Abstract
Glycerin-based fluids are proposed as a promising alternative to inhibited fluids in the drilling of highly-reactive formations. However, even with the use of these fluids, it is still possible to observe the occurrence of problems related to the balling of drill bits and drill pipes, such as the agglomeration and accretion of cuttings. This study aims to analyze how the interaction between expandable minerals from reactive formations and glycerin-based drilling fluids affects the stability of oil wells, focusing on the occurrence and extent of the accretion phenomenon. For this purpose, bentonite pellets were characterized regarding their mineralogical composition and plastic behavior. In addition, accretion tests were performed in order to evaluate the interaction between bentonite pellets and glycerin-based drilling fluids containing different types of inhibitors. The results revealed that the pellets were predominantly composed of interstratified illite–smectite (IS) clay minerals and presented highly plastic properties with a high degree of expansion. Furthermore, it was found that the accretion percentages were significant for all the fluids studied, at higher than 58%. Therefore, it was found that using glycerin in drilling fluids did not stabilize expandable minerals in reactive formations, even with different expansion inhibitors, which were ineffective in reducing the rock expansibility.
1. Introduction
The instability of oil wells, resulting from the presence of geological formations that are difficult to drill, represents one of the main adversities faced during drilling operations. The behavior of these formations is influenced by a complex combination of mechanical, chemical, thermal, and electrical processes, which are interconnected and can-not be understood in isolation [1].
Clay minerals found in sedimentary rocks can be categorized into three main groups: smectite, kaolinite, and illite. Each of these groups can lead to various issues in wells, including fine migration, increase in pore tortuosity, formation swelling, and decreased penetration rate [2].
Bentonite is a rock that generally presents a high concentration of expandable clay minerals, normally belonging to the smectite group. These smectite clays are characterized by the easy hydration capacity of cations and interlayer surfaces, giving them a remarkable expansion property. Due to this property, they are often called expansive clays [3].
The understanding of the agglomeration and accretion of cuttings remains limited due to the lack of field data, diverse drilling practices, and variations in the lithology of the formations. According to Reid and co-authors [4], theories based on the plasticity of clays have been proposed to explain this phenomenon. These theories are based on the idea that the reduced penetration of water into the rock fragments generated by the action of the bit decreases the hydration rate, resulting in a prolonged plasticity of the fragments. It is believed that this extended plasticity state of the clays allows the cuttings to be molded into the bottom hole assembly (BHA) components and incorporated close to the well wall.
In drilling operations where the formation presents a high degree of reactivity, it is necessary to use inhibited fluids. Most of the studies related to the prevention of agglomeration and accretion focus on the performance of additives in the formulation in which the continuous phase is composed of water. However, due to the thermal and oxidative stability characteristics and low interaction with clays during swelling tests, glycerin-based fluids have emerged as a promising alternative to inhibited fluids, potentially improving wellbore stability. Pormerlau [5] highlights several advantages of glycerin-based drilling fluids. However, even with the use of these fluids, it is still possible to observe the occurrence of problems related to the balling of drill bits and drill pipes, that can result in increased non-productive time during the drilling process.
In this context, the present study aims to analyze how the interaction between expandable minerals existing in reactive formations and glycerin-based drilling fluids affects the stability of oil wells, focusing on the occurrence and extension of the accretion phenomenon.
2. Materials and Methods
2.1. Materials
To carry out this work, a sample of pellets was used, which corresponded to the compacted form of bentonite. This sample was donated by Bentonite União Nordeste—BUN (Boa Vista, Brazil).
The glycerin-based drilling fluids were formulated with additives in the amounts specified in Table 1. All additives were supplied by Leopoldo Américo Miguez de Mello Research and Development Center—CENPES/PDDP/FCE from PETROBRAS (Rio de Janeiro, Brazil).

Table 1.
Composition of the glycerin-based drilling fluid.
Three different types of clay swelling inhibitors, named I1, I2 and I3, were incorporated into the fluid composition. In total, seven glycerin-based fluid formulations were prepared, for which there was variation in the use of inhibitors, as described in Table 2.

Table 2.
Identification used for the drilling fluids studied.
2.2. Methods
2.2.1. Pellets’ Characterization
X-ray diffraction (XRD) was used to identify the mineralogical constituents of the samples using a Rigaku (Miniflex 600) X-ray diffractometer. The quantitative determination of the mineral composition was carried out by the Rietveld method, which performs a complete simulation of the diffractogram, refining the geometric parameters of the phases present, and taking into account the crystallographic aspects. Quantification is determined by comparing a calculated diffraction pattern with the observed pattern, performing a point-by-point analysis, and adjusting the differences found using the least squares method.
In addition, measurements of the liquid limit and plasticity limits of the bentonite pellets were carried out to evaluate their plastic behavior in the different liquid media used in the formulations of the drilling fluids under study: water, glycerin, and water with glycerin. The liquid limit was obtained in the Casagrande apparatus, according to the procedures of NBR 6459, while the plastic limit was obtained according to NBR 7180 [6,7]. The plasticity index of the pellets was calculated using Equation (1).
where PI = plasticity index; LL = liquid limit, and PL = plastic limit.
PI = (LL − PL) × 100%,
2.2.2. Drilling Fluids’ Preparation
The drilling fluids used in this study were prepared in aliquots of 350 mL, using a Silverson L5M-A equipped with an emulsifying screen rotor/stator assembly, at a rotational speed of 8720 rpm.
The additives were added in the order presented in Table 1, with 5 min of homogenization for each one. The final homogenization time was 10 min.
2.2.3. Interaction Between Expandable Minerals and Drilling Fluids: Accretion Test
The accretion test was performed following the methodology established by Cliffe and Young [8]. For that, solid steel bars were positioned in the center of the test cell, which was filled with drilling fluid until reaching 50% of its volume. Subsequently, samples containing 50 g of pellets with a narrow particle size distribution (3.35–4.75 mm) were added to the cell. Gentle agitation was then applied with a spatula to ensure an even distribution of the solids in the fluid uniformly. Finally, the cell was completely filled with the fluid, sealed, and inverted 3 to 4 times to minimize the chances of pellets sticking to the bottom of the cell.
The test was conducted using a Rollen Over model 705S from Fann. During the procedure, the cells were rotated at predetermined time intervals (10, 20, 30, 40, and 50 min), maintaining room temperature. Figure 1 illustrates the test procedure.

Figure 1.
Equipment and methodology for accretion tests using bentonite pellets and inhibited drilling fluids.
After the rolling step, the bar was removed from the test cell, and the added solids were properly removed, finishing with a quick wash of the bar using a pipette containing water, in order to minimize any sample loss. Then, the solids were subjected to drying at 110 °C until they reached a constant mass. The accretion rate was then calculated according to Equation (2).
where A = accretion; W2 = weight of solids, in grams, after drying at 110 °C, and M1 = initial water content of the pellets sample, not exposed to the fluid.
3. Results and Discussion
3.1. Pellets’ Characterization
3.1.1. Mineralogical Composition Section
Figure 2a displays the X-ray diffractogram of the bentonite pellet (total powder). To enhance the interpretation of the diffractogram, particularly for identifying clay minerals, the clay fraction underwent additional analyses, which included heating at 500 °C for 2 h and treatment with ethylene glycol, as shown in Figure 2b. These techniques were employed based on the principle that ethylene glycol treatment expands the basal spacing of smectites to 17Å, while heating at 500 °C dehydrates kaolinites and reduces the basal spacing of smectites. Therefore, the mineral identifications presented in Figure 2a incorporate the results from these additional tests.
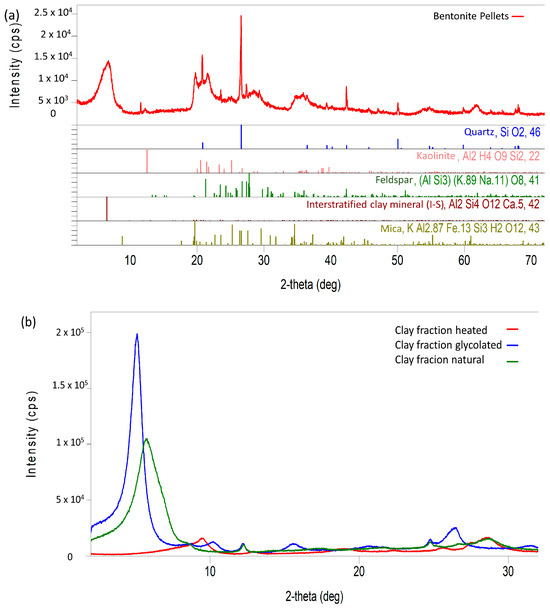
Figure 2.
X-ray diffractogram of the pellet sample (total powder) (a) and XRD pattern of the heated, glycolated, and natural clay fraction of the pellet sample (b).
In general, it is possible to observe, through the X-ray diffractogram, peaks indicative of the presence of interstratified clay minerals illite–smectite (IS), evidencing the reactive nature of the bentonite pellet sample with a high degree of expansion. Additionally, peaks associated with the presence of mica, feldspar, and quartz can be identified, as well as moderate peaks that denote the existence of clay minerals corresponding to kaolinite.
Figure 3 presents the qualitative and quantitative results of the X-ray diffraction analysis.
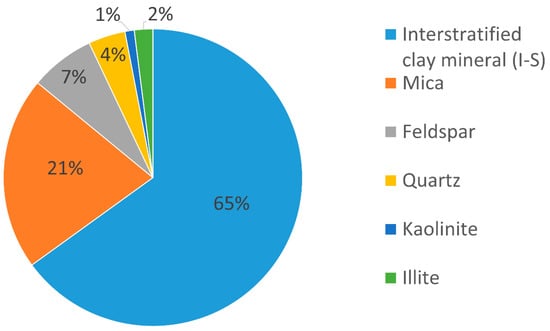
Figure 3.
Qualitative and quantitative results of X-ray diffraction of the pellets (quantitative evaluation parameter: 0 ≤ 1% = possible presence; 1% ≤ 5% = traces; >5% = presence).
In the quantitative analysis, it was found that the sample was predominantly composed of an expandable clay mineral of illite interstratified with smectite, whose content reached 65%, evidencing the result presented in the diffractogram shown in Figure 1. The interstratified clay minerals are partial products resulting from a process of synthesis of the phyllosilicate layers, in which there is no predominance of a specific crystal, therefore presenting characteristics common to two or more clay minerals. According to Wilson and co-authors [9], the presence of smectite is directly related to the reactivity of the formation. Thus, due to the presence of smectite in the synthesized layer, the bentonite pellets have exhibited a notable sensitivity to the presence of water.
Based on the data presented in Figure 2, it is also possible to verify that significant proportions of accessory minerals, such as quartz and feldspar, were observed. The mineral quartz, whose characteristics include a non-plastic nature and a brittle structure, presented a composition of 4%. For feldspar, a concentration of 7% was observed. According to Macedo et al. (2008), feldspathic accessory minerals have a direct influence on the plastic properties of the formations, since, together with quartz, they generally play a role in reducing plasticity [10]. However, Apolônio and co-authors [11] carried out a study on the reactivity of shales in the Northeast region of Brazil and found that the predominance of Na-smectite and Na-feldspar confers greater plasticity to the clay formations.
3.1.2. Atterberg Limits
The moisture contents of the pellet sample, in relation to each liquid medium that makes up the bases of the drilling fluids (water, glycerin, and water with glycerin), was investigated, as well as the number of drops (revolutions) used to determine the liquid limit. Those parameters were correlated and visually represented in the graphs shown in Figure 4. The highlighted points were obtained through a linearization of the other points, thus corresponding to the liquid limit for each medium.
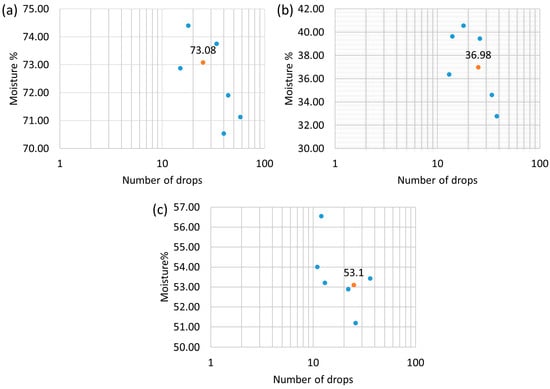
Figure 4.
Liquid limit for the pellet sample with (a) water, (b) glycerin, and (c) water with glycerin.
The results in Figure 3 reveal that, in the presence of water, the pellets obtained a liquid limit of 73.08%, which indicates very high plasticity, according to the classification proposed by Bell [12] (Table 3). With glycerin, the pellets presented a liquid limit of 36.98%, and its plasticity was classified as intermediate. In the presence of water with glycerin, the pellet sample reached a liquid limit of 53.1%, which corresponded to the classification of high plasticity. Therefore, the pellets demonstrated liquid limit values that classify them as plastic materials in the three bases used in the drilling fluids.

Table 3.
Plasticity classification according to the liquid limit [12].
The liquid limit is associated with the saturation point of the hydration state of the formations, at which the rock begins to behave like a liquid and loses the properties that would allow it to agglomerate in the BHA. Although the rock loses its fluid characteristics when it reaches the liquid limit, it still can be easily molded, being in a state known as plastic [13]. Therefore, a high liquid limit favors the permanence of the formation in the plastic state, increasing its plasticity.
The plasticity index, determined by the difference between the liquid limit and the plasticity limit values, was used as a reference for classifying the sample in relation to its degree of plasticity, following the classification described in Table 4 [14].

Table 4.
Classification according to the degree of plasticity.
Table 5 presents the results of the plasticity index, together with the classification of the pellets according to their degree of plasticity.

Table 5.
Plasticity index of pellets and their classifications according to the degree of plasticity.
The results shown in Table 5 reveal that the pellets presented a plasticity index of 36% in the presence of water, which means that they are highly plastic in this medium. In glycerin, the plasticity index was 5%, resulting in a classification of weakly plastic. In water with glycerin, the plasticity index reached 22%, characterizing them as highly plastic in this medium. According to van Oort et al. (2000), the reactivity of the formations is more pronounced in rocks that present plastic characteristics [15].
Thus, the data obtained from X-ray diffraction (Figure 1 and Figure 2) and the Atterberg limits reveal that the pellets analyzed were predominantly composed of the clay mineral smectite, being classified as highly plastic when exposed to water and water with glycerin. In this sense, the pellets can be considered as representative of reactive formations for drilling fluids composed of these bases. These results corroborate the studies of Hajjaji et al. (2010), who observed that formations in which smectite is the dominant clay mineral have a greater predisposition to reactivity in terms of their plastic characteristics [16]. Additionally, Fontoura (2002) observed that the rock–fluid system is directly influenced by the physicochemical properties of the clay–water system, since clays have a series of characteristics that make them reactive when in contact with water-based drilling fluids, such as high specific area and the expansibility of these minerals [17].
3.2. Interaction Between Expendable Minerals and Drilling Fluids
Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11 show the appearance of the pellets accreted to the steel bar, for each fluid studied, for times of 10, 20, 30, 40, and 50 min.
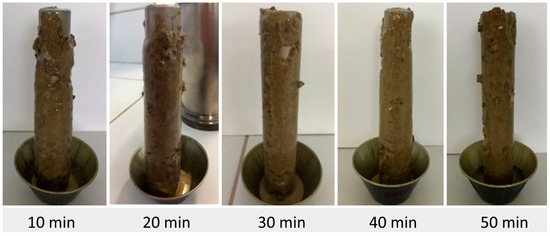
Figure 5.
Pellet accretion using fluid F1 (without inhibitor).
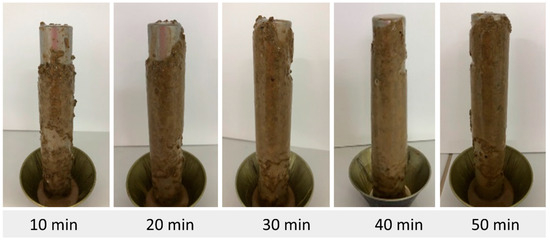
Figure 6.
Accretion of pellets using fluid F2 (added with I1).
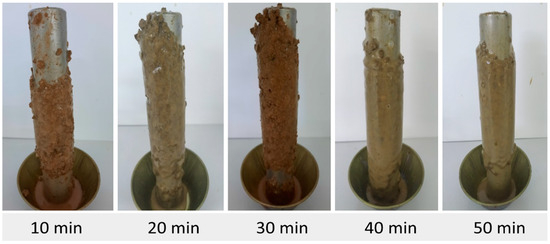
Figure 7.
Accretion of pellets using fluid F3 (added with I1, I2, and I3).
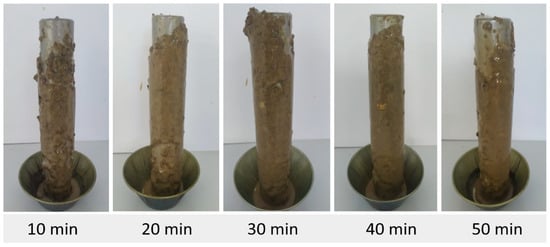
Figure 8.
Accretion of pellets using fluid F4 (added with I1 and I2).
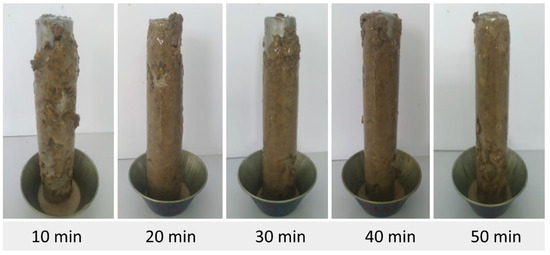
Figure 9.
Accretion of pellets using fluid F5 (with I2).
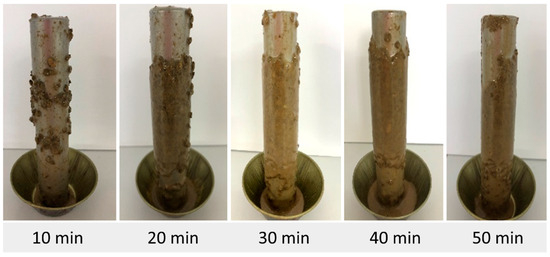
Figure 10.
Accretion of pellets using fluid F6 (with I1 and I3).

Figure 11.
Accretion of pellets using fluid F7 (with I3).
It is possible to see in Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 that the pellet accretion process was more evident after a period of 10 min, with the highest accretion percentages occurring between 20 and 50 min. The pellets used underwent a deformation process before aggregating and formed a more uniform layer, thus resulting in the accretion phenomenon.
Table 6 presents the pellets accretion values as a function of time, after performing the test with the analyzed drilling fluids.

Table 6.
Pellet accretion percentages.
According to the results, the accretion of the pellets using fluid F5 was the most significant, reaching 83.39% after 20 min of exposure. Fluid F7 showed a maximum accretion of 81.70% after 10 min of exposure. The maximum accretion values for fluids F1 and F3 were 79.92% and 71.27%, respectively. Fluid F4 showed a maximum accretion of 66.89% after 30 min. Samples F2 and F6 showed the lowest accretions, with maximum values of 61.93% and 58.91%, respectively, after 40 min of exposure.
After the pellets reached the maximum accretion value, they formed a consistent layer around the bars, which reduced the amount of water moving into the cuttings. This slowed down the rate at which the formation absorbed water, keeping it in a plastic state. Over time, the accretion values decreased for all fluids after reaching their peak, indicating a prolonged period of hydration for the pellets. This caused the pellets to exceed their liquid limits, resulting in reduced accretion percentages for all drilling fluids.
Studies conducted by Pivovarski and co-authors [18] revealed that as the time of exposure of bentonite to water increased, a reduction in the measured swelling rate was observed. This means that, in the initial stages in which the pellets absorbed a small amount of water, a significant volumetric increase occurred per unit of time. However, over time, this volumetric increase decreased exponentially.
The relationship between pellet behavior in the accretion test and time can be understood through the clay plasticity and Atterberg limits. Clay plasticity arises from the interaction of electrical charges of expandable mineral particle and water, which also lubricates these particles. The plastic state occurs when sufficient water allows mineral surfaces to slide and align under tangential stress. Higher clay content in a formation enhances its plastic properties, increasing the likelihood of the accretion phenomenon.
The interaction between glycerin and pellets is largely due to significant electrostatic interactions between the hydroxyl groups of glycerin and the ionic and polar sites in clay materials [19,20]. Glycerol molecules preferentially adsorb to the active sites of clay, displacing water molecules [21,22]. This adsorption may influence the aggregation and dispersion states of the system. In addition to well-known hydrogen bond interactions with water, relevant electrostatic interactions occur between the hydroxyl groups of glycerin and clay’s ionic and polar sites.
The addition of glycerin—a tri-alcohol—into water-based mud increases the interlayer spacing of aluminosilicate, similar to ethylene glycol. The hydroxyl groups in glycerin interact strongly with clay lamellae and water molecules, with each molecule capable of forming hydrogen bonds with up to eight water molecules. This “glycerin–water” complex penetrates the interlayer space, resulting in expansion and enhanced hydration due to its volume.
The accretion test conducted with fluid F1, without inhibitors, demonstrated the pronounced reactivity of the pellets in relation to their plastic characteristics, as expected due to the high levels of expandable minerals present. In addition, it was observed that the glycerin-based drilling fluid was not able to stabilize the reactive formation, resulting in the accretion phenomenon.
From the comparative analysis between fluids F1 and F2, it was found that the inclusion of inhibitor I1 showed a partial effectiveness, promoting a decrease in the accretion levels. However, despite the action of this inhibitor, accretion phenomena still occurred in considerable proportions.
Considering the analysis of fluids F6 and F7, it can be concluded that the use of inhibitor I3 in the fluid composition was not effective, resulting in the second highest accretion rate (81.70%) among all tests performed. However, when adding inhibitor I1 to the composition, a 22.79% reduction in the accretion percentage was observed. This same behavior was noted in fluids F4 and F5. Using inhibitor I2, the highest accretion value for the study was obtained (83.39%), evidencing the inefficiency of this inhibitor in preventing expansion. However, when adding inhibitor I1 to the fluid, there was a 16.5% reduction in the accretion rate.
For fluid F3, which contained the three inhibitors, an accretion percentage of approximately 71% was observed at 20 and 30 min. These results reinforce the findings obtained when using other fluids, indicating that inhibitors I1, I2, and I3, either individually or together, were not able to effectively inhibit the swelling of the expandable minerals present in the pellets, thus allowing the occurrence of the accretion phenomenon.
4. Conclusions
With the aim of analyzing how the interaction between expandable minerals existing in reactive formations and glycerin-based drilling fluids affected the stability of oil wells, focusing on the occurrence and extension of the accretion phenomenon, the following was concluded:
- The characterization of bentonite pellets revealed a predominance of clay minerals of the smectite group in their composition, which provides a strong indication of their reactivity with a high degree of expansion;
- According to the liquid limit, the pellets were categorized as presenting very high plasticity in the presence of water, high plasticity in the presence of water with glycerin, and intermediate plasticity in the presence of glycerin;
- Based on the degree of plasticity, it was found that the pellets were classified as highly plastic both in contact with water and water with glycerin;
- The accretion percentages were considerable for all drilling fluids at different times of the accretion test, indicating that the addition of glycerin to the fluids was not efficient in stabilizing the reactive formations;
- The use of inhibitors I1, I2, and I3, alone or together, did not demonstrate effectiveness in reducing the accretion phenomenon;
- For future research, it is recommended to investigate the compositions of fluids with varying glycerin concentrations, as well as to evaluate additional commercial inhibitors beyond those already analyzed.
Based on the above, it can be seen that the drilling fluid with added glycerin was not effective in preventing the accretion phenomenon. When used to drill highly plastic formations, where smectite clay minerals are predominant, such as reactive shales, there will be a greater probability of instability problems, such as the agglomeration and accretion of cuttings. Furthermore, the addition of clay swelling inhibitors to the fluids has not been shown to have a positive effect in preventing or reducing the accretion process.
Author Contributions
Conceptualization, R.C.A.M.N. and L.V.A.; methodology, A.P.O.S. and M.C.S.L.; software, A.P.O.S.; validation, L.V.A. and W.R.P.C.; formal analysis, R.C.A.M.N. and M.C.S.L.; investigation, A.P.O.S.; resources, L.V.A. and R.C.A.M.N.; data curation, M.C.S.L. and W.R.P.C.; writing—original draft preparation, A.P.O.S.; writing—review and editing, L.V.A., W.R.P.C., J.M.P.Q.D. and A.G.B.L.; visualization, A.P.O.S., J.M.P.Q.D. and A.G.B.L.; supervision, R.C.A.M.N.; project administration, L.V.A.; funding acquisition, R.C.A.M.N., L.V.A., J.M.P.Q.D. and A.G.B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors A. P. O. Sousa, M. C. S. Lima, W. R. P. Costa, R. C. A. M. Nascimento and L. V. Amorim would like to acknowledge Bentonit União Nordeste (Boa Vista, Brazil) and Petrobras (Brazil), grant numbers 0050.0120134.21.9 and 0050.0126178.23.9, for the support for this research. J.M.P.Q. Delgado is grateful to the Research Unit CONSTRUCT funded by national funds through the FCT/MCTES (PIDDAC) and FCT through the individual Scientific Employment Stimulus 2020.00828.CEECIND/CP1590/CT0004, with DOI: 10.54499/2020.00828.CEECIND/CP1590/CT0004.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frydman, M.; Fontoura, S.A.B. Modeling Aspects of Wellbore Stability in Shales. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 25–28 March 2001. [Google Scholar]
- Grim, R.E. Clay Mineralogy, 2nd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Fuenkajorn, K.; Daemen, J.J.K. Drilling-Induced Fractures in Borehole Walls. J. Pet. Technol. 1992, 44, 210–216. [Google Scholar] [CrossRef]
- Reid, P.I.; Minton, R.C.; Twynam, A. Field Evaluation of a Novel Inhibitive Water-Based Drilling Fluid for Tertiary Shales. In Proceedings of the European Petroleum Conference, Cannes, France, 16–18 November 1992. [Google Scholar]
- Pormerlau, D.G. Glycerol Based Drilling Fluids. U.S. Patent No. 2009/0143254 A1, 4 June 2009. [Google Scholar]
- Associação Brasileira de Normas Técnicas (ABNT). NBR 6459: Solo-Determinação do Limite de Liquidez; ABNT: Rio de Janeiro, Brasil, 2016. [Google Scholar]
- Associação Brasileira de Normas Técnicas (ABNT). NBR 7180: Solo-Determinação do Limite de Plasticidade; ABNT: Rio de Janeiro, Brasil, 2016. [Google Scholar]
- Cliffe, S.; Young, S. Agglomeration and Accretion of Drill Cuttings in Water-based Fluids. In Proceedings of the AADE Fluids Conference and Exhibition, Houston, TX, USA, 8–9 April 2008. [Google Scholar]
- Wilson, M.J.; Wilson, L.; Patey, I. The influence of individual clay minerals on formation damage of reservoir sandstones: A critical review with some new insights. Clay Min. 2014, 49, 147–164. [Google Scholar] [CrossRef]
- Macedo, R.S.; Menezes, R.R.; Neves, G.A.; Ferreira, H.C. Study of clays used in red ceramic. Cerâmica 2008, 54, 411–417. [Google Scholar] [CrossRef]
- Apolônio, T.G.; Amorim, L.V.; Leal, C.A. Correlação entre a Composição Química e Mineralógica e as Características Plásticas de Folhelhos do Nordeste do Brasil. Rev. Eletrônica Mater. Process. 2020, 15, 102–109. [Google Scholar]
- Bell, F.G. Engineering Geology, 2nd ed.; Elsevier: London, UK, 2007. [Google Scholar]
- Couto, B.O.C.; Pereira, E.L.; Gomes, R.C.; Ferreira, L.D. Correlação entre os Valores do Limite de Liquidez Obtidos pelos Métodos de Casagrande e Cone de Queda Livre para Diferentes Materiais. In Proceedings of the XVIII Brazilian Congress of Soil Mechanics and Geotechnical Engineering–COBRAMSEG, Belo Horizonte, Brazil, 19–22 October 2016. [Google Scholar]
- Caputo, H.M. Mecânica dos Solos e Suas Aplicações, 6th ed.; LTC: Rio de Janeiro, Brazil, 1988. [Google Scholar]
- Van Oort, E.; Bland, R.; Pessier, R. Drilling more Stable Wells Faster and Cheaper with PDC Bits and Water Based Muds. In Proceedings of the IADC/SPE Drilling Conference, New Orleans, LA, USA, 23–25 February 2000. [Google Scholar]
- Hajjaji, W.; Moussi, B.; Hachani, M.; Mounir, M.; Galindo, A.; Rocha, F.; Labrincha, J.; Jamoussi, F. The Potential Use of Tithonian–Barremian Detrital Deposits from Central Tunisia as Raw Materials for Ceramic Tiles and Pigments. Appl. Clay Sci. 2010, 48, 552–560. [Google Scholar] [CrossRef]
- Fontoura, S.A.B. Lade and Modified Lade 3D Rock Strength Criteria. Rock Mech. Rock Eng. 2012, 45, 1001–1006. [Google Scholar] [CrossRef]
- Pivovarski, R.G.; Daroz, V.; Lugarini, A.; Franco, A.T.; Loureiro, S.; Waldmann, A.T.A.; Martins, A.L. I Testes de Hidratação Estáticos e Dinâmicos de Pellets de Bentonita para P&A. In Proceedings of the Encontro Nacional de Construção de Poços de Petróleo e Gás, Serra Negra, Brazil, 9–22 August 2019. [Google Scholar]
- Campos, L.F.A.; Macedo, R.S.; Kiyohara, P.K.; Ferreira, H.C. Plasticity characteristics of clays for use in structural clay products. Cerâmica 1999, 45, 140–145. [Google Scholar] [CrossRef]
- Steiger, R.P. Nontoxic, Nonchloride, Water-Base, Inhibitive Fluid to Stabilize Water Sensitive Shale 1993. US Patent No. 5198415, 30 March 1993. [Google Scholar]
- Bradley, W.F. Molecular Associations between Montmorillonite and Some Polyfunctional Organic Liquids. J. Am. Chem. Soc. 1945, 67, 975–981. [Google Scholar] [CrossRef]
- Bloys, B.; Davis, N.; Smolen, B.; Bailey, L.; Houwen, O.; Reid, P.; Sherwood, J.; Fraser, L.; Hodder, M. Designing and managing drilling fluid. Oilfield Rev. 1994, 6, 33–43. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).