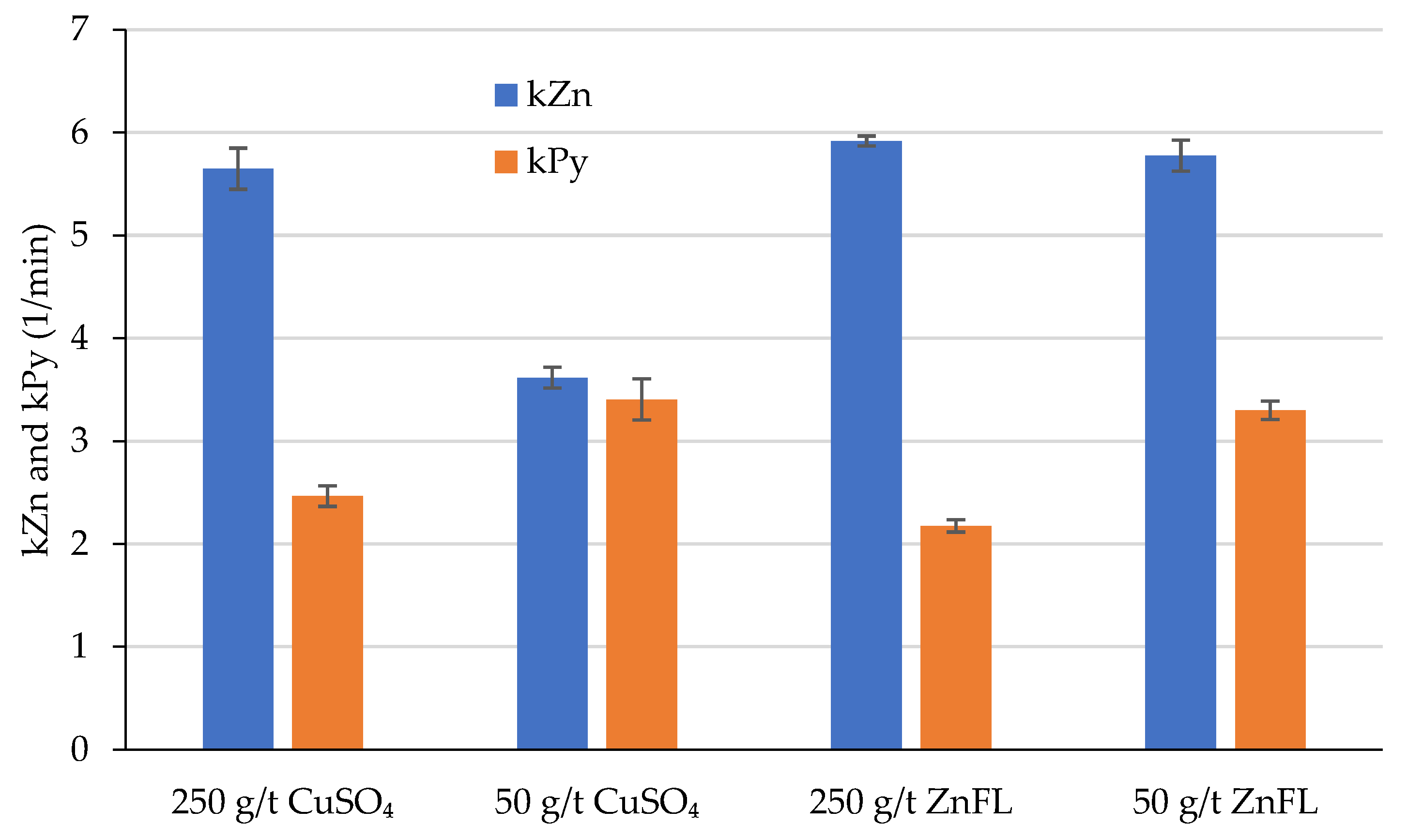1. Introduction
Activation is a critical step in the flotation of sphalerite, the most abundant zinc mineral in ore deposits. Unlike many other sulfide minerals, sphalerite exhibits poor floatability with short-chain collectors and therefore requires surface activation to achieve high recoveries [
1]. Sphalerite can be activated by several heavy metal ions such as Cu
2+, Pb
2+, Ag
1+, Au
1+, Cd
2+ and Fe
2+; however, Cu
2+ derived from copper sulfate (CuSO
4) remains the most widely used activator in sphalerite and marmatite flotation [
2,
3,
4,
5,
6]. The activation mechanism of sphalerite (ZnS) and marmatite (Zn,Fe)S by CuSO
4 has been extensively studied [
7,
8,
9,
10,
11,
12,
13,
14,
15] and is generally represented by the following reaction which is given in Equation (1) [
16,
17,
18]:
In this process, Cu2+ ions replace Zn2+ on the mineral surface, forming a CuS-like layer that interacts strongly with xanthate collectors. This mechanism underpins the widespread industrial application of CuSO4 as a sphalerite activator.
Despite its effectiveness, CuSO
4 presents several operational and environmental limitations. Firstly, relatively high dosages are required, increasing reagent costs and introducing large quantities of sulfate ions into the flotation circuit. Elevated sulfate concentrations, combined with lime addition, can promote scaling and corrosion and complicate recycled water management. Secondly, CuSO
4 requires a separate conditioning stage prior to collector addition; otherwise, premature reaction with xanthates produces inactive copper xanthate species that reduce flotation efficiency [
19]. Finally, variability in sphalerite mineralogy, particularly the presence of iron-rich varieties such as marmatite, can complicate its activation behavior [
20].
Alternative activators have been investigated in recent decades to overcome these limitations. For instance, ammoniacal copper solutions (ACSs) have demonstrated improved activation of marmatite and sphalerite in weakly alkaline environments. Marmatite is an iron-rich variety of sphalerite that typically contains up to 25% Fe and is also Mn-rich, and it is formed at relatively high temperatures. Activation of marmatite by copper ions is a challenging process and requires high dosages of copper sulfate and long pre-conditioning times. ACS has been found to be more effective in activating marmatite in a weakly alkaline environment than copper sulfate, ammonium chloride and lead nitrate [
20]. ACS has also been used commercially at the Dulong Mine in Yunnan Province, in southern China [
21]. Despite successful applications, few alternative reagents have been adopted on an industrial scale. Research into novel activators remains limited compared to the extensive literature on CuSO
4.
This study investigated the use of ZnFL (Zn Flooter), a copper–amine-based complex developed by Metal-Kim in Türkiye, as a potential alternative to CuSO4 for sphalerite activation. ZnFL differs from conventional CuSO4 in three important respects:
It is supplied as a liquid reagent containing stabilized ionic copper over a wide pH range, including strongly alkaline conditions.
It contains substantially less sulfate (approximately 15%) than CuSO4 (approximately 38%), which reduces potential environmental and water chemistry impacts.
Due to its stability, ZnFL can be added without pre-conditioning, potentially simplifying flotation circuits and lowering operating costs.
The objective of this study was to systematically evaluate the activation performance of ZnFL relative to that of conventional CuSO4 in two sphalerite-bearing ores of distinct mineralogy. Contact angle measurements were conducted to assess changes in mineral hydrophobicity, while batch rougher kinetic flotation tests quantified reagent effectiveness in terms of zinc grade, recovery and froth stability. Additional experiments investigated the effect of reagent addition order on flotation performance. By integrating surface wettability and metallurgical data, this work provides the first comprehensive comparison between ZnFL and CuSO4 and assesses ZnFL’s potential as a practical and more sustainable activator for industrial sphalerite flotation.
2. Materials and Methods
2.1. Materials
Two sphalerite-bearing ores from different regions in northwestern Türkiye were used in this study. Ore 1 is a lead–zinc complex sulfide ore containing 2.86% Pb, 3.57% Zn, 5.19% Fe and 63 ppm Ag. The major sulfide minerals are galena, sphalerite and pyrite, with the latter occurring mainly as gangue. Ore 2 is a copper–zinc complex sulfide ore containing 0.76% Cu, 2.76% Zn, 38.64% Fe and 26.9 ppm Ag. The major sulfide minerals are chalcopyrite, sphalerite and pyrite.
The pyrite content of this ore (~70%) is much higher than that of Ore 1. These ores were selected to represent two different mineralogical contexts (Pb–Zn sulfide ore vs. Cu–Zn sulfide ore), enabling evaluation of activator performance across contrasting systems.
ZnFL is a proprietary liquid copper–amine complex developed by Metal-Kim Metallurgy and Chemical Industry Ltd. in Türkiye. It remains stable in solution across the full pH range, unlike conventional copper salts that tend to hydrolyze or precipitate. It is a dark-blue, viscous, odorless liquid containing emulsified surfactants, with approximately 9% active copper, 15% sulfate, a pH of 10–11 and a bulk density of 1.05–1.25 g/cm3. In ZnFL, copper is found in ionic form at a wide range of pH values, particularly in strongly alkaline solutions. The pH value of the ZnFL solution is between 10 and 11. ZnFL has lesser amounts of sulfate (approximately 15%) present in the formula compared to copper sulfate (approximately 38%). It is in liquid form and stable across a wide pH range. It can replace copper salts that tend to precipitate upon hydration at certain pH levels.
2.2. Contact Angle Measurements
Contact angle experiments were conducted to evaluate sphalerite’s hydrophobicity with different activators and collectors. Zinc concentrates from both ores were purified by flotation, washed with dilute acid and acetone to remove organic residuals, and mounted in epoxy resin. The results of chemical analyses of the concentrates are given in
Table 1. The compositions shown in
Table 1 correspond to the nearly pure sphalerite specimens produced from the ore concentrates and therefore differ from the flotation feed grades reported in the text.
Zinc concentrate samples were mounted on a cold hardened epoxy resin to form a polished section with a flat surface. The surface was then polished in several stages using diamond-impregnated liquids and a rotary lap built into the Buehler Power Pro 4000 Automatic Abrasive Polishing Machine.
The experimental setup is shown in
Figure 1. It consists of a light source, a polished-section sample, a 12 × 6 × 5 cm rectangular glass cell containing the test solution, a microburette, an optical lens, a mirror positioned at a 45° angle and a frosted glass screen onto which the image is reflected. An air bubble is generated using the microburette, and the resulting contact angle formed on the polished sample is projected onto the paper by means of the light source, optical lens and mirror system. The contact angle is then measured manually by using a goniometer. This setup was developed by Can in 1997 [
22] and also used by Talan in 2019 [
23] in her MSc thesis.
Contact angles were measured in deionized water, with ZnFL alone, with CuSO
4 alone, and with ZnFL + SIPX and CuSO
4 + SIPX together. All solutions were prepared at a 10
−4 M concentration. Measurements were carried out in a rectangular glass cell equipped with a light source, optical lens and 45° mirror [
22,
23]. Each condition was tested in triplicate, and mean values with standard deviations are reported.
2.3. Flotation Tests
Representative ore samples were crushed to -2 mm and split into batches of 1.0 kg (Ore 1) and 1.7 kg (Ore 2). The sample mass used in the flotation tests differed between the two ores due to their distinct head grades. Ore 1 had higher metal grades, and floating 1.0 kg in a 2.5 L cell produced sufficient concentrate for analysis. Ore 2 had lower grades, so 1.7 kg of sample was floated in a 4.5 L cell to obtain enough concentrate for the planned chemical and particle size measurements. Grinding was performed in a 30 × 30 cm stainless steel ball mill using high-chrome balls at 60% w/w pulp density. The liberation characteristics of the ore samples and, hence, the particle size of the flotation feed were different. The particle sizes applied in the flotation plants of each ore were used in the tests. All flotation test conditions were kept constant except the activator type and dosage. Ore 1 was ground for 6 min to obtain an 80% passing size of 180 µm and Ore 2 was ground for 30 min to obtain an 80% passing size of 38 µm for flotation. Base conditions for the ores were taken from the operating plant data of the ores.
Flotation tests for Ore 1 were performed in a Denver flotation machine at 30% w/w pulp density. A flotation cell of 2.5 L volume was used for rougher flotation tests. A quantity of 2 kg/t ZnSO4 was added in the grinding stage to depress sphalerite in the Pb rougher section. The pulp pH was adjusted to 9 with lime. PEX (Potassium Ethyl Xanthate) was used as the collector and Dow-froth 250 as the frother. In the zinc flotation stage, the pulp pH was raised to 11.5 using lime. PAX (Potassium Amyl Xanthate) was used as the collector and Dow-froth 250 as the frother. Before the addition of the collector and frother, CuSO4 or ZnFL was added as the activator at equivalent dosages. A quantity of 250 g/t CuSO4 was considered as the base or standard dosage.
Flotation tests for Ore 2 were performed in a 4.5-L Denver flotation cell at 30% w/w pulp density. The depressants 0.5 kg/t Na2S (sodium disulfide), 1 kg/t ZnSO4 (zinc sulfate) and 3 kg/t SMBS (N2S2O5, sodium metabisulfate) were added in the grinding stage for the depression of sphalerite and pyrite in the copper flotation stage. Pre-flotation was carried out for 5 min before copper flotation to remove the naturally floatable gangue minerals, mainly talc. Sodium Aero-float (NaAF) is a type of collector consisting of sodium diethyl dithiophosphate, and it (90 g/t) was used as the collector in the copper circuit. MIBC (a synthetic alcohol type of frother) at 25 g/t was used as the frother in the copper flotation stage. In the zinc flotation stage, the pulp pH was raised to over 11.5 using lime. SIPX (sodium isopropyl xanthate) at 50 g/t was used as the collector for sphalerite, and 10 g/t MIBC was used as the frother. The CuSO4 dosage was determined as 500 g/t for sphalerite activation.
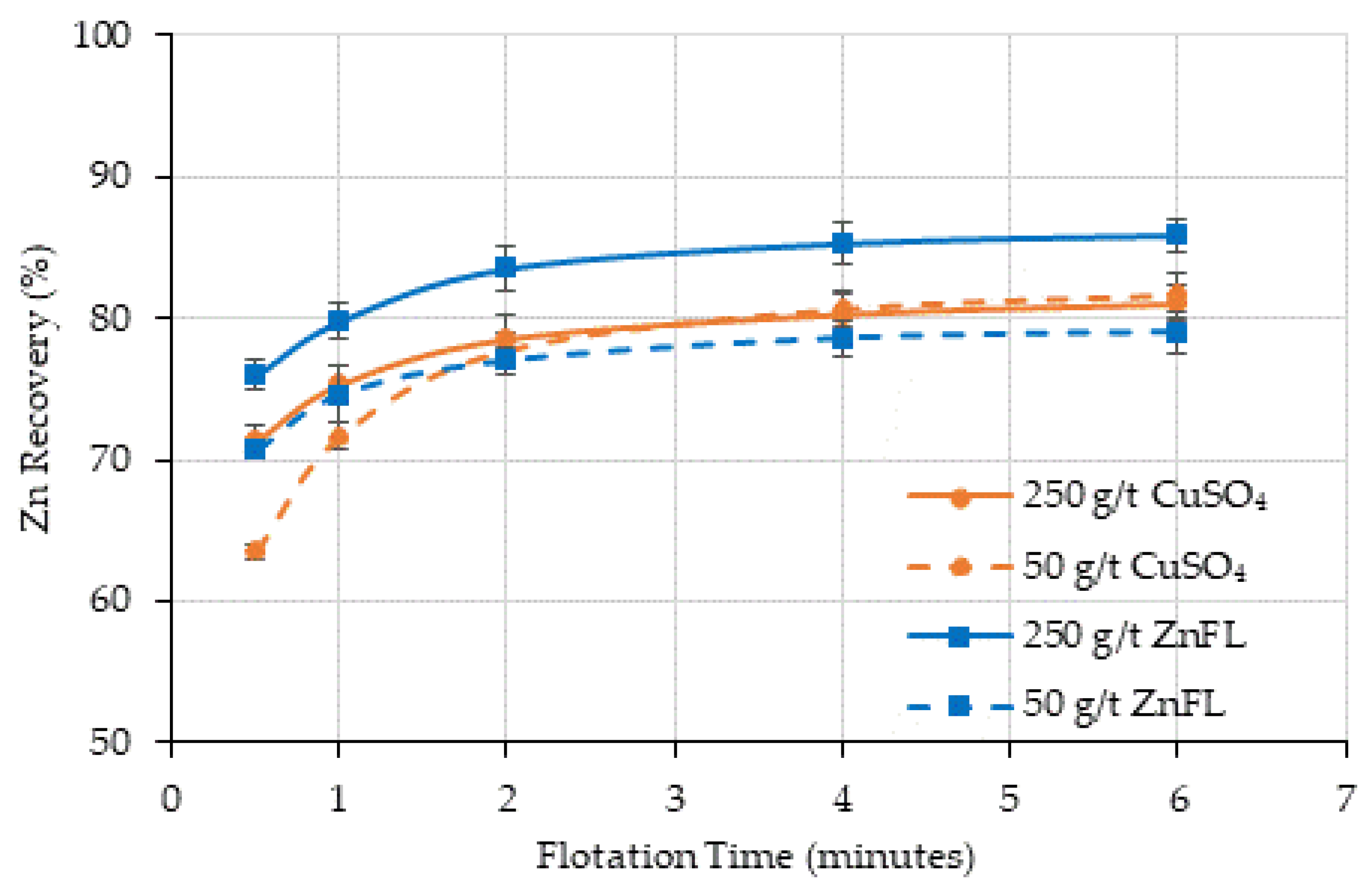
In the flotation tests, CuSO4 was used at dosages of 250 g/t (Ore 1) and 500 g/t (Ore 2) for sphalerite activation. For a direct comparison, ZnFL was applied at the same dosages: 250 g/t for Ore 1 and 500 g/t for Ore 2. To assess performance at lower additions, we also evaluated 50 g/t and 100 g/t, corresponding to one-fifth of the standard activator dosages for Ore 1 and Ore 2, respectively. ZnFL and CuSO4 differ in their total active Cu content; therefore, comparisons were made on an equal-reagent-dosage basis.
The flotation products were assayed for Cu, Fe, Pb and Zn using a Thermo Fisher Scientific Niton XL series X-ray fluorescence (XRF) device and a Varian AA 240 FS model Atomic Absorption Spectrometer.
4. Discussion
The effect of ZnFL and CuSO4 on sphalerite was studied with contact angle measurements and rougher kinetic batch-scale flotation tests by using two different ore samples. Ore 1 contains lead and zinc sulfides with pyrite minerals as gangue, whereas Ore 2 consists of copper and zinc sulfates with talc and pyrite acting as gangue minerals. ZnFL, a copper–amine complex reagent, demonstrated equivalent or enhanced activation efficiency at significantly lower dosages, together with improved operational flexibility.
Contact angle measurements confirmed that ZnFL produced higher hydrophobicity on sphalerite surfaces than did CuSO4, despite containing less active copper. This suggests that the amine-complexed copper species in ZnFL exhibited stronger adsorption on sphalerite.
Activation of sphalerite is commonly caused by Cu
2+, which then enables xanthate adsorption and flotation [
1]. Surface analyses (XPS/electrochemical) indicated the formation of Cu–S species (CuS/Cu
2S-like layers) and enhanced xanthate adsorption on Cu-activated surfaces [
2]. ZnFL is a copper–amine complex. Ammoniacal copper (cupric ammine complexes, e.g., [Cu(NH
3)
4]
2+) has been shown in several studies to activate sphalerite and in some cases perform better than simple CuSO
4 because ammonia keeps copper soluble at higher pH (dissolves Cu(OH)
2 into ammine complexes) [
20].
In alkaline pH, introducing ammonia into the activation system significantly increases the kinetics of the activation and the copper uptake. This may be because ammonia forms a complex with copper such that the copper hydroxide precipitate will dissolve and form Cu(NH
3)
4 2+, which can readily react with ZnS to form copper sulfide through a reaction Equation (5) like
The activation kinetics at near-neutral and alkaline pHs can be significantly improved by the addition of a small amount of ammonia to the activating solution. In acidic and alkaline solution, the xanthates formed at open circuit potential render the mineral surface hydrophobic; at near-neutral pH, the mineral surface is hydrophilic due to the co-existence of copper hydroxy species with the xanthate surface product [
18,
19].
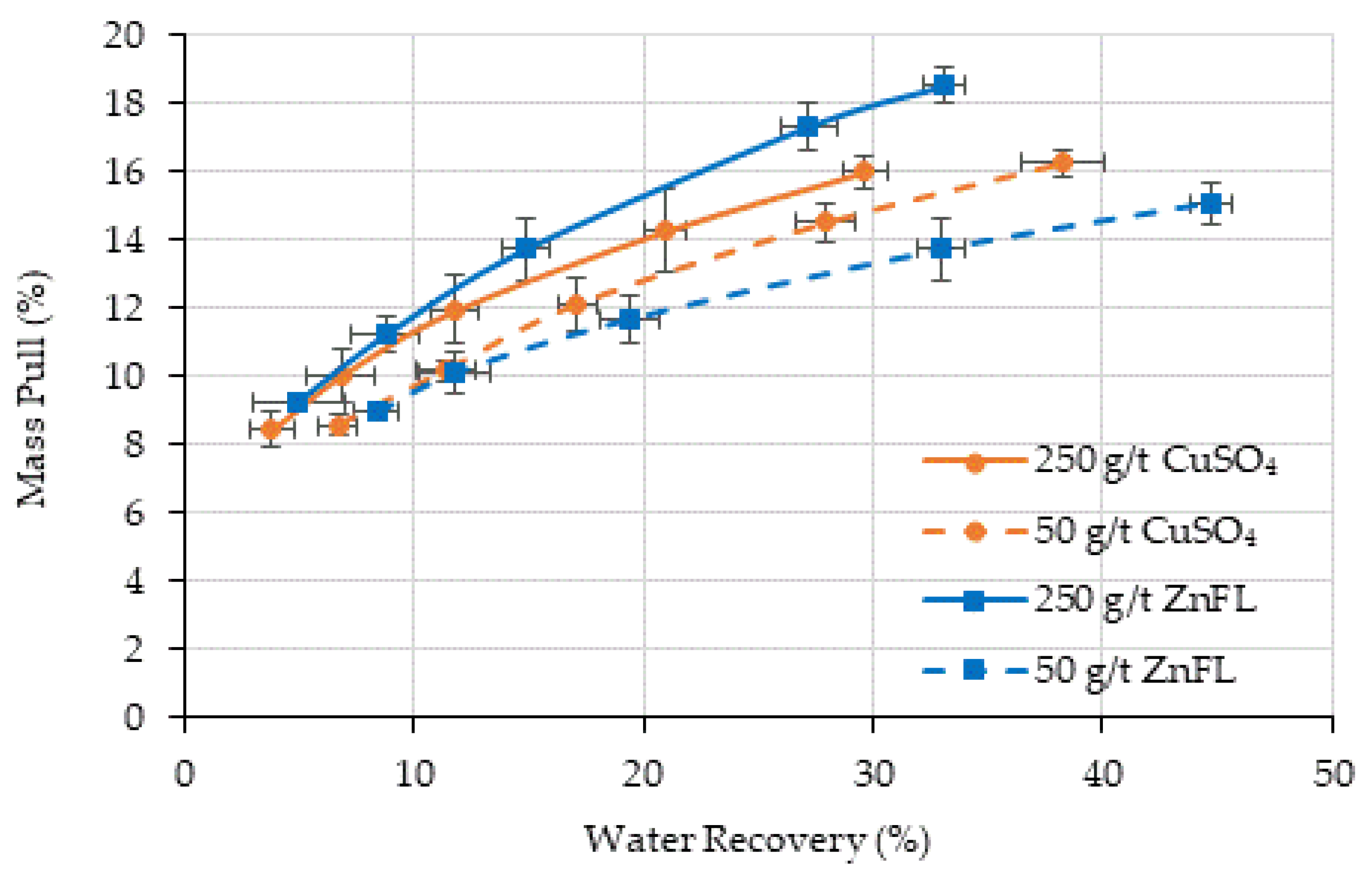
The effects of CuSO
4 and ZnFL on the surface hydrophobicity can also be determined by decoupling the recovery by true flotation and entrainment. The recovery by entrainment was calculated using the water recovery and the non-sulfide gangue recovery [
25,
26,
27,
28]. Partitioning of recoveries into true flotation and entrainment components confirmed that both activators predominantly promoted true flotation. For Ore 1 (P
80 = 180 µm), entrainment was minimal due to the coarser particle size, while for Ore 2 (P
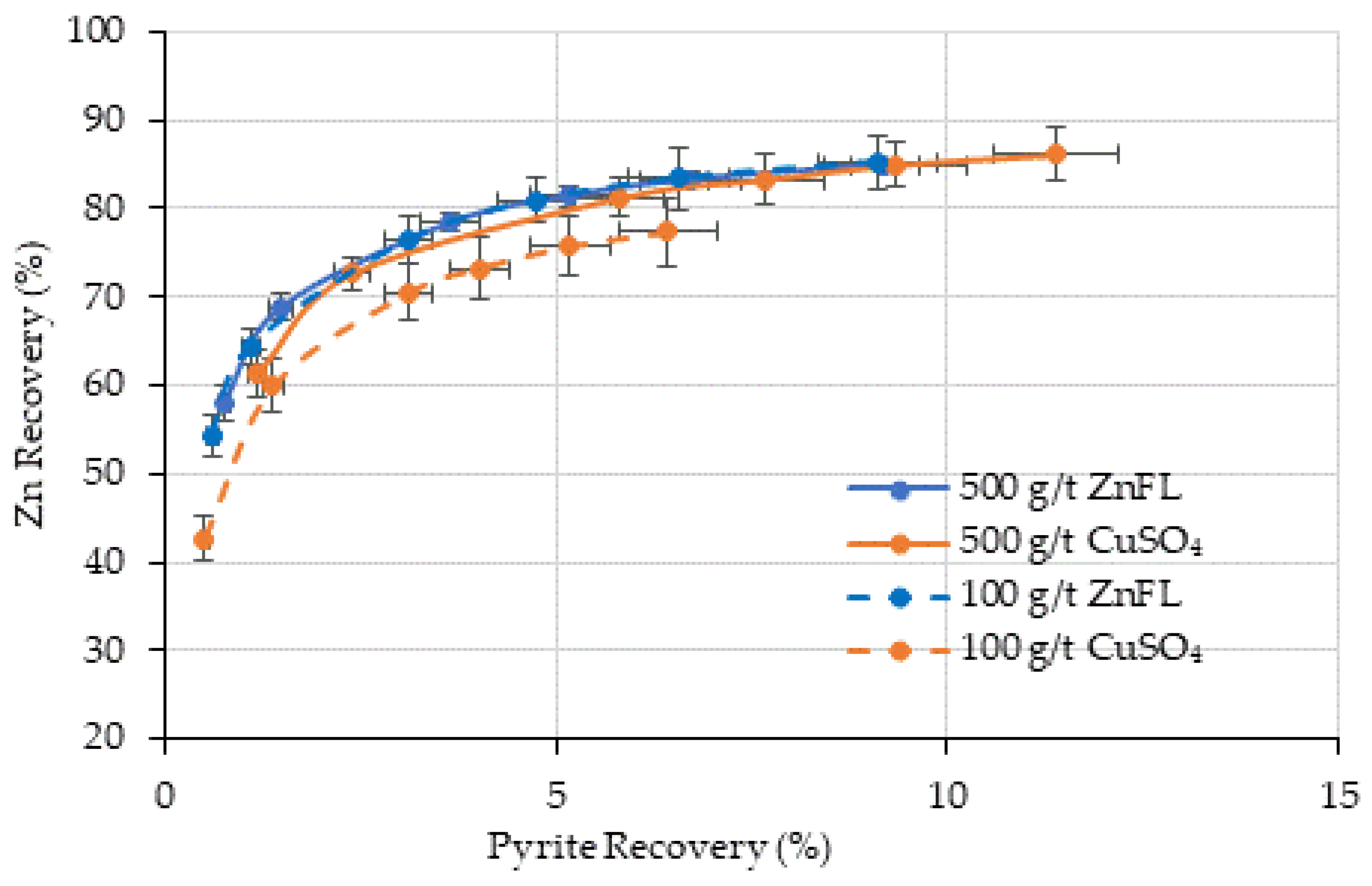
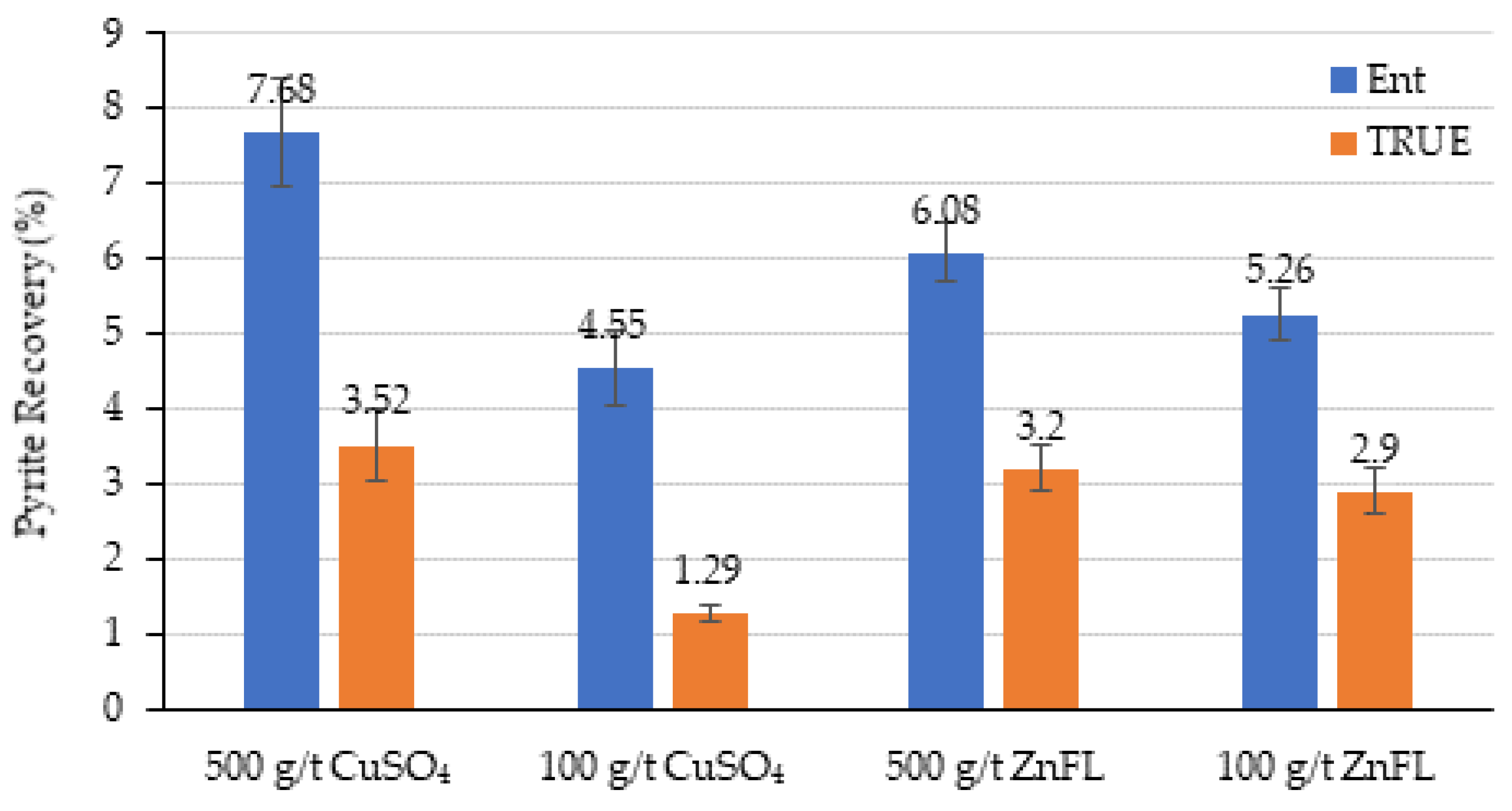
80 = 38 µm), entrainment was more significant but consistent across reagents. It is obvious that both activators can be used to activate sphalerite, and 90% of sphalerite recovery was achieved by true flotation (
Figure 13). The recovery by true flotation at 250 g/t ZnFL was slightly higher than that under the other conditions in Ore 1. The recovery by entrainment was close in all conditions. In Ore 2, the recovery by true flotation was about 71% in all conditions, except 100 g/t CuSO
4. It is obvious that 100 g/t CuSO
4 was not enough to provide the same degree of hydrophobicity as 100 g/t ZnFL. It seems that 100 g/t ZnFL could provide the same degree of hydrophobicity as 500 g/t ZnFL and 500 g/t CuSO
4. These results indicate that ZnFL achieved slightly higher true flotation recoveries at equivalent dosages, supporting its stronger activation effect.
Ammoniacal copper solution has been used for the activation of sphalerite [
20] and pentlandite [
29]. Yang recently used ammonium sulfate as a complexing agent in the activation and flotation of sphalerite and marmatite [
9]. ZnFL can be considered similar to ammoniacal copper solution; hence, their activation mechanisms on sphalerite are also considered similar. Yang has shown that the activation occurs in two sequential stages: dissolution of zinc from the mineral surface by ammonium ions in the form of zinc–ammonia complexes followed by copper ion adsorption. Dissolution of zinc reduces the zinc content on the mineral surface, and the mineral lattice develops pores, enhancing the surface activity of the mineral. The copper ions readily occupy the voids on the mineral surface and penetrate the mineral lattice, forming Zn
xCu
1−xS. Consequently, the addition of ammoniacal copper solution enhances copper activation compared to CuSO
4, thereby improving flotation performance.
The presence of ammonium ions may also affect the pyrite surface and its flotation behavior. There are several research studies conducted on the effects of ammonia/ammonium species on pyrite surface chemistry and flotation. Depending on the pH and whether pyrite is Cu-activated or lime-depressed, ammonia ions can activate or depress pyrite [
30,
31,
32]. Ammonia exists mainly as NH
4+ in acidic-to-neutral pH and as NH
3 in alkaline pH. The flotation tests were conducted at a strongly alkaline pH for pyrite depression. Therefore, ammonia was in NH
3 form, which can form complexes with Fe
2+/Fe
3+ ions released from pyrite surfaces. Fe-NH
3 complexes reduce the precipitation of iron hydroxides on the surface and keep the surface more active and hydrophilic. This may reduce the extent of dixanthogen formation on pyrite, which can depress pyrite flotation.
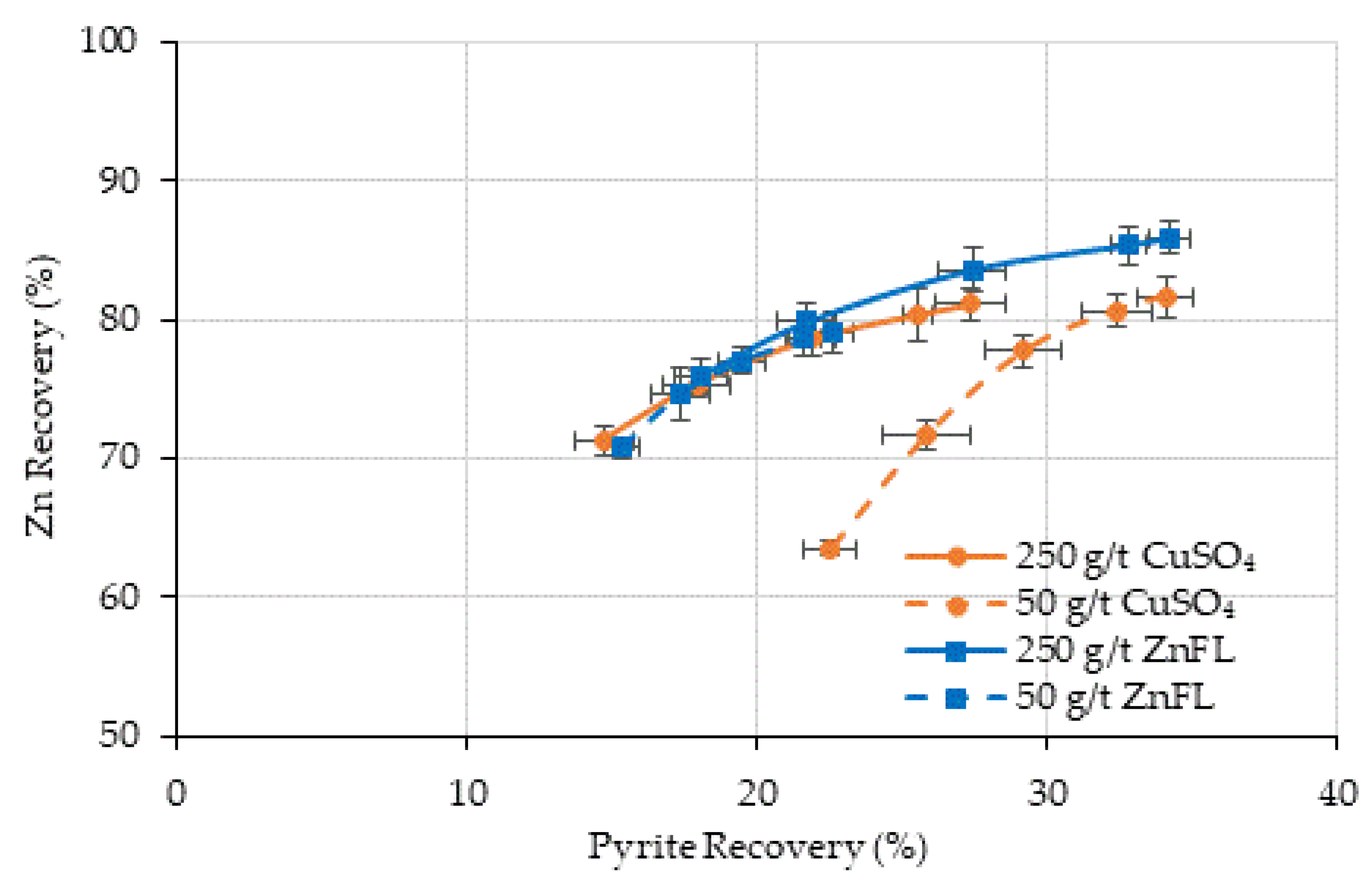
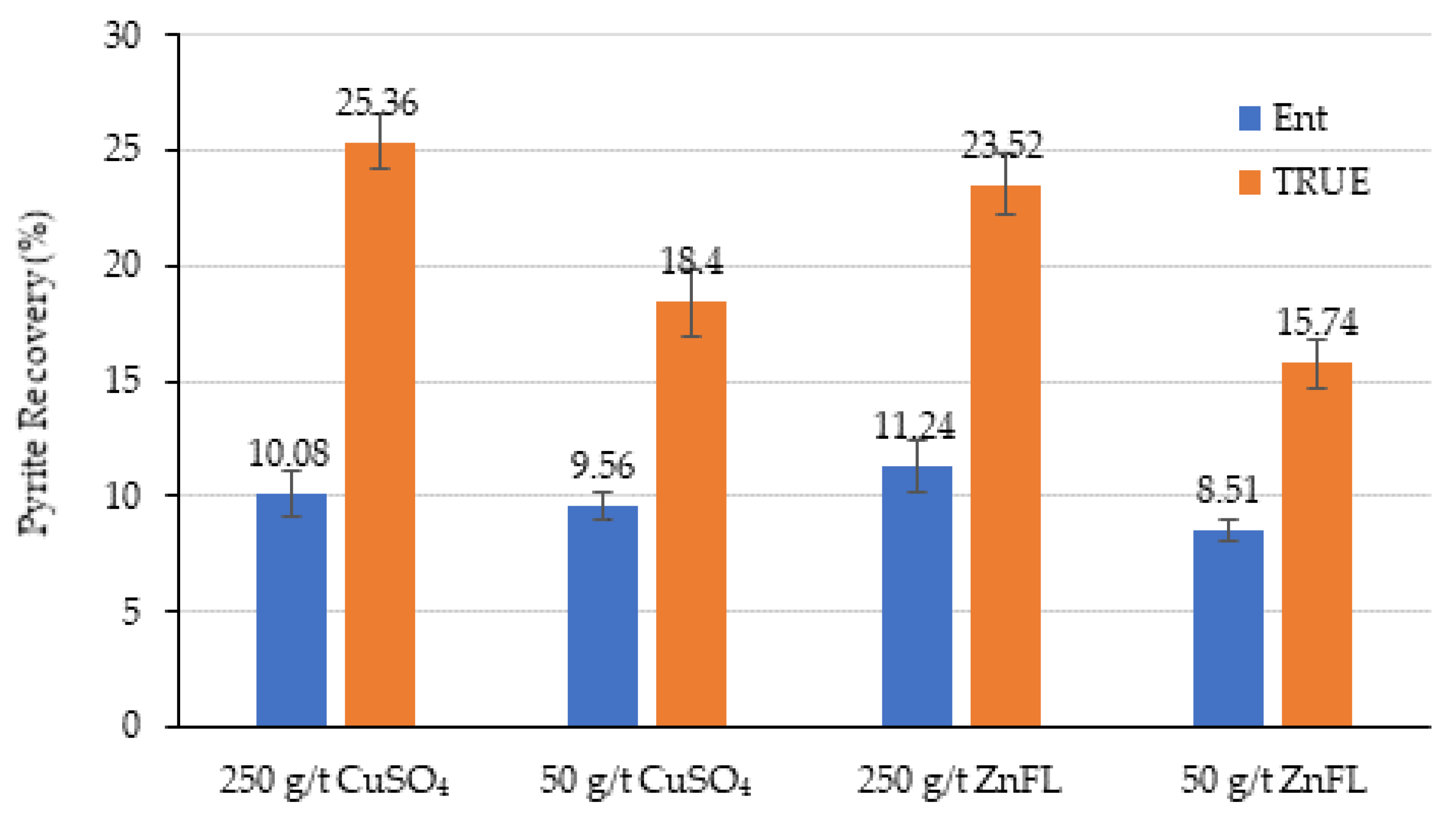
Figure 14 and
Figure 15 show the pyrite recovery by true flotation and entrainment for Ore 1 and Ore 2, respectively.
The grind size was d80 =180 µm for Ore 1, and recovery by entrainment should be low due to the coarse particle size distribution. The recovery by true flotation was higher at the 250 g/t dosage for both activators. The activation by copper sulfate was slightly higher than that by ZnFL. These results suggest that ZnFL does not enhance pyrite activation and may even contribute to mild depression through this mechanism.
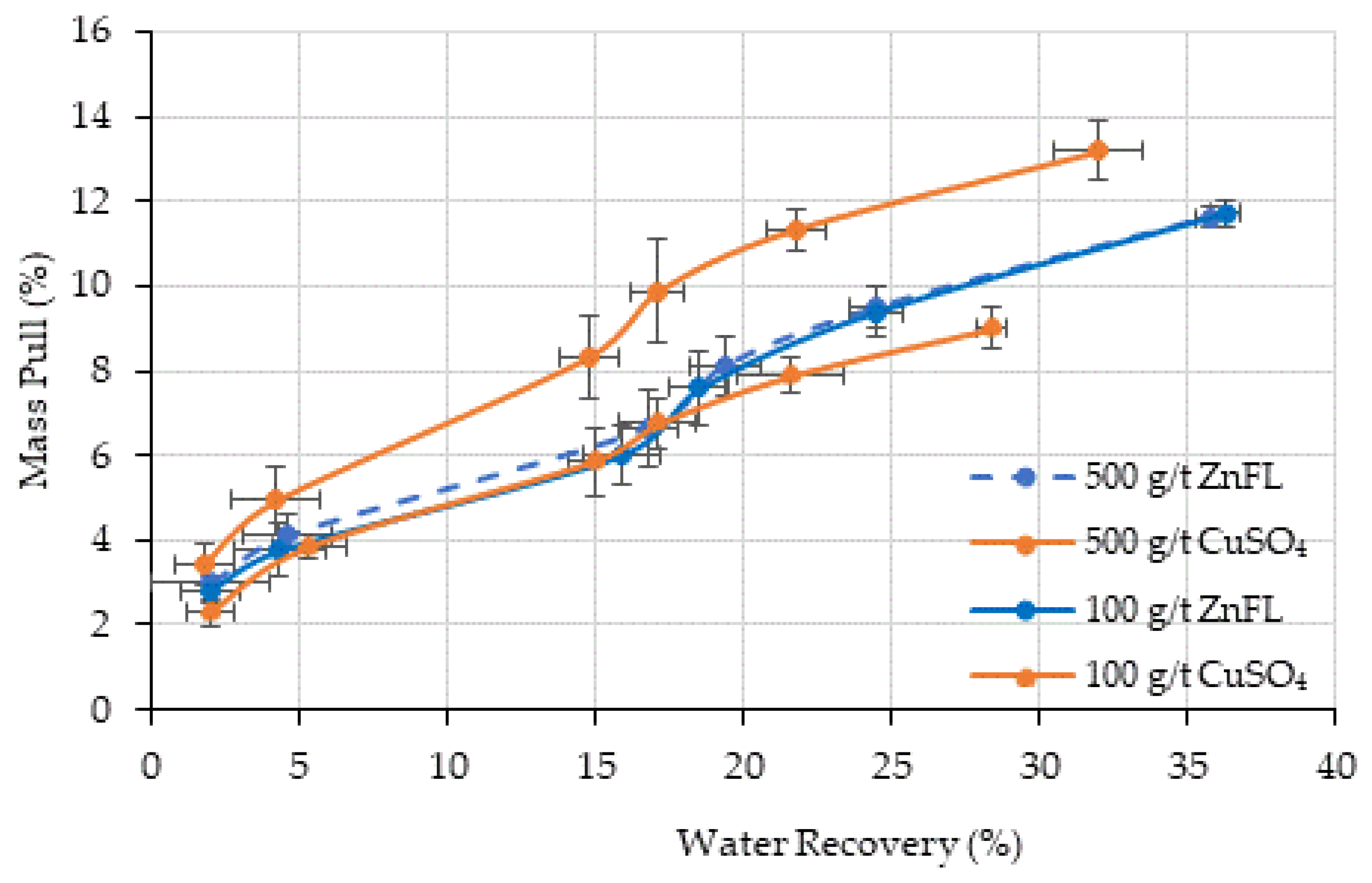
The grind size for Ore 2 was d80 = 38 µm, much finer than that for Ore 1. The recovery by entrainment was higher than that by true flotation under all test conditions. The recovery by true flotation was similar in all tests, except with 100 g/t CuSO4, where the water recovery and mass pull were lower than those in the other tests. Therefore, it was concluded that ZnFL did not show any activation or extra depression effects on pyrite in the Ore 2 sample. Both activators have similar effects.
Both activators were applied on an equal-reagent-dosage basis rather than on an equivalent-copper-content basis. Because the total active copper content differs between ZnFL and CuSO4—and ZnFL contains less active Cu—any comparable or superior performance by ZnFL at the same dosage indicates higher activation efficiency per unit of copper.
An important operational advantage of ZnFL is its stability across a wide pH range (10–11) and its compatibility with collectors during simultaneous addition. Tests showed that ZnFL could be added directly with SIPX without reducing the zinc recovery or grade, whereas CuSO4 required a pre-conditioning stage to avoid premature reaction with xanthate Equation (4). This feature simplifies plant operation by potentially eliminating the need for a separate activator conditioning tank and reducing the residence time.
The combination of lower dosage requirements, absence of pre-conditioning and reduced sulfate content (~15% vs. ~38% for CuSO4) makes ZnFL a technically and environmentally attractive alternative activator. A lower sulfate input can mitigate scaling, corrosion and water-recycling issues in flotation circuits. Together, these features point toward a more sustainable and simplified reagent regime for industrial sphalerite flotation.
5. Conclusions
This study evaluated the performance of a copper–amine complex reagent (ZnFL) as an alternative activator to copper sulfate (CuSO4) in the flotation of sphalerite-bearing ores of different mineralogy. The key findings are as follows:
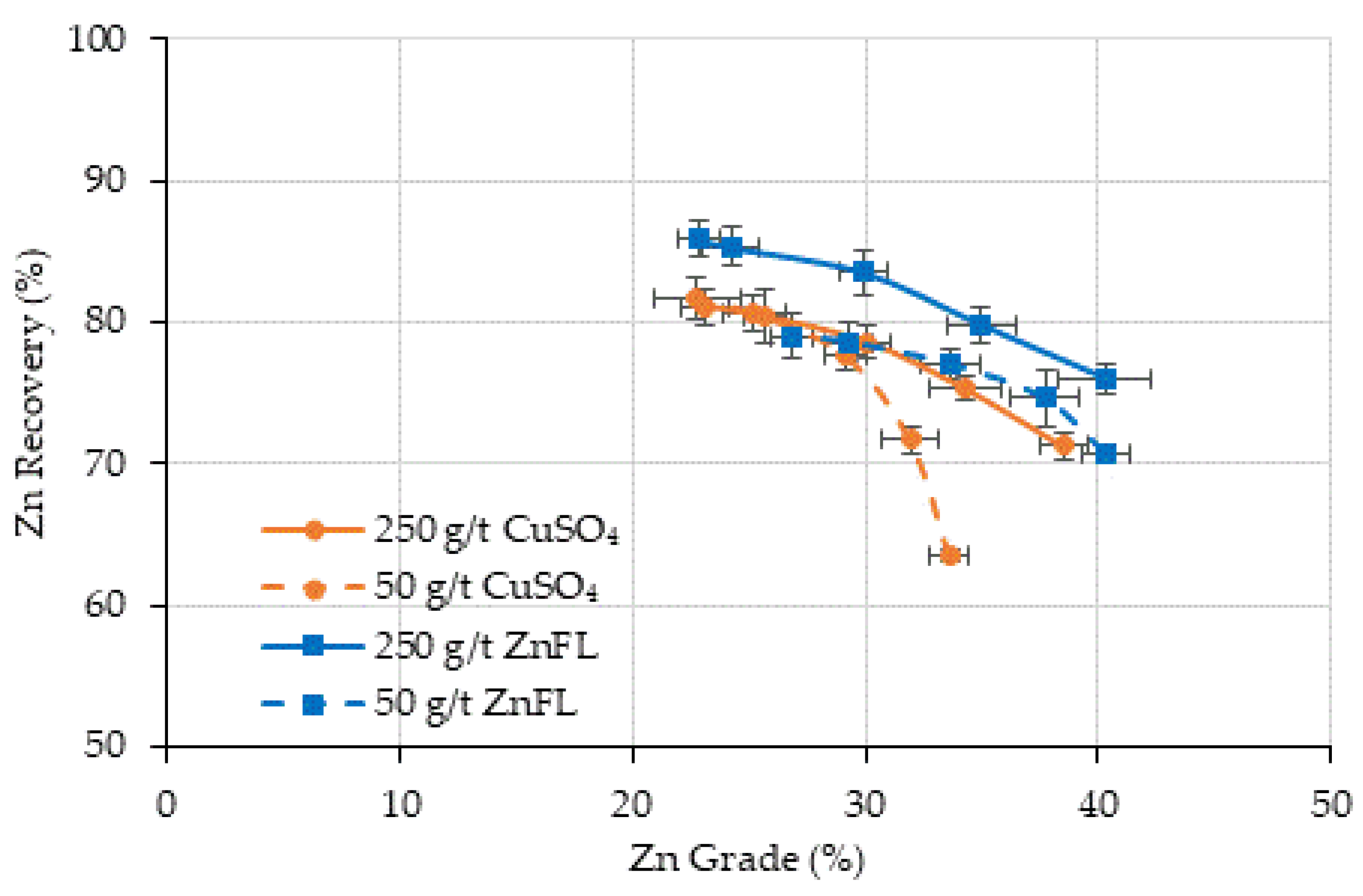
Under alkaline flotation conditions (pH 11–12) with SIPX collector and MIBC frother, ZnFL produced sphalerite recoveries equal to or higher than those obtained with CuSO4 at standard activator dosages, while maintaining or improving concentrate zinc grades.
Contact angle measurements confirmed that ZnFL induced higher sphalerite hydrophobicity (68–71°) than CuSO4 (61–66°), indicating stronger or more stable xanthate adsorption on ZnFL-activated surfaces.
ZnFL allowed simultaneous addition with the collector without loss of recovery or grade, eliminating the need for the pre-conditioning stage required by CuSO4. This can reduce the conditioning time and equipment requirements in plant operation.
ZnFL yielded better sphalerite/pyrite selectivity, particularly in the Cu–Zn ore, likely due to lower incidental activation of pyrite under strongly alkaline conditions.
Froth stability and water recovery trends indicated that ZnFL did not adversely affect flotation hydrodynamics, and metallurgical results were not compromised by froth drainage effects.
Overall, ZnFL provides a technically effective and operationally simpler alternative to CuSO4 for sphalerite activation, offering potential reductions in reagent dosage, sulfate load and process complexity. Further studies are recommended to elucidate its adsorption mechanism, interaction with recycled process water and applicability across different sphalerite ore types to support broader industrial adoption.