The Composition of Volatiles in Quartz and Pyrite from the Konduyak Gold Deposit (Yenisei Ridge, Russia)
Abstract
1. Introduction
2. The Geological Setting
3. Samples and Methods
3.1. Sampling and Preparation
3.2. Raman Spectroscopy Analysis
3.3. Gas Chromatography–Mass Spectrometry Analysis
4. Results
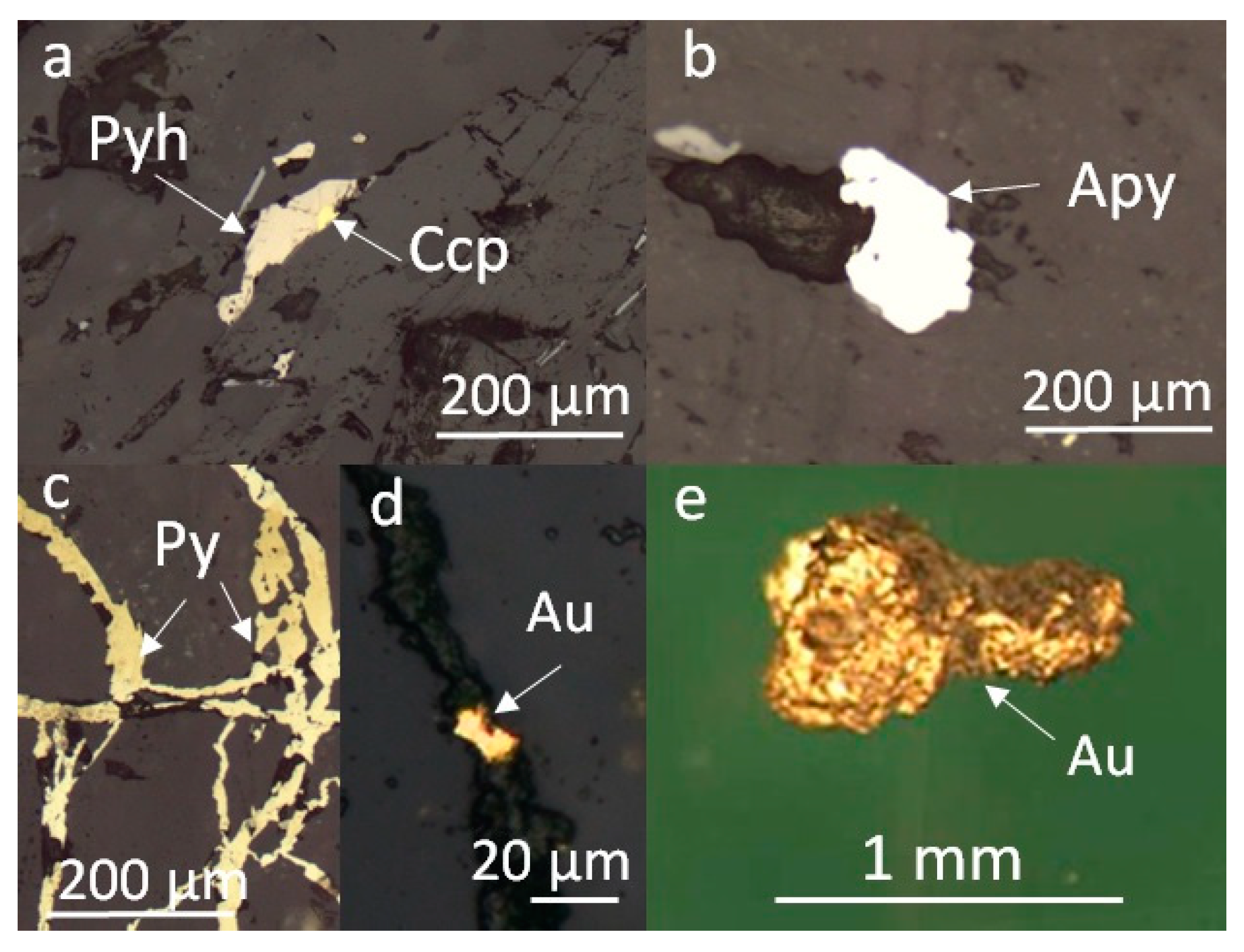
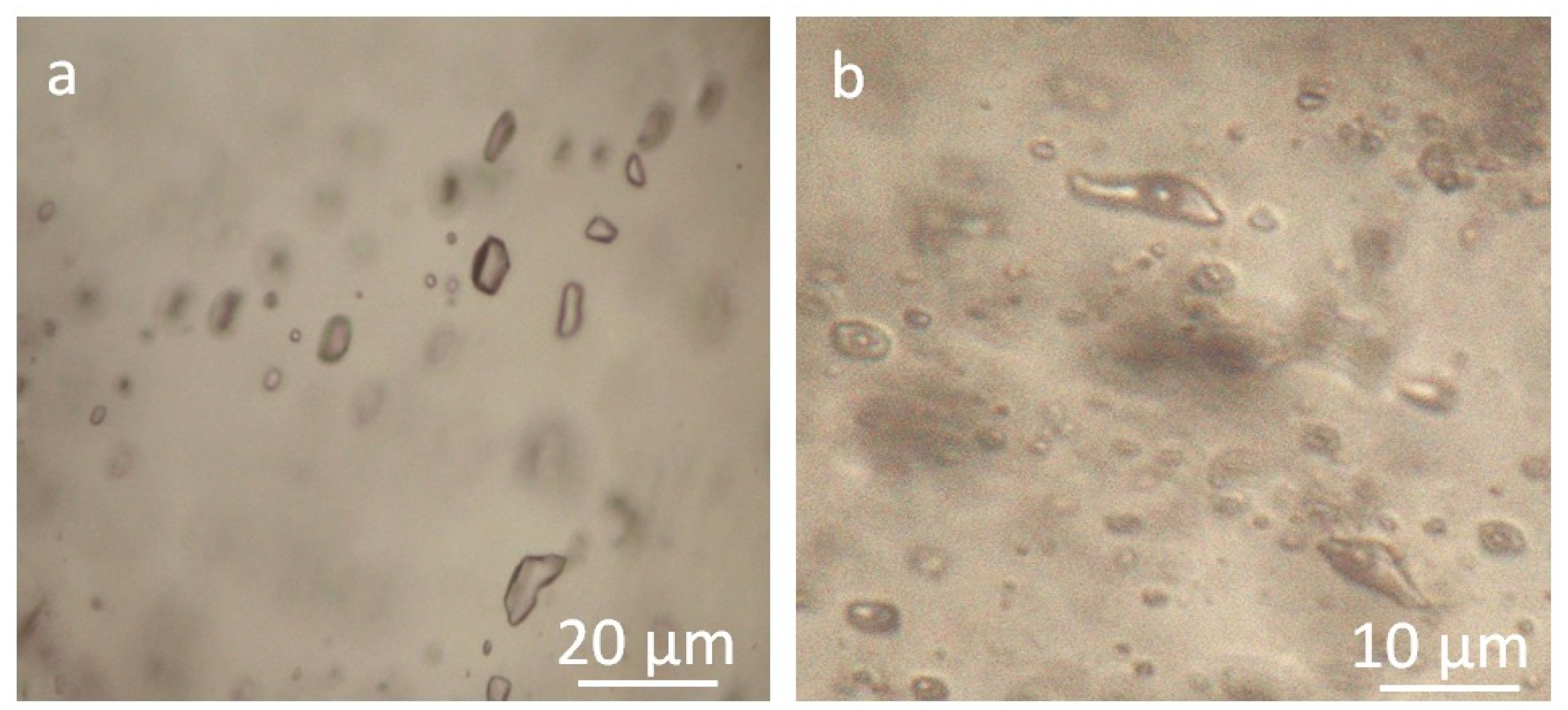
4.1. Fluid Inclusion Assemblages
4.2. Raman Spectroscopy Data
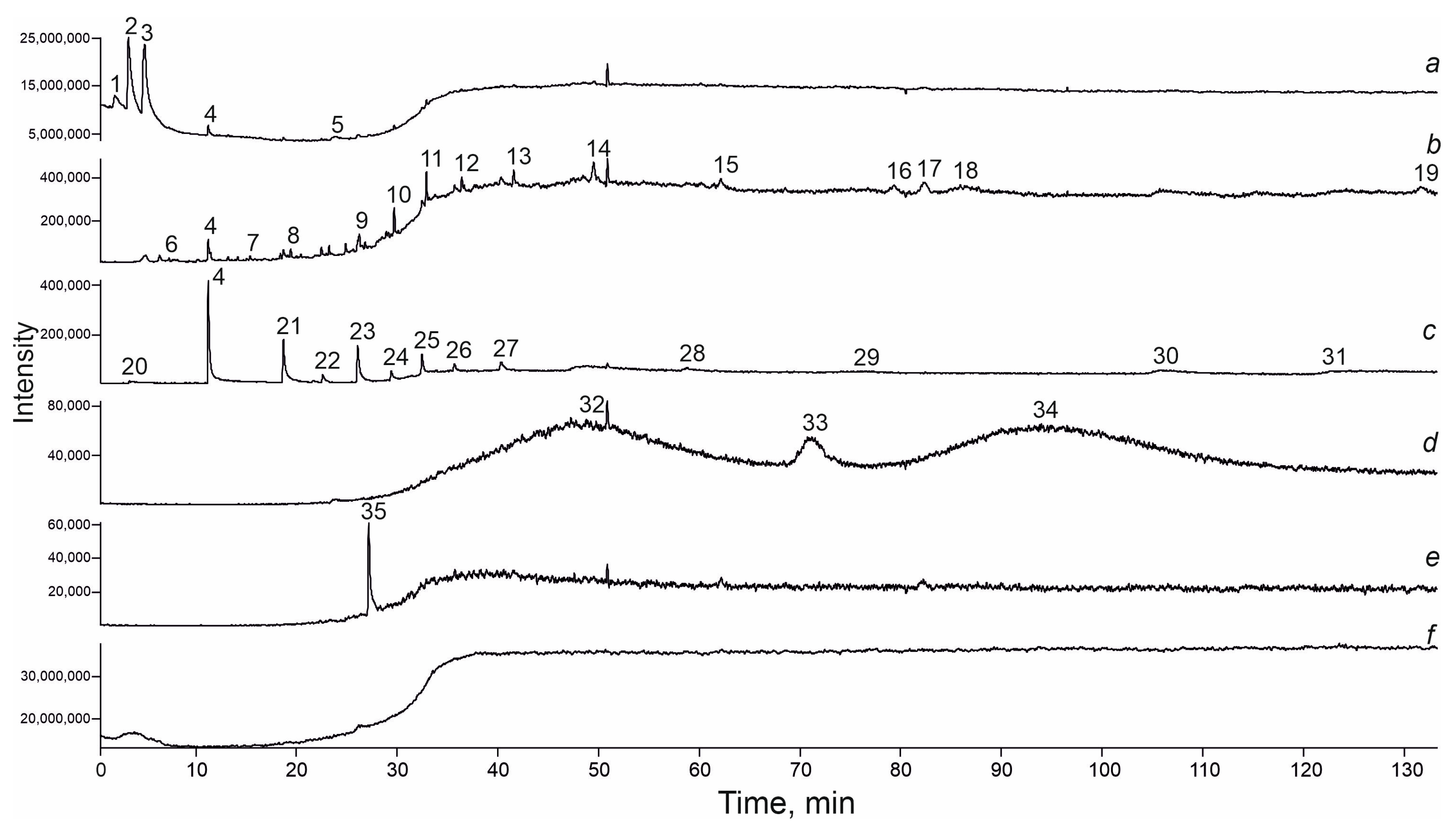
4.3. GC-MS Data
5. Discussion
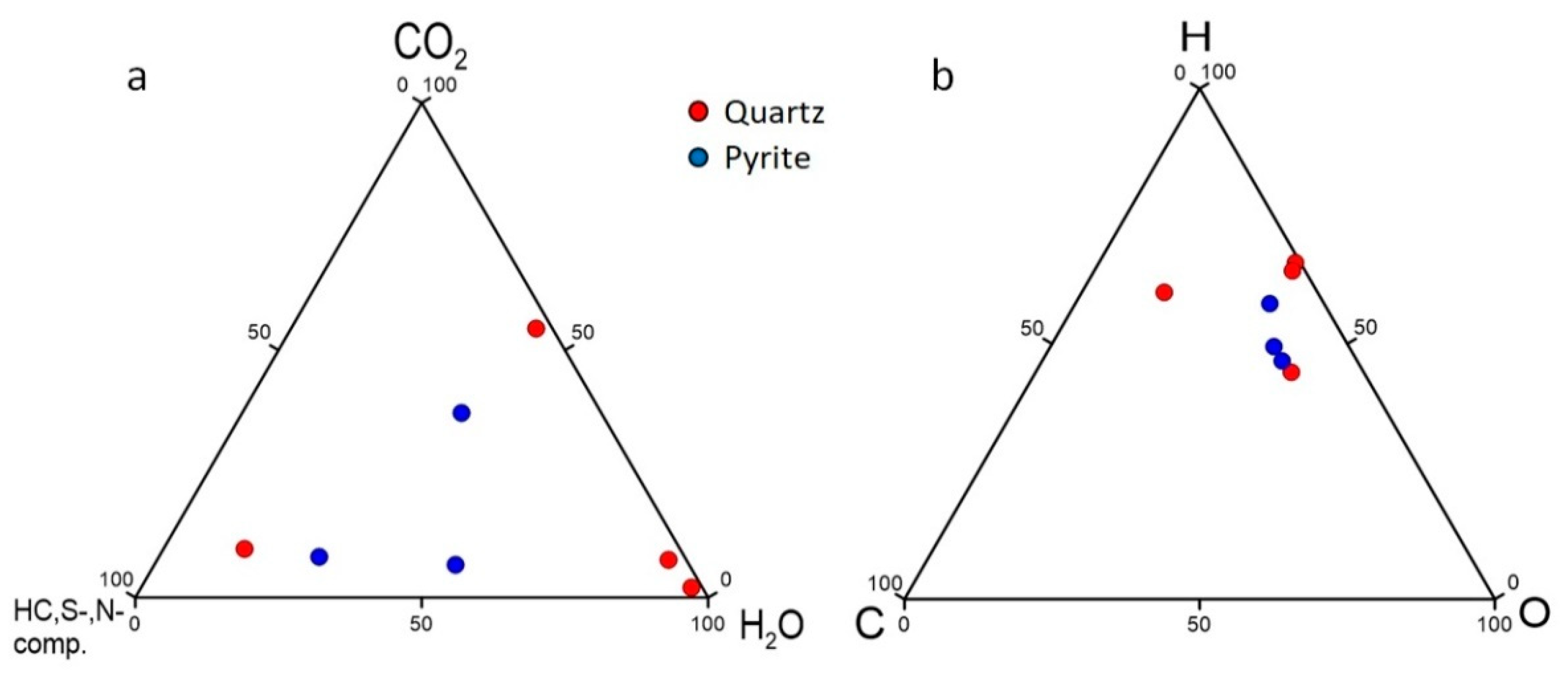
5.1. Fluid Composition
5.2. The Role of Various Volatiles in Ore Formation
5.3. Potential Fluid Origin
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ermakov, N.P.; Dolgov, Y.A. Thermobarogeochemistry; Nedra: Moscow, Russia, 1979; 271p. (In Russian) [Google Scholar]
- Roedder, E.; Bodnar, R.J. Fluid inclusion studies of hydrothermal deposits. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 657–698. [Google Scholar]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common vs. evolving fluid and metal sources through time. Lithos 2015, 223, 2–26. [Google Scholar] [CrossRef]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common problems and pitfalls in fluid inclusion study: A review and discussion. Minerals 2020, 11, 7. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Santosh, M. The conjunction of factors that lead to formation of giant gold provinces and deposits in non-arc settings. Geosci. Front. 2016, 7, 303–314. [Google Scholar] [CrossRef]
- Shaparenko, E.; Gibsher, N.; Tomilenko, A.; Sazonov, A.; Bul’bak, T.; Ryabukha, M.; Khomenko, M.; Silyanov, S.; Nekrasova, N.; Petrova, M. Ore-Bearing Fluids of the Blagodatnoye Gold Deposit (Yenisei Ridge, Russia): Results of Fluid Inclusion and Isotopic Analyses. Minerals 2021, 11, 1090. [Google Scholar] [CrossRef]
- Kryazhev, S.G.; Fridovsky, V.Y. The Fluid Regime of Orogenic Gold Deposit Formation in the Yana-Kolyma Belt. Russ. J. Pac. Geol. 2023, 17, 622–634. [Google Scholar] [CrossRef]
- Williams-Jones, A.; Migdisov, A.; Archibald, S.; Xiao, Z. Vapor-transport of ore metals. Geochem. Soc. Spec. Publ. 2002, 7, 279–305. [Google Scholar]
- Heinrich, C.A.; Candela, P.A. Fluids and Ore Formation in the Earth’s Crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 1–28. [Google Scholar] [CrossRef]
- Burke, E.A. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Pankrushina, E.A.; Krupenin, M.T.; Shchapova, Y.V.; Kobuzov, A.S.; Garaeva, A.A.; Votyakov, S.L. The Study of Fluid Inclusion Salinity in Minerals by Raman Spectroscopy Revisited. In Minerals: Structure, Properties, Methods of Investigation; Votyakov, S., Kiseleva, D., Grokhovsky, V., Shchapova, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 175–183. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Gibsher, N.A.; Dublaynsky, Y.V.; Dallai, L. Geochemical and isotopic properties of fluid from gold-bearing and barren quartz veins of the Sovetskoye deposit (Siberia, Russia). Econ. Geol. 2010, 105, 375–394. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Akinfiev, N.N.; Selektor, S.L. Gas mixing with aqueous solution in the ore-forming hydrothermal process: An example of gold. Geochem. Int. 2016, 54, 403–414. [Google Scholar] [CrossRef]
- Volkov, A.V.; Prokofiev, V.Y.; Aristov, V.V.; Sidorova, N.V. Conditions of Formation of the Pavlik Gold–Sulfide–Quartz Deposit (Northeast of Russia) According to a Study of Fluid Inclusions. Geol. Ore Depos. 2024, 66, 191–202. [Google Scholar] [CrossRef]
- Fridovsky, V.; Kryazhev, S.; Polufuntikova, L.; Kudrin, M.; Anisimova, G. Geology, fluid inclusions, mineral and (S-O) isotope chemistry of the Badran orogenic Au deposit, Yana-Kolyma belt, eastern Siberia: Implications for ore genesis. Front. Earth Sci. 2024, 12, 1340112. [Google Scholar] [CrossRef]
- Gaboury, D.; Keita, M.; Guha, J.; Lu, H.-Z. Mass spectrometric analysis of volatiles in fluid inclusions decrepitated by controlled heating under vacuum. Econ. Geol. 2008, 103, 439–443. [Google Scholar] [CrossRef]
- Gaboury, D. Volatile Composition of Fluid Inclusions in Gold-Bearing Quartz Veins Analyzed by Solid-Mass Spectrometry: Method and Contributions to the Orogenic Metallogenic Model and Exploration. In Ore Geology [Working Title]; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Gibsher, N.A.; Sazonov, A.M.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O.; Sil’yanov, S.A.; Nekrasova, N.A. Hydrocarbons in Fluid Inclusions from Native Gold, Pyrite, and Quartz of the Sovetskoe Deposit (Yenisei Ridge, Russia) According to Pyrolysis-Free Gas Chromatography-Mass Spectrometry Data. Russ. Geol. Geophys. 2020, 61, 1260–1282. [Google Scholar] [CrossRef]
- Han, Y.; Noah, M.; Lüders, V.; Körmös, S.; Schubert, F.; Poetz, S.; Horsfield, B.; Mangelsdorf, K. Fractionation of hydrocarbons and NSO-compounds during primary oil migration revealed by high resolution mass spectrometry: Insights from oil trapped in fluid inclusions. Int. J. Coal Geol. 2022, 254, 103974. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Bulk analysis of volatiles in fluid inclusions. In Fluid Inclusions: Analysis and Interpretation; Samson, I., Anderson, A., Marshall, D., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 2003; pp. 279–305. [Google Scholar] [CrossRef]
- Akande, W.G. A Review of Experimental Procedures of Gas Chromatography-Mass Spectrometry (GC-MS) and Possible Sources of Analytical Errors. Earth Sci. 2012, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Gibsher, N.A.; Tomilenko, A.A.; Bul’bak, T.A.; Ryabukha, M.A.; Khomenko, M.O.; Shaparenko, E.O.; Sazonov, A.V.; Sil’yanov, S.A.; Nekrasova, N.A. The Olimpiadinskoe gold deposit (Yenisei ridge): Temperature, pressure, composition of ore-forming fluids, 34S in sulfides, 3He/4He of fluids, Ar-Ar age, and duration of formation. Russ. Geol. Geophys. 2019, 60, 1043–1059. [Google Scholar] [CrossRef]
- Shaparenko, E.; Gibsher, N.; Khomenko, M.; Tomilenko, A.; Sazonov, A.; Bul’bak, T.; Silyanov, S.; Petrova, M.; Ryabukha, M. Parameters for the Formation of the Dobroe Gold Deposit (Yenisei Ridge, Russia): Evidence from Fluid Inclusions and S–C Isotopes. Minerals 2023, 13, 11. [Google Scholar] [CrossRef]
- Nozhkin, A.D.; Likhanov, I.I. Gold in the Precambrian rocks of the Yenisei ridge (East Siberia) and geological and geochemical prerequisites for the formation of gold mineralization in the central metallogenic belt of the region. Geospheric Res. 2023, 2, 49–70. [Google Scholar] [CrossRef]
- Kuzmichev, A.B.; Sklyarov, E.V. The Precambrian of Transangaria, Yenisei Ridge (Siberia): Neoproterozoic microcontinent, Grenville-age orogen, or reworked margin of the Siberian craton? J. Asian Earth Sci. 2016, 115, 419–441. [Google Scholar] [CrossRef]
- Bershtein, P.S. Structural factors determining the patterns of placement and nature of gold mineralization of the Yenisei Ridge. In Proceedings of the NIGRI-Gold Institute; OBTI: Moscow, Russia, 1951. (In Russian) [Google Scholar]
- Bernstein, P.S.; Petrovskaya, N.V. Sovetskoe Gold-Ore Deposit (Yenisei Ridge); Geology of the Major Gold-Ore Deposits of the USSR; Nauka: Moscow, Russia, 1954; Volume 6, 162p. (In Russian) [Google Scholar]
- Petrovskaya, N.V. Gold Mineralization of the Yenisei Ridge and Features of the Processes of Formation of Gold-Bearing Ores. Ph.D. Thesis, NIGRIZOLOTO, Moscow, Russia, 1954; 86p. (In Russian). [Google Scholar]
- Li, L.V.; Datsenko, V.M. The position of granitoid formations and the place of gold mineralization in the history of the development of the Yenisei Ridge. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 1970, 239, 60–65. (In Russian) [Google Scholar]
- Serdyuk, S.S.; Zonov, V.A. Predictive assessment of gold content of the Ayakhtinsky ore cluster (South Yenisei gold-bearing region). In Geology and Mineral Resources of Central Siberia; KNIIGiMS: Krasnoyarsk, Russia, 2003; pp. 96–103. (In Russian) [Google Scholar]
- Serdyuk, S.S. Gold-Bearing Provinces of Central Siberia: Geology, Minerageny and Development Prospects; KNIIGiMS: Krasnoyarsk, Russia, 2004; 480p. (In Russian) [Google Scholar]
- Serdyuk, S.S.; Komorovsky, Y.E.; Zverev, A.I.; Oyaber, V.K.; Vlasov, V.S.; Babushkin, V.E.; Kirillenko, V.A.; Zemlyansky, S. Models of Gold Deposits in Yenisei Siberia; Siberian Federal University: Krasnoyarsk, Russia, 2010; 584p. (In Russian) [Google Scholar]
- Pitulko, V.M.; Mkrtychyan, A.K.; Yurkevich, L.G. Theory and Practice of Intensive Technology of Geochemical Works in Forecasting and Prospecting for Gold Ore Deposits; Nestor-History: St. Petersburg, Russia, 2014; 424p. (In Russian) [Google Scholar]
- Maltzeva, G.D.; Nikanuk, T.S. Conditions of ore formation of some hydrothermal gold deposits. Proc. Sib. Dep. Sect. Earth Sci. Russ. Acad. Nat. Sciences. Geol. Explor. Dev. Miner. Depos. 2011, 2, 25–31. (In Russian) [Google Scholar]
- Maltzeva, G.D.; Nikanuk, T.S. Gold morphology of certain hydrothermal deposits of Siberia and Far East. Proc. Sib. Dep. Sect. Earth Sci. Russ. Acad. Nat. Sciences. Geol. Explor. Dev. Miner. Depos. 2010, 2, 21–26. (In Russian) [Google Scholar]
- Tomilenko, A.A.; Bul’bak, T.A.; Timina, T.Y.; Shaparenko, E.O.; Simonov, V.A.; Laptev, Y.V. Composition of volatiles of sulfide deposits and carbonate structures in submarine hydrothermal fields of the Mid-Atlantic Ridge. Mar. Geol. 2022, 444, 106713. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions; Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 12, 644p. [Google Scholar]
- Gibsher, N.A.; Tomilenko, A.A.; Sazonov, A.M.; Bul’bak, T.A.; Khomenko, M.O.; Ryabukha, M.A.; Shaparenko, E.O.; Sil’yanov, S.A.; Nekrasova, N.A. Ore–bearing fluids of the Eldorado gold deposit (Yenisei Ridge, Russia). Russ. Geol. Geophys. 2018, 59, 983–996. [Google Scholar] [CrossRef]
- Petrella, L.; Thébaud, N.; Fougerouse, D.; Evans, K.; Quadir, Z.; Laflamme, C. Colloidal gold transport: A key to high-grade gold mineralization. Miner. Depos. 2020, 55, 1247–1254. [Google Scholar] [CrossRef]
- Hastie, E.C.G.; Schindler, M.; Kontak, D.J.; Lafrance, B. Transport and coarsening of gold nanoparticles in an orogenic deposit by dissolution–reprecipitation and Ostwald ripening. Commun. Earth Environ. 2021, 2, 57. [Google Scholar] [CrossRef]
- Petrella, L.; Thébaud, N.; Fougerouse, D.; Tattitch, B.; Martin, L.; Turner, S.; Suvorova, A.; Gain, S. Nanoparticle suspensions from carbon-rich fluid make high-grade gold deposits. Nat. Commun. 2022, 13, 3795. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Pitcairn, I. Orogenic gold: Is a genetic association with magmatism realistic? Miner. Depos. 2023, 58, 5–35. [Google Scholar] [CrossRef]
- Prokofiev, V.Y.; Banks, D.A.; Lobanov, K.V.; Selektor, S.L.; Milichko, V.A.; Borovikov, A.A.; Chicherov, M.V. Transport of Au–Ag Nanoparticles in Dense Carbon Dioxide Fluid of the Middle Crust. Minerals 2024, 14, 1224. [Google Scholar] [CrossRef]
- Phillips, G.N.; Evans, K.A. Role of CO2 in the formation of gold deposits. Nature 2004, 429, 860–863. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Guo, X.; Xu, H.; Williams-Jones, A.E.; Sun, C.J.; Vasyukova, O.; Sugiyama, I.; Fuchs, S.; Pearce, K.; Roback, R. Hydrocarbons as ore fluids. Geochem. Perspect. Lett. 2017, 5, 47–52. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, X.; Xu, X.; Ling, S.; Xie, Z. Likely gold transportation via organic complexes in sediment-hosted orogenic gold deposits in southern Tibet: From the perspective of thermodynamic modelling. J. Asian Earth Sci. 2024, 264, 106068. [Google Scholar] [CrossRef]
- Greenwood, P.F.; Brocks, J.J.; Grice, K.; Schwark, L.; Jaraula, C.; Dick, J.M.; Evans, K.A. Organic geochemistry and mineralogy. I. Characterisation of organic matter associated with metal deposits. Ore Geol. Rev. 2013, 50, 1–27. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Weng, W.; Dong, C.; Yang, L.; Wang, X.; Deng, J. Co-precipitation of gold and base metal sulfides during fluid boiling triggered by fault-valve processes in orogenic gold deposits. Ore Geol. Rev. 2022, 149, 105090. [Google Scholar] [CrossRef]
- Lyakhov, Y.V.; Pavlun’, N.N. Some geological and geochemical features of gold concentration processes in metamorphic-hydrothermal and magmatic-hydrothermal mineral-forming systems. In Ore-Forming Processes: From Genetic Concepts to Forecasting and Discovery of New Ore Provinces and Deposits; IGEM RAS: Moscow, Russia, 2013; p. 144. (In Russian) [Google Scholar]
- Haghi, A.; Raissi, H.; Hashemzadeh, H.; Farzad, F. On the role of alkanethiol Au complex in the formation of gold deposits; an in-silico approach. Chem. Geol. 2022, 610, 121101. [Google Scholar] [CrossRef]
- Kryazhev, S.G.; Berkovsky, E.M. The Regime of the Olimpiada Gold–Sulfide Deposit Formation. Geol. Ore Depos. 2023, 65 (Suppl. 2), 221–231. [Google Scholar] [CrossRef]
- Gaboury, D. The Neglected Involvement of Organic Matter in Forming Large and Rich Hydrothermal Orogenic Gold Deposits. Geosciences 2021, 11, 344. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, X.; Yi, J.; Zhang, X.; Mo, R.; Zhou, F.; Wei, H.; Zeng, Q. Geology, geochemistry, and genesis of orogenic gold-antimony mineralization in the Himalayan Orogen, South Tibet, China. Ore Geol. Rev. 2014, 58, 68–90. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, X.; Hu, S.; Chen, H.; Li, D.; Fu, Y.; Xu, L.; Wu, Z.; Huang, F. Role of carbonaceous material in gold precipitation for orogenic gold deposits: A case study of the Bangbu gold deposit in southern Tibet, China. Ore Geol. Rev. 2022, 152, 105231. [Google Scholar] [CrossRef]
- Xia, Q.-X.; Zheng, Y.-F.; Zhou, L.-G. Dehydration and melting during continental collision: Constraints from element and isotope geochemistry of low-T/UHP granitic gneiss in the Dabie orogen. Chem. Geol. 2008, 247, 36–65. [Google Scholar] [CrossRef]
- Groppo, C.; Roflo, F.; Frezzotti, M.L. CO2 outgassing during collisional orogeny is facilitated by the generation of immiscible fluids. Commun. Earth Environ. 2022, 3, 13. [Google Scholar] [CrossRef]
- Stewart, E.M.; Penman, D.E. Enhanced metamorphic CO2 release on the Proterozoic Earth. Proc. Natl. Acad. Sci. USA 2024, 121, e2401961121. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, V.J.; Heinonen, J.S.; Märki, L.; Galvez, M.E.; Molnár, F. Sedimentary and metamorphic processes priming black shale for magmatic assimilation of sulfur: An example from the Virginia Formation, Minnesota, United States. Miner. Depos. 2024, 60, 303–319. [Google Scholar] [CrossRef]
- Coldebella, B.; LaFlamme, C.; Malta, I.S.; Guilmette, C.; Barré, G.; Beaudoin, G.; Martin, L.; Savard, D. The chemical and isotopic characterization of the pyrite to pyrrhotite desulfidation reaction across the metamorphic gradient of a metasedimentary basin. Geochim. Cosmochim. Acta, 2025; in press. [Google Scholar] [CrossRef]
- Hunt, J.M.; Philp, R.P.; Kvenvolden, K.A. Early developments in petroleum geochemistry. Org. Geochem. 2002, 33, 1025–1052. [Google Scholar] [CrossRef]
- Katz, B.J. Microbial processes and natural gas accumulations. Open Geol. J. 2011, 5, 75–83. [Google Scholar] [CrossRef]
- Mazurova, K.; Miyassarova, A.; Eliseev, O.; Stytsenko, V.; Glotov, A.; Stavitskaya, A. Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts 2023, 13, 1215. [Google Scholar] [CrossRef]
- Cho, Y.S.; Oh, I.-H.; Kim, J.H.; Kim, J.I.; Lee, K.-B.; Nah, I.W. Production of abiotic or biogenic hydrocarbons on rock particles in the presence of H2O and carbon compounds. Sci. Rep. 2025, 15, 2085. [Google Scholar] [CrossRef]
- Konopleva, I. Possible application of bicyclic sesquiterpanes to evaluate the thermal maturity of oils. A study of Kamchatka and Chukotka oils from Cenozoic deposits, Russia. Org. Geochem. 2024, 187, 104717. [Google Scholar] [CrossRef]




| Components | MW * | 504/120 Quartz | 510/88.7 Quartz | 510/88.7 Pyrite | 588/150 Quartz | 588/150 Pyrite | 597/145.3 Quartz | 597/145.3 Pyrite |
|---|---|---|---|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||||||
| Paraffins (alkanes) (CH4–C18H38) | 16–254 | 0.07 (20) | 0.73 (23) | 0.52 (15) | 0.13 (20) | 0.15 (17) | 3.57 (20) | 0.57 (19) |
| Olefins (alkenes) (C2H2–C17H34) | 26–238 | 0.11 (37) | 0.13 (37) | 0.45 (26) | 0.11 (35) | 0.21 (34) | 8.71 (42) | 0.60 (32) |
| Cyclic hydrocarbons | ||||||||
| Cycloalkanes, cycloalkenes, arenes, PAH (C4H6–C15H24) | 54–204 | 0.09 (47) | 0.13 (46) | 0.93 (29) | 0.08 (40) | 0.41 (29) | 3.39 (40) | 0.95 (29) |
| Oxygenated hydrocarbons | ||||||||
| Alcohols (CH4O–C8H10O3) | 32–214 | 0.09 (12) | 0.06 (10) | 0.61 (7) | 0.14 (18) | 3.90 (14) | 2.69 (13) | 0.69 (8) |
| Ethers and esters (C4H8O–C14H26O2) | 72–234 | 0.58 (28) | 0.24 (23) | 4.17 (17) | 0.43 (32) | 0.44 (21) | 31.16 (29) | 6.30 (19) |
| Aldehydes (CH2O–C18H36O) | 30–268 | 0.22 (27) | 0.38 (28) | 2.61 (26) | 0.36 (28) | 1.23 (26) | 8.74 (26) | 1.90 (28) |
| Ketones (C3H6O–C16H32O) | 58–240 | 0.12 (23) | 0.23 (23) | 1.59 (21) | 0.19 (25) | 0.99 (22) | 3.75 (23) | 3.61 (24) |
| Carboxylic acids (CH2O2–C14H28O2) | 46–228 | 0.30 (14) | 0.52 (15) | 2.90 (16) | 0.57 (27) | 1.33 (16) | 6.45 (13) | 2.12 (16) |
| Heterocyclic compounds | ||||||||
| Dioxanes, furans (C4H4O–C13H22O) | 68–194 | 0.01 (25) | 0.02 (27) | 0.19 (23) | 0.02 (27) | 0.07 (21) | 0.47 (28) | 0.17 (30) |
| Nitrogenated compounds | ||||||||
| N2, ammonia, nitriles (N2–C10H21NO) | 17–185 | 0.22 (20) | 0.51 (22) | 4.28 (24) | 0.57 (31) | 0.85 (21) | 5.62 (24) | 0.91 (27) |
| Sulfur compounds | ||||||||
| H2S, SO2, CS2, COS, thiophenes (H2S–C12H20S) | 34–196 | 0.11 (25) | 0.12 (25) | 45.40 (25) | 0.11 (25) | 14.80 (20) | 1.30 (15) | 22.92 (25) |
| Inorganic compounds | ||||||||
| CO2 | 44 | 2.07 | 7.54 | 8.31 | 54.44 | 37.31 | 9.82 | 6.62 |
| H2O | 18 | 96.02 | 89.38 | 28.00 | 42.83 | 38.32 | 14.25 | 52.64 |
| Ar | 40 | <0.01 | <0.01 | 0.04 | <0.01 | 0.01 | 0.09 | 0.02 |
| Number of components | 281 | 282 | 232 | 302 | 244 | 276 | 260 | |
| CO2/(CO2 + H2O) | 0.02 | 0.08 | 0.23 | 0.56 | 0.49 | 0.41 | 0.11 | |
| H/(H + O) | 0.67 | 0.66 | 0.57 | 0.51 | 0.53 | 0.81 | 0.64 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaparenko, E.; Bul’bak, T.; Tomilenko, A.; Sazonov, A.; Petrova, M.; Silyanov, S.; Gibsher, N.; Khomenko, M. The Composition of Volatiles in Quartz and Pyrite from the Konduyak Gold Deposit (Yenisei Ridge, Russia). Minerals 2025, 15, 278. https://doi.org/10.3390/min15030278
Shaparenko E, Bul’bak T, Tomilenko A, Sazonov A, Petrova M, Silyanov S, Gibsher N, Khomenko M. The Composition of Volatiles in Quartz and Pyrite from the Konduyak Gold Deposit (Yenisei Ridge, Russia). Minerals. 2025; 15(3):278. https://doi.org/10.3390/min15030278
Chicago/Turabian StyleShaparenko, Elena, Taras Bul’bak, Anatoly Tomilenko, Anatoly Sazonov, Marina Petrova, Sergey Silyanov, Nadezhda Gibsher, and Margarita Khomenko. 2025. "The Composition of Volatiles in Quartz and Pyrite from the Konduyak Gold Deposit (Yenisei Ridge, Russia)" Minerals 15, no. 3: 278. https://doi.org/10.3390/min15030278
APA StyleShaparenko, E., Bul’bak, T., Tomilenko, A., Sazonov, A., Petrova, M., Silyanov, S., Gibsher, N., & Khomenko, M. (2025). The Composition of Volatiles in Quartz and Pyrite from the Konduyak Gold Deposit (Yenisei Ridge, Russia). Minerals, 15(3), 278. https://doi.org/10.3390/min15030278






