Modified Analyses of Trace Elements in Glass Beads by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Application for Particular Silicate Rocks
Abstract
1. Introduction
2. Non-Magmatic Melt Rocks: General Characteristics
3. Experimental
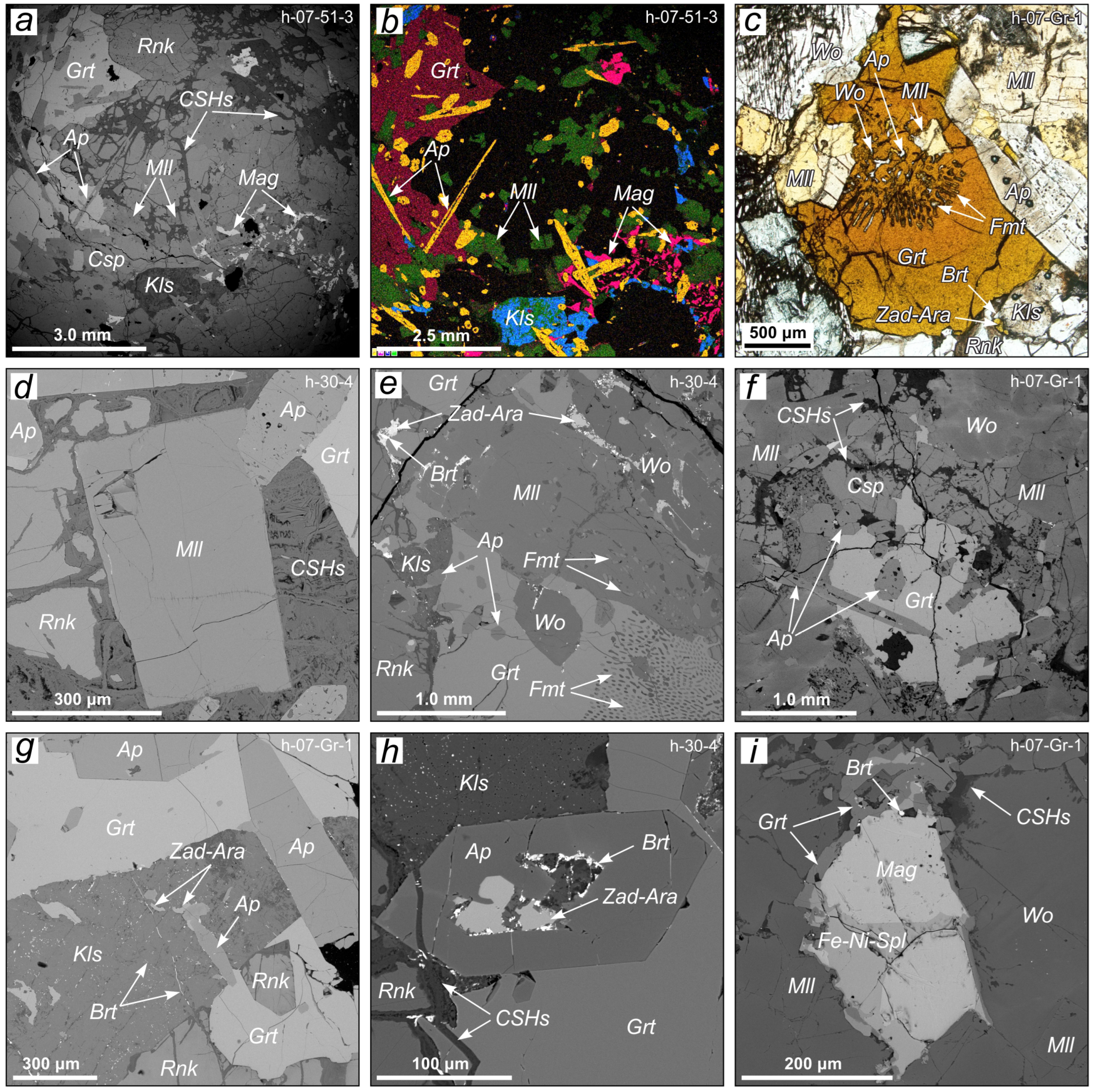
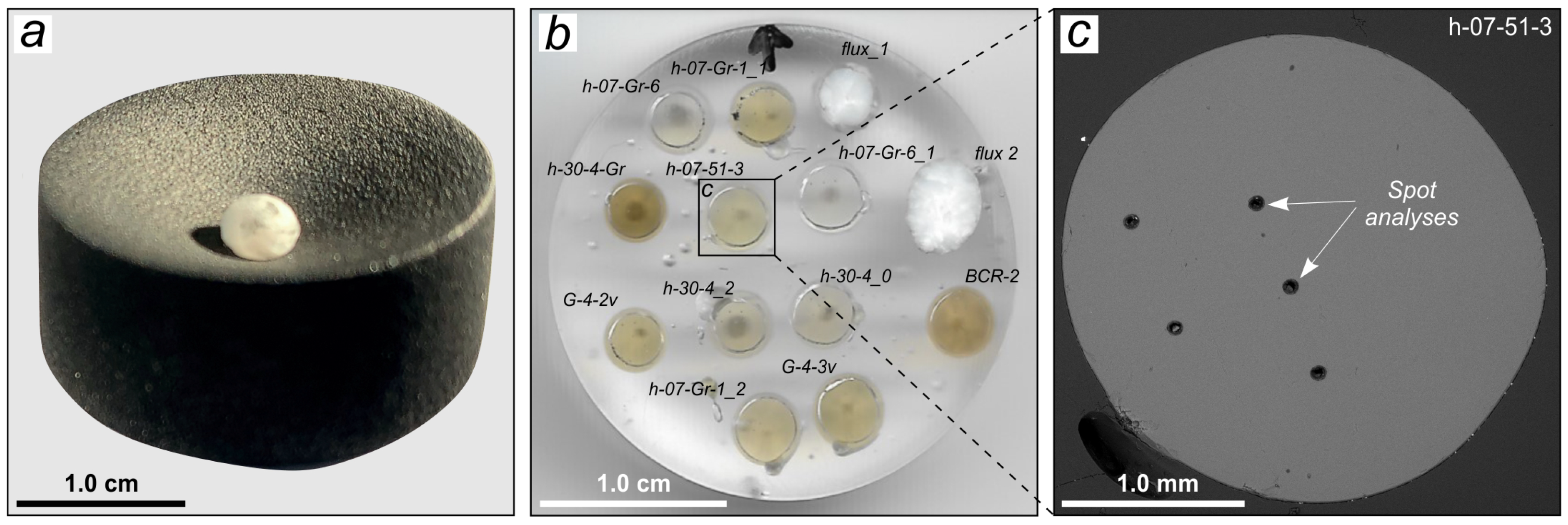
3.1. Samples and Sample Preparation
3.2. Methods
4. Results and Discussion
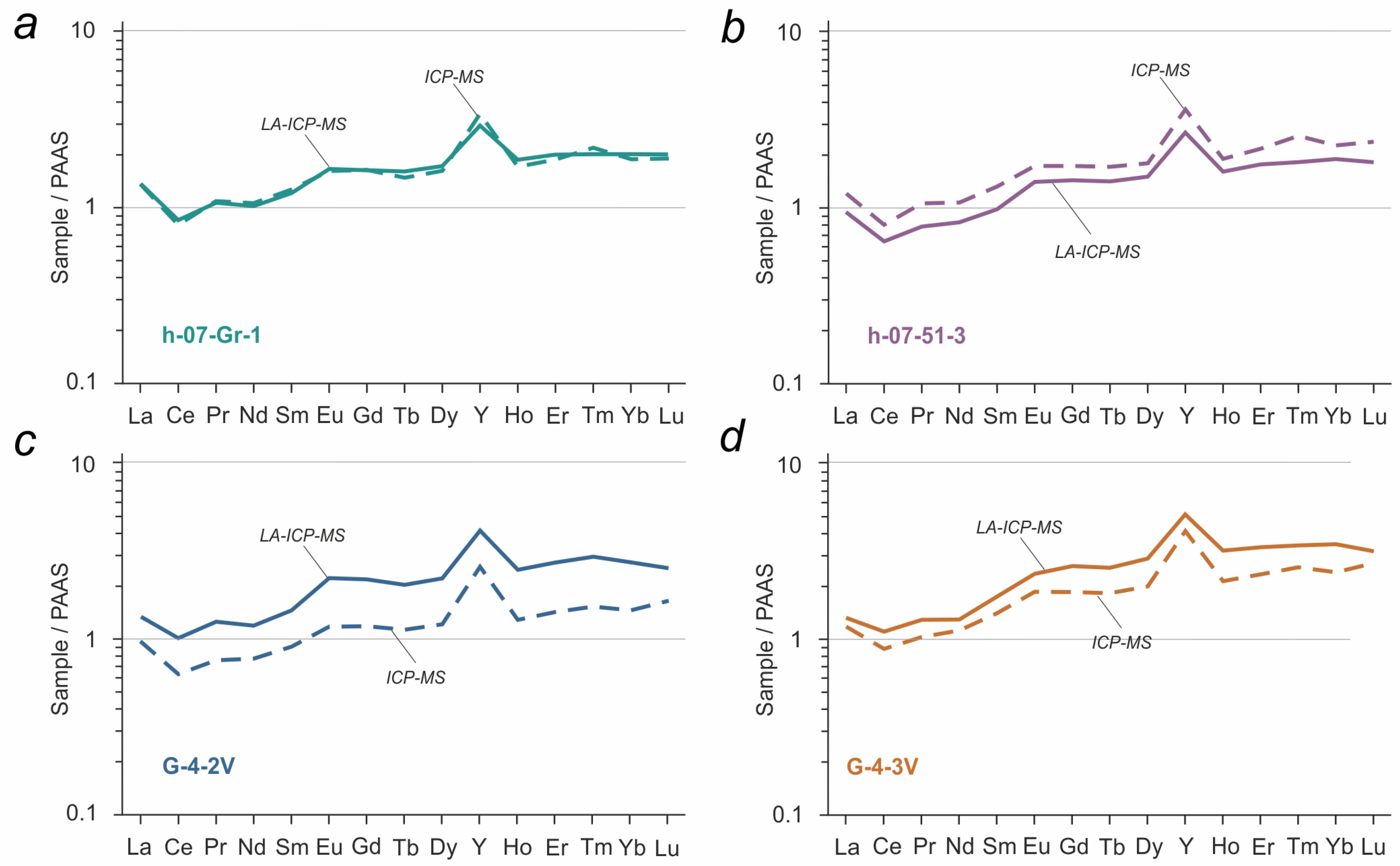
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sylvester, P.J. Trace element analysis of fused whole rock glasses by laser ablation ICPMS. In Laser Ablation ICP-MS in the Earth Sciences, Principles and Applications; Mineralogical Association of Canada: Gatineau, QC, USA, 2001; pp. 147–162. [Google Scholar]
- Yu, Z.; Norman, M.D.; Robinson, P. Major and trace element analysis of silicate rocks by XRF and laser ablation ICP-MS using lithium borate fused glasses: Matrix effects, instrument response and results for international reference materials. Geostand. Newsl. 2003, 27, 67–89. [Google Scholar] [CrossRef]
- Eggins, S.M. Laser ablation ICP-MS analysis of geological materials prepared as lithium borate glasses. Geostand. Newsl. 2003, 27, 147–162. [Google Scholar] [CrossRef]
- Orihashi, Y.; Hirata, T. Rapid quantitative analysis of Y and REE abundances in XRF glass bead for selected GSJ reference rock standards using Nd-YAG 266 nm UV laser ablation ICP-MS. Geochem. J. 2003, 37, 401–412. [Google Scholar] [CrossRef]
- Kil, Y.; Jung, H. LA-ICP-MS analysis of natural rock samples using XRF glass beads. Geosci. J. 2015, 19, 45–52. [Google Scholar] [CrossRef]
- Park, C.S.; Shin, H.S.; Oh, H.; Cho, H.; Cheong, A.C.-s. Trace element analysis of whole-rock glass beads of geological reference materials by Nd: YAG UV 213 nm LA-ICP-MS. J. Anal. Sci. Technol. 2016, 7, 1–8. [Google Scholar] [CrossRef][Green Version]
- Fedorowich, J.; Richards, J.; Jain, J.; Kerrich, R.; Fan, J. A rapid method for REE and trace-element analysis using laser sampling ICP-MS on direct fusion whole-rock glasses. Chem. Geol. 1993, 106, 229–249. [Google Scholar] [CrossRef]
- Nakayama, K.; Ichikawa, S.; Nakamura, T. Glass bead with minimized amount (11 mg) of sample for X-ray fluorescence determination of archaeological ceramics. X-Ray Spectrom. 2012, 41, 16–21. [Google Scholar] [CrossRef]
- Ichikawa, S.; Onuma, H.; Nakamura, T. Development of undersized (12.5 mm diameter) low-dilution glass beads for X-ray fluorescence determination of 34 components in 200 mg of igneous rock for applications with geochemical and archeological silicic samples. X-Ray Spectrom. 2016, 45, 34–47. [Google Scholar] [CrossRef]
- Gross, S. The Mineralogy of the Haturim Formation, Israel; Geological Survey of Israel: Jerusalem, Israel, 1977.
- Sharygin, V.; Vapnik, Y.; Sokol, E.; Kamenetsky, V.; Shagam, R. Melt inclusions in minerals of schorlomite-rich veins of the Hatrurim Basin, Israel: Composition and homogenization temperatures. In Proceedings of the ACROFI I Program with Abstracts, Nanjing, China, 26–28 May 2006; pp. 189–192. [Google Scholar]
- Sokol, E.; Novikov, I.; Zateeva, S.; Sharygin, V.; Vapnik, Y. Pyrometamorphic rocks of the spurrite-merwinite facies as indicators of hydrocarbon discharge zones (the Hatrurim Formation, Israel). Dokl. Earth Sci. 2008, 420, 608. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kozmenko, O.A.; Khoury, H.N.; Kokh, S.N.; Novikova, S.A.; Nefedov, A.A.; Sokol, I.A.; Zaikin, P. Calcareous sediments of the Muwaqqar Chalk Marl Formation, Jordan: Mineralogical and geochemical evidences for Zn and Cd enrichment. Gondwana Res. 2017, 46, 204–226. [Google Scholar] [CrossRef]
- Krzątała, A.; Krüger, B.; Galuskina, I.; Vapnik, Y.; Galuskin, E. Walstromite, BaCa2(Si3O9), from rankinite paralava within gehlenite hornfels of the Hatrurim Basin, Negev Desert, Israel. Minerals 2020, 10, 407. [Google Scholar] [CrossRef]
- Sokol, E.; Novikov, I.; Zateeva, S.; Vapnik, Y.; Shagam, R.; Kozmenko, O. Combustion metamorphism in the Nabi Musa dome: New implications for a mud volcanic origin of the Mottled Zone, Dead Sea area. Basin Res. 2010, 22, 414–438. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Sokol, E.V.; Kokh, S.N. Natural pseudowollastonite: Crystal structure, associated minerals, and geological context. Lithos 2012, 134, 75–90. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Almogi-Labin, A.; Bein, A.; Sass, E. Late Cretaceous upwelling system along the southern Tethys margin (Israel): Interrelationship between productivity, bottom water environments, and organic matter preservation. Paleoceanography 1993, 8, 671–690. [Google Scholar] [CrossRef]
- Fleurance, S.; Cuney, M.; Malartre, F.; Reyx, J. Origin of the extreme polymetallic enrichment (Cd, Cr, Mo, Ni, U, V, Zn) of the Late Cretaceous–Early Tertiary Belqa Group, central Jordan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 369, 201–219. [Google Scholar] [CrossRef]
- Abed, A.M.; Sadaqah, R.M. Enrichment of uranium in the uppermost Al-Hisa Phosphorite Formation, Eshidiyya basin, southern Jordan. J. Afr. Earth Sci. 2013, 77, 31–40. [Google Scholar] [CrossRef]
- Ali Hussein, M.; Alqudah, M.; Blessenohl, M.; Podlaha, O.G.; Mutterlose, J. Depositional environment of Late Cretaceous to Eocene organic-rich marls from Jordan. GeoArabia 2015, 20, 191–210. [Google Scholar] [CrossRef]
- Goren, O. Distribution and mineralogical residence of trace elements in the Israeli carbonate oil shales. Fuel 2015, 143, 118–130. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Sharygin, V.V.; Danilovsky, V.A.; Seryotkin, Y.V.; Liferovich, R.; Deviatiiarova, A.S.; Nigmatulina, E.N.; Karmanov, N.S. Mineralogical diversity of Ca2SiO4-bearing combustion metamorphic rocks in the Hatrurim Basin: Implications for storage and partitioning of elements in oil shale clinkering. Minerals 2019, 9, 465. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Schwager, B.; Stoll, B.; Wilson, S.A.; Haug, G.H.; Andreae, M.O.; Enzweiler, J. Reference values following ISO guidelines for frequently requested rock reference materials. Geostand. Geoanal. Res. 2015, 40, 333–350. [Google Scholar] [CrossRef]
- Amosova, A.A.; Panteeva, S.V.; Chubarov, V.M.; Finkelshtein, A.L. Determination of major elements by wavelength-dispersive X-ray fluorescence spectrometry and trace elements by inductively coupled plasma mass spectrometry in igneous rocks from the same fused sample (110 mg). Spectrochim. Acta Part B At. Spectrosc. 2016, 122, 62–68. [Google Scholar] [CrossRef]
- Nikolaeva, I.; Palesskii, S.; Kozmenko, O.; Anoshin, G. Analysis of geologic reference materials for REE and HFSE by inductively coupled plasma-mass spectrometry (ICP-MS). Geochem. Int. 2008, 46, 1016–1022. [Google Scholar] [CrossRef]
- Weis, P.; Beck, H.P.; Günther, D. Characterizing ablation and aerosol generation during elemental fractionation on absorption modified lithium tetraborate glasses using LA-ICP-MS. Anal. Bioanal. Chem. 2005, 381, 212–224. [Google Scholar] [CrossRef]
- Jenner, F.E.; Arevalo Jr, R.D. Major and trace element analysis of natural and experimental igneous systems using LA–ICP–MS. Elements 2016, 12, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Yang, Y.; Hu, Z. Calibration and correction of LA-ICP-MS and LA-MC-ICP-MS analyses for element contents and isotopic ratios. Solid Earth Sci. 2016, 1, 5–27. [Google Scholar] [CrossRef]
- Deviatiiarova, A.; Karputin, I.; Ragozin, A.; Sokol, E. Trace-element fractionation in minerals of high-calcium combustion metamorphic rocks: Case study of garnet-rich paralavas of the Hatrurim formation. In Proceedings of the XIII Russian Youth Scientific and Practical School, Moscow, Russia, 12–13 April 2024; pp. 67–71. [Google Scholar]
- Esbensen, K.H. Materials properties: Heterogeneity and appropriate sampling modes. J. AOAC Int. 2015, 98, 269–274. [Google Scholar] [CrossRef]
- Grant, C.; Pelton, P. Role of Homogeneity in Powder Sampling; ASTM International: West Conshohocken, PA, USA, 1973. [Google Scholar]
- Stanley, C.R. On the special application of ThompsonHowarth error analysis to geochemical variables exhibiting a nugget effect. Geochem. Explor. Environ. Anal. 2006, 6, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Hoboken, NJ, USA, 1985. [Google Scholar]


| Sample | h-07-Gr-1 | h-07-51-3 | G-4-2V | G-4-3V |
|---|---|---|---|---|
| SiO2 | 35.18 | 33.95 | 33.14 | 36.09 |
| TiO2 | 1.66 | 1.41 | 1.50 | 2.03 |
| Al2O3 | 7.63 | 8.26 | 9.76 | 7.28 |
| Fe2O3 | 8.82 | 7.61 | 8.15 | 8.54 |
| MnO | 0.13 | 0.12 | 0.11 | 0.11 |
| MgO | 0.54 | 0.60 | 0.71 | 0.54 |
| CaO | 40.76 | 41.41 | 39.88 | 40.57 |
| Na2O | 0.40 | 0.35 | 0.44 | 0.34 |
| K2O | 1.12 | 0.41 | 0.63 | 1.25 |
| P2O5 | 1.81 | 1.78 | 1.59 | 1.87 |
| SO3 | 0.09 | 0.18 | 0.38 | 0.12 |
| LOI | 2.43 | 4.50 | 4.99 | 2.22 |
| Total | 100.57 | 100.58 | 101.28 | 100.96 |
| CO2 | 0.89 | 1.42 | 1.42 | 0.62 |
| H2O | 1.54 | 3.08 | 3.57 | 1.60 |
| F | 0.42 | 0.48 | 0.47 | 0.23 |
| Sample | NIST SRM 612 | BCR-2 | AGV-2 | Flux * | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | [24] | U [24] |
Mean, n = 6 | SD |
RSD, % | U | [25] | U [25] |
Mean, n = 10 | SD |
RSD, % | U | [25] | U [25] |
Mean, n = 5 | SD |
RSD, % | U |
Mean, n = 3 |
| Al | 10,741 | 212 | 9645 | 814 | 8.44 | 854 | 71,327 | 635 | 81,403 | 4810 | 5.91 | 3438 | 90,111 | 635 | 87,076 | 4412 | 5.07 | 5485 | 5.54 |
| Sc | 39.9 | 2.50 | 39.2 | 1.10 | 2.81 | 1.16 | 33.5 | 0.40 | 33.6 | 1.28 | 3.81 | 0.91 | 13.0 | 0.31 | 13.2 | 0.94 | 7.12 | 1.16 | 0.17 |
| V | 38.8 | 1.20 | 37.2 | 2.52 | 6.77 | 2.64 | 418 | 4.50 | 391 | 25.81 | 6.60 | 18.45 | 119 | 1.20 | 113 | 6.35 | 5.62 | 7.89 | 0.90 |
| Cr | 36.4 | 1.50 | 35.3 | 3.35 | 9.50 | 3.52 | 15.9 | 0.38 | 15.5 | 1.91 | 12.32 | 1.37 | 16.2 | 0.72 | 15.4 | 1.46 | 9.48 | 1.82 | 4.79 |
| Mn | 38.7 | 0.90 | 36.1 | 1.69 | 4.68 | 1.77 | 1523 | 23.23 | 1554 | 118.7 | 7.64 | 84.83 | 778 | 20.14 | 629 | 43.09 | 6.85 | 53.58 | 0.53 |
| Co | 35.3 | 1.00 | 33.9 | 1.62 | 4.77 | 1.70 | 37.3 | 0.37 | 33.9 | 1.54 | 4.54 | 1.10 | 15.5 | 0.50 | 13.2 | 0.64 | 4.85 | 0.79 | 0.06 |
| Ni | 38.8 | 0.20 | 38.8 | 3.04 | 7.85 | 3.19 | 12.6 | 0.30 | 14.3 | 1.03 | 7.20 | 0.74 | 18.9 | 0.41 | 17.5 | 0.75 | 4.29 | 0.94 | 0.14 |
| Cu | 37.8 | 1.50 | 36.8 | 3.19 | 8.67 | 3.34 | 19.7 | 0.72 | 27.3 | 4.94 | 18.10 | 3.53 | 51.5 | 0.65 | 42.1 | 5.96 | 14.16 | 7.40 | 0.05 |
| Zn | 39.1 | 1.70 | 39.7 | 2.46 | 6.18 | 2.58 | 130 | 1.80 | 127 | 9.84 | 7.75 | 7.03 | 86.7 | 1.20 | 66.3 | 2.56 | 3.86 | 3.18 | 1.59 |
| Ga | 36.9 | 1.50 | 35.9 | 2.31 | 6.42 | 2.42 | 22.1 | 0.19 | 30.6 | 1.85 | 6.05 | 1.33 | 20.4 | 0.17 | 32.4 | 2.00 | 6.17 | 2.49 | 0.01 |
| Rb | 31.4 | 0.40 | 31.7 | 2.19 | 6.90 | 2.29 | 46.0 | 0.56 | 40.9 | 2.28 | 5.57 | 1.63 | 67.8 | 0.66 | 52.1 | 2.02 | 3.88 | 2.51 | 0.04 |
| Sr | 78.4 | 0.20 | 73.0 | 6.91 | 9.46 | 7.25 | 337 | 6.70 | 362 | 16.18 | 4.47 | 11.56 | 660 | 5.70 | 573 | 32.76 | 5.72 | 40.73 | 1.05 |
| Y | 38.3 | 1.40 | 29.6 | 2.22 | 7.52 | 2.33 | 36.1 | 0.37 | 34.8 | 2.02 | 5.80 | 1.44 | 19.1 | 0.84 | 14.5 | 0.93 | 6.41 | 1.16 | 0.03 |
| Zr | 37.9 | 1.20 | 37.6 | 2.48 | 6.59 | 2.60 | 187 | 1.50 | 180 | 11.25 | 6.25 | 8.04 | 232 | 2.30 | 188 | 14.30 | 7.61 | 17.78 | 0.38 |
| Nb | 38.9 | 2.10 | 38.1 | 2.39 | 6.27 | 2.51 | 12.4 | 0.20 | 12.6 | 0.94 | 7.46 | 0.67 | 14.1 | 0.22 | 12.3 | 1.37 | 11.14 | 1.17 | 0.03 |
| Ba | 39.3 | 0.90 | 38.3 | 2.17 | 5.66 | 2.28 | 684 | 4.70 | 684 | 31.66 | 4.63 | 22.63 | 1134 | 8.00 | 991 | 50.50 | 5.10 | 62.78 | 2.44 |
| La | 36.0 | 0.70 | 36.9 | 2.27 | 6.15 | 2.38 | 25.1 | 0.16 | 25.2 | 0.88 | 3.49 | 0.63 | 38.2 | 0.38 | 32.8 | 1.33 | 4.05 | 1.65 | 0.03 |
| Ce | 38.4 | 0.70 | 35.4 | 2.82 | 7.96 | 2.96 | 53.1 | 0.33 | 50.9 | 2.13 | 4.18 | 1.53 | 69.4 | 0.57 | 57.6 | 4.03 | 7.00 | 5.01 | 0.06 |
| Pr | 37.9 | 1.00 | 36.8 | 2.55 | 6.94 | 2.68 | 6.83 | 0.04 | 7.09 | 0.56 | 7.90 | 0.40 | 8.17 | 0.08 | 7.03 | 0.27 | 3.84 | 0.34 | 0.02 |
| Nd | 35.5 | 0.70 | 35.8 | 2.57 | 7.16 | 2.69 | 28.3 | 0.37 | 30.1 | 1.83 | 6.08 | 1.30 | 30.5 | 0.47 | 27.0 | 1.41 | 5.22 | 1.75 | 0.002 |
| Sm | 37.7 | 0.80 | 35.4 | 2.46 | 6.95 | 2.58 | 6.55 | 0.05 | 6.87 | 0.62 | 9.02 | 0.44 | 5.51 | 0.08 | 4.61 | 0.41 | 8.89 | 0.50 | 0.004 |
| Eu | 35.6 | 0.80 | 35.3 | 2.03 | 5.73 | 2.13 | 1.99 | 0.02 | 2.06 | 0.13 | 6.31 | 0.09 | 1.55 | 0.02 | 1.36 | 0.07 | 5.16 | 0.09 | 0.003 |
| Gd | 37.3 | 0.90 | 37.7 | 2.08 | 5.52 | 2.19 | 6.81 | 0.08 | 6.52 | 0.47 | 7.21 | 0.33 | 4.68 | 0.06 | 3.66 | 0.40 | 10.93 | 0.49 | <0.002 |
| Tb | 37.6 | 1.10 | 36.1 | 2.64 | 7.31 | 2.77 | 1.08 | 0.03 | 1.00 | 0.05 | 5.00 | 0.04 | 0.65 | 0.01 | 0.48 | 0.03 | 6.25 | 0.04 | <0.002 |
| Dy | 35.5 | 0.70 | 32.1 | 2.71 | 8.46 | 2.84 | 6.42 | 0.06 | 6.28 | 0.60 | 9.55 | 0.43 | 3.55 | 0.03 | 2.82 | 0.12 | 4.26 | 0.15 | 0.003 |
| Ho | 38.3 | 0.80 | 37.1 | 2.99 | 8.06 | 3.13 | 1.31 | 0.01 | 1.26 | 0.08 | 6.35 | 0.06 | 0.68 | 0.01 | 0.54 | 0.05 | 9.26 | 0.06 | 0.003 |
| Er | 38.0 | 0.90 | 36.5 | 2.73 | 7.47 | 2.86 | 3.67 | 0.04 | 3.52 | 0.13 | 3.69 | 0.09 | 1.83 | 0.01 | 1.45 | 0.14 | 9.66 | 0.18 | 0.005 |
| Tm | 36.8 | 0.60 | 34.6 | 2.68 | 7.74 | 2.81 | 0.53 | 0.01 | 0.51 | 0.04 | 7.84 | 0.03 | 0.26 | 0.004 | 0.18 | 0.02 | 11.11 | 0.02 | 0.002 |
| Yb | 39.2 | 0.90 | 34.5 | 3.01 | 8.72 | 3.16 | 3.39 | 0.04 | 3.40 | 0.33 | 9.71 | 0.24 | 1.65 | 0.01 | 1.40 | 0.11 | 8.86 | 0.14 | <0.002 |
| Lu | 37.0 | 0.90 | 35.6 | 2.69 | 7.58 | 2.83 | 0.50 | 0.01 | 0.51 | 0.03 | 5.88 | 0.02 | 0.25 | 0.003 | 0.20 | 0.01 | 5.00 | 0.01 | 0.002 |
| Hf | 36.7 | 1.20 | 35.1 | 2.74 | 7.81 | 2.88 | 4.97 | 0.03 | 4.87 | 0.23 | 4.72 | 0.17 | 5.10 | 0.06 | 4.15 | 0.31 | 7.47 | 0.38 | 0.005 |
| Ta | 37.6 | 1.90 | 37.7 | 2.34 | 6.21 | 2.46 | 0.79 | 0.02 | 0.73 | 0.05 | 6.85 | 0.04 | 0.87 | 0.02 | 0.71 | 0.05 | 7.04 | 0.06 | 0.003 |
| Pb | 38.6 | 0.20 | 38.3 | 2.82 | 7.37 | 2.96 | 10.8 | 0.17 | 6.40 | 0.33 | 5.16 | 0.24 | 13.1 | 0.15 | 8.44 | 0.16 | 1.90 | 0.20 | 0.07 |
| Th | 37.8 | 0.08 | 36.1 | 3.15 | 8.75 | 3.31 | 5.83 | 0.05 | 5.81 | 0.21 | 3.61 | 0.15 | 6.17 | 0.06 | 5.25 | 0.46 | 8.76 | 0.58 | 0.01 |
| U | 37.4 | 0.08 | 38.7 | 2.96 | 7.65 | 3.11 | 1.68 | 0.02 | 1.49 | 0.06 | 4.03 | 0.05 | 1.89 | 0.02 | 1.69 | 0.13 | 7.69 | 0.16 | 0.002 |
| Sample | G-4-2V | G-4-3V | h-07-51-3 | h-07-Gr-1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | LA-ICP-MS | ICP-MS | LA-ICP-MS | ICP-MS | LA-ICP-MS | ICP-MS | LA-ICP-MS | ICP-MS | ||||||||
| Element | Mean, n = 5 | SD | RSD, % | Mean, n = 5 | SD | RSD, % | Mean, n = 5 | SD | RSD, % | Mean, n = 20 | SD | RSD, % | ||||
| Al | 39,904 | 1883 | 4.72 | n.a. | 29,027 | 962 | 3.31 | n.a. | 27,727 | 1771 | 6.39 | n.a. | 33,585 | 4389 | 13.07 | n.a. |
| Sc | 41.5 | 1.39 | 3.35 | 30.7 | 51.9 | 2.49 | 4.80 | 51.0 | 31.5 | 1.16 | 3.68 | n.a. | 30.2 | 3.64 | 12.05 | n.a. |
| V | 435 | 27.85 | 6.40 | 358 | 392 | 13.74 | 3.51 | 393 | 281 | 17.62 | 6.27 | n.a. | 439 | 51.85 | 11.81 | n.a. |
| Cr | 669 | 34.71 | 5.19 | 614 | 633 | 14.69 | 2.32 | 586 | 628 | 61.54 | 9.80 | n.a. | 452 | 11.82 | 2.62 | n.a. |
| Mn | 201 | 8.92 | 4.44 | 162 | 170 | 2.99 | 1.76 | 167 | 142 | 6.38 | 4.49 | n.a. | 215 | 29.83 | 13.87 | n.a. |
| Co | 20.1 | 1.47 | 7.31 | 17.5 | 16.3 | 0.75 | 4.60 | 15.6 | 13.9 | 0.51 | 3.67 | n.a. | 19.2 | 3.44 | 17.92 | n.a. |
| Ni | 580 | 16.49 | 2.84 | 474 | 542 | 17.88 | 3.30 | 435 | 504 | 37.89 | 7.52 | n.a. | 647 | 197.15 | 30.47 | n.a. |
| Cu | 406 | 29.39 | 7.24 | 299 | 401 | 37.63 | 9.38 | 350 | 297 | 46.74 | 15.74 | n.a. | 525 | 161.72 | 30.80 | n.a. |
| Rb | 49.6 | 1.99 | 4.01 | 33.1 | 37.5 | 0.88 | 2.35 | 55.6 | 12.3 | 1.45 | 11.79 | 16.6 | 46.9 | 5.76 | 12.28 | 54.1 |
| Sr | 2810 | 121.79 | 4.33 | n.a. | 1997 | 76.50 | 3.83 | n.a. | 1655 | 117.98 | 7.13 | 1679 | 2401 | 403.64 | 16.81 | 1887 |
| Y | 108 | 1.74 | 1.61 | 67.6 | 135 | 5.04 | 3.73 | 109 | 72.2 | 3.87 | 5.36 | 97.9 | 79.3 | 11.64 | 14.68 | 91.4 |
| Zr | 318 | 11.56 | 3.64 | 195 | 423 | 13.40 | 3.17 | 295 | 200 | 11.25 | 5.63 | 211 | 233 | 38.16 | 16.38 | 199 |
| Nb | 34.2 | 0.77 | 2.25 | 25.2 | 33.3 | 1.42 | 4.26 | 23.2 | 20.9 | 0.83 | 3.97 | 22.6 | 34.8 | 7.05 | 20.26 | 32.4 |
| Ba | 2327 | 91.74 | 3.94 | n.a. | 2397 | 97.85 | 4.08 | n.a. | 466 | 13.97 | 3.00 | 625 | 3255 | 360.24 | 11.07 | 3261 |
| La | 50.5 | 0.75 | 1.49 | 36.5 | 50.1 | 2.06 | 4.11 | 44.7 | 36.1 | 1.49 | 4.13 | 46.3 | 51.7 | 7.29 | 14.10 | 52.6 |
| Ce | 79.6 | 3.35 | 4.21 | 49.8 | 86.6 | 4.76 | 5.50 | 69.0 | 51.3 | 2.21 | 4.31 | 63.5 | 67.2 | 10.16 | 15.12 | 63.6 |
| Pr | 10.9 | 0.45 | 4.13 | 6.62 | 11.2 | 0.56 | 5.00 | 8.95 | 6.88 | 0.25 | 3.63 | 9.37 | 9.49 | 1.49 | 15.70 | 9.64 |
| Nd | 39.8 | 0.92 | 2.31 | 25.8 | 42.9 | 0.95 | 2.21 | 36.6 | 28.0 | 1.67 | 5.96 | 36.2 | 35.0 | 3.07 | 8.77 | 36.0 |
| Sm | 8.00 | 0.32 | 4.00 | 5.01 | 9.56 | 0.59 | 6.17 | 7.72 | 5.46 | 0.73 | 13.37 | 7.35 | 6.82 | 0.68 | 9.97 | 7.07 |
| Eu | 2.35 | 0.19 | 8.09 | 1.26 | 2.50 | 0.18 | 7.20 | 1.99 | 1.51 | 0.15 | 9.93 | 1.87 | 1.82 | 0.17 | 9.34 | 1.75 |
| Gd | 9.99 | 0.33 | 3.30 | 5.44 | 11.9 | 0.83 | 6.97 | 8.54 | 6.68 | 0.44 | 6.59 | 8.09 | 7.58 | 1.14 | 15.04 | 7.75 |
| Tb | 1.55 | 0.05 | 3.23 | 0.87 | 1.94 | 0.11 | 5.67 | 1.40 | 1.09 | 0.09 | 8.26 | 1.32 | 1.23 | 0.19 | 15.45 | 1.15 |
| Dy | 10.1 | 0.29 | 2.87 | 5.60 | 13.2 | 0.71 | 5.38 | 9.19 | 7.00 | 0.27 | 3.86 | 8.37 | 8.03 | 0.98 | 12.20 | 7.59 |
| Ho | 2.42 | 0.12 | 4.96 | 1.27 | 3.11 | 0.22 | 7.07 | 2.08 | 1.60 | 0.06 | 3.75 | 1.87 | 1.86 | 0.23 | 12.37 | 1.70 |
| Er | 7.71 | 0.36 | 4.61 | 4.06 | 9.51 | 0.62 | 6.52 | 6.67 | 5.11 | 0.17 | 3.33 | 6.20 | 5.78 | 0.63 | 10.90 | 5.46 |
| Tm | 1.15 | 0.01 | 0.87 | 0.60 | 1.34 | 0.08 | 5.97 | 1.01 | 0.73 | 0.04 | 5.48 | 1.02 | 0.80 | 0.07 | 8.75 | 0.87 |
| Yb | 7.61 | 0.52 | 6.83 | 4.04 | 9.60 | 0.45 | 4.69 | 6.67 | 5.32 | 0.37 | 6.95 | 6.38 | 5.70 | 0.36 | 6.32 | 5.46 |
| Lu | 1.07 | 0.03 | 2.80 | 0.70 | 1.34 | 0.08 | 5.97 | 1.15 | 0.79 | 0.04 | 5.06 | 1.03 | 0.87 | 0.07 | 8.05 | 0.83 |
| Hf | 8.41 | 0.20 | 2.38 | 4.64 | 10.1 | 0.62 | 6.14 | 6.95 | 5.45 | 0.20 | 3.67 | 6.77 | 5.59 | 0.64 | 11.45 | 5.60 |
| Ta | 2.42 | 0.14 | 5.79 | 1.57 | 2.60 | 0.12 | 4.62 | 1.75 | 1.40 | 0.09 | 6.43 | 1.61 | 2.01 | 0.32 | 15.92 | 2.08 |
| Pb | 19.4 | 1.63 | 8.40 | 7.47 | 20.7 | 1.27 | 6.14 | 13.5 | 8.55 | 0.32 | 3.74 | n.a. | 19.5 | 5.36 | 27.49 | n.a. |
| Th | 8.41 | 0.10 | 1.19 | 5.68 | 9.82 | 0.30 | 3.05 | 7.14 | 6.24 | 0.33 | 5.29 | 7.87 | 8.27 | 1.03 | 12.45 | 7.94 |
| U | 78.3 | 1.89 | 2.41 | 78.2 | 60.2 | 3.01 | 5.00 | 74.5 | 65.3 | 4.85 | 7.43 | 88.9 | 105 | 12.64 | 12.04 | 109 |
| Beads | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Mean, n = 10 | SD | RSD, % | Mean, n = 5 | SD | RSD, % | Mean, n = 5 | SD | RSD, % |
| Al | 38,023 | 2663.16 | 7.00 | 33,486 | 791.32 | 2.36 | 29,246 | 991.98 | 3.39 |
| Sc | 26.1 | 1.31 | 5.02 | 32.6 | 1.35 | 4.14 | 32.0 | 0.84 | 2.63 |
| V | 389 | 14.14 | 3.63 | 493 | 25.94 | 5.26 | 434 | 12.20 | 2.81 |
| Cr | 439 | 24.79 | 5.65 | 454 | 83.97 | 18.50 | 463 | 30.09 | 6.50 |
| Mn | 184 | 17.02 | 9.25 | 243 | 27.42 | 11.28 | 216 | 13.57 | 6.28 |
| Co | 15.3 | 0.56 | 3.66 | 22.0 | 1.05 | 4.77 | 20.2 | 0.97 | 4.80 |
| Ni | 425 | 19.27 | 4.53 | 802 | 53.50 | 6.67 | 715 | 40.17 | 5.62 |
| Cu | 393 | 106.97 | 27.22 | 705 | 264.96 | 37.58 | 476 | 27.53 | 5.78 |
| Zn | 2258 | 192.70 | 8.53 | 2763 | 204.94 | 7.42 | 2287 | 84.47 | 3.69 |
| Ga | 56.5 | 2.49 | 4.41 | 27.6 | 1.21 | 4.38 | 24.4 | 0.99 | 4.06 |
| Rb | 41.3 | 2.97 | 7.19 | 52.8 | 2.67 | 5.06 | 46.6 | 1.79 | 3.84 |
| Sr | 1968 | 100.62 | 5.11 | 2766 | 88.21 | 3.19 | 2469 | 173.94 | 7.04 |
| Y | 67.1 | 5.41 | 8.06 | 90.3 | 3.38 | 3.74 | 80.6 | 1.39 | 1.72 |
| Zr | 196 | 6.88 | 3.51 | 272 | 16.27 | 5.98 | 230 | 8.73 | 3.80 |
| Nb | 27.9 | 1.49 | 5.34 | 42.0 | 2.27 | 5.40 | 34.6 | 1.77 | 5.12 |
| Ba | 2926 | 93.61 | 3.20 | 3640 | 188.81 | 5.19 | 3200 | 153.06 | 4.78 |
| La | 46.4 | 1.95 | 4.20 | 60.0 | 3.40 | 5.67 | 48.6 | 1.66 | 3.42 |
| Ce | 58.4 | 6.38 | 10.92 | 78.3 | 5.38 | 6.87 | 64.9 | 2.46 | 3.79 |
| Pr | 8.18 | 0.21 | 2.57 | 11.1 | 0.39 | 3.51 | 9.18 | 0.41 | 4.47 |
| Nd | 32.5 | 1.34 | 4.12 | 38.4 | 1.55 | 4.04 | 33.9 | 1.61 | 4.75 |
| Sm | 6.51 | 0.30 | 4.61 | 7.60 | 0.46 | 6.05 | 6.36 | 0.32 | 5.03 |
| Eu | 1.67 | 0.11 | 6.59 | 2.00 | 0.12 | 6.00 | 1.78 | 0.11 | 6.18 |
| Gd | 6.40 | 0.41 | 6.41 | 8.67 | 0.33 | 3.81 | 7.66 | 0.31 | 4.05 |
| Tb | 1.06 | 0.14 | 13.21 | 1.43 | 0.05 | 3.50 | 1.21 | 0.09 | 7.44 |
| Dy | 6.96 | 0.50 | 7.18 | 8.88 | 0.26 | 2.93 | 8.26 | 0.25 | 3.03 |
| Ho | 1.60 | 0.10 | 6.25 | 2.06 | 0.12 | 5.83 | 1.90 | 0.12 | 6.32 |
| Er | 5.16 | 0.62 | 12.02 | 6.42 | 0.32 | 4.98 | 5.74 | 0.29 | 5.05 |
| Tm | 0.73 | 0.10 | 13.70 | 0.85 | 0.05 | 5.88 | 0.82 | 0.04 | 4.88 |
| Yb | 5.35 | 0.39 | 7.29 | 6.07 | 0.29 | 4.78 | 5.68 | 0.33 | 5.81 |
| Lu | 0.82 | 0.04 | 4.88 | 0.95 | 0.07 | 7.37 | 0.84 | 0.07 | 8.33 |
| Hf | 5.05 | 0.44 | 8.71 | 6.29 | 0.21 | 3.34 | 5.42 | 0.35 | 6.46 |
| Ta | 1.75 | 0.13 | 7.43 | 2.37 | 0.10 | 4.22 | 1.92 | 0.05 | 2.69 |
| Pb | 13.6 | 0.76 | 5.59 | 24.0 | 1.49 | 6.21 | 20.9 | 0.54 | 2.58 |
| Th | 7.49 | 0.49 | 6.54 | 9.45 | 0.53 | 5.61 | 7.88 | 0.36 | 4.57 |
| U | 103 | 5.84 | 5.67 | 119 | 7.05 | 5.92 | 93.8 | 2.00 | 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozmenko, O.A.; Deviatiiarova, A.S.; Ragozin, A.L.; Sokol, E.V.; Karputin, I.S.; Sokol, A.G. Modified Analyses of Trace Elements in Glass Beads by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Application for Particular Silicate Rocks. Minerals 2025, 15, 129. https://doi.org/10.3390/min15020129
Kozmenko OA, Deviatiiarova AS, Ragozin AL, Sokol EV, Karputin IS, Sokol AG. Modified Analyses of Trace Elements in Glass Beads by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Application for Particular Silicate Rocks. Minerals. 2025; 15(2):129. https://doi.org/10.3390/min15020129
Chicago/Turabian StyleKozmenko, Olga A., Anna S. Deviatiiarova, Alexey L. Ragozin, Ella V. Sokol, Ivan S. Karputin, and Alexander G. Sokol. 2025. "Modified Analyses of Trace Elements in Glass Beads by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Application for Particular Silicate Rocks" Minerals 15, no. 2: 129. https://doi.org/10.3390/min15020129
APA StyleKozmenko, O. A., Deviatiiarova, A. S., Ragozin, A. L., Sokol, E. V., Karputin, I. S., & Sokol, A. G. (2025). Modified Analyses of Trace Elements in Glass Beads by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Application for Particular Silicate Rocks. Minerals, 15(2), 129. https://doi.org/10.3390/min15020129







