Recycling Copper (Cu) from Waste Automotive Printed Circuit Boards (WPCBs) After Characterization and Liberation Study by Mineral Processing Techniques
Abstract
1. Introduction
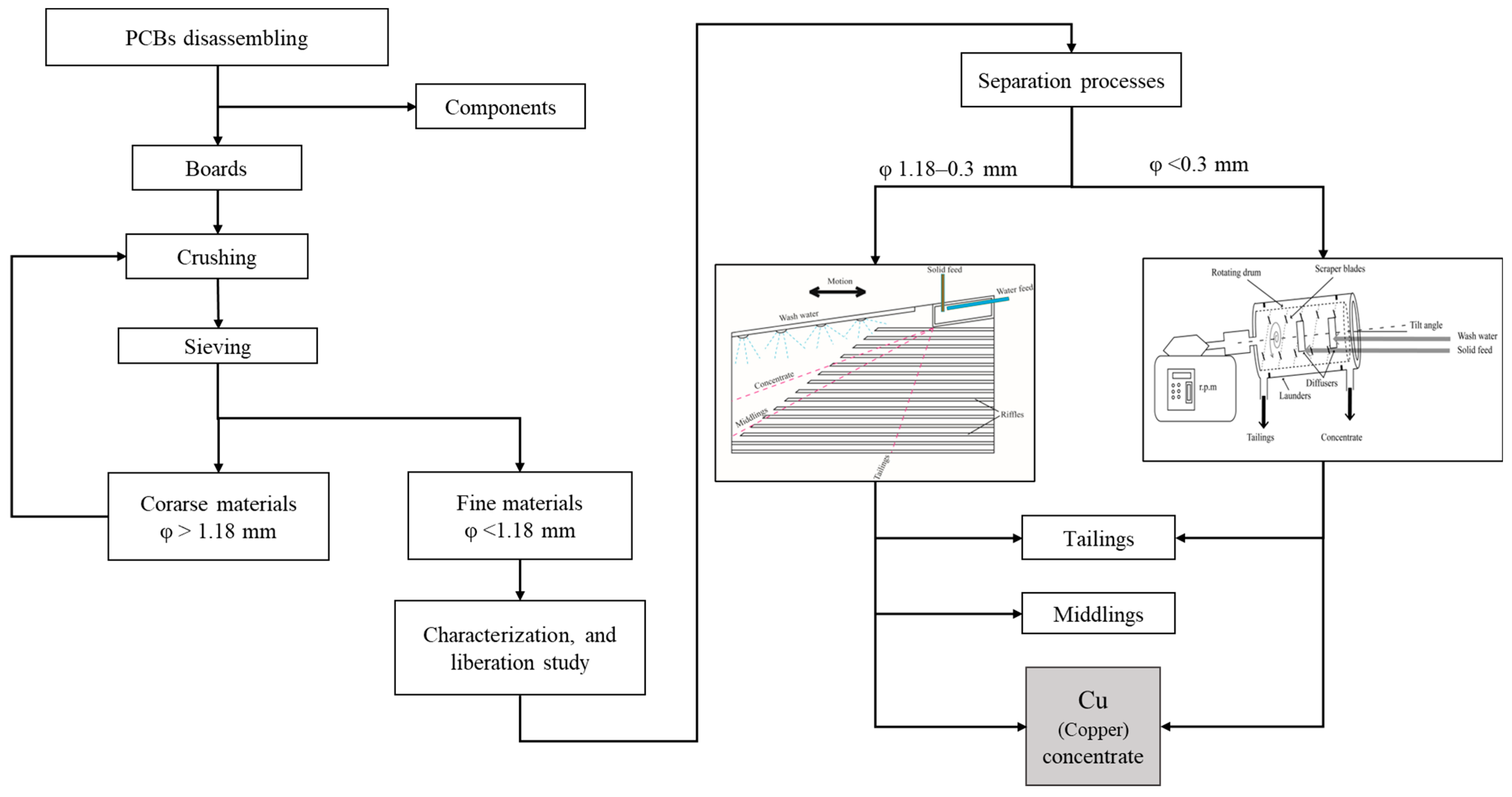
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Comminution
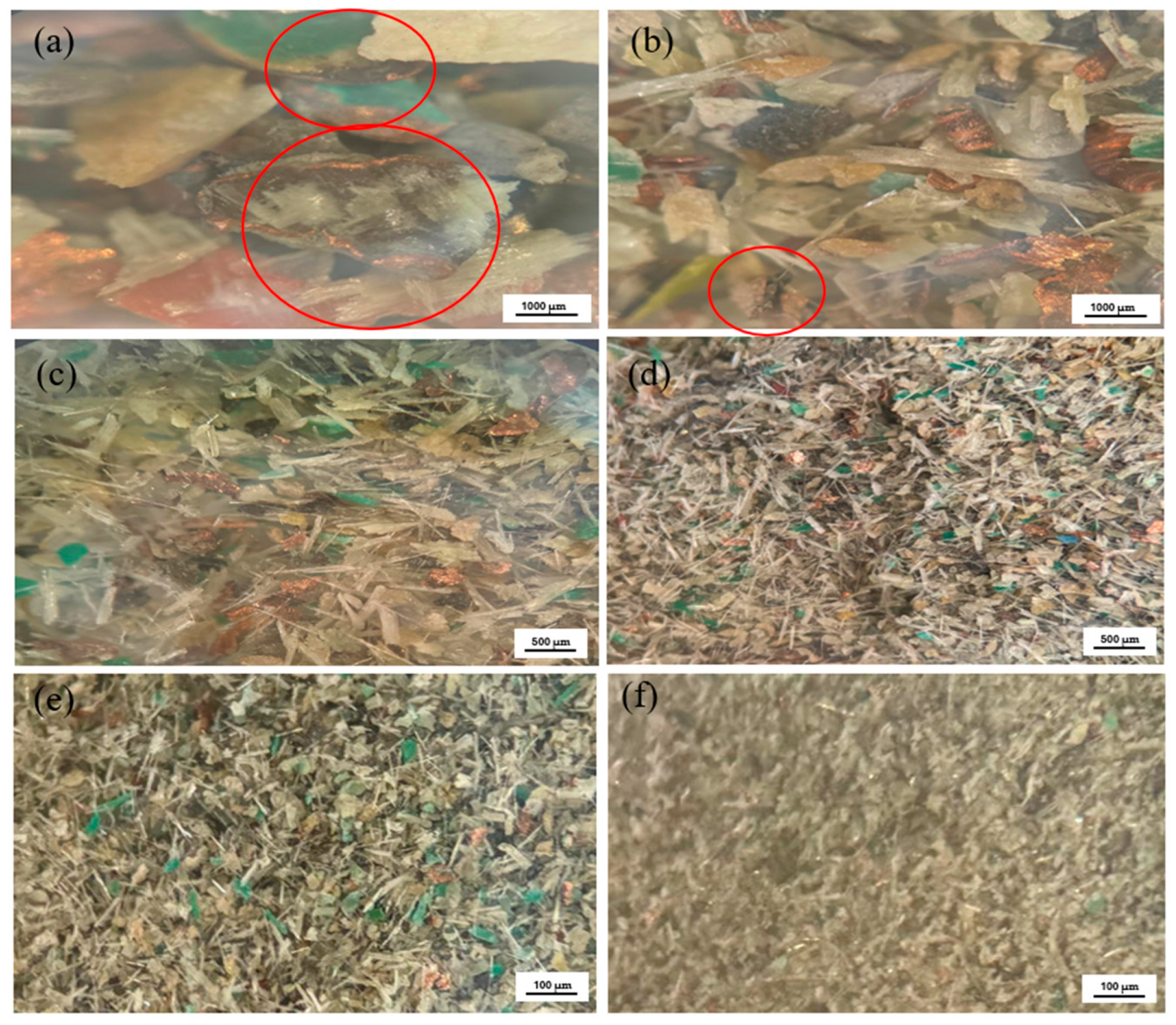
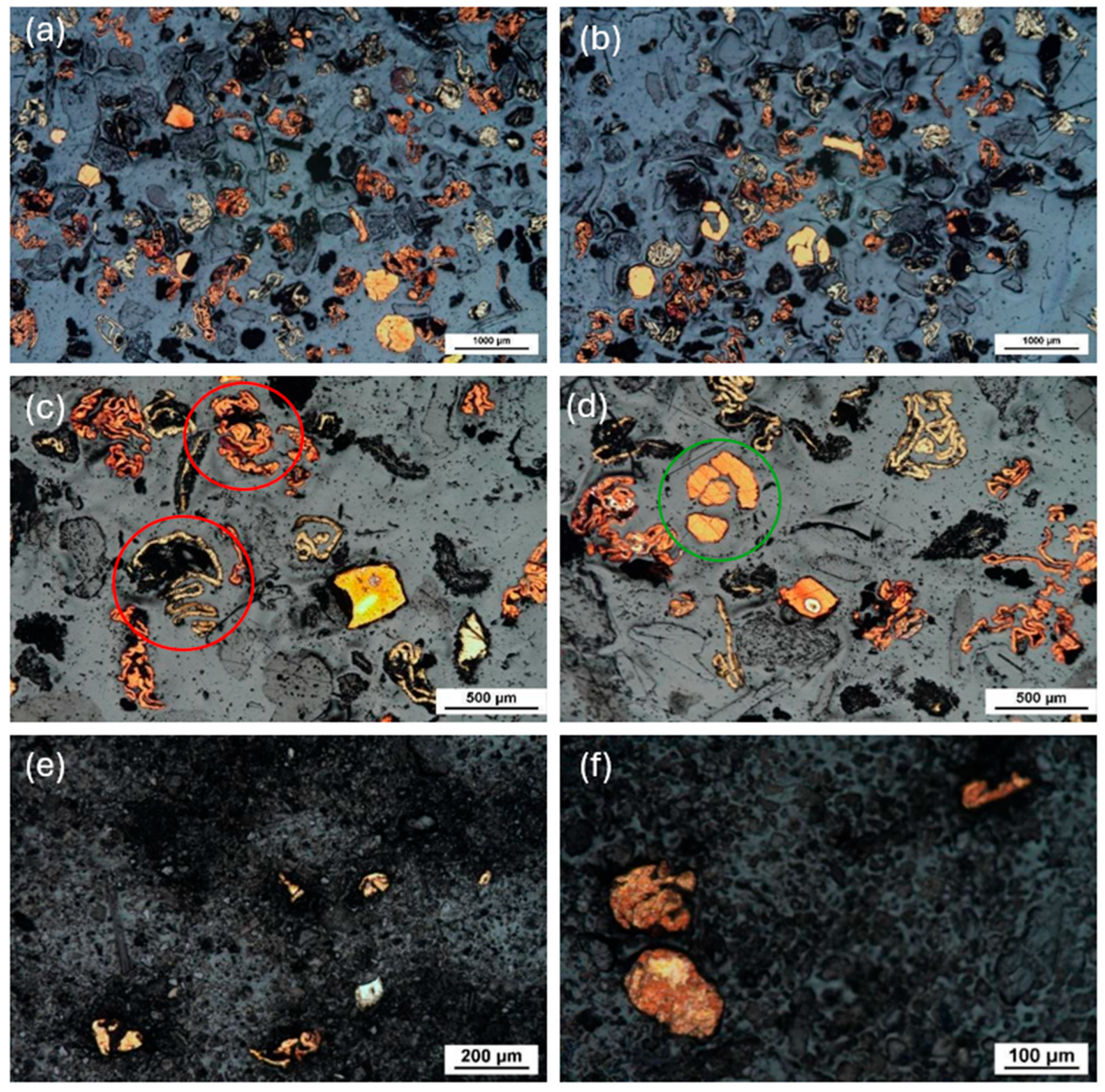
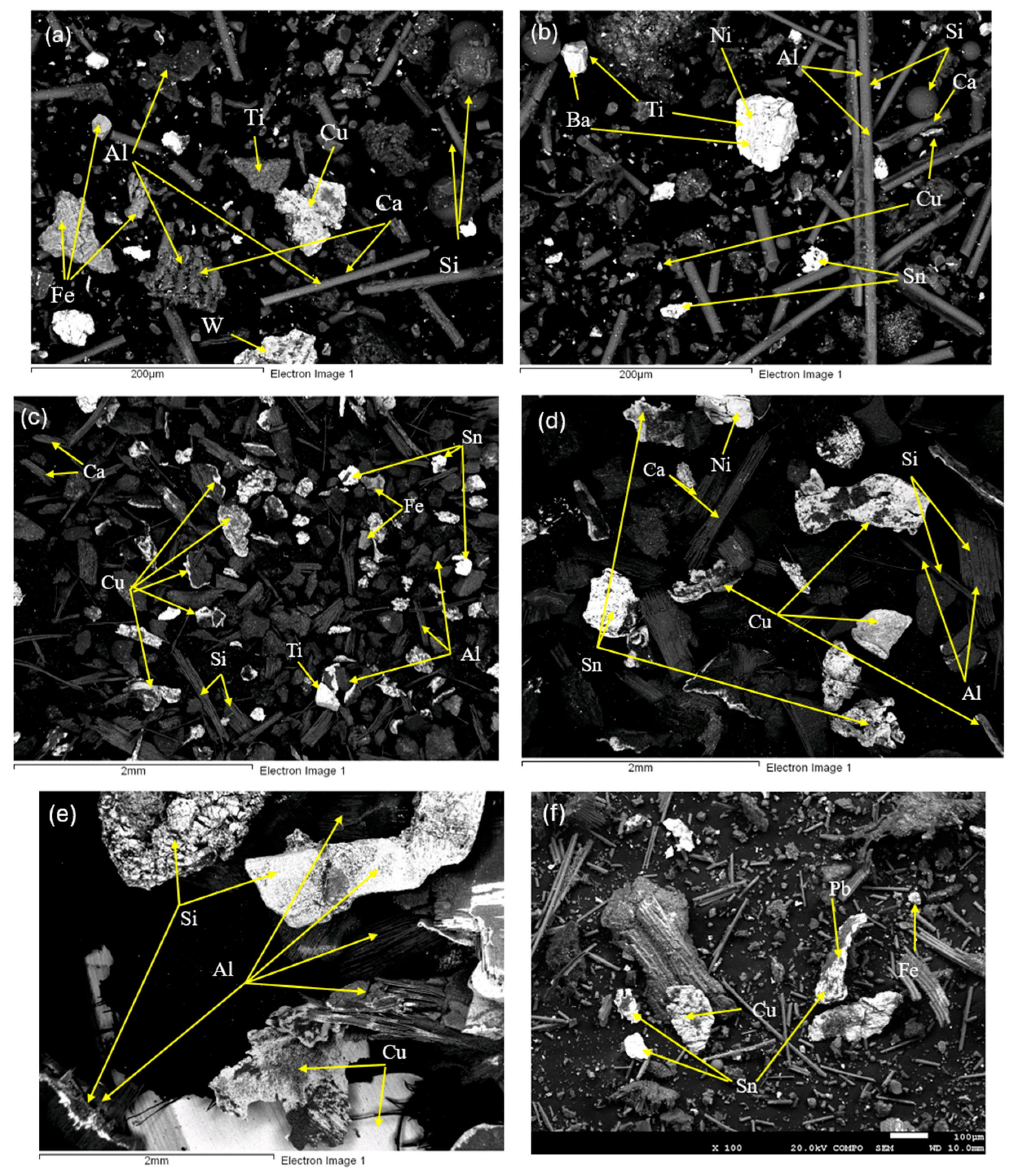
2.2.2. Characterization and Liberation Study
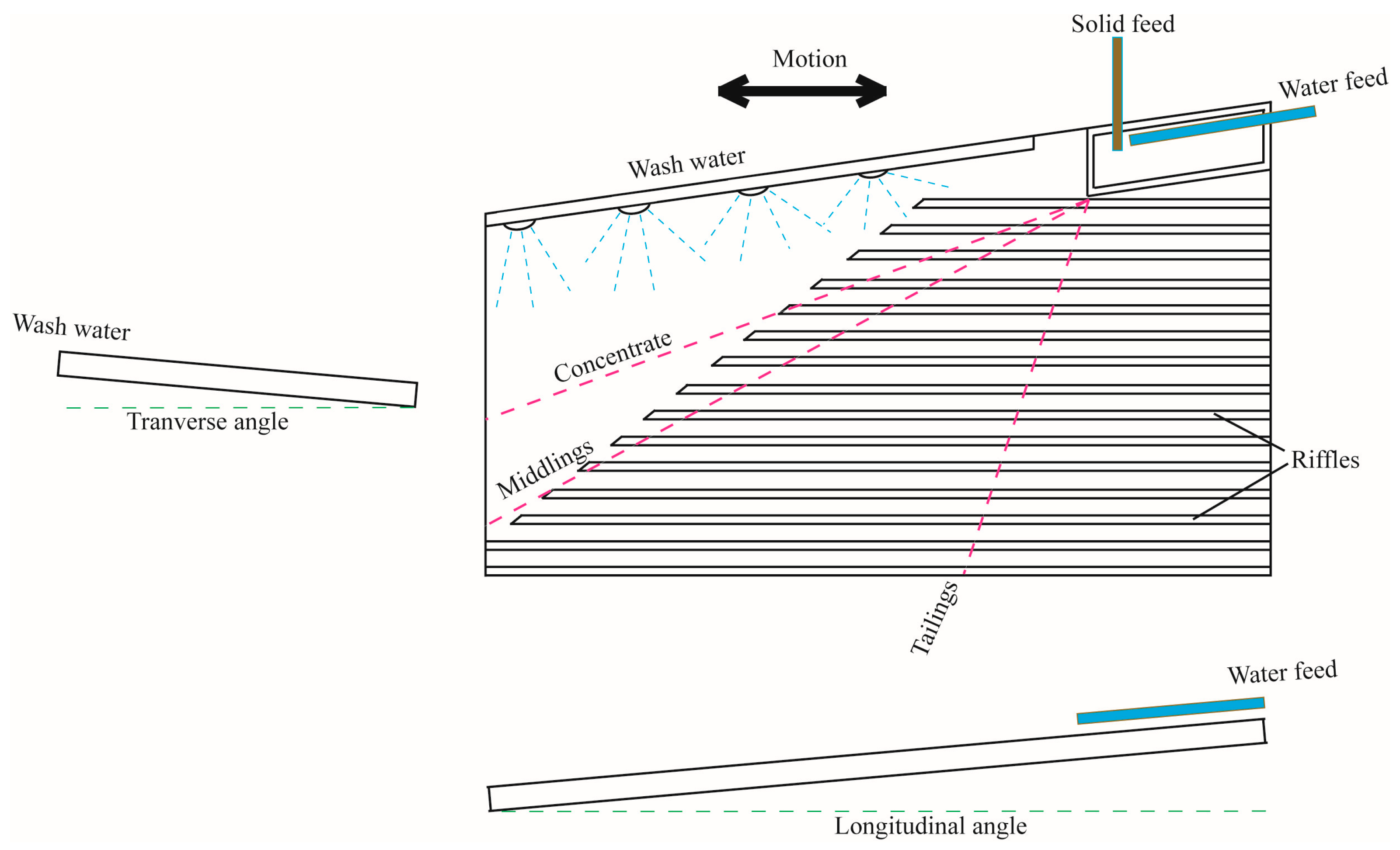
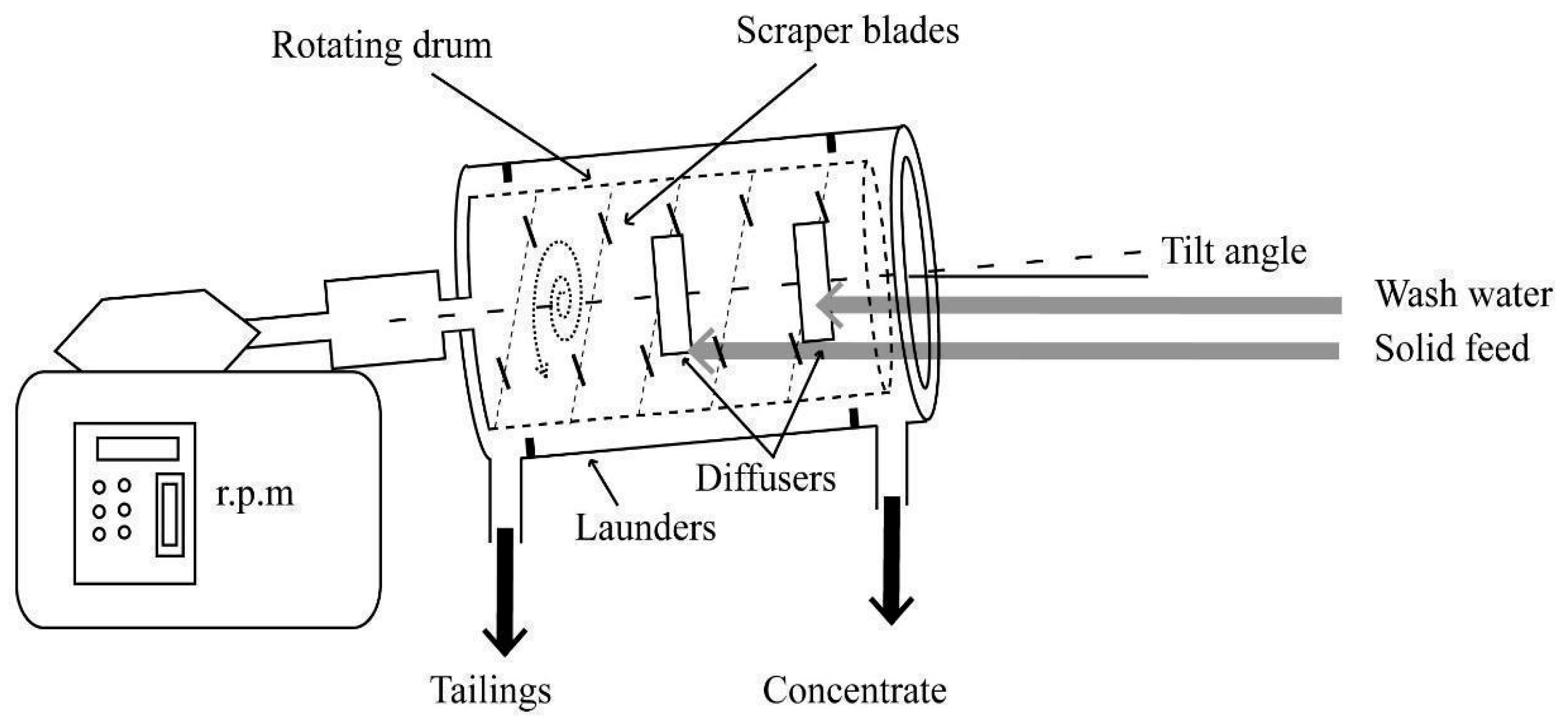
2.2.3. Separation
3. Results and Discussion
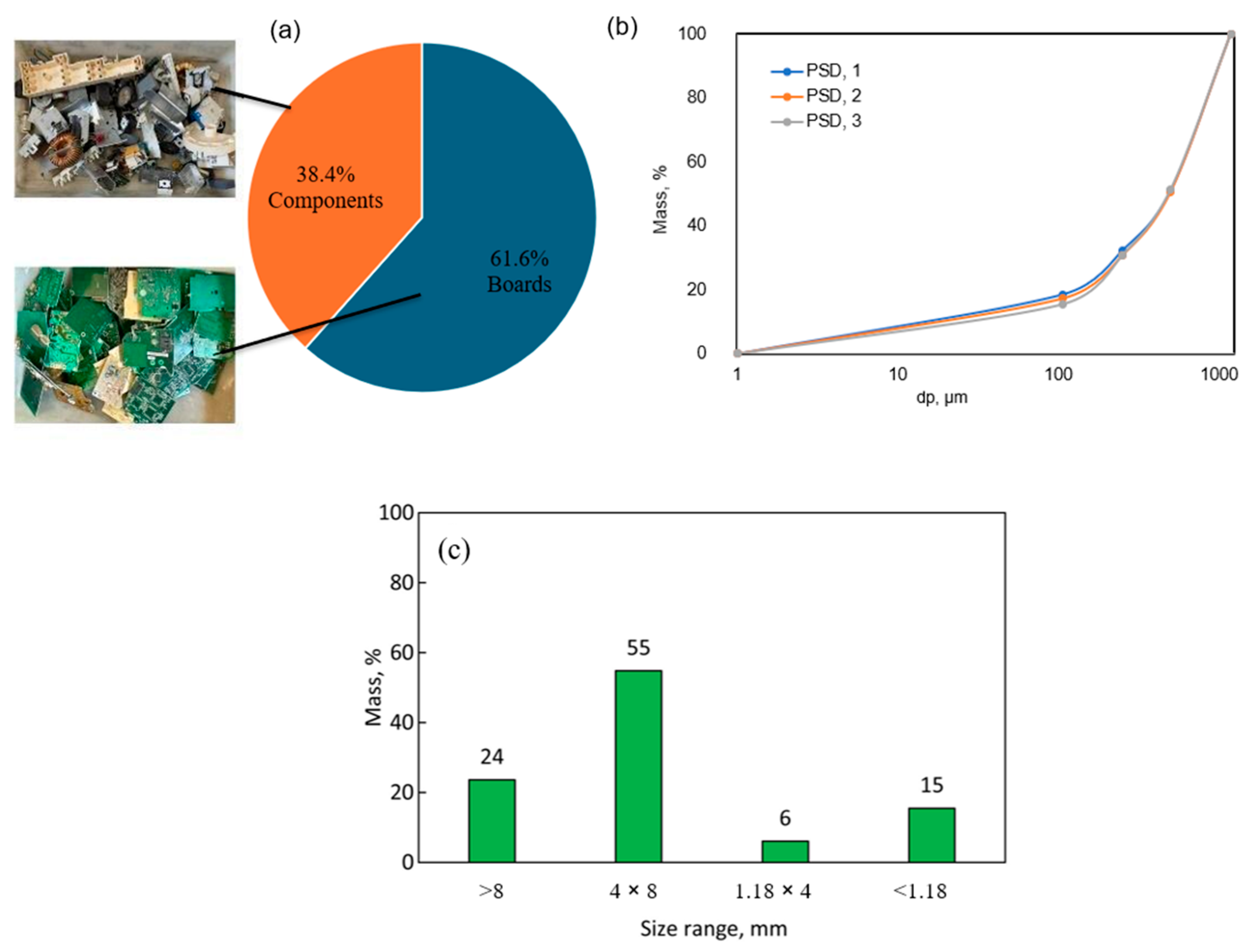
3.1. Sample Preparation
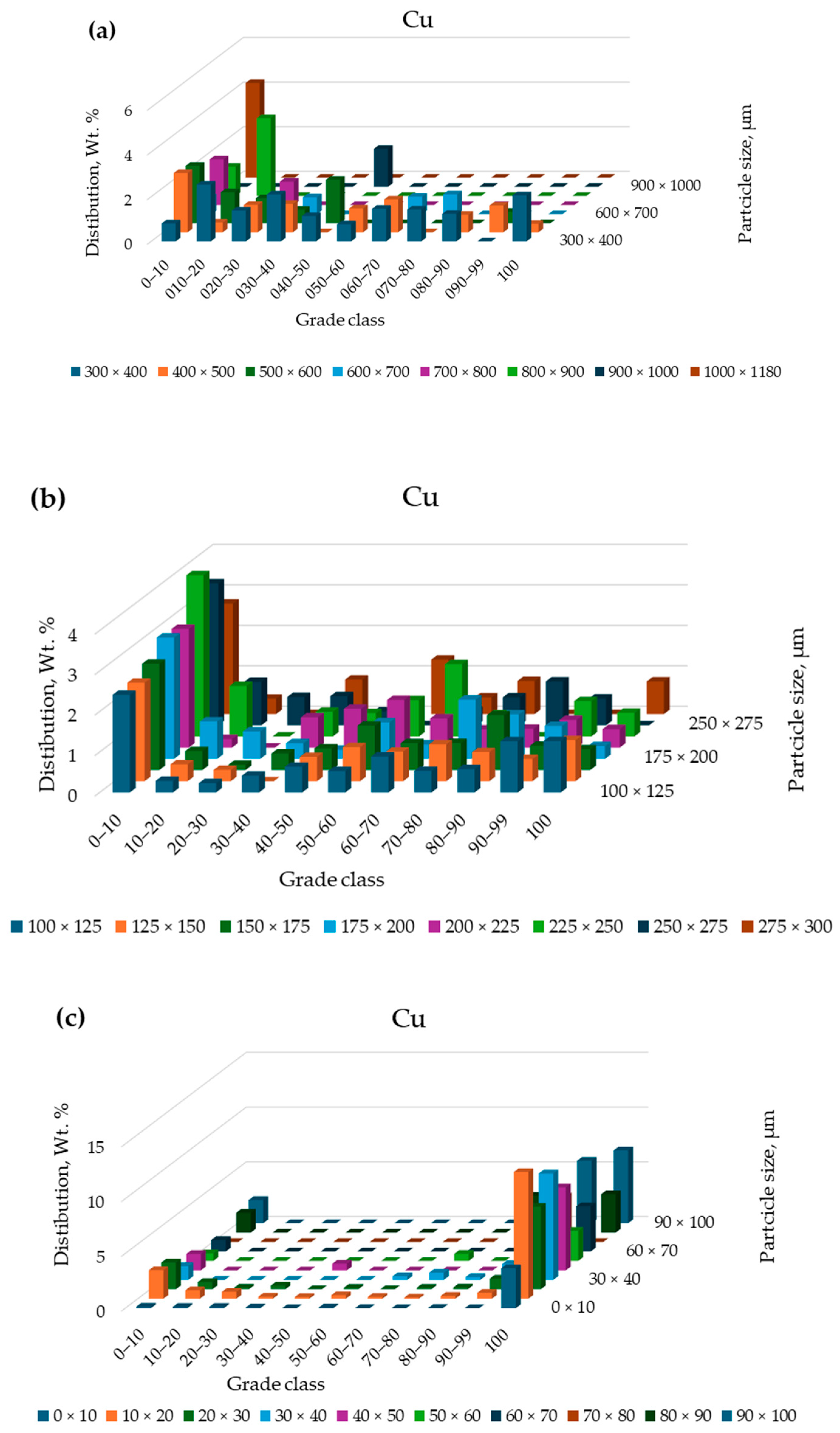
3.2. Characterization
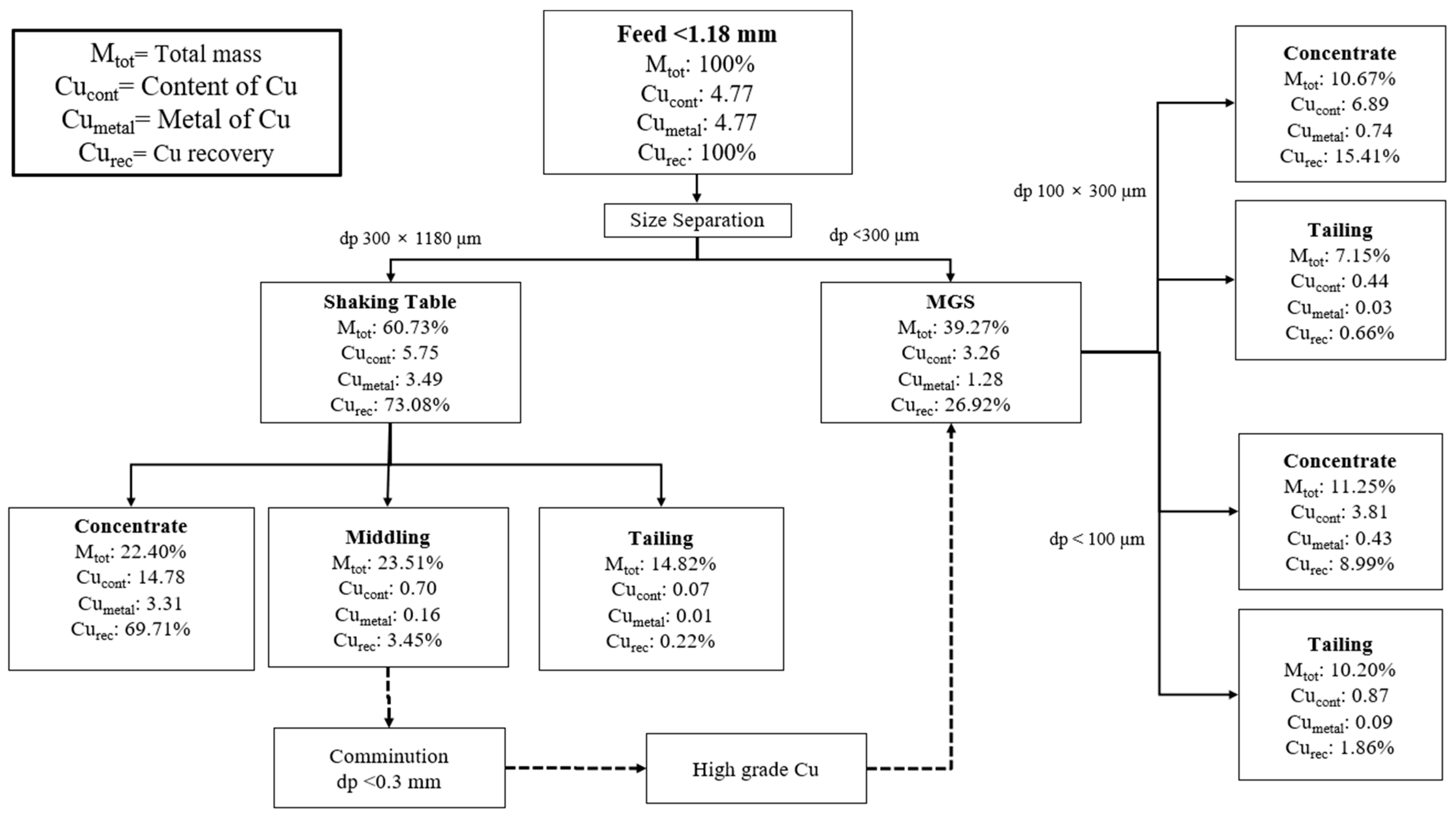
3.3. Separation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Holuszko, M.E.; Janke, T. Characterization of the non-metal fraction of the processed waste printed circuit boards. Waste Manag. 2018, 75, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammad, M.; Oliva, J.; Anticoi, H.; Hoffmann Sampaio, C.; Alfonso, P.; Valderrama, C.; Cortina Pallas, J.L. The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques. Recycling 2025, 10, 54. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Sheedy, C.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. A comparison of methods for the characterization of waste-printed circuit boards. Metals 2021, 11, 1935. [Google Scholar] [CrossRef]
- Otsuki, A.; Mensbruge, L.D.L.; King, A.; Serrano, S.; Fiore, L.; Bonifazi, G. Non-destructive characterization of mechanically processed waste printed circuit boards—Particle liberation analysis. Waste Manag. 2020, 102, 510–519. [Google Scholar] [CrossRef]
- Abbadi, A.; Rácz, A.; Bokányi, L. Exploring the comminution process of waste printed circuit boards in recycling; a review. J. Mater. Cycles Waste Manag. 2024, 26, 1326–1348. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Wan, A.; Yu, S.; Yao, Z. Bioleaching of Typical Electronic Waste—Printed Circuit Boards (PCBs): A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 7508. [Google Scholar] [CrossRef]
- Mori de Oliveira, C.; Bellopede, R.; Tori, A.; Zanetti, G.; Marini, P. Gravity and Electrostatic Separation for Recovering Metals from Obsolete Printed Circuit Board. Materials 2022, 15, 1874. [Google Scholar] [CrossRef]
- Sarvar, M.; Slarirad, M.M.; Shabani, M.M. Characterisation and mechanical separation of metals from computer Printed Circuit Board (PCBs) based on mineral processing methods. Waste Manag. 2015, 45, 246–257. [Google Scholar] [CrossRef]
- Palmieri, R.; Bonifazi, G.; Serranti, S. Recycling-oriented characterization of plastic frames and printed circuit boards from mobile phones by electronic and chemical imaging. Waste Manag. 2014, 34, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Vidyadhar, A.; Mehrotra, S.P. A novel flowsheet for the recovery of metal values from waste printed circuit boards. Resour. Conserv. Recycl. 2009, 53, 464–469. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, H. Discarded e-waste/printed circuit boards: A review of their recent methods of disassembly, sorting and environmental implications. J. Mater. Cycles Waste Manag. 2024, 26, 1277–1293. [Google Scholar] [CrossRef]
- Keleş, B.; Dinç, N.I.; Dursun, H.N.; Burat, F.; Ulusoy, U. The effect of particle geometry (size & shape) on the recovery of gold and copper metallic particles from end-of-life random access memory cards by flotation. Waste Manag. 2024, 179, 66–76. [Google Scholar] [CrossRef]
- Otsuki, A.; Gonçalves, P.P.; Stieghorst, C.; Révay, Z. Non-destructive characterization of mechanically processed waste printed circuit boards: X-ray fluorescence spectroscopy and prompt gamma activation analysis. J. Compos. Sci. 2019, 3, 54. [Google Scholar] [CrossRef]
- Touze, S.; Guignot, S.; Hubau, A.; Devau, N.; Chapron, S. Sampling waste printed circuit boards: Achieving the right combination between particle size and sample mass to measure metal content. Waste Manag. 2020, 118, 380–390. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Sun, X.; Wang, L. Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep. Sci. Technol. 2018, 54, 1302–1311. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, J.; Yoo, K.; Lee, J.; Kim, W. Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill. Waste Manag. 2009, 29, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Veit, H.M.; Juchnes, N.C.; Scherer, J. Use of Gravity Separation in Metals Concentration from Printed Circuit Board Scraps. Metallurgy and materials. Rem Revista Escola de Minas 2014, 67, 73–79. [Google Scholar] [CrossRef]
- Burat, F.; Özer, M. Physical separation route for printed circuit boards. Physicochem. Probl. Miner. Process. 2018, 54, 554–566. [Google Scholar] [CrossRef]
- Keglevich de Buzin, P.J.W.; Ambrós, W.M.; Schadach de Brum, I.A.; Candiota Tubino, R.M.; Hoffmann Sampaio, C.; Oliva Moncunill, J. Development of a Physical Separation Route for the Concentration of Base Metals from Old Wasted Printed Circuit Boards. Minerals 2021, 11, 1014. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Dong, S.; Kou, W.; Nie, C.; Lyu, X.; Qiu, J.; Li, L.; Liu, Z.; Wu, P. Mechanical activation to enhance the natural floatability of waste printed circuit boards. Waste Manag. 2020, 109, 222–230. [Google Scholar] [CrossRef]
- Ogunniyi, I.; Vermaak, M.; Groot, D. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Content evaluation of different waste PCBs to enhance basic metals recycling. Resour. Conserv. Recycl. 2018, 139, 298–306. [Google Scholar] [CrossRef]
- Hubau, A.; Chagnes, A.; Minier, M.; Touzé, S.; Chapron, S.; Guezennec, A.G. Recycling-oriented methodology to sample and characterize the metal composition of waste Printed Circuit Boards. Waste Manag. 2019, 91, 62–71. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient separation of precious metals from computer waste printed circuit boards by hydrocyclone and dilution-gravity methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Ichikawa, S.; Hirokawa, Y.; Kurisaki, T.; Nakamura, T. Characterization of a printed-circuit board by X-ray fluorescence and X-ray diffraction analyses for metal recovery. Spectrochim. Acta Part B At. Spectrosc. 2023, 210, 106819. [Google Scholar] [CrossRef]
- Agbim, A.; Schumacher, K.A.; Sharp, N.; Paul, R.; Corzo, R. Elemental characterization of electronic waste: A review of research methodologies and applicability to the practice of e-waste recycling. Waste Manag. 2024, 187, 91–100. [Google Scholar] [CrossRef]
- Gonçalves, P.P.; Otsuki, A. Determination of liberation degree of mechanically processed waste printed circuit boards by using the digital microscope and SEM-EDS analysis. Electronics 2019, 8, 1202. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Zhang, T.; Wang, H.; Zhu, X. Recovery of residual metals from fine nonmetallic fractions of waste printed circuit boards using a vibrated gas-solid fluidized bed. Sep. Purif. Technol. 2018, 207, 321–328. [Google Scholar] [CrossRef]
- Escalante, P.; Oliva, J.; Anticoi, H.; Hoffmann Sampaio, C.; Mohanty, K. Characterization of mineralogical impurities in a carbonate-rich material using MLA. Miner. Eng. 2025, 230, 109409. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Gugala, E.; Blannin, R.; Bachmann, K.; Serna-Guerrero, R.; Rudolp, M. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 2021, 169, 106924. [Google Scholar] [CrossRef]
- Donnelly, L.; Pirrie, D.; Power, M.; Menzies, A. Phase Characterisation for Recycling of Shredded Waste Printed Circuit Boards. Recycling 2025, 10, 157. [Google Scholar] [CrossRef]
- Sun, Z.H.I.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Visser, G.; Yang, Y. Characterisation of metals in the electronic waste of complex mixtures of end-of-life ICT products for development of cleaner recovery technology. Waste Manag. 2015, 35, 227–235. [Google Scholar] [CrossRef]
- Lancaster, S.T.; Sahlin, E.; Oelze, M.; Ostermann, M.; Vogl, J.; Laperche, V.; Touze, S.; Ghestem, J.; Dalencourt, C.; Gendre, R.; et al. Evaluation of X-ray fluorescence for analysing critical elements in three electronic waste matrices: A comprehensive comparison of analytical techniques. Waste Manag. 2024, 190, 496–505. [Google Scholar] [CrossRef]
- Gok, Ö.; Akar Sen, G. Recovery of copper from printed circuit boards (PCBs) using shaking table. J. Serbian Chem. Soc. 2023, 88, 1039–1053. [Google Scholar] [CrossRef]
- Duana, C.; Wen, X.; Shi, C.; Zhao, Y.; Wen, B.; He, Y. Recovery of metals from waste printed circuit boards by a mechanical method using a water medium. J. Hazard. Mater. 2009, 166, 478–482. [Google Scholar] [CrossRef]
- Estrada-Ruiz, R.H.; Flores-Campos, R.; Gámez-Altamirano, H.A.; Velarde-Sánchez, E.J. Separation of the metallic and non-metallic fraction from printed circuit boards employing green technology. J. Hazard. Mater. 2016, 311, 91–99. [Google Scholar] [CrossRef]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.S.; Bernardes, A.M.; Teno’rio, J.A.S. Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Manag. 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Farajzadeh, S.; Chelgani, S.C. Gravity separation by falcon concentrator- an over review. Sep. Sci. Technol. 2022, 57, 2145–2164. [Google Scholar] [CrossRef]
- Guo, C.; Wang, H.; Liang, W.; Fu, J.; Yi, X. Liberation characteristic and physical separation of printed circuit board (PCB). Waste Manag. 2011, 31, 2161–2166. [Google Scholar] [CrossRef]
- Tanısalı, E.; Özer, M.; Burat, F. Precious Metals Recovery from Waste Printed Circuit Boards by Gravity Separation and Leaching. Miner. Process. Extr. Metall. Rev. 2020, 42, 24–37. [Google Scholar] [CrossRef]
- Palav, S.D.; Torres, A.; Biegler, L.T. Systematic conceptual design strategies for the recovery of metals from E-waste. Front. Chem. Eng. 2024, 6, 1388456. [Google Scholar] [CrossRef]
- Lopez-Paneque, A.M.; Gallardo Garcia-Orta, V.H.; Gallardo, J.M.; Sepulveda-Ferrer, R.E.; Chicardi, E. The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs. Metals 2025, 15, 826. [Google Scholar] [CrossRef]
- Abbadi, A.; Bokanyi, L. Flotation of comminuted waste printed circuit boards particles—A review. Miner. Eng. 2025, 233, 109642. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.E.; Janke, T. Assessing the Applicability of Gravity Separation for Recycling of Non-Metal Fraction from Waste Printed Circuit Boards. Adv. Sustain. Syst. 2022, 6, 2000231. [Google Scholar] [CrossRef]
- Das, S.K.; Ellamparuthy, G.; Kundu, T.; Angadi, S.I.; Rath, S.S. A comprehensive review of the mechanical separation of waste printed circuit boards. Process Saf. Environ. Prot. 2024, 187, 221–239. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Hegarty, P.; Fergusson, K.; Rollinson, G.; Xie, W.; Mildren, T. Optimisation of a Multi-Gravity Separator with Novel Modifications for the Recovery of Ferberite. Minerals 2018, 8, 191. [Google Scholar] [CrossRef]
- Bhaskar, K.U.; Rao1, K.K.; Govindarajan1, B.; Barnwal1, J.P.; Rao, T.C. Studies on multi-gravity separator for rejection of acid insoluble in a copper concentrate. Eur. J. Miner. Process. Environ. Prot. 2005, 5, 35–47. [Google Scholar]
- Mori de Oliveira, C.; Bellopede, R.; Tori, A.; Marini, M.P. Study of Metal Recovery from Printed Circuit Boards by Physical-Mechanical Treatment Processes. Mater. Proc. 2021, 5, 121. [Google Scholar] [CrossRef]
- Burt, R.O. Gravity Concentration Technology; Developments in Mineral Processing; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1984; Volume 5. [Google Scholar]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operations: An Introduction, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Franke, D.M.; Suponik, T.; Nuckowski, P.M.; Dubaj, J. Evaluation of the efficiency of metal recovery from printed circuit boards using gravity processes. Physicochem. Probl. Miner. Process. 2021, 57, 63–77. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Lee, J. Disassembly and physical separation of electric/electronic components layered in printed circuit boards (PCB). J. Hazard. Mater. 2012, 241–242, 387–394. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chou, S.L.; Lo, S.L. Gold recovery from waste printed circuit boards of mobile phones by using microwave pyrolysis and hydrometallurgical methods. Sustain. Environ. Res. 2022, 32, 6. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Zhao, Y.; Zhou, Y.; Ma, E.; Bai, J.; Wang, J. Recycling gold from printed circuit boards gold-plated layer of waste mobile phones in “mild aqua regia” system. J. Clean. Prod. 2021, 278, 123597. [Google Scholar] [CrossRef]
- Cardenas-Vera, A.; Hesse, M.; Möckel, R.; Merker, R.G.; Heinig, T.; Phan, Q.V. Investigation of Sensor-Based sorting and selective comminution for pre-concentration of an unusual parisite-rich REE ore, South Namxe, Vietnam. Miner. Eng. 2022, 177, 107371. [Google Scholar] [CrossRef]
- Sandmann, D.; Gutzmer, J. Use of Mineral Liberation Analysis (MLA) in the Characterization of Lithium-Bearing Micas. J. Miner. Mater. Charact. Eng. 2013, 1, 285–292. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, K.; He, J.; Zhu, L.; Zhao, Y.; Bai, Q. Efficient recovery of valuable metals in the disposal of waste printed circuit boards via reverse flotation. J. Clean. Prod. 2021, 284, 124805. [Google Scholar] [CrossRef]
- Magoda, K.; Nomngongo, P.N.; Mekuto, L. Two-Step Bio-Dissolution of Metals from Printed Circuit Boards Using Acidophilic Iron- and Sulfur-Oxidizing Mesophiles. Recycling 2024, 9, 6. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, L.; Yu, M.; Li, J. An innovative method of recycling metals in printed circuit board (PCB) using solutions from PCB production. J. Hazard. Mater. 2020, 390, 121892. [Google Scholar] [CrossRef]
- Ajiboye, A.E.; Olasehinde, F.E.; Adebayo, O.A.; Ajayi, O.J.; Ghosh, M.K.; Basu, S. Extraction of Copper and Zinc from Waste Printed Circuit Boards. Recycling 2019, 4, 36. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G. Investigation of froth flotation for beneficiation of printed circuit board comminution fines. Miner. Eng. 2009, 22, 378–385. [Google Scholar] [CrossRef]
- Chen, L.; He, J.; Zhu, L.; Yao, Q.; Sun, Y.; Guo, C.; Chen, H.; Yang, B. Efficient recovery of valuable metals from waste printed circuit boards via ultrasound-enhanced flotation. Process Saf. Environ. Prot. 2023, 169, 869–878. [Google Scholar] [CrossRef]
- He, J.; Duan, C. Recovery of metallic concentrations from waste printed circuit boards via reverse floatation. Waste Manag. 2017, 60, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, I.O.; Vermaak, M.K.G. Froth Flotation for Beneficiation of Printed Circuit Boards Comminution Fines: An Overview. Miner. Process. Extr. Met. Rev. 2009, 30, 101–121. [Google Scholar] [CrossRef]
- Ismail Mohamed, A.T.; Schimperna, G.; Cantoni, G.; Demichelis, F.; Fino, D.; Perucchini, S.; Rubertelli, F.; Laviano, F. Effect of Solvent Pre-Treatment on the Leaching of Copper During Printed Circuit Board Recycling. Recycling 2025, 10, 80. [Google Scholar] [CrossRef]
- Magoda, K.; Mekuto, L. Biohydrometallurgical Recovery of Metals from Waste Electronic Equipment: Current Status and Proposed Process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Xian, Y.; Tao, Y.; Ma, F.; Zhou, Y. Recovery of Metals from Heat-Treated Printed Circuit Boards via an Enhanced Gravity Concentrator and High-Gradient Magnetic Separator. Materials 2021, 14, 4566. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comprehensive characterization of printed circuit boards of various end-of-life electrical and electronic equipment for beneficiation investigation. Waste Manag. 2018, 75, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, G.; Zhang, C. Process and systematic study of gold recovery from flexible printed circuit boards (FPCBs) using a choline chloride–ethylene glycol system. Waste Manag. 2024, 178, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Ilkhani, Z.; Aiouache, F. Bioleaching of Gold from Printed Circuit Boards: Potential Sustainability of Thiosulphate. Recycling 2025, 10, 87. [Google Scholar] [CrossRef]
- Martinez-Ballesteros, G.; Valenzuela-García, G.L.; Gómez-Alvarez, A.; Encinas-Romero, M.A.; Mejía-Zamudio, F.A.; Rosas-Durazo, A.D.J.; Valenzuela-Frisby, R. Recovery of Ag, Au, and Pt from Printed Circuit Boards by Pressure Leaching. Recycling 2021, 6, 67. [Google Scholar] [CrossRef]
- Marques, A.C.; Marrero, J.M.C.; Malfatti, C.F. A review of the recycling of non-metallic fractions of printed circuit boards. SpringerPlus 2013, 2, 521. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Bhagavatula, S.G.K.; Karingamanna, J.; Madhavan, M.K. An Experimental Investigation into the Performance of Concrete and Mortar with Partial Replacement of Fine Aggregate by Printed Circuit Board (PCB) E-Waste. Recycling 2025, 10, 138. [Google Scholar] [CrossRef]
- Flores-Campos, R.; Deaquino-Lara, R.; Rodríguez-Reyes, M.; Martínez-Sánchez, R.; Estrada-Ruiz, R.H. The Use of Nonmetallic Fraction Particles with the Double Purpose of Increasing the Mechanical Properties of Low-Density Polyethylene Composite and Reducing the Pollution Associated with the Recycling of Metals from E-Waste. Recycling 2024, 9, 56. [Google Scholar] [CrossRef]
- Primary Copper Smelting: National Emissions Standards for Hazardous Air Pollutants (NESHAP), EPA Unitaed States Environmental Protection Agency. Available online: https://www.epa.gov/stationary-sources-air-pollution/primary-copper-smelting-national-emissions-standards-hazardous-air#:~:text=Rule%20Summary,-Alert&text=Primary%20copper%20smelting%20is%20the,50%20percent%20from%20these%20sources (accessed on 14 November 2025).
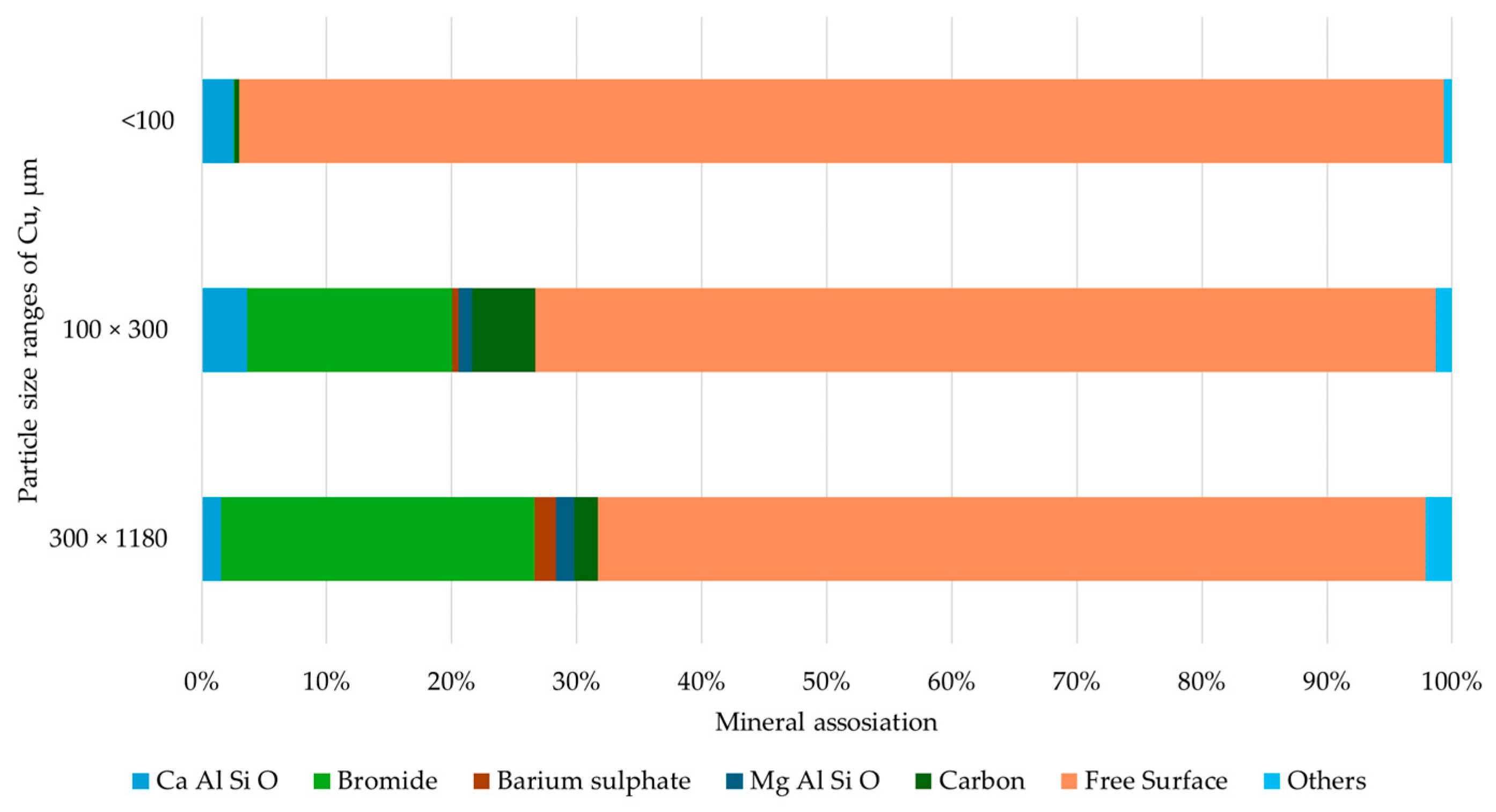
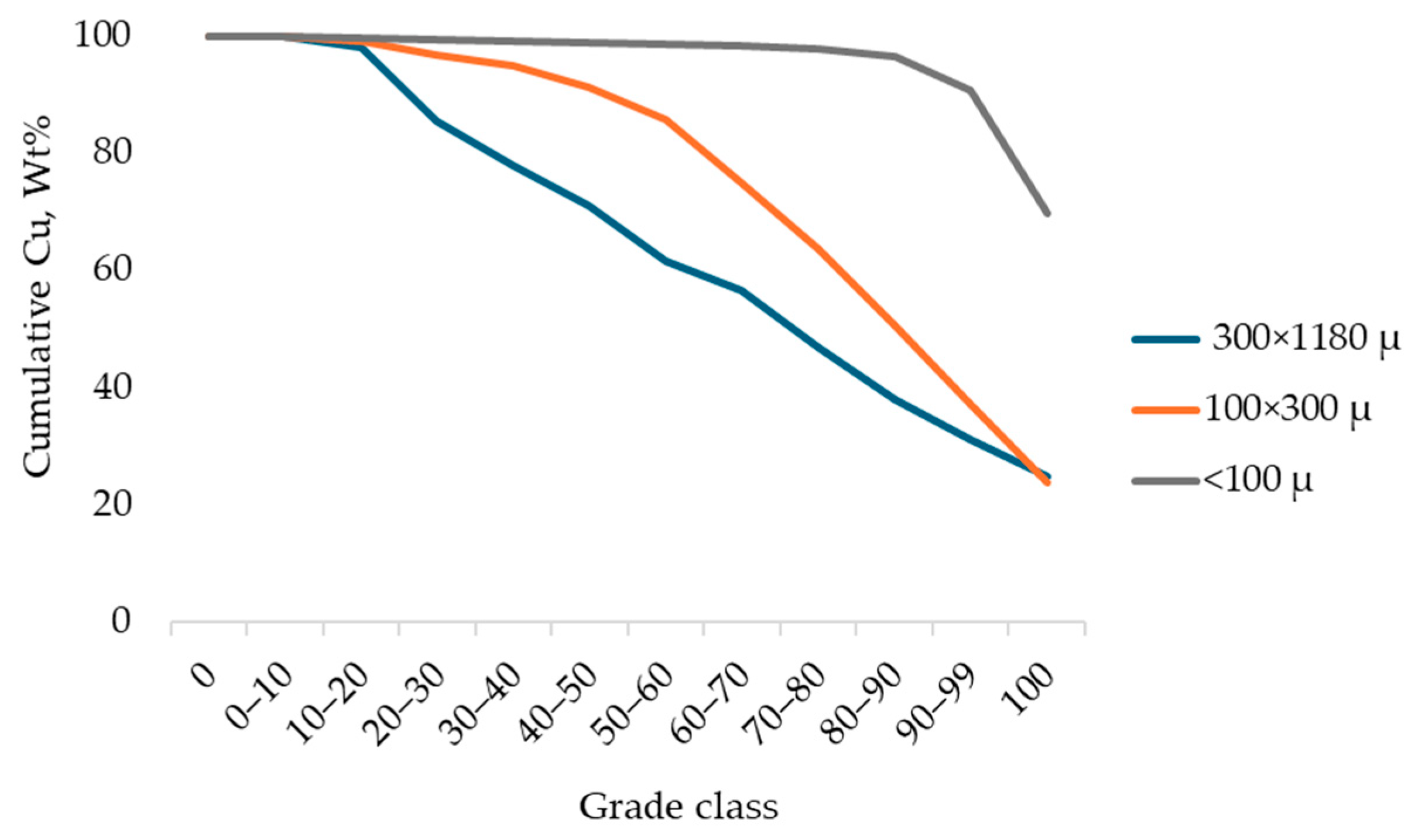
| <100, µm | 100 × 250, µm | 250 × 500, µm | 500 × 1180, µm | Feed, µm | |
|---|---|---|---|---|---|
| Al2O3 | 6.8 | 7.4 | 7.9 | 8.9 | 6.4 |
| CaO | 15.0 | 11.6 | 10.6 | 8.7 | 13.6 |
| SiO2 | 36.1 | 25.7 | 23.2 | 19.9 | 35.0 |
| Fe2O3 | 2.9 | 1.4 | 0.7 | 0.5 | 1.6 |
| Cu | 4.8 | 13.2 | 13.0 | 18.9 | 11.0 |
| Br | 5.7 | 12.9 | 13.0 | 10.9 | 9.4 |
| Sb | 0.16 | 0.21 | 0.23 | 0.25 | 0.22 |
| Ba | 1.4 | 0.9 | 0.3 | 0.2 | 0.5 |
| W | 5.9 | 4.5 | 3.5 | 2.1 | 3.8 |
| <100, µm | 100 × 250, µm | 250 × 500, µm | 500 × 1180, µm | Feed, µm | |
|---|---|---|---|---|---|
| ppm | |||||
| Au | 846.79 | 149.38 | 176.32 | 69.45 | 400.64 |
| Ag | 116.68 | 236.79 | 185.84 | 397.74 | 236.84 |
| Pb | 455.14 | 640.74 | 889.85 | 714.07 | 747.33 |
| Co | 23.23 | 13.60 | 25.13 | 27.01 | 38.99 |
| % | |||||
| Sn | 1.60 | 2.33 | 3.36 | 4.23 | 3.24 |
| Ni | 0.42 | 0.40 | 0.58 | 1.12 | 0.65 |
| Ti | 0.19 | 0.14 | 0.07 | 0.08 | 0.12 |
| Particle Size Ranges, µm | wt% |
|---|---|
| 300 × 1180 | 9.65 |
| 100 × 300 | 4.58 |
| <100 | 4.25 |
| Concentration | Middlings | Waste | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass, g | Deck Angle, Ɵ | Frequency, Hz | Wash Water, L/min | Process Water, L/min | Mass, % | Cu, % | Cu R, % | Mass, % | Cu, % | Cu R, % | Mass, % | Cu, % | Cu R, % |
| 296.4 | 1 | 60 | 12 | 8 | 37.9 | 30.7 | 94.6 | 53.4 | 0.9 | 4.1 | 8.7 | 1.9 | 1.4 |
| 298.2 | 2 | 60 | 12 | 8 | 36.9 | 40.1 | 95 | 38.7 | 1.8 | 4.5 | 24.4 | 0.3 | 0.5 |
| 312.5 | 3 | 60 | 12 | 8 | 43.6 | 47.7 | 73.2 | 39.9 | 18.4 | 26 | 16.5 | 1.5 | 0.8 |
| 322.9 | 4 | 40 | 2 | 4 | 45.2 | 42 | 83 | 46.5 | 7.8 | 15.9 | 8.4 | 3 | 1.1 |
| 300.9 | 5 | 50 | 2 | 4 | 44.7 | 47.8 | 82.1 | 23.5 | 17.3 | 15.7 | 31.8 | 1.8 | 2.3 |
| 319.2 | 5 | 60 | 4 | 4 | 29.4 | 56.7 | 60.9 | 40.3 | 24.5 | 36.1 | 30.3 | 2.7 | 3 |
| Concentration | Waste | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mass, g | Wash Water, L/min | Drum Revolutions, rpm | Deck Angle, Ɵ | Mass, % | Cu, % | Cu R, % | Mass, % | Cu, % | Cu R, % |
| <100 µm | |||||||||
| 106.2 | 0.5 | 300 | 2.4 | 52.4 | 7.3 | 81.5 | 47.6 | 1.8 | 18.5 |
| 203.3 | 0.5 | 295 | 3 | 17.8 | 18.4 | 65.3 | 82.2 | 2.1 | 34.7 |
| 195.8 | 0.9 | 290 | 2.94 | 8.5 | 19.2 | 40.9 | 91.5 | 2.6 | 59.1 |
| 100 × 300 µm | |||||||||
| 86.7 | 0.5 | 300 | 2.4 | 59.8 | 11.5 | 94 | 40.1 | 1.1 | 6 |
| 153.4 | 0.5 | 300 | 3.4 | 21.4 | 40.1 | 80.4 | 78.6 | 2.7 | 19.6 |
| 202.7 | 0.5 | 305 | 3 | 17.2 | 37.9 | 56.9 | 82.8 | 6 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourmohammad, M.; Oliva, J.; Anticoi, H.; Sampaio, C.H.; Alfonso, P.; Valderrama, C.; Cortina, J.L.; Escalante, P. Recycling Copper (Cu) from Waste Automotive Printed Circuit Boards (WPCBs) After Characterization and Liberation Study by Mineral Processing Techniques. Minerals 2025, 15, 1259. https://doi.org/10.3390/min15121259
Pourmohammad M, Oliva J, Anticoi H, Sampaio CH, Alfonso P, Valderrama C, Cortina JL, Escalante P. Recycling Copper (Cu) from Waste Automotive Printed Circuit Boards (WPCBs) After Characterization and Liberation Study by Mineral Processing Techniques. Minerals. 2025; 15(12):1259. https://doi.org/10.3390/min15121259
Chicago/Turabian StylePourmohammad, Mahsa, Josep Oliva, Hernan Anticoi, Carlos Hoffmann Sampaio, Pura Alfonso, César Valderrama, Jose Luis Cortina, and Percy Escalante. 2025. "Recycling Copper (Cu) from Waste Automotive Printed Circuit Boards (WPCBs) After Characterization and Liberation Study by Mineral Processing Techniques" Minerals 15, no. 12: 1259. https://doi.org/10.3390/min15121259
APA StylePourmohammad, M., Oliva, J., Anticoi, H., Sampaio, C. H., Alfonso, P., Valderrama, C., Cortina, J. L., & Escalante, P. (2025). Recycling Copper (Cu) from Waste Automotive Printed Circuit Boards (WPCBs) After Characterization and Liberation Study by Mineral Processing Techniques. Minerals, 15(12), 1259. https://doi.org/10.3390/min15121259













