Gold and Silver Recovery from a Refractory Pyritic Concentrate by Roasting and Alkaline Pressure Oxidation
Abstract
1. Introduction
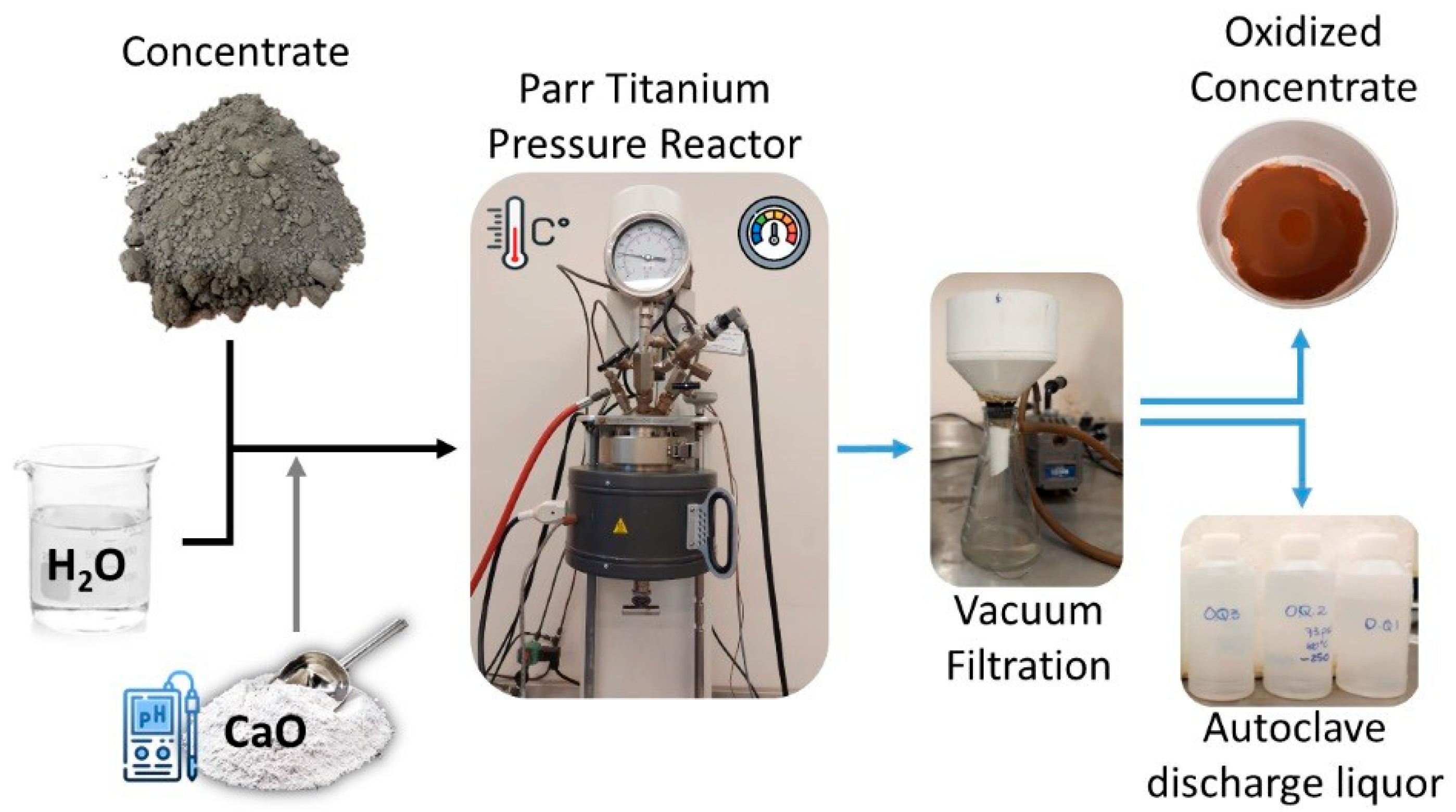
2. Materials and Methods
3. Results
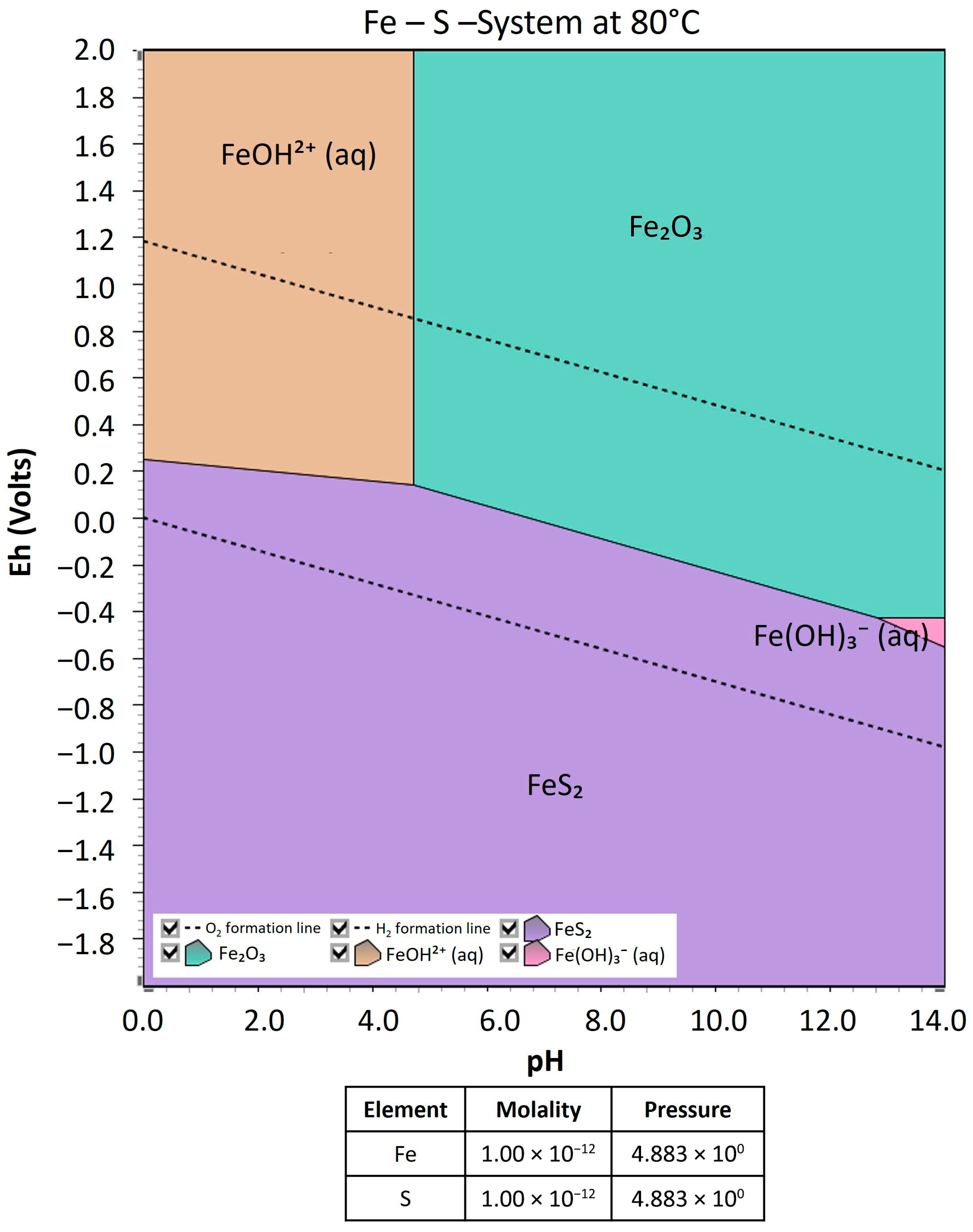
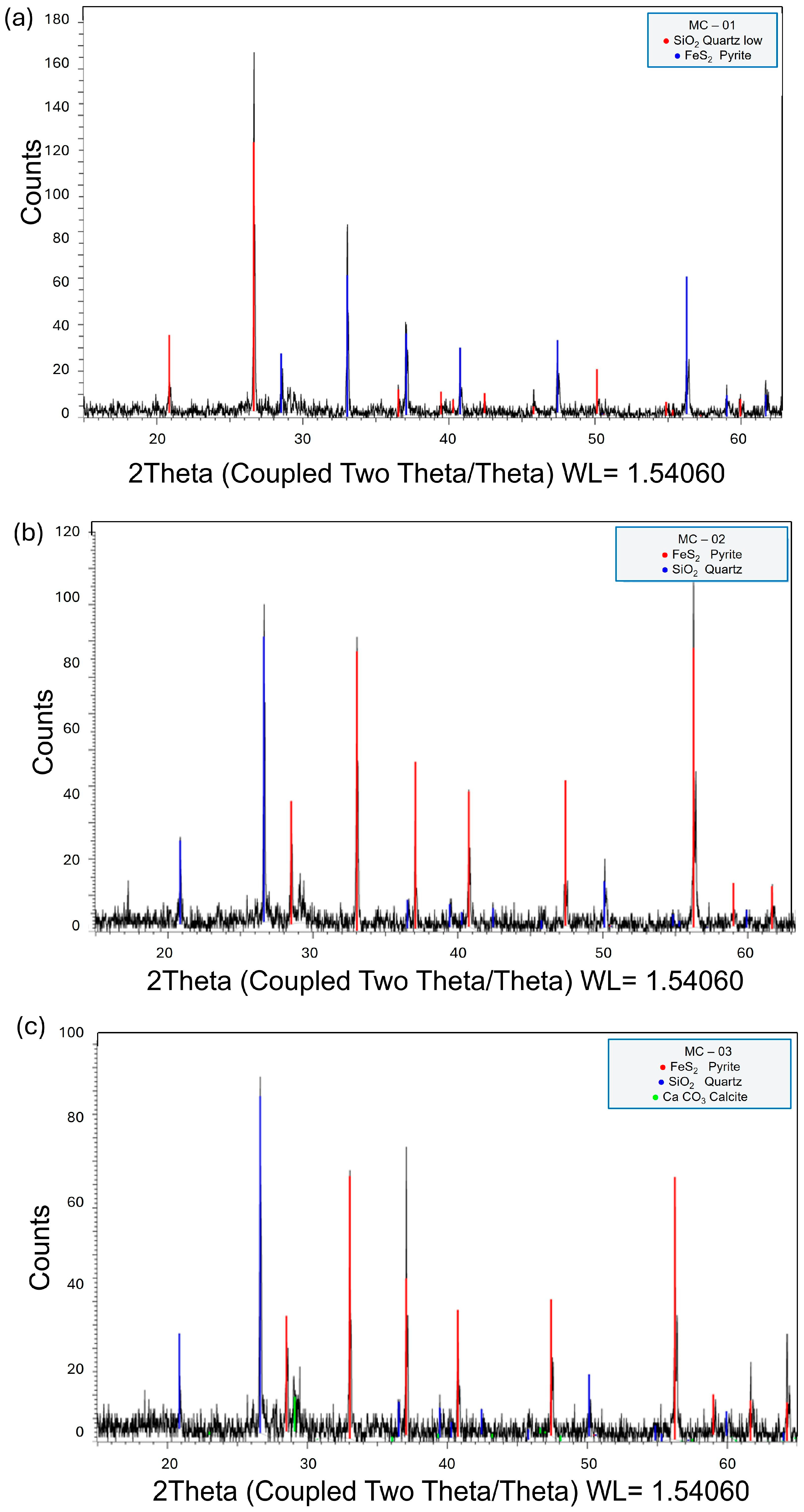
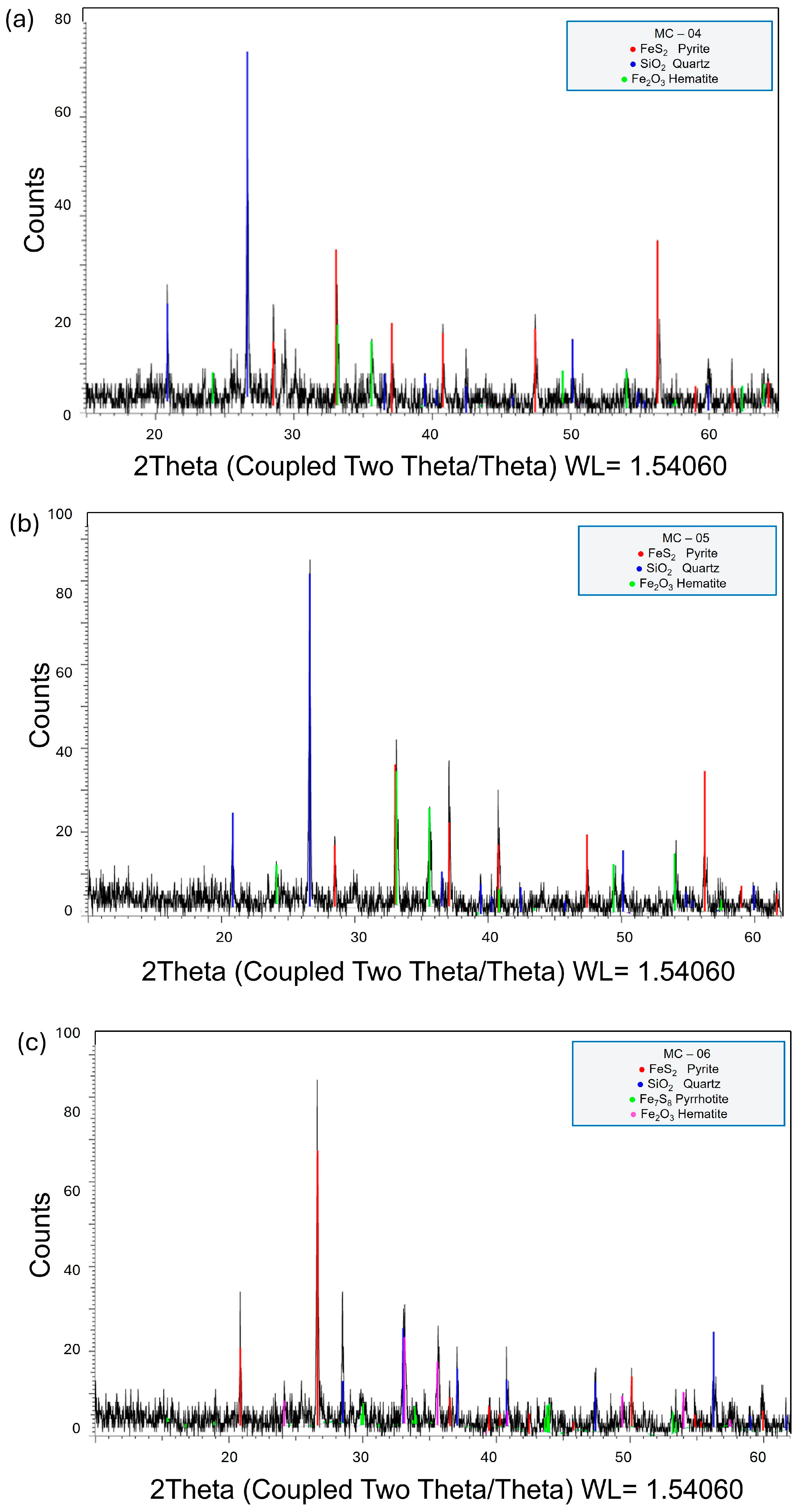
3.1. Mineralogical Characterization
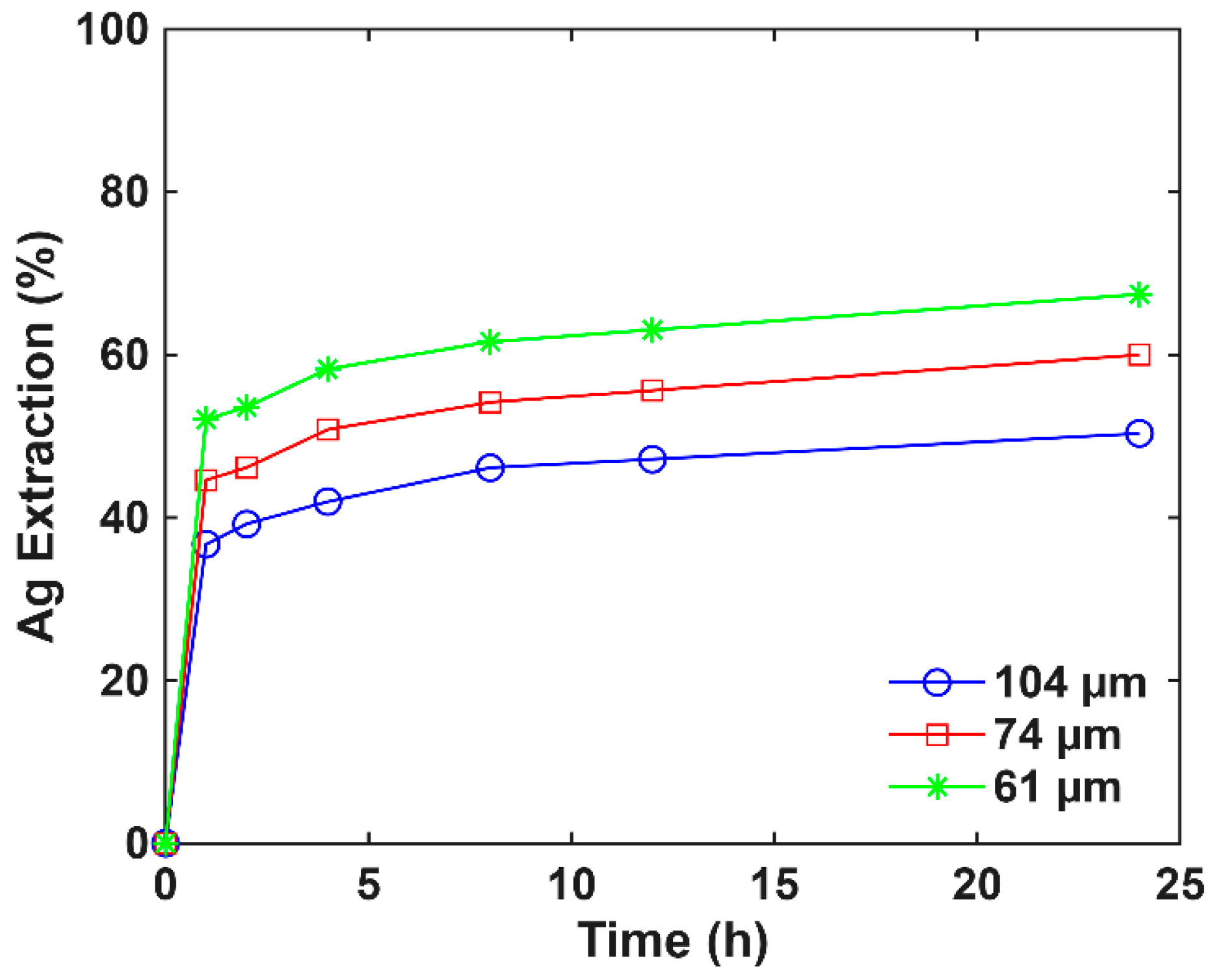
3.2. Conventional Cyanidation Test
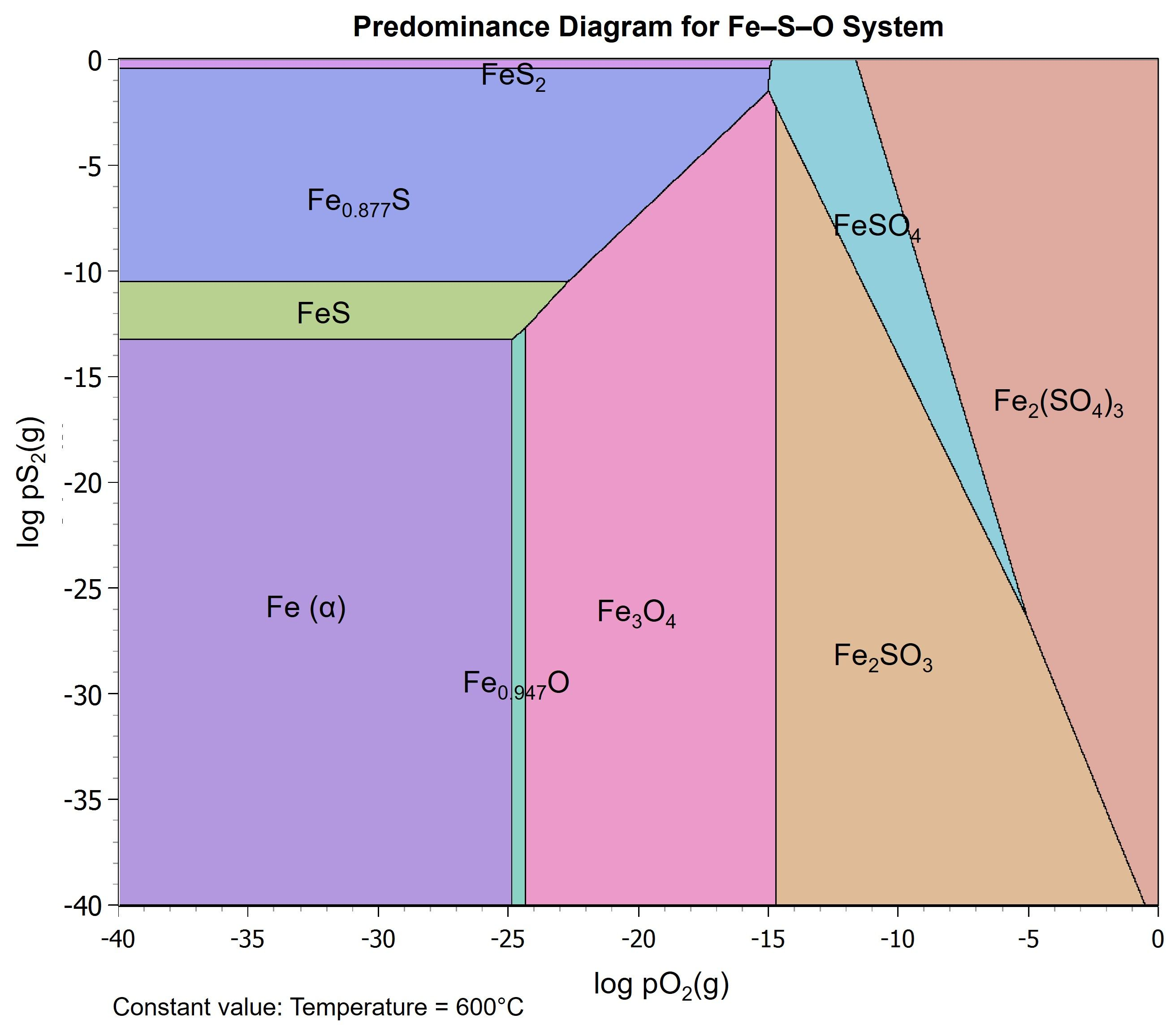
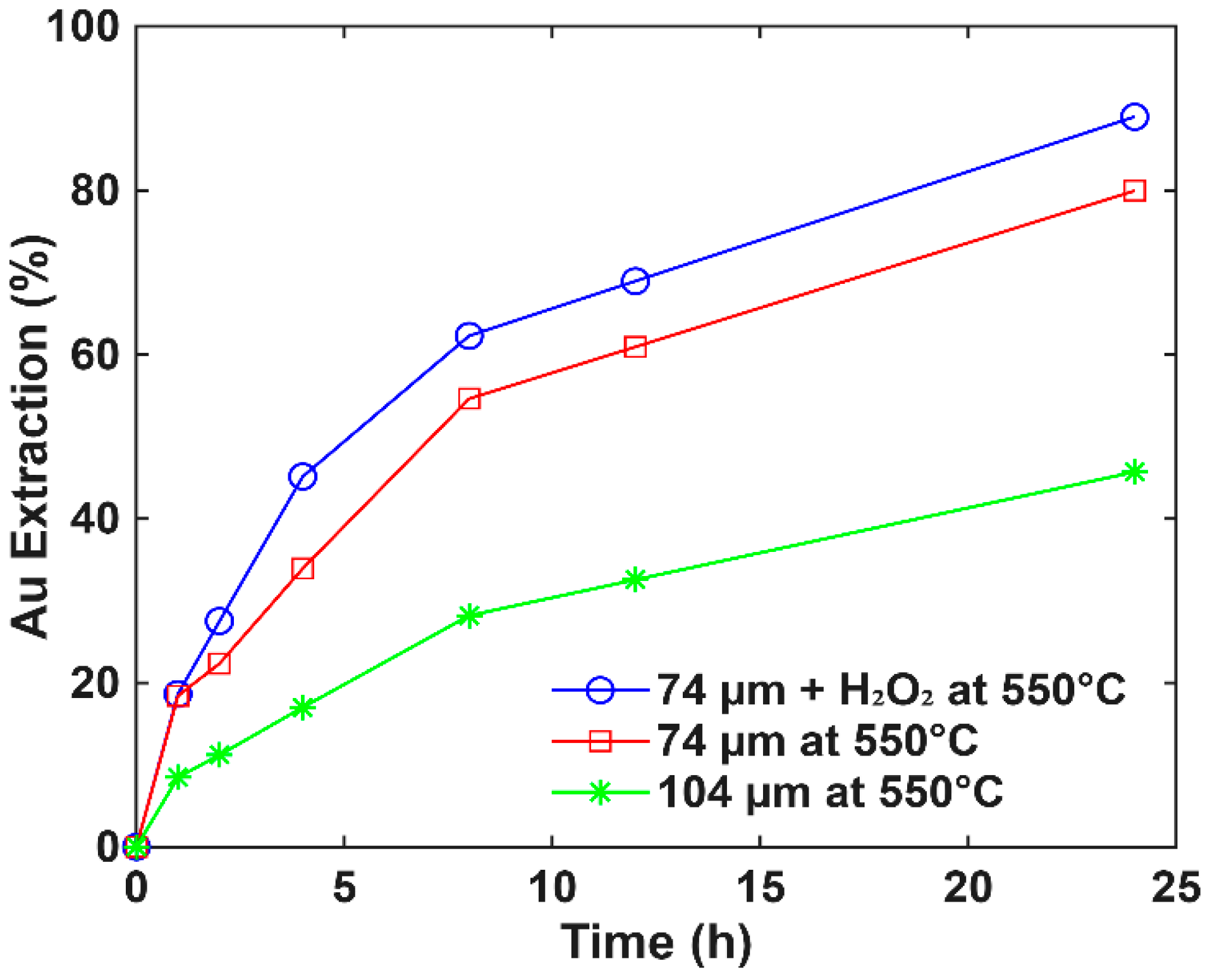
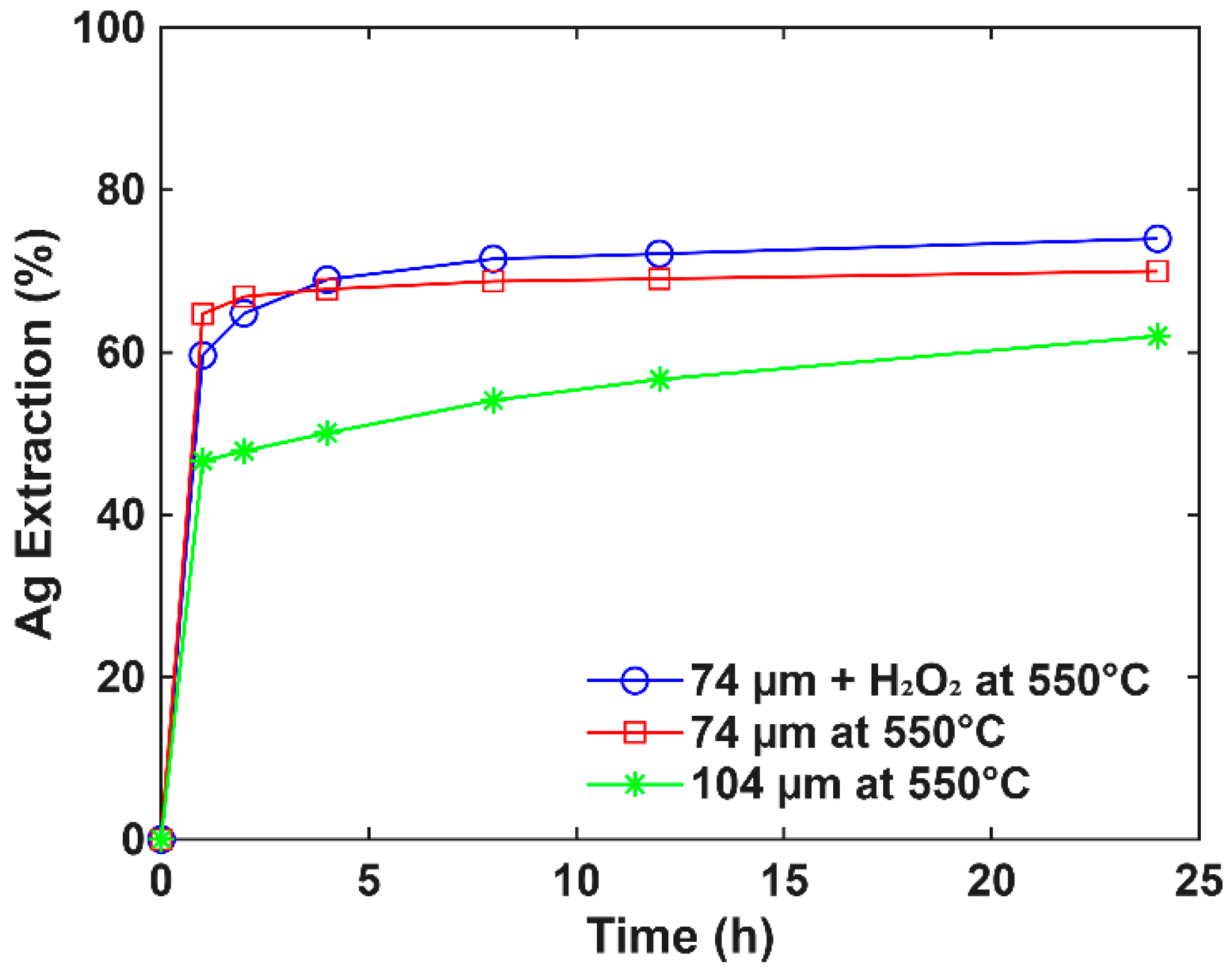
3.3. Roasting Effect on Extraction
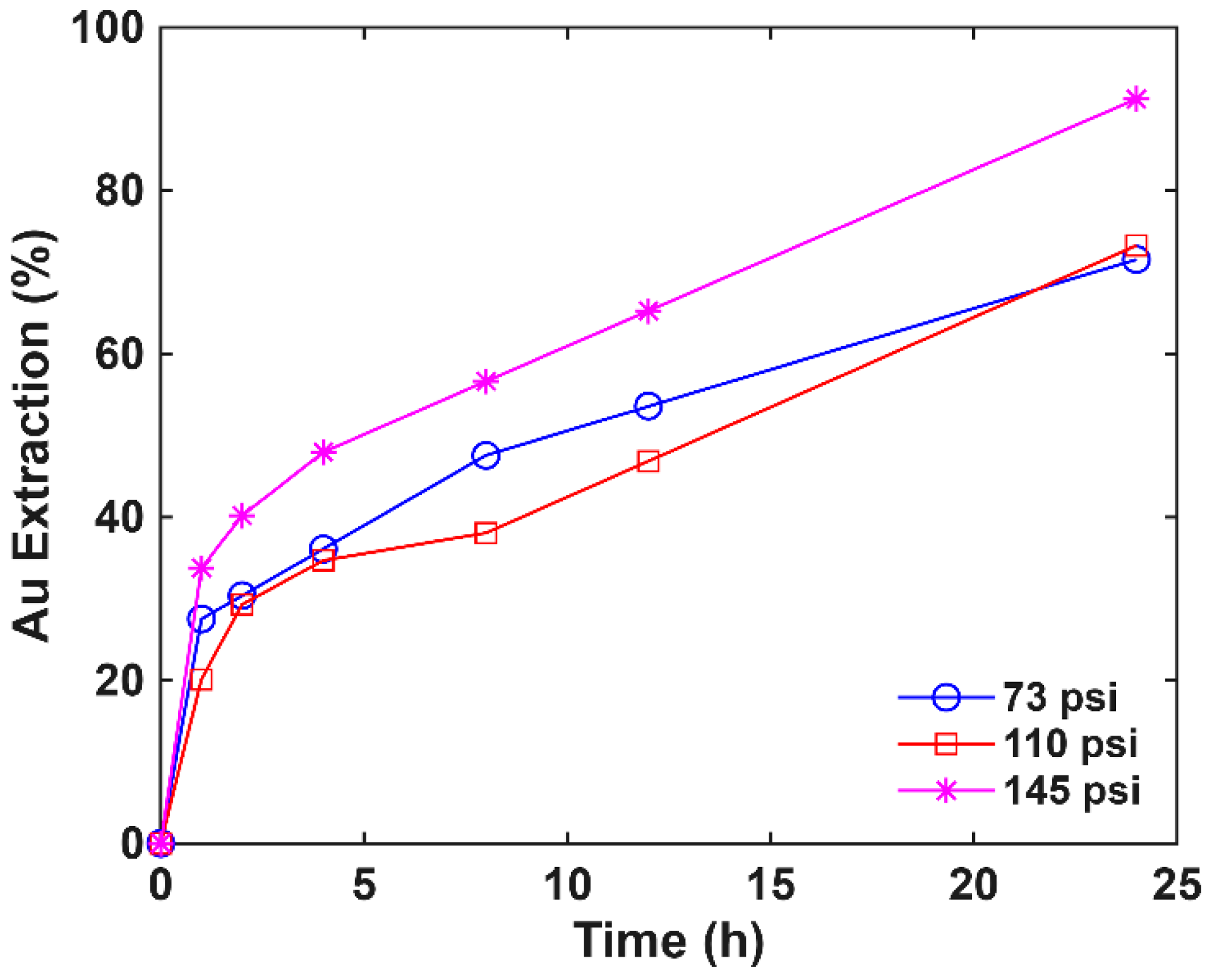
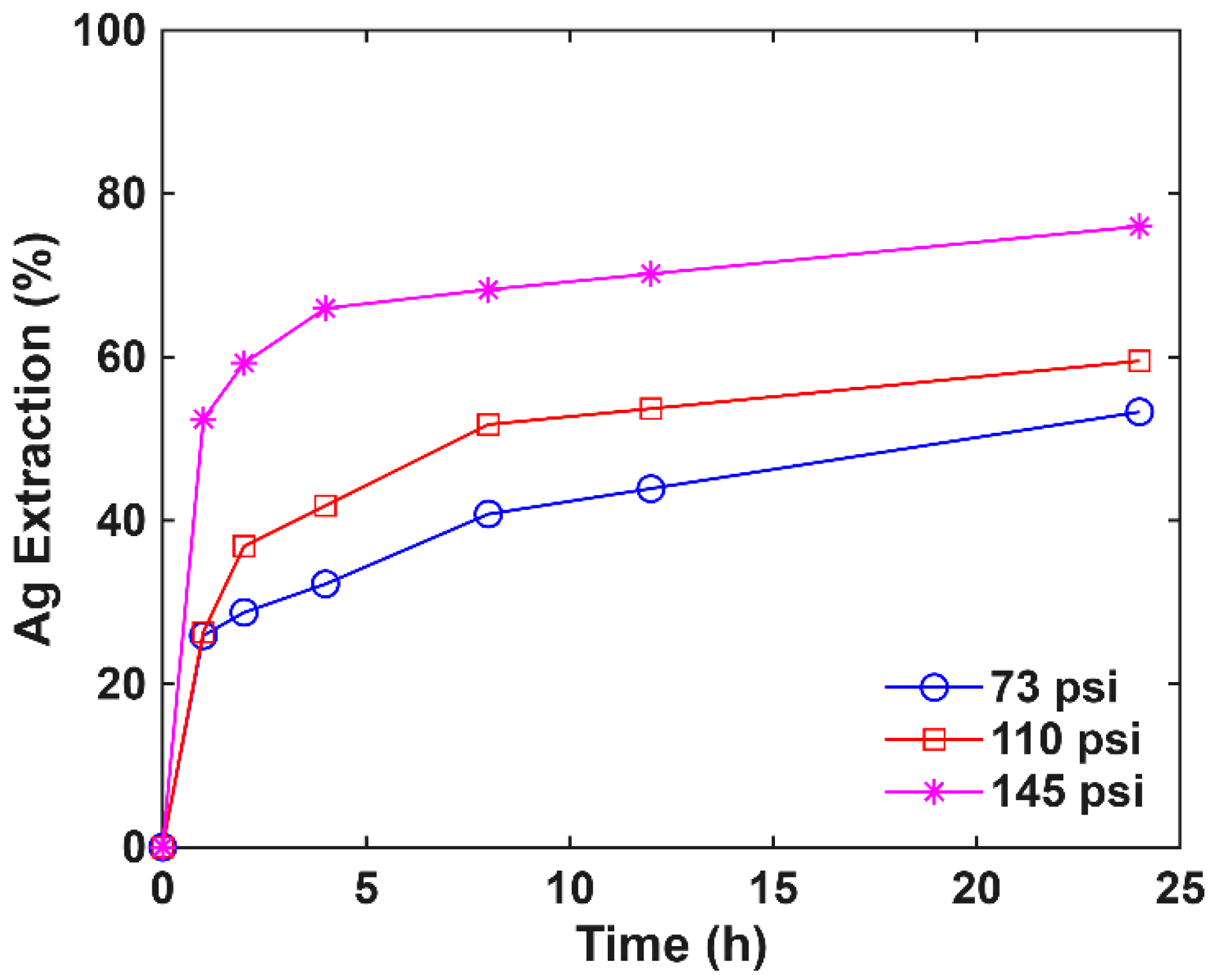
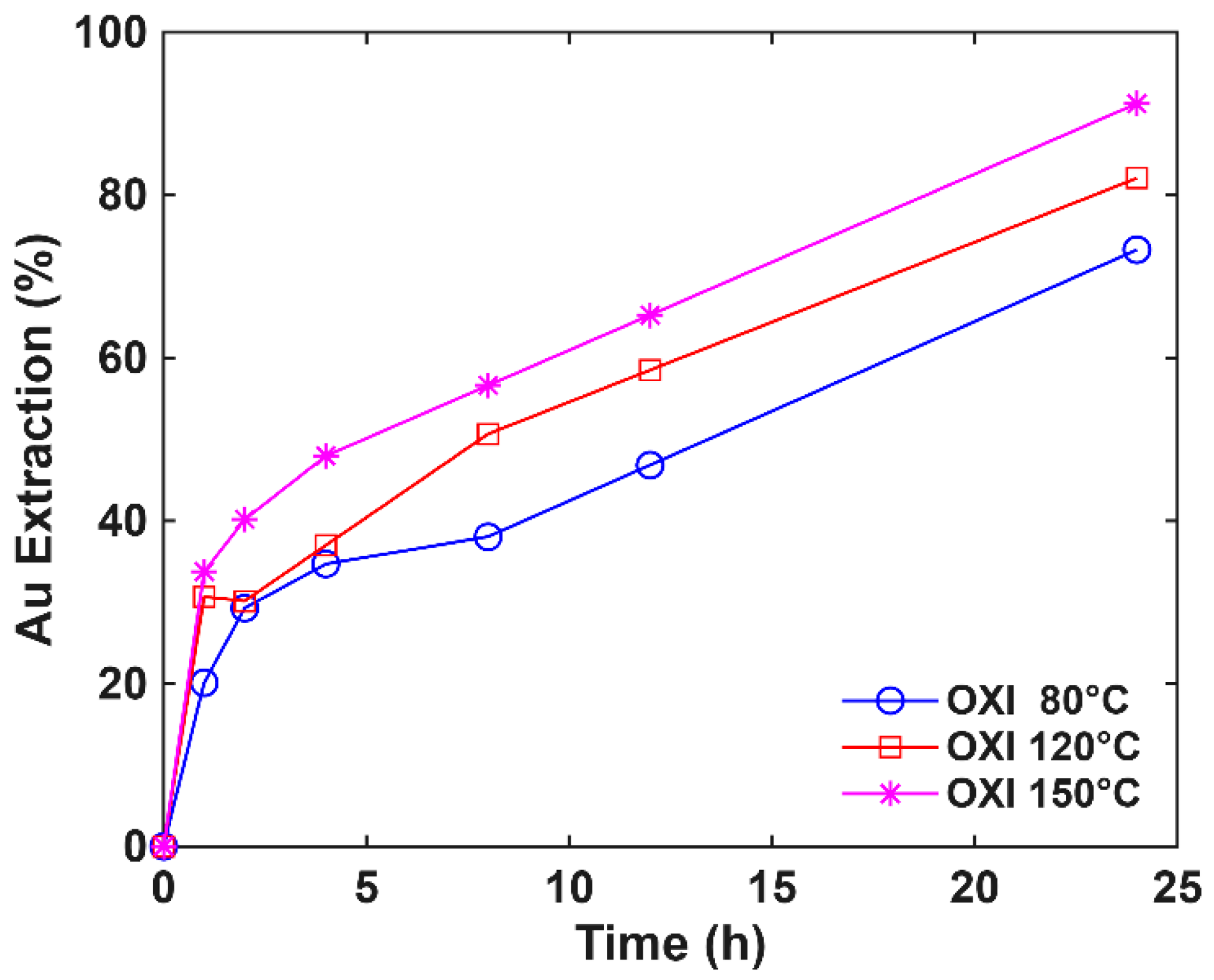
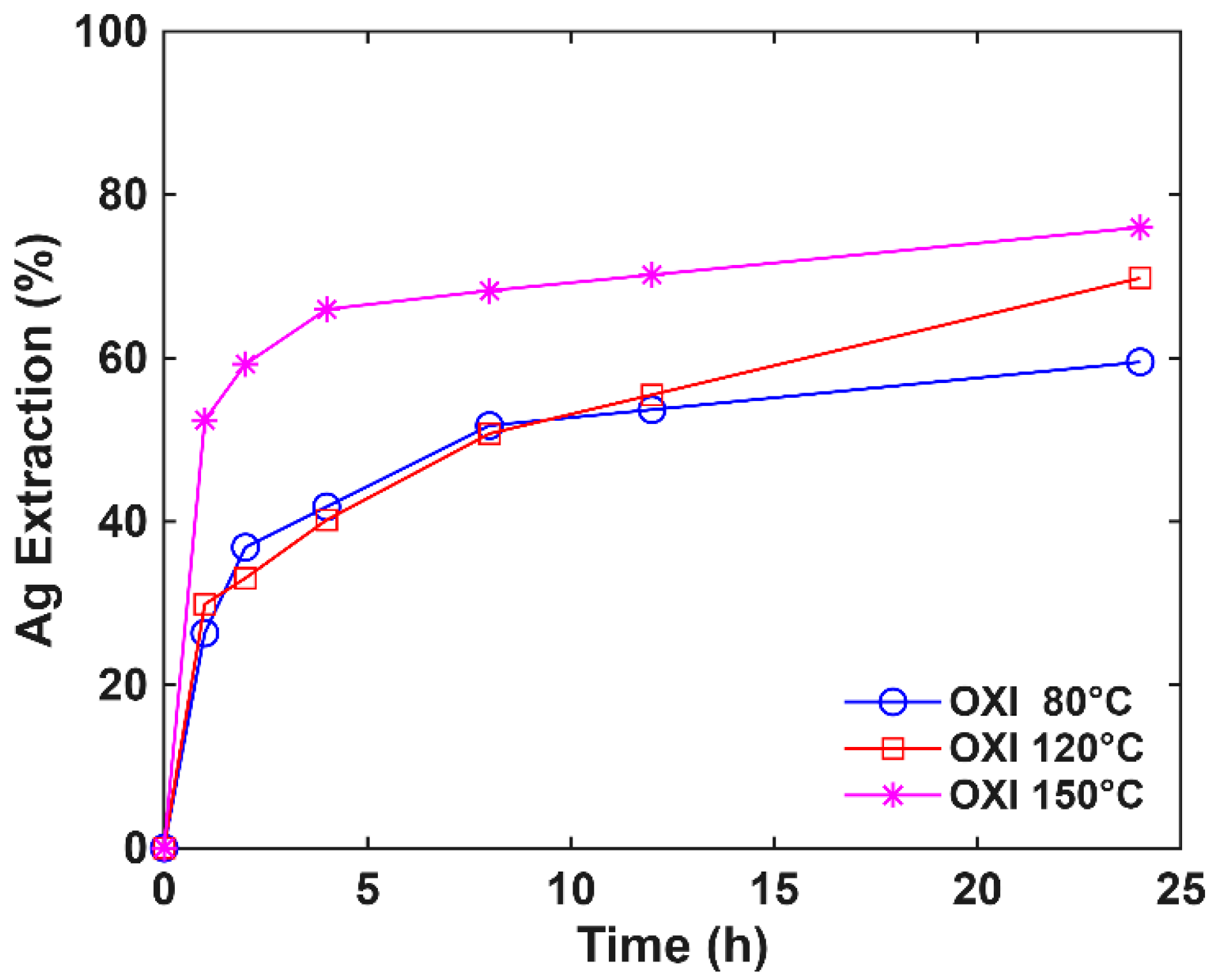
3.4. Pressure Effect on Oxidative Pretreatment
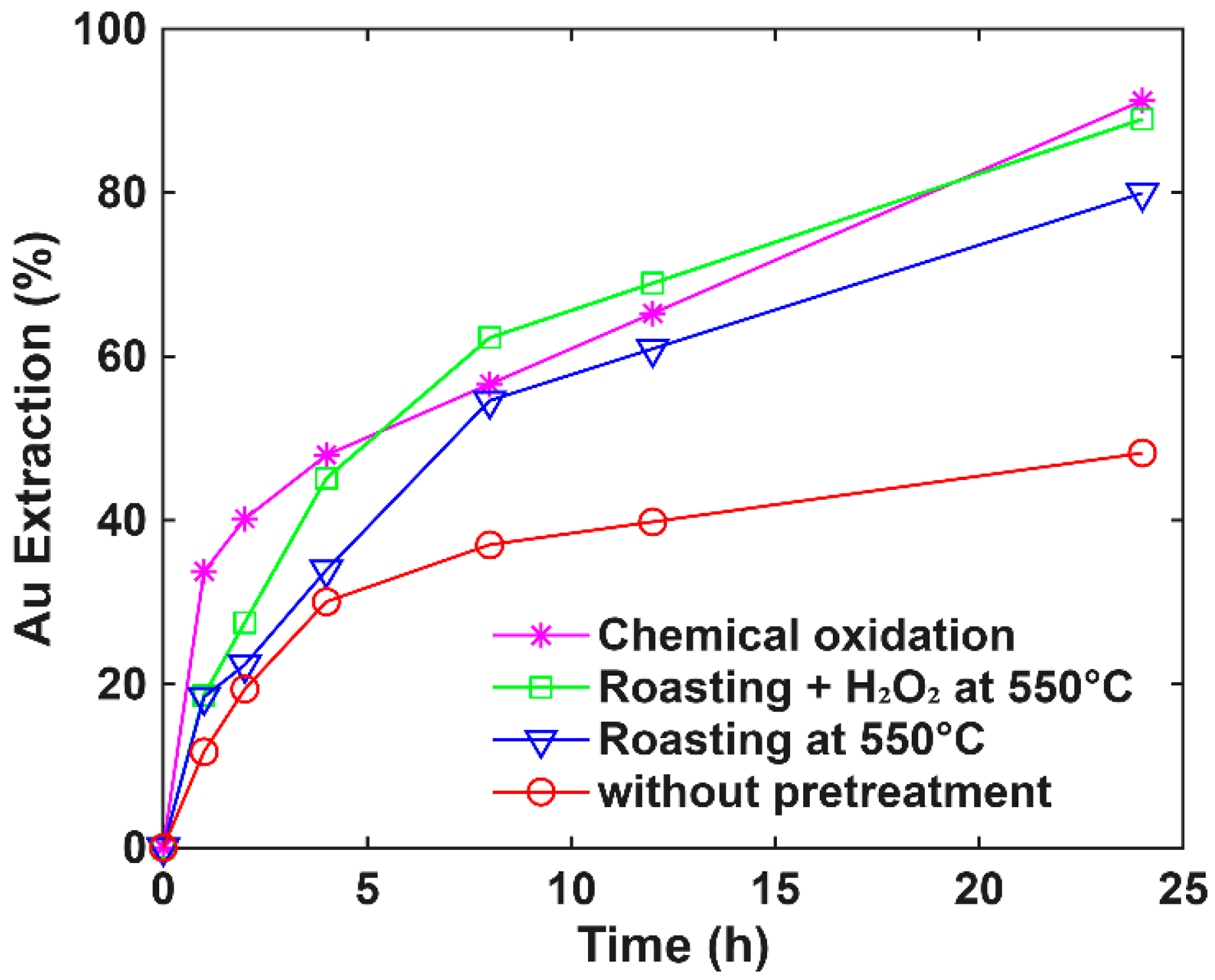
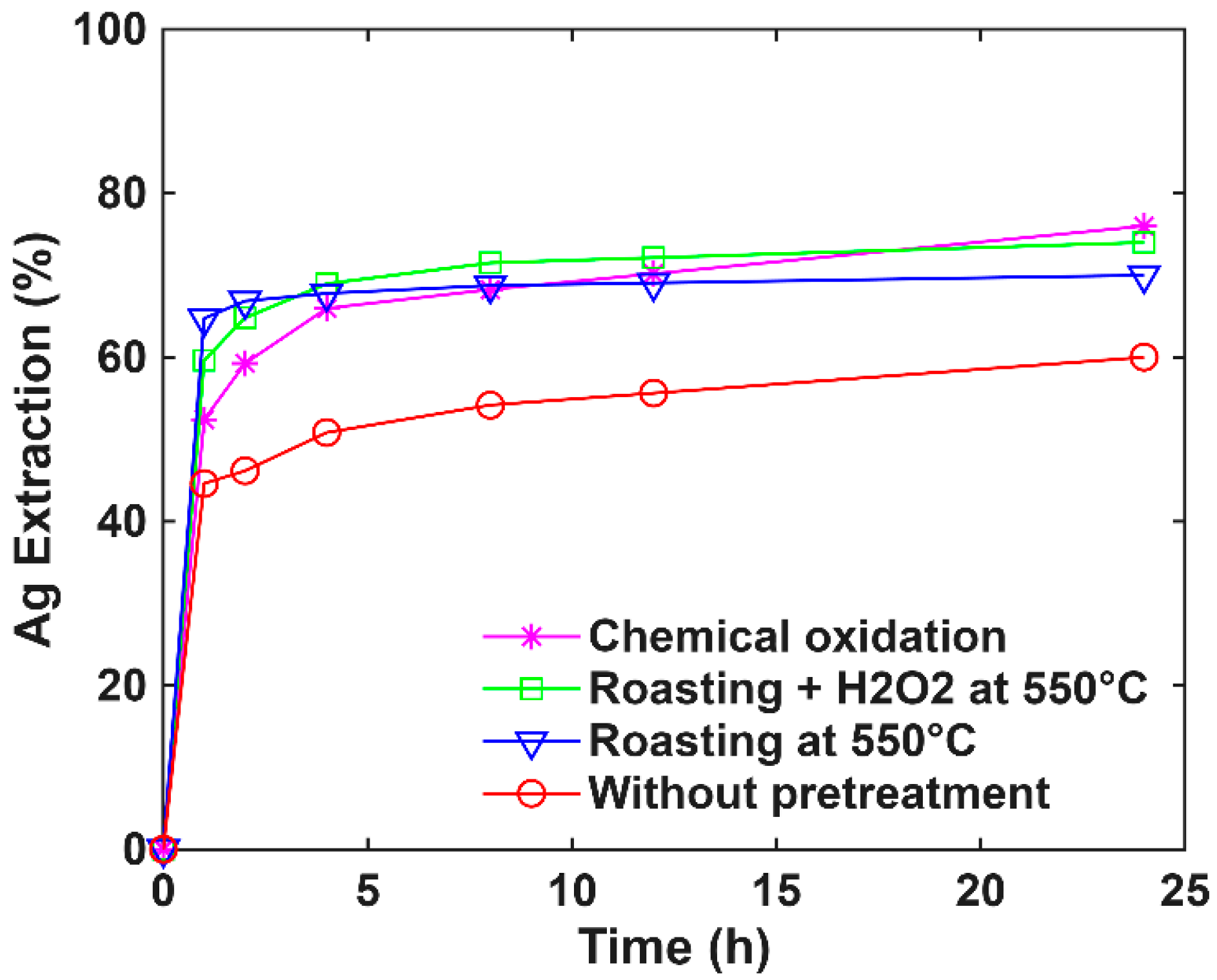
3.5. Comparative Evaluation of Oxidative Pretreatments for Gold and Silver Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parga, J.R.; Valenzuela, J.L.; Cepeda, T.F. Pressure cyanide leaching for precious metals recovery. JOM 2007, 59, 43–47. [Google Scholar] [CrossRef]
- Gómez Santiago, M.; Martínez-Ponce, M.Á.; Ortiz Lara, N.; Escudero García, R.; Cholico-González, D. Ultrafine grinding of a refractory ore and its effect on the gold and silver leaching. MRS Adv. 2025, 10, 868–873. [Google Scholar] [CrossRef]
- Saba, M.; MohammadYousefi, A.; Rashchi, F.; Moghaddam, J. Diagnostic pre-treatment procedure for simultaneous cyanide leaching of gold and silver from a refractory gold/silver ore. Miner. Eng. 2012, 24, 1703. [Google Scholar] [CrossRef]
- Coronado, J.H.; Encinas, M.A.; Leyva, J.C.; Valenzuela, J.L.; Valenzuela, A.; Munive, G.T. Tostación de un concentrado refractario de oro y plata. Rev. De Metal. 2012, 48, 165–174. [Google Scholar] [CrossRef]
- Marsden, J.; House, I. The Chemistry of Gold Extraction; SME: Littleton, CO, USA, 2006. [Google Scholar]
- Valenzuela, A.; Valenzuela, J.L.; Parga, J.R. Effect of Pretreatment of Sulfide Refractory Concentrate with Sodium H ypochlorite, Followed by Extraction of Gold by Pressure Cyanidation, o n Gold Removal. Adv. Chem. Eng. Sci. 2013, 3, 171–177. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Jiao, F. Pretreatment and Extraction of Gold from Refractory Gold Ore in Acidic Conditions. Minerals 2025, 15, 340. [Google Scholar] [CrossRef]
- Quijada-Noriega, Y.R.; Valenzuela-Garcia, J.L.; Salazar-Campoy, M.M.; Tiburcio-Munive, G.; Vazquez-Vazquez, V.M.; Parga-Torres, J.R. Gold Extraction from a Refractory Ore Using Calcium Hypochlorite at Moderate Pressure and Temperature before Cyanidation. ACS Eng. Au 2025, 5, 89–97. [Google Scholar] [CrossRef]
- Barbouchi, A.; Ayadi, S.; Idouhli, R.; Khadiri, M.E.; Abouelfida, A.; Barfoud, L.; Faqir, H.; Benzakour, I.; Benzakour, J. Highly efficient pretreatment for refractory gold ores using persulfate, catalyst and free radical based advanced oxidation processes to improve cyanidation. Hydrometallurgy 2025, 235, 106488. [Google Scholar] [CrossRef]
- Gui, Q.; Fu, L.; Hu, Y.; Di, H.; Liang, M.; Wang, S.; Zhang, L.; Dong, E. Oxidative pretreatment of refractory gold ore using persulfate under ultrasound for efficient leaching of gold by a novel eco-friendly lixiviant: Demonstration of the effect of particle size and economic benefits. Hydrometallurgy 2023, 221, 106110. [Google Scholar] [CrossRef]
- Barbouchi, A.; Louarrat, M.; Mikali, M.; Barfoud, L.; El Alaoui-Chrifi, M.A.; Faqir, H.; Benzakour, I.; Idouhli, R.; Khadiri, M.E.; Benzakour, J. Advancements in improving gold recovery from refractory gold ores/concentrates: A review. Can. Metall. Q. 2025, 64, 2370–2387. [Google Scholar] [CrossRef]
- Soto-Uribe, J.C.; Valenzuela-Garcia, J.L.; Salazar-Campoy, M.M.; Parga-Torres, J.R.; Tiburcio-Munive, G.; Encinas-Romero, M.A.; Vazquez-Vazquez, V.M. Gold Extraction from a Refractory Sulfide Concentrate by Simultaneous Pressure Leaching/Oxidation. Minerals 2023, 13, 116. [Google Scholar] [CrossRef]
- Xiao, H.; Jin, J.; He, F.; Han, Y.; Sun, Y. Strengthening gold extraction from carbonaceous gold ore based on decarburization by two-stage fluidized oxidation roasting. Minerals 2022, 12, 1620. [Google Scholar] [CrossRef]
- Hapid, A.; Zullaikah, S.; Kawigraha, A.; Sudiyanto, Y.; Nareswari, R.B.; Quitain, A.T. Oxidation of sulfide mineral and metal extraction analysis in the microwave-assisted roasting pretreatment of refractory gold ore. Arab. J. Chem. 2024, 17, 105447. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Jin, J.; Han, Y.; Li, Y. Pore Evolution in Refractory Gold Ore Formed by Oxidation Roasting and the Effect on the Cyanide Leaching Process. ACS Omega 2022, 7, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Barbouchi, A.; El Mouden, A.A.; Labbilta, T.; Anasser, I.; Idouhli, R.; Khadiri, M.E.; Abouelfida, A.; Faqir, H.; Benzakour, I.; Benzakour, J. Optimization of Single-Stage Roasting Process for Pretreatment of Refractory Gold Concentrate Using Experimental Design. J. Sustain. Metall. 2025, 11, 2650–2662. [Google Scholar] [CrossRef]
- Yang, Y.-b.; Li, Q.; Jiang, T.; Guo, Y.-f.; Li, G.-h.; Xu, B. Co-intensification of gold leaching with heavy metals and hydrogen peroxide. Trans. Nonferrous Met. Soc. China 2010, 20, 903–909. [Google Scholar] [CrossRef]
- Lemos, F.D.A.; Nascimento, M.; Moreira Júnior, G.R.; Andrade, V.R.D.; Pinto, P.C.; Salles, A.J.G. Recovery of gold from refractory ore employing pressure oxidation. REM-Int. Eng. J. 2025, 78, e230116. [Google Scholar] [CrossRef]
- Lee, S.; Sadri, F.; Ghahreman, A. Enhanced gold recovery from alkaline pressure oxidized refractory gold ore after its mechanical activation followed by thiosulfate leaching. J. Sustain. Metall. 2022, 8, 186–196. [Google Scholar] [CrossRef]
- Guzman, L.; Segarra, M.; Chimenos, J.M.; Fernandez, M.A.; Espiell, F. Gold cyanidation using hydrogen peroxide. Hydrometallurgy 1999, 52, 21–35. [Google Scholar] [CrossRef]
- Nunan, T.O.; Viana, I.L.; Peixoto, G.C.; Ernesto, H.; Verster, D.M.; Pereira, J.H.; Bonfatti, J.M.; Teixeira, L.A.C. Improvements in gold ore cyanidation by pre-oxidation with hydrogen peroxide. Miner. Eng. 2017, 108, 67–70. [Google Scholar] [CrossRef]
- Liu, Y.; Sung, N.G.W.; Chen, M. Thermodynamic analysis of iron and arsenic species distribution and phase variation of pyrite and arsenopyrite under acidic oxidation relevant to refractory gold ores. Hydrometallurgy 2025, 236, 106496. [Google Scholar] [CrossRef]
- Mbuya, B.; Fosso-Kankeu, E.; Bongaerts, J.; Mulaba-Bafubiandi, A.F. Thermodynamic Investigation on the Impact of Oxidized Copper–Cobalt and Copper Sulfide Ores Stream Mixture Toward the Dissolution of Cu and Co. J. Sustain. Metall. 2025, 11, 1299–1318. [Google Scholar] [CrossRef]
- Hinojosa, O. Oxidación de Sulfuros: Importante Proceso de Pretratamiento; Revista Metalúrgica UTO: Oruro, Bolivia, 2002; p. 31. [Google Scholar]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Aracena, A.; Jerez, O. Mechanism and kinetics of pyrite transformation at elevated temperatures. Physicochem. Probl. Miner. Process. 2021, 57, 131–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Fang, Y.; Cabrera, A.R.; Peng, C.; López-Valdivieso, A. Roasting temperature effect on the recovery of refractory gold and silver in pyrite concentrates. J. Min. Metall. Sect. B Metall. 2021, 57, 19. [Google Scholar] [CrossRef]
- Deschênes, G.; Prud, P.J.H. Cyanidation of a copper-gold ore. Int. J. Miner. Process. 1997, 50, 127–141. [Google Scholar] [CrossRef]
- Larrabure, G.; Rodríguez-Reyes, J.C.F. A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process. Miner. Eng. 2021, 173, 107194. [Google Scholar] [CrossRef]
- Bas, A.D.; Zhang, W.; Ghali, E.; Choi, Y. A study of the electrochemical dissolution and passivation phenomenon of roasted gold ore in cyanide solutions. Hydrometallurgy 2015, 158, 1–9. [Google Scholar] [CrossRef]
- Alvarez, A.G. Manual de Métodos Analíticos Para Rocas y Minerales; Edit. UniSon: Hermosillo, Mexico, 2004; ISBN 9706892060. [Google Scholar]
- Celep, O.; Yazici, E.; Deveci, H.; Mercimek, M.; Gonen, A.; Demirel, C.; Cingoz, M.; Komurcu, H.; Cakmak, S. Leaching behaviour of base and precious metals during cyanide leaching of a pyrite concentrate. Physicochem. Probl. Miner. Process. 2025, 61, 203328. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching chalcopyrite concentrate with oxygen and sulfuric acid using a low-pressure reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Aydin, B.; Basturkcu, H.; Gul, A. The influence of pre-aeration on cyanide leaching of a non-refractory sulphide gold and silver ore. Physicochem. Probl. Miner. Process. 2015, 51, 647–660. [Google Scholar]
- Bas, A.D.; Larachi, F.; Laflamme, P. The effect of pyrite particle size on the electrochemical dissolution of gold during cyanidation. Hydrometallurgy 2018, 175, 367–375. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D.B. Leaching of silver sulfide with ferricyanide–cyanide solution. Hydrometallurgy 2007, 88, 98–108. [Google Scholar] [CrossRef]
- Adams, M.D. Advances in Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Yessengarayev, Y.K.; Surimbayev, B.N.; Baimbetov, B.S.; Mamyachenkov, S.V.; Kanaly, T.S. Ore treatment hydrogen peroxide during heap leaching of gold. Kompleks. Ispolz. Miner. Syra = Complex Use Miner. Resour. 2021, 316, 5–14. [Google Scholar] [CrossRef]
- Bayat, O.; Vapur, H.; Akyol, F.; Poole, C. Effects of oxidising agents on dissolution of Gumuskoy silver ore in cyanide solution. Miner. Eng. 2003, 16, 395–398. [Google Scholar] [CrossRef]
- Rusanen, L.; Aromaa, J.; Forsen, O. Pressure oxidation of pyrite-arsenopyrite refractory gold concentrate. Physicochem. Probl. Miner. Process. 2013, 49, 101–109. [Google Scholar] [CrossRef]
- Parga, J.R.; Carrillo, F.R. Avances en los métodos de recuperación de oro y plata de minerales refractarios. Rev. De Metal. 1996, 32, 254–261. [Google Scholar] [CrossRef]
- Adams, M.; Lawrence, R.; Bratty, M. Biogenic sulphide for cyanide recycle and copper recovery in gold–copper ore processing. Min. Eng. 2008, 21, 509–517. [Google Scholar] [CrossRef]
- Hurtado, R.; Suazo, A. Lixiviación de plata a temperatura alta en mineral complejo de pirita. Rev. Soc. Química Perú 2019, 85, 97–108. [Google Scholar]
- Bidari, E.; Aghazadeh, V. Pyrite from Zarshuran Carlin-type gold deposit: Characterization, alkaline oxidation pretreatment, and cyanidation. Hydrometallurgy 2018, 179, 222–231. [Google Scholar] [CrossRef]
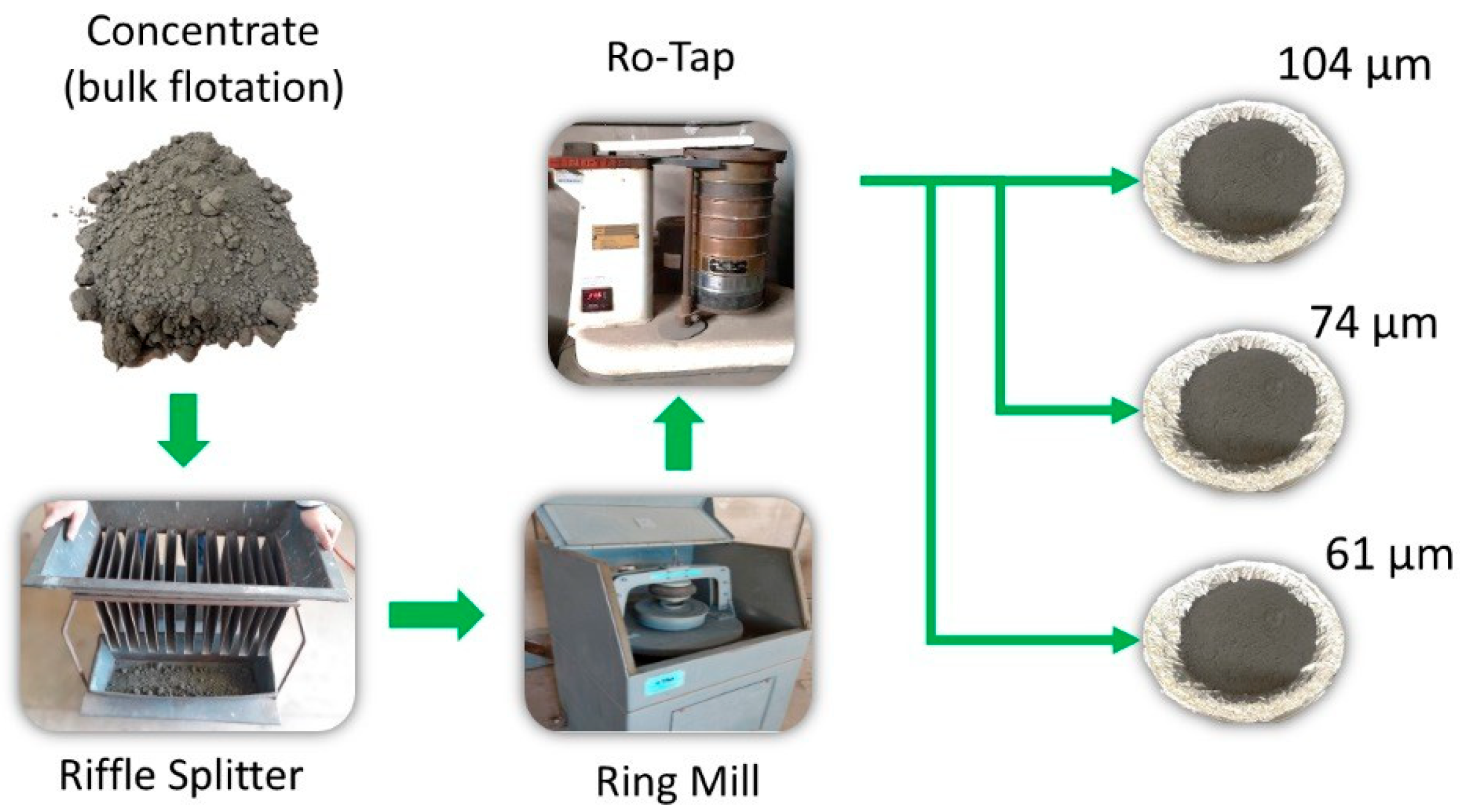
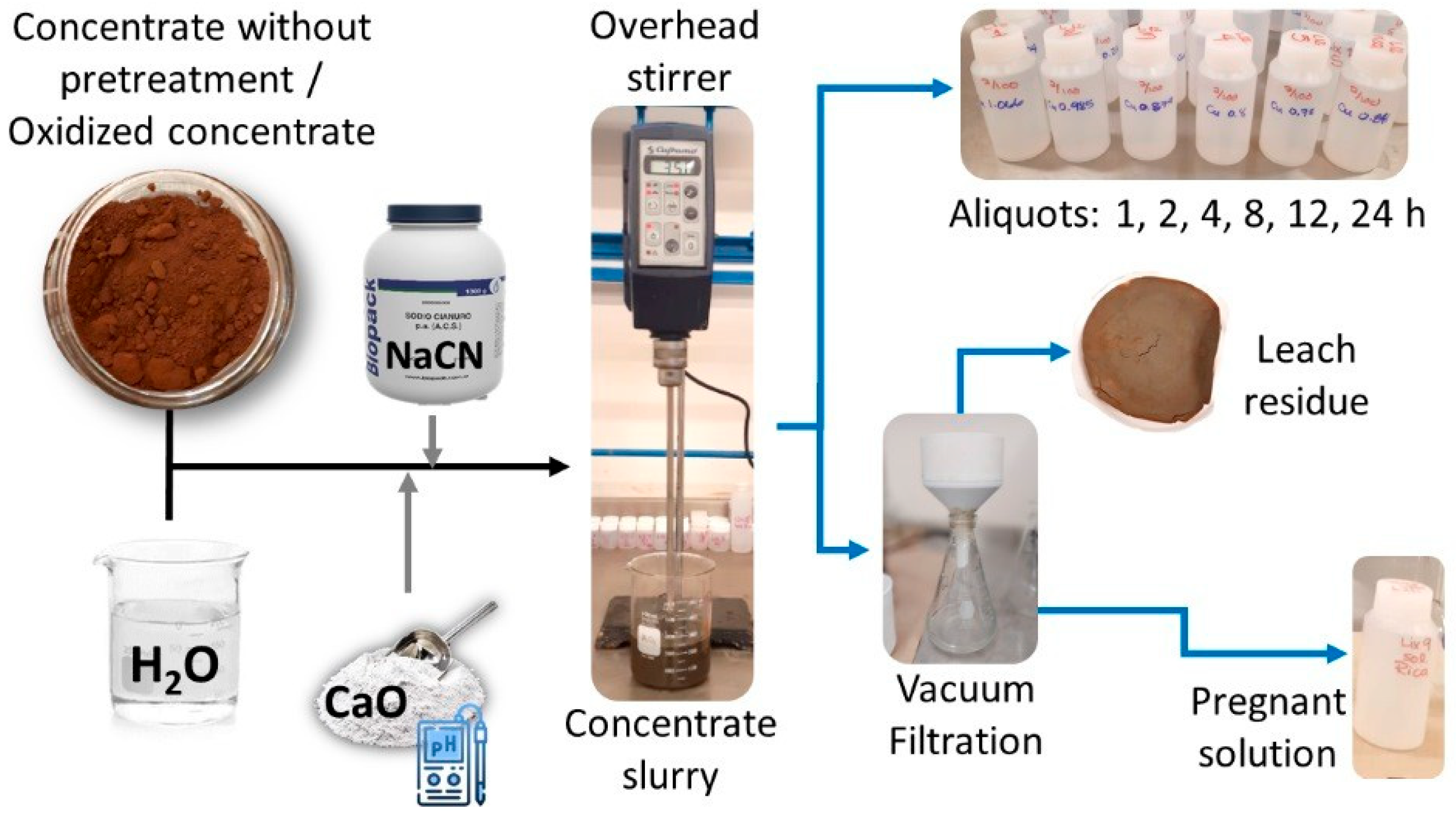



| Parameter | Value |
|---|---|
| Particle size, µm | 104, 74, and 61 |
| Temperature, °C | ambient |
| Pressure | atmospheric |
| NaCN, g/L | 5 |
| Solids wt.% | 20 |
| Stirring speed, rpm | 350 |
| pH | ≥11 |
| Time (h) | 24 |
| H2O2, mL | 50 |
| Parameter | Value |
|---|---|
| Particle size, µm | 104, 74, and 61 |
| Temperature, °C | ambient |
| Pressure | atmospheric |
| NaCN, g/L | 5 |
| CaO, Kg/t | 45 |
| Solids wt.% | 20 |
| Agitation speed, rpm | 350 |
| pH | ≥11 |
| Time (h) | 24 |
| Parameter | Value |
|---|---|
| Particle size, µm | 104, 74, 61 |
| Temperature, °C | 500, 550, 600 |
| Time (h) | 1 |
| Parameter | Value |
|---|---|
| Oxygen pressure, MPa | 0.5, 0.75, and 1 |
| Temperature, °C | 80, 120, and 150 |
| Particle size, µm | 104, 74, and 61 |
| CaO, Kg/t | 44.23 |
| Solids wt.% | 20 |
| Agitation speed, rpm | 600 |
| Time (h) | 1 |
| Compounds | wt.% |
|---|---|
| Pyrite, FeS2 | 43.6 |
| Chalcopyrite, CuFeS2 | 1.8 |
| Sphalerite, ZnS | 2.3 |
| Quartz, SiO2 | 49.3 |
| Calcite, CaCO3 | 3.3 |
| Total | 100.0 |
| Au (g/t) | Ag (g/t) | Cu wt. (%) | Fe wt. (%) | Zn wt. (%) |
|---|---|---|---|---|
| 25.56 | 5654.33 | 0.62 | 20.82 | 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Martínez, A.M.; Valenzuela-García, J.L.; Salazar-Campoy, M.M.; Encinas-Romero, M.A.; Martínez-Ballesteros, G.; Parga Torres, J.R. Gold and Silver Recovery from a Refractory Pyritic Concentrate by Roasting and Alkaline Pressure Oxidation. Minerals 2025, 15, 1260. https://doi.org/10.3390/min15121260
Espinoza-Martínez AM, Valenzuela-García JL, Salazar-Campoy MM, Encinas-Romero MA, Martínez-Ballesteros G, Parga Torres JR. Gold and Silver Recovery from a Refractory Pyritic Concentrate by Roasting and Alkaline Pressure Oxidation. Minerals. 2025; 15(12):1260. https://doi.org/10.3390/min15121260
Chicago/Turabian StyleEspinoza-Martínez, Ana María, Jesús Leobardo Valenzuela-García, María Mercedes Salazar-Campoy, Martín Antonio Encinas-Romero, Guadalupe Martínez-Ballesteros, and José Refugio Parga Torres. 2025. "Gold and Silver Recovery from a Refractory Pyritic Concentrate by Roasting and Alkaline Pressure Oxidation" Minerals 15, no. 12: 1260. https://doi.org/10.3390/min15121260
APA StyleEspinoza-Martínez, A. M., Valenzuela-García, J. L., Salazar-Campoy, M. M., Encinas-Romero, M. A., Martínez-Ballesteros, G., & Parga Torres, J. R. (2025). Gold and Silver Recovery from a Refractory Pyritic Concentrate by Roasting and Alkaline Pressure Oxidation. Minerals, 15(12), 1260. https://doi.org/10.3390/min15121260







