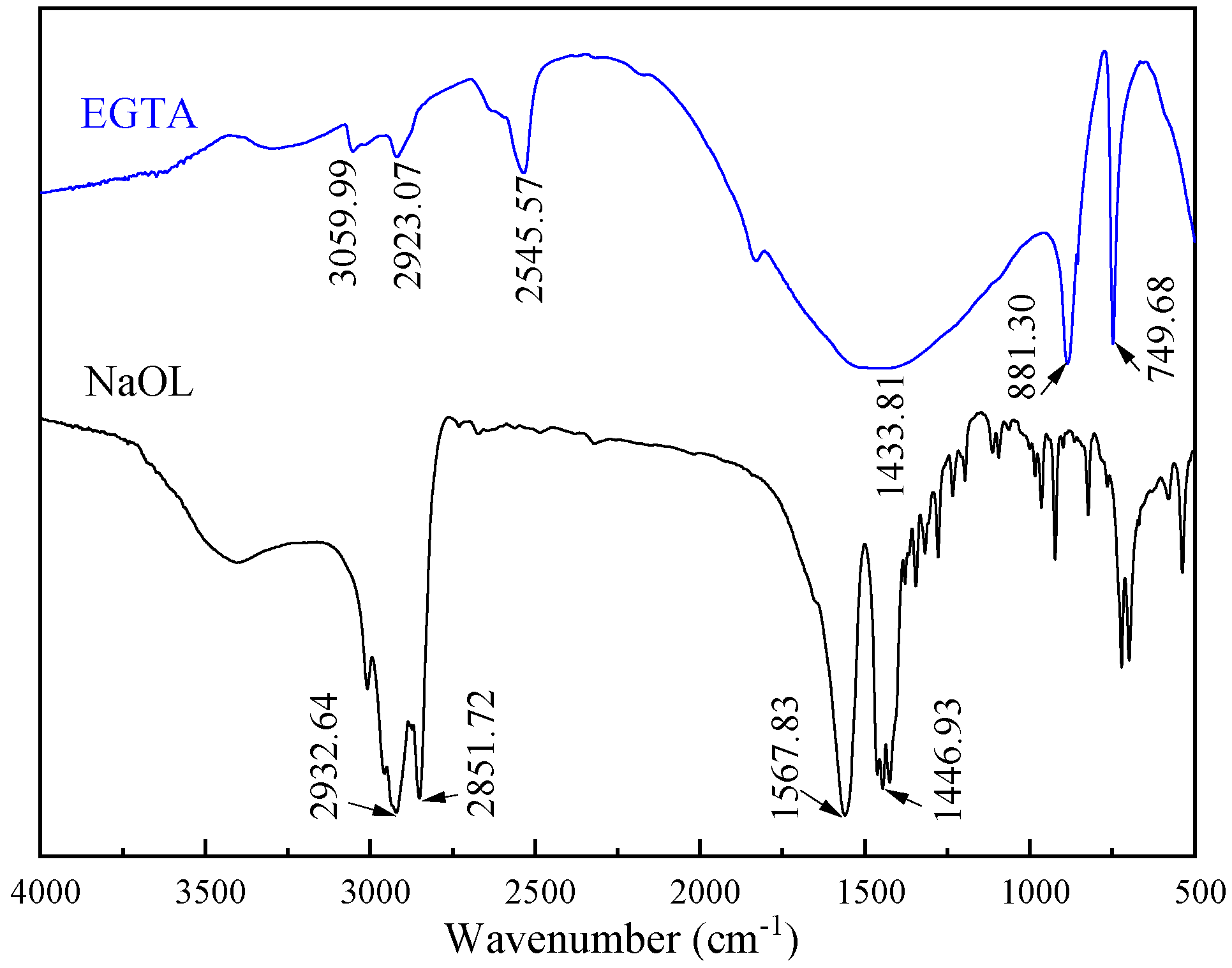
3.3. FTIR Detection
3.4. Zeta Potential Detection
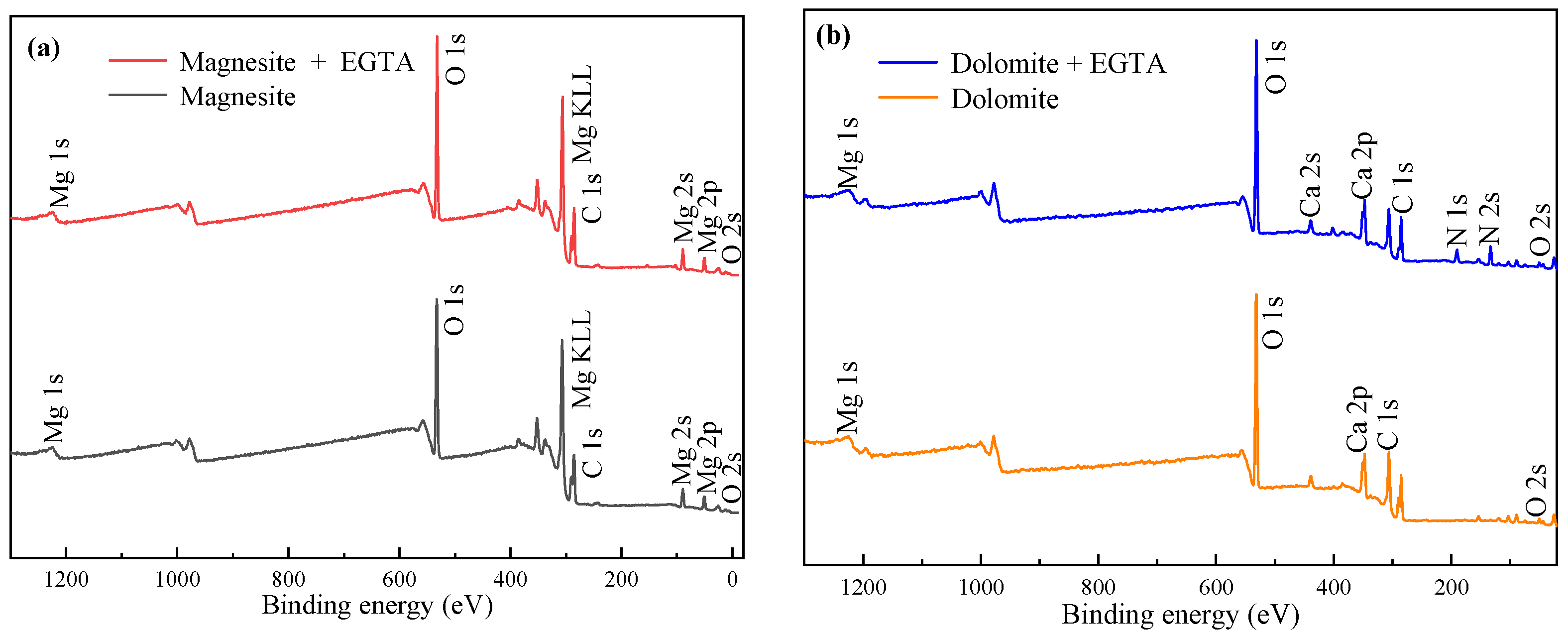
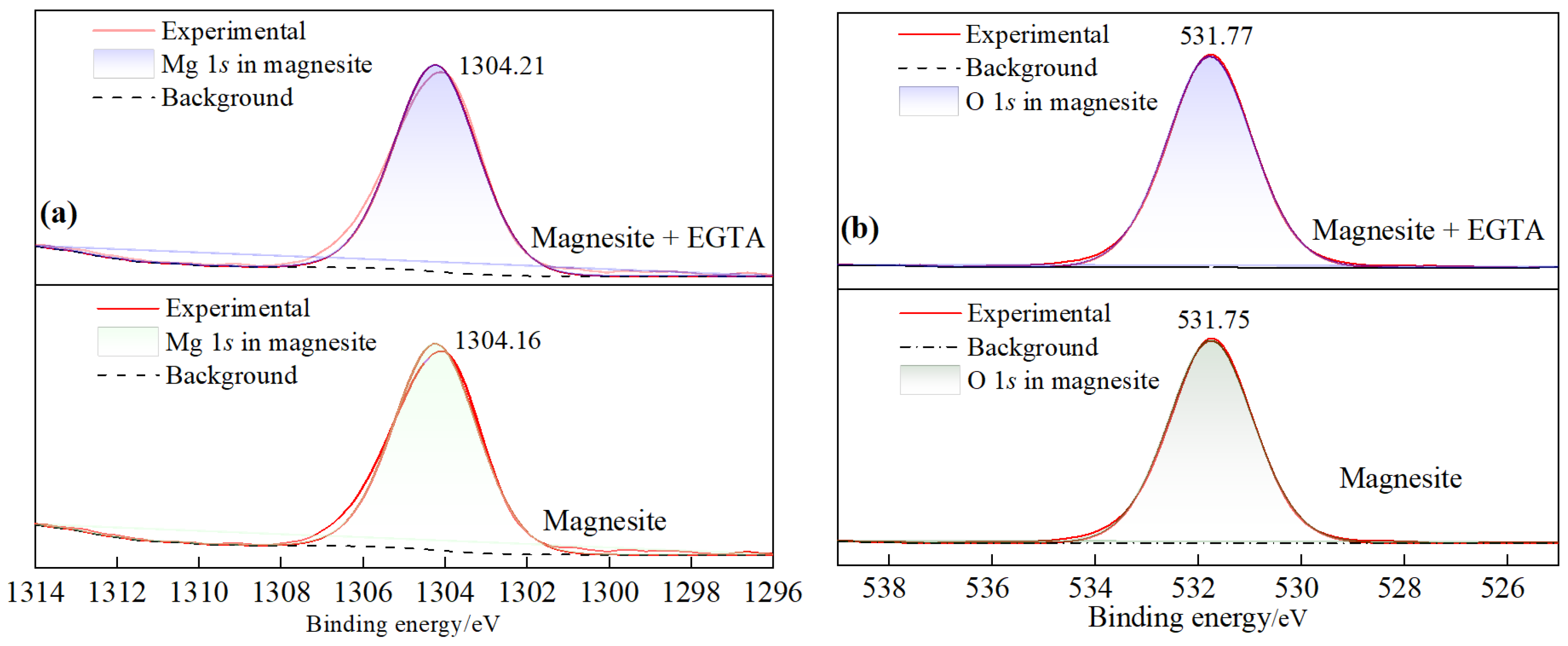
3.5. XPS Analysis
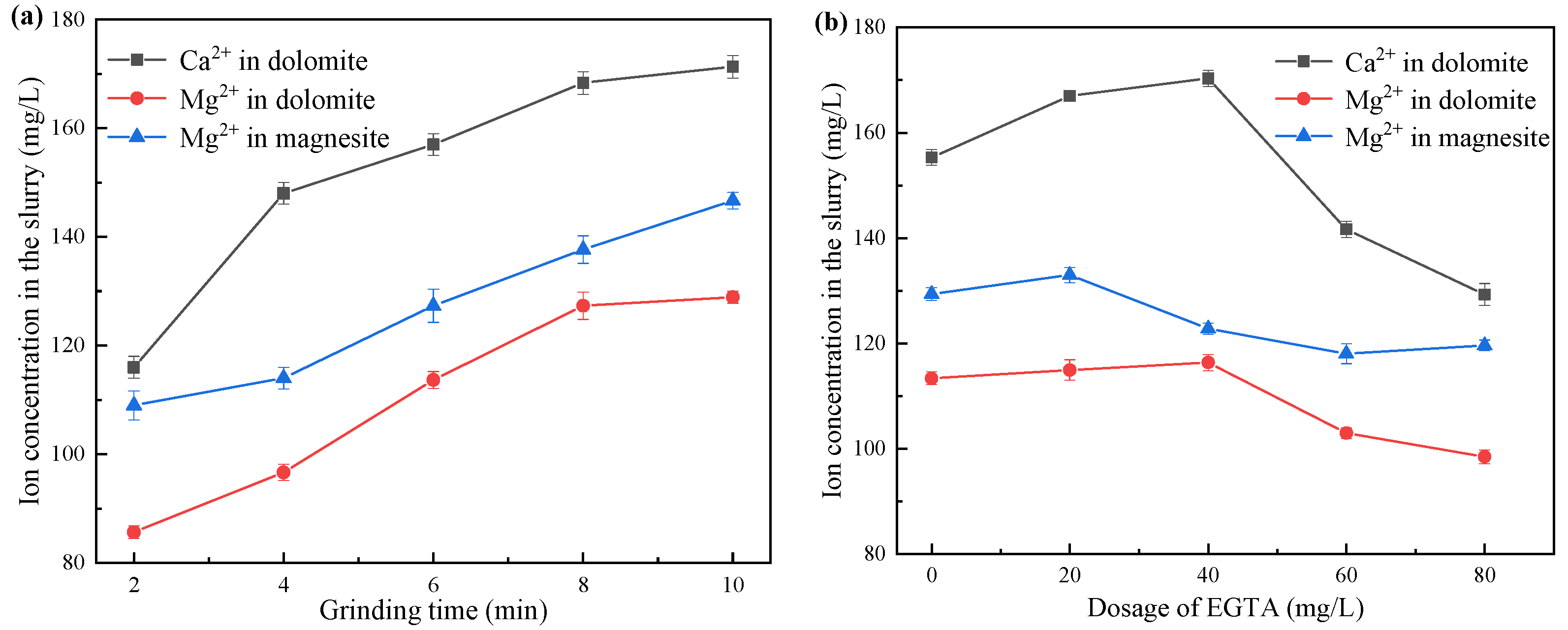
3.6. ICP-OES Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, Y.; Liu, W.; Liu, W.; Shen, Y.; Zhao, Q. An environmentally friendly depressant for magnesite and calcite flotation separation: Selective depression and adsorption mechanism. J. Environ. Chem. Eng. 2024, 12, 114499. [Google Scholar] [CrossRef]
- ANeisiani, A.; Saneie, R.; Mohammadzadeh, A.; Wonyen, D.G.; Chelgani, S.C. Polysaccharides-based pyrite depressants for green flotation separation: An overview. Int. J. Min. Sci. Technol. 2023, 33, 1229–1241. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, C.; Chen, Q.; Li, Q.; He, D.; Li, Z.; Fu, Y. Effect of a novel environmental-friendly chelating depressant DTPMP on the flotation separation of magnesite and dolomite. Appl. Surf. Sci. 2025, 690, 162650. [Google Scholar] [CrossRef]
- Cheng, Q.; Mei, G.; Xu, W.; Yuan, Q. Flotation of quartz using imidazole ionic liquid collectors with different counterions. Miner. Eng. 2022, 180, 107491. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Wang, X.; Gu, X.; Sun, W.; Peng, X.; Yue, H. Investigation on flotation separation of bastnaesite from calcite and barite with a novel surfactant: Octylamino-bis-(butanohydroxamic acid). Sep. Purif. Technol. 2021, 256, 117792. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, Y.; Han, Y.; Li, Y.; Yuan, S. Flotation performance and adsorption mechanism of a new collector 2-(carbamoylamino) lauric acid on quartz surface. Miner. Eng. 2020, 153, 106343. [Google Scholar] [CrossRef]
- Han, W.; Zhu, Y.; Ge, W.; Liu, J.; Li, Y. Curdlan as a new depressant of hematite for quartz-hematite reverse flotation separation. Miner. Eng. 2022, 185, 107708. [Google Scholar] [CrossRef]
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Jović, B.; Panić, M.; Radnović, N.; Živojević, K.; Mladenović, M.; Crnojević, V.; Knežević, N. Investigation of the surface interactions of selected amides with mesoporous silica using FTIR spectroscopy and hyperspectral imaging. J. Mol. Struct. 2020, 1219, 128562. [Google Scholar] [CrossRef]
- Li, M.; Yang, C.; Wu, Z.; Gao, X.; Tong, X.; Yu, X.; Long, H. Selective depression action of taurine in flotation separation of specularite and chlorite. Int. J. Min. Sci. Technol. 2022, 32, 637–644. [Google Scholar] [CrossRef]
- Li, R.; Luo, X.; Wen, S.; Li, C.; Wei, D.; Yang, W.; Zhang, Y.; Zhu, Y.; Wang, Y. Three-phase froth stability in hematite flotation using DDA as a collector. Miner. Eng. 2023, 195, 108023. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Dai, S.; Yang, T.; Li, Z.; Fang, P. Enhancing the purity of magnesite ore powder using an ethanolamine-based collector: Insights from experiment and theory. J. Mol. Liq. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Zhao, Q.; Peng, X.; Wang, B.; Zhou, S.; Zhao, L. Investigating the performance of a novel polyamine derivative for separation of quartz and hematite based on theoretical prediction and experiment. Sep. Purif. Technol. 2020, 237, 116370. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, W.; Liu, W.; Shen, Y.; Zhao, Q. Exploring the behavior of an alcoholic amine grinding aid in grinding-flotation system: Separation of quartz from magnesite. J. Mol. Liq. 2024, 411, 125783. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Tong, K.; Shen, Y.; Zhao, Q.; Zhao, S.; Sun, W. Novel polyhydroxy cationic collector N-(2,3-propanediol)-N-dodecylamine: Synthesis and flotation performance to hematite and quartz. Int. J. Min. Sci. Technol. 2023, 33, 115–122. [Google Scholar] [CrossRef]
- Liu, W.; Mao, Y.; Zheng, J.; Wang, Z.; Shang, C.; Liu, W.; Zhao, Q.; Zhao, S.; Shen, Y. Enhancing the flotation separation of magnesite and dolomite by introducing a phosphonic acid depressant during grinding. Sep. Purif. Technol. 2025, 361, 131412. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Z.; Liu, W.; Tian, P. Effect of TIPA/TEA combined grinding aid on the behavior of quartz flotation in DDA system. Powder Technol. 2022, 406, 117570. [Google Scholar] [CrossRef]
- Prziwara, P.; Kwade, A. Grinding aid additives for dry fine grinding processes—Part II: Continuous and industrial grinding. Powder Technol. 2021, 394, 207–213. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, P.; Mao, Y. Effects of grinding aids on the grinding kinetics and surface morphological characterization of quartz. Adv. Powder Technol. 2023, 34, 104142. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, G.; Zuo, Q.; Xiao, W.; Kang, X.; Liang, X.; Zhu, S. Activation of ilmenite flotation by sodium chlorite in the sodium oleate system. Sep. Purif. Technol. 2023, 305, 122506. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Zhai, Q.; Xie, Z.; Sun, W.; Li, P.; Wang, Z. Exploring the effect of pulp aeration and lime-aid grinding on pyrrhotite-rich type copper sulfide ore flotation separation. Sep. Purif. Technol. 2023, 311, 123268. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Xu, S.; Ayedzi, L.D.; Addai-Mensah, J.; Skinner, W. Flotation recovery of monazite from kaolinite using sodium oleate collector: Understanding mineral–collector interaction. Miner. Eng. 2024, 209, 108605. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Meng, Q.; Zhang, Y.; Xu, Y. Investigation of selective adsorption of sodium oleate during separation of ilmenite from titanaugite via surface magnetization. Miner. Eng. 2021, 171, 107128. [Google Scholar] [CrossRef]
- Banu, J.R.; Eswari, A.P.; Saratale, G.D.; Rani, R.U.; Kaliappan, S.; Yeom, I. Enhancing biomethanation from dairy waste activated biomass using a novel EGTA mediated microwave disintegration. J. Environ. Manag. 2018, 223, 644–651. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Huang, Y.; Tan, X.; Zeng, G.; Hu, X.; Yang, Z. Fast adsorption of Cd2+ and Pb2+ by EGTA dianhydride (EGTAD) modified ramie fiber. J. Colloid. Interface Sci. 2014, 434, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Yin, D.; Sillanpää, M.E.T. Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J. Colloid. Interface Sci. 2013, 409, 174–182. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, Z.; Wang, L.; Li, Z.; Han, X.; Sun, W. An eco-friendly approach to purify natural magnesite and to densify sintered magnesia. Open Ceram. 2024, 17, 100549. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Wang, L.; Liu, W.; Sun, W.; Zhang, N. A novel depressant N,N-bis(phosphonomethyl)glycine for magnesite-dolomite separation and its mechanism. Miner. Eng. 2023, 202, 108281. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Huang, L.; Zhu, Y.; Li, F. Flotation separation of magnesite from dolomite with gellan gum as depressant and its depression mechanism. Miner. Eng. 2024, 212, 108718. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Hu, H.; Liu, S.; Xie, X.; Huang, J.; Han, Z. Boosting synergistic recovery of ammonia nitrogen and phosphate from phosphorus chemical wastewater by co-pyrolyzing the biomass and magnesite. Sep. Purif. Technol. 2024, 347, 127645. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Yin, W.; Yin, X.; Ban, X.; Wang, Y. Effect of acid corrosion on the surface roughness and floatability of magnesite and dolomite. Green Smart Min. Eng. 2024, 1, 118–125. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, H.; Zhao, L.; Dong, H.; Zhang, Z. Effect of swirling gas inlet design on particle motion and decomposition in magnesite flash calciner. Chem. Eng. Res. Des. 2024, 206, 386–396. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Ma, Y.; Chen, K. Effects of ultrasonic treatment on the flotation behavior of magnesite and dolomite in a sodium oleate system. Green Smart Min. Eng. 2024, 1, 76–84. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Shan, J.; Wu, C.; Lichtfouse, E.; Liu, H. Efficient recovery of phosphate from urine using magnesite modified corn straw biochar and its potential application as fertilizer. J. Environ. Chem. Eng. 2024, 12, 111925. [Google Scholar] [CrossRef]
- Gokcen, H.S.; Cayirli, S.; Ucbas, Y.; Kayaci, K. The effect of grinding aids on dry micro fine grinding of feldspar. Int. J. Miner. Process. 2015, 136, 42–44. [Google Scholar] [CrossRef]
- Zhu, H.; Shao, S.; Guo, M.; Zhang, S.; Zhang, Y. Engineering properties and sustainability evaluation of crushed low grade magnesite mortars. J. Clean. Prod. 2023, 425, 138979. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Chen, M.; Hu, H.; Huang, J.; Jiang, T.; Zhang, Q. Enhanced Cd2+ removal from aqueous solution using olivine and magnesite combination: New insights into the mechanochemical synergistic effect. J. Environ. Sci. 2025, 147, 714–725. [Google Scholar] [CrossRef]
- Chen, G.; Ma, Y.; López-Valdivieso, A.; Xiao, W.; Yin, W. Selective depression of phenoxyacetyl chloride on magnesite: Implications for effective flotation separation of magnesite from dolomite. Miner. Eng. 2024, 218, 109017. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Yin, W.; Yu, J.; Yang, B.; Wang, Y. Investigation of the flotation behavior and interaction characteristics of micro-fine quartz and magnesite in a dodecylamine system under ultrasonic treatment. Particuology 2024, 94, 339–386. [Google Scholar] [CrossRef]
- Xue, Z.; Feng, Y.; Li, H. Enhancement mechanism of polysorbate surfactant at solid/liquid and gas/liquid interfaces in magnesite tailings flotation desilication via MD and DFT calculations. J. Environ. Chem. Eng. 2024, 12, 113085. [Google Scholar] [CrossRef]
- Telesca, A.; Ibris, N.; Marroccoli, M.; Tregambi, C.; Solimene, R.; Di Lauro, F.; De Ballesteros, O.R.; Salatino, P.; Montagnaro, F. Evaluation of the technical properties of reactive-MgO cements produced by solar calcination of magnesite in a fluidized bed reactor. Renew. Energy 2024, 225, 120231. [Google Scholar] [CrossRef]
- Arya, P.C.; Nambaje, C.; Kiran, S.; Satish-Kumar, M.; Sajeev, K. Himalayan magnesite records abrupt cyanobacterial growth that plausibly triggered the Neoproterozoic Oxygenation Event. Precambrian Res. 2023, 395, 107129. [Google Scholar] [CrossRef]
- Tian, D.; Yin, W.; Xie, Y.; Liu, J.; Zhu, Z.; Yao, J. Influence of surface roughness of magnesite on bubble-particle energy barrier: Analysis based on a new simplified model. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132860. [Google Scholar] [CrossRef]
- Xue, Z.; Feng, Y.; Li, H. Investigation of Alkane-Assisted dodecylamine on flotation desilication of magnesite Tailings: Molecular dynamics simulations and model optimization. J. Mol. Liq. 2024, 400, 124504. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Liu, W.; Zhang, R.; Wang, S. Application of novel ionic liquids in flotation separation of quartz and magnesite and its mechanism. Powder Technol. 2024, 447, 120218. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Fekry, A.M.; Fornasiero, D. Investigation of the effect of phosphoric acid as an acidic medium in flotation separation of dolomite from magnesite. Miner. Eng. 2023, 198, 108079. [Google Scholar] [CrossRef]
- Raudsepp, M.J.; Wilson, S.; Zeyen, N.; Arizaleta, M.L.; Power, I.M. Magnesite everywhere: Formation of carbonates in the alkaline lakes and playas of the Cariboo Plateau, British Columbia, Canada. Chem. Geol. 2024, 648, 121951. [Google Scholar] [CrossRef]
- Montes-Hernandez, G. Magnesite formation from nesquehonite slurry at 90 °C using some soluble Mg salts: Eitelite as an atypical transient mineral phase. Chem. Eng. Sci. 2024, 287, 119776. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Zhao, X.; Qi, Z.; Yang, B.; Yin, W.; Wang, Y. Effect of ultrasonic treatment on the surface roughness and floatability of magnesite and dolomite. J. Mol. Liq. 2024, 404, 125002. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Liu, W.; Li, P.; Chen, X.; Tong, K.; Kou, W. Adsorption study of potential collector polyoxyethylene ether phosphate on magnesite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131282. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Han, H.; Sun, W.; Zhang, X. The enhanced flotation separation of magnesite and dolomite by introducing chelating reagent EDTA. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132969. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 126–138. [Google Scholar] [CrossRef]





| Reagents | Products | Yield/% | Grade/% | Recovery/% | Separation Efficiency/% | ||
|---|---|---|---|---|---|---|---|
| MgO | CaO | MgO | CaO | ||||
| Without EGTA | Concentrate | 79.12 | 45.75 | 2.77 | 81.12 | 66.82 | 80.97 |
| Tailing | 20.88 | 40.35 | 5.21 | 18.88 | 33.18 | ||
| Feed | 100.00 | 44.62 | 3.28 | 100 | 100 | ||
| Added EGTA during grinding | Concentrate | 88.54 | 46.47 | 1.64 | 92.21 | 44.42 | 83.60 |
| Tailing | 11.46 | 30.33 | 15.91 | 7.79 | 55.58 | ||
| Feed | 100.00 | 44.62 | 3.28 | 100.00 | 100.00 | ||
| Minerals | Equations for Dissolution Reactions | Equilibrium Constant |
|---|---|---|
| Magnesite | 3.40 | |
| Dolomite | 19.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, C.; Fan, W.; Mao, Y.; Liu, W. An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism. Minerals 2025, 15, 289. https://doi.org/10.3390/min15030289
Wang B, Liu C, Fan W, Mao Y, Liu W. An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism. Minerals. 2025; 15(3):289. https://doi.org/10.3390/min15030289
Chicago/Turabian StyleWang, Benying, Changfeng Liu, Wenyu Fan, Yong Mao, and Wengang Liu. 2025. "An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism" Minerals 15, no. 3: 289. https://doi.org/10.3390/min15030289
APA StyleWang, B., Liu, C., Fan, W., Mao, Y., & Liu, W. (2025). An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism. Minerals, 15(3), 289. https://doi.org/10.3390/min15030289







