1. Introduction
Ceramics have been necessary to human civilization for millennia. Archaeological evidence indicates that clay-based ceramics, including fired clay bricks and tiles, were utilized as structural building materials in ancient Mesopotamia, Egypt, and Rome [
1]. Sun-dried and kiln-fired bricks have been used since at least the 4th millennium BC, providing durable construction units when stone was scarce [
2]. This long legacy underpins the dominance of red ceramics (structural clay products) in construction worldwide, with masonry bricks remaining among the most prevalent building techniques today. Global production of fired clay bricks, in 2020, was estimated at 2.18 Gt [
3], translating to nearly a trillion bricks per year, illustrating that red ceramics remain a cornerstone of the global construction materials sector. Besides their historical importance, structural red ceramics also have contemporary relevance for infrastructure and housing applications. However, this demands a significant volume of raw material and has an environmental impact, driving current efforts to improve the sustainability of ceramic production. Traditional ceramic manufacturing is energy-intensive and has a substantial environmental footprint. Firing clay-based structural products require high temperatures (usually between 900 and 1150 °C), commonly achieved by burning fossil fuels (i.e., coal, oil, natural gas) in kilns. As a result, the ceramics industry contributes notably to anthropogenic CO
2 emissions globally, on the order of 19 Mt per year, arising from, e.g., fuel combustion for drying and firing, decomposition of mineral carbonates in the clay during firing, and indirect emissions from electricity use [
4]. Only the breakdown of accessory carbonate minerals (e.g., CaCO
3 → CaO + CO
2) in red clays contributes to ≈17% of total ceramic CO
2 emissions. The reliance on carbon-intensive fuels, combined with process-related CO
2 release, makes decarbonization a pressing challenge for the ceramic sector. In recent years, there has been a strong motivation toward sustainable practices and decarbonization of the ceramic industry, exploring different strategies to reduce the CO
2 footprint, such as improving energy efficiency of kilns, transitioning to renewable or lower-carbon energy sources (e.g., biomass, hydrogen-fueled kilns, optimizing firing schedules to lower peak temperatures).
An important aspect of sustainable ceramic production is the use of locally available raw materials, which aligns with the principles of circular economy. Sourcing clays and other materials locally offers benefits. Economically, it decreases dependence on imported or distant raw materials and lowers transportation costs, while also supporting local economies, preserving traditional knowledge, and promoting employment, contributing to socio-economic sustainability. Also, environmentally, shorter transport distances reduce the carbon footprint of the supply chain [
5]. A recent assessment highlighted that utilizing residual clay deposits from a nearby source can substantially reduce transport-related CO
2 emissions and energy consumption. The incorporation of locally sourced residual clays or low-value rocks, such as clay schists, into ceramic products encourages the efficient use of natural resources by valorizing them in ceramic formulations. By carefully characterizing clays and blending them appropriately, the raw material demand of a specific location can be reduced, and the need for materials from other locations can be minimized. Clay-rich schist, such as the ones from Barrancos considered a geotechnical byproduct of dimension stone quarrying, showed suitability to be a component for brick clays when properly processed [
6]. The valorization of such local materials offers a low-impact alternative to traditional ceramic clays, promoting circular use of materials often considered as byproducts. In addition to environmental gains, this strategy diversifies the raw material base and can stimulate regional development, as local industries emerge to process and supply these materials.
Local georesources for red ceramics have been studied, reflecting a global interest in the sustainable use of indigenous materials. Correia et al. [
7] reported that clayey soils used as raw material for red ceramics meet the strength and quality requirements for brick and tile production, and that, after proper processing, they can yield ceramic products compliant with construction standards, underscoring the viability of local clays. Sirbu-Radasanu et al. [
8] characterized residual clays derived from weathered sericite schists in the Eastern Carpathians, finding an illite-rich composition with suitable mineralogical composition for ceramic applications. Kouonang et al. [
9] reported that two local clayey raw materials (one more smectitic-plastic, and one more silty) studied for brick production, produced bricks with the characteristic red-orange color, undergoing the expected mineral transformations on firing, and developed phases such as hematite (Fe
2O
3) and mullite (Al
6Si
2O
13), showing proper vitrification and strength development. The linear shrinkage and water absorption of these bricks fell in acceptable ranges for structural use, confirming that those local alluvial and residual clays could be used to produce viable red ceramic products with standard firing procedures.
São Pedro do Corval (southern Portugal) is a region renowned for its pottery, which utilizes rich, residual clays formed by in situ chemical weathering of ancient metasedimentary rocks. The mineralogy of these clays reflects the parent lithologies, i.e., typically mixtures of illite and kaolinite, with variable amounts of smectite, especially in clays derived from more mafic or hydrothermally altered protoliths. Recent detailed studies on Corval clays [
10] identified distinct clay samples with varying grain size distributions and clay mineral compositions. In Barrancos (southern Portugal), metamorphic clay schist raw materials were studied [
6,
10]. Unlike sedimentary clays, these raw materials are essentially a soft argillaceous rock, rich in Σphyllosilicates (the total content of all phyllosilicate minerals, such as illite, smectite, kaolinite, and chlorite), due to retrograde metamorphism and weathering. Studies revealed that these schists can be used as an alternative clay source for red ceramics. Mineralogically, the Barrancos clay schist was dominated by illite with secondary kaolinite, and also presented a good amount of natural flux components (feldspars) and fillers (quartz, dolomite); however, the schist alone showed that it can be improved for extrusion. Simple technological adjustments, such as blending with more plastic clays or adjusting the particle size distribution, can overcome these limitations. The aim of this study is to evaluate the firing behavior and ceramic performance of two specific ceramic batches, using the studied residual clays from Corval and the Barrancos schist, after being subjected to industrial firing. Based on prior analyses, 1100 and 1150 °C were selected as the firing temperatures, as these fall within the typical range for structural red ceramics and are known to induce significant phase transformations (e.g., mullite crystallization) in illitic–smectitic clays. The study examined how each batch responded to firing in terms of key technological properties: linear shrinkage, bulk density, water absorption, and flexural (bending) strength of the fired bodies, as well as microstructural and phase developments. This study ultimately seeks to contribute to demonstrate that locally sourced formulations can produce high-quality ceramic products, while advancing the goals of resource efficiency and CO
2 reduction in the ceramics industry [
11].
3. Results and Discussion
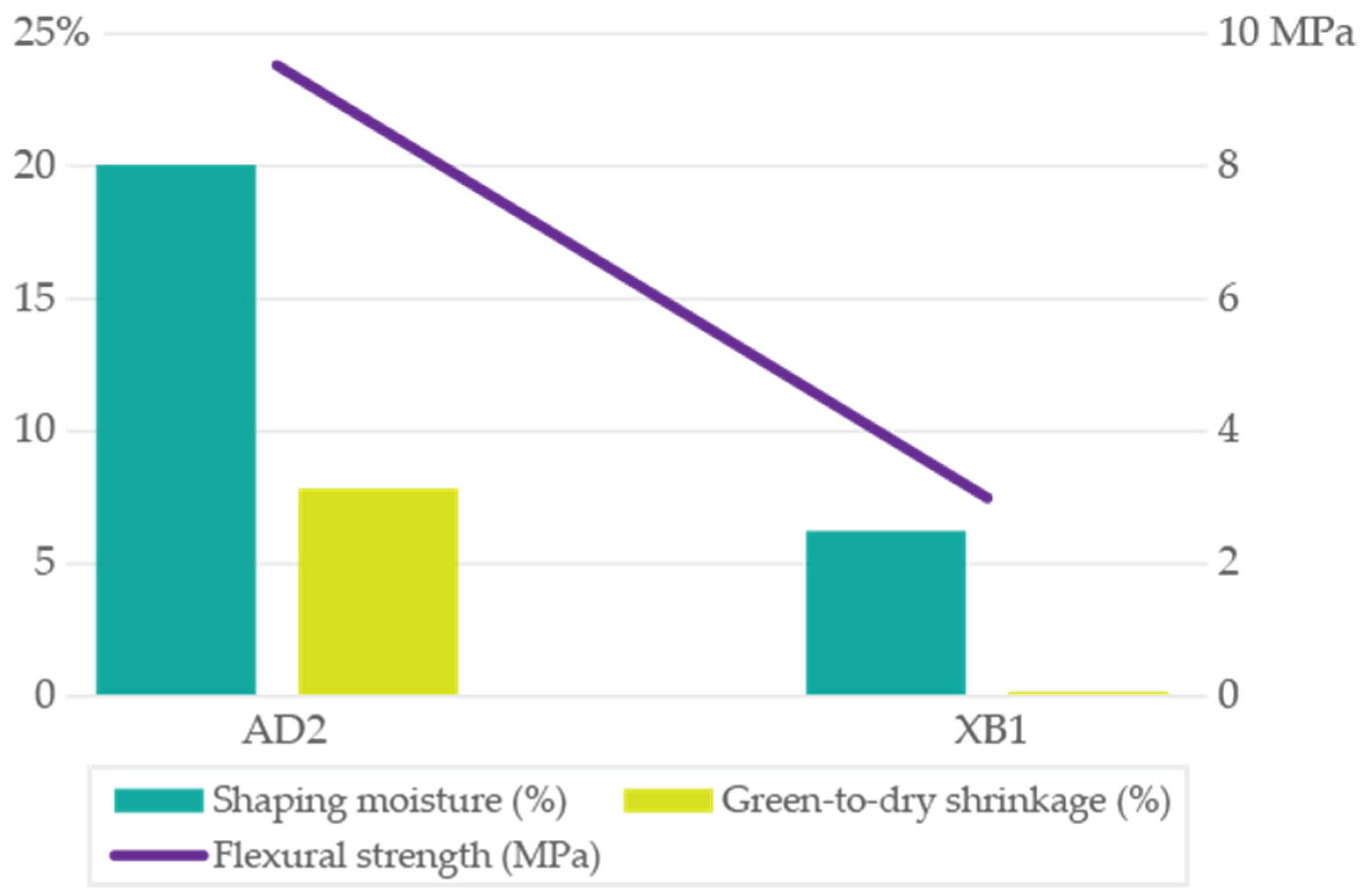
After blending and drying (110 °C), batch AD2 (1:1 residual clays A2 and D2) [
10] showed a relatively high SM (SM; 20.04%) requirement (water content for workable extrusion), reflecting the fine particle size (51% <0.002 mm) of raw A2 clay and smectite content of A2 (71%) and D2 (83%) clays. Nevertheless, its green-to-dry shrinkage (GDS) upon drying at 110 °C (7.82 ± 0.18%) was lower than that of raw A2 clay (9.63 ± 0.15%), as the inclusion of raw D2 (58% >0.063 mm size) and lower PI acted as a temper, improving dimensional stability. The AD2 dried green FS (9.33 ± 0.82 MPa/95.17 ± 8.39 kgf/cm
2) (
Figure 2;
Table S5) was high, benefitting from the interparticle cohesion of the smectite-rich raw clays, although FS was slightly reduced when compared to A2 (FS 13.13 ± 0.48 MPa/133.93 ± 4.93 kgf/cm
2) due to D2 (FS 6.84 ± 0.51 MPa/69.76 ± 5.24 kgf/cm
2) dilutive effect. The abundant smectite content disclosed high PI and water retention, leading to greater SM demand. However, the D2 coarser fraction and lower specific surface area (SSA; 14.1 m
2/g vs. A2, 22.2 m
2/g) limited water uptake and shrinkage. Thus, dried AD2 batch achieved a balance between workability and drying behavior, requiring significant water for shaping due to smectitic clay bonding, but showed controlled drying shrinkage and maintained strong dry strength, from the tempering effect of D2 non-clay components and the cohesive matrix provided by A2. Results highlighted that blending a highly plastic, smectite-rich clay with a less plastic, coarser clay (rich in fillers) can improve drying behavior (lowering GDS) without compromising green strength, as evidenced in AD2 (
Figure 2). Nandi et al. [
26] on a study about fast-drying materials, proposed a mixture design strategy that optimize plasticity, drying stability, and strength, supporting the rationale to combine smectitic and illitic fractions and to engineer the silt/coarser window for robust extrusion and rapid drying. Such strategies include: (i) partial substitution with an illitic coarser phase (e.g., 10%–30% schist or feldspathic sand); (ii) lower peak temperature with longer oxidation holds (≤1050–1100 °C), what will avoid over-firing; and (iii) efflorescence control (raw-mix washing or Ba-carbonate at low levels, when possible). These actions were consistent with A2 over-firing susceptibility and D2 limited densification.
Batch XB1 (residual clay B1 and clay schist 1:1 blend) [
6,
10], after drying at 110 °C, showed lower required SM (6.22%) than raw clay B1 (23.6%). The illite-dominated schist (85%) contributed to lower plasticity and lower water demand, thereby reducing the SM of XB1 (
Figure 2;
Table S5). Consistently, the GDS was minimal (0.15 ± 0.06%), indicating good dimensional stability during drying. This behavior was attributed to the prevalence of non-expanding clay minerals in the blend (85% illite in the shist, and 55% kaolinite from B1), reflecting the low expandability (Exp) and cation exchange capacity (CEC) of the raw materials. Batch XB1 exhibited limited shrink–swell upon drying, thereby mitigating the risk of cracking. The FS after drying was lower in XB1 (2.93 ± 0.49 MPa/29.91 ± 5.03 kgf/cm
2) than in B1 (8.11 ± 0.31 MPa/82.76 ± 3.15 kgf/cm
2), reflecting the reduced smectite content (B1 15%) and the lower green cohesion in the XB1 batch. Raw B1 clay green FS was diluted by the schist component, which yielded only low green strength, with illite providing structural stability but not a high bond strength, as with smectite. Even so, XB1 dried FS remained adequate for handling, benefiting from the B1 clay minerals content that enhanced dry strength and ensured a low moisture requirement and shrinkage during drying, due to the illite dimensional stability, while maintaining sufficient mechanical strength for safe handling. Results showed the effectiveness of incorporating a low-CEC, illite-rich schist as a temper to improve drying behavior in ceramic batches without introducing efflorescence or warping issues. Assunção et al. [
27] reported that higher illite content can enhance FS in red-ceramic bodies, through better packing and earlier viscous sintering, suggesting that the addition of illitic or feldspathic phases to the blend can compensate for the strength deficit without raising shrinkage excessively. Boukili et al. [
28,
29] demonstrated that clay raw materials, which exhibit low PI or poor firing response, can be optimized through blending and/or minor additions, such as bentonite or feldspar, thereby achieving adequate strength while controlling shrinkage and efflorescence. Gu and Ling [
30] proposed that quartz–kaolinite–illite assemblages, characterized by moderate Fe
2O
3, minor alkalis, and limited carbonates, tend to exhibit predictable vitrification and firing strength gain with increasing temperature, consistent with the B1 and schist trends.
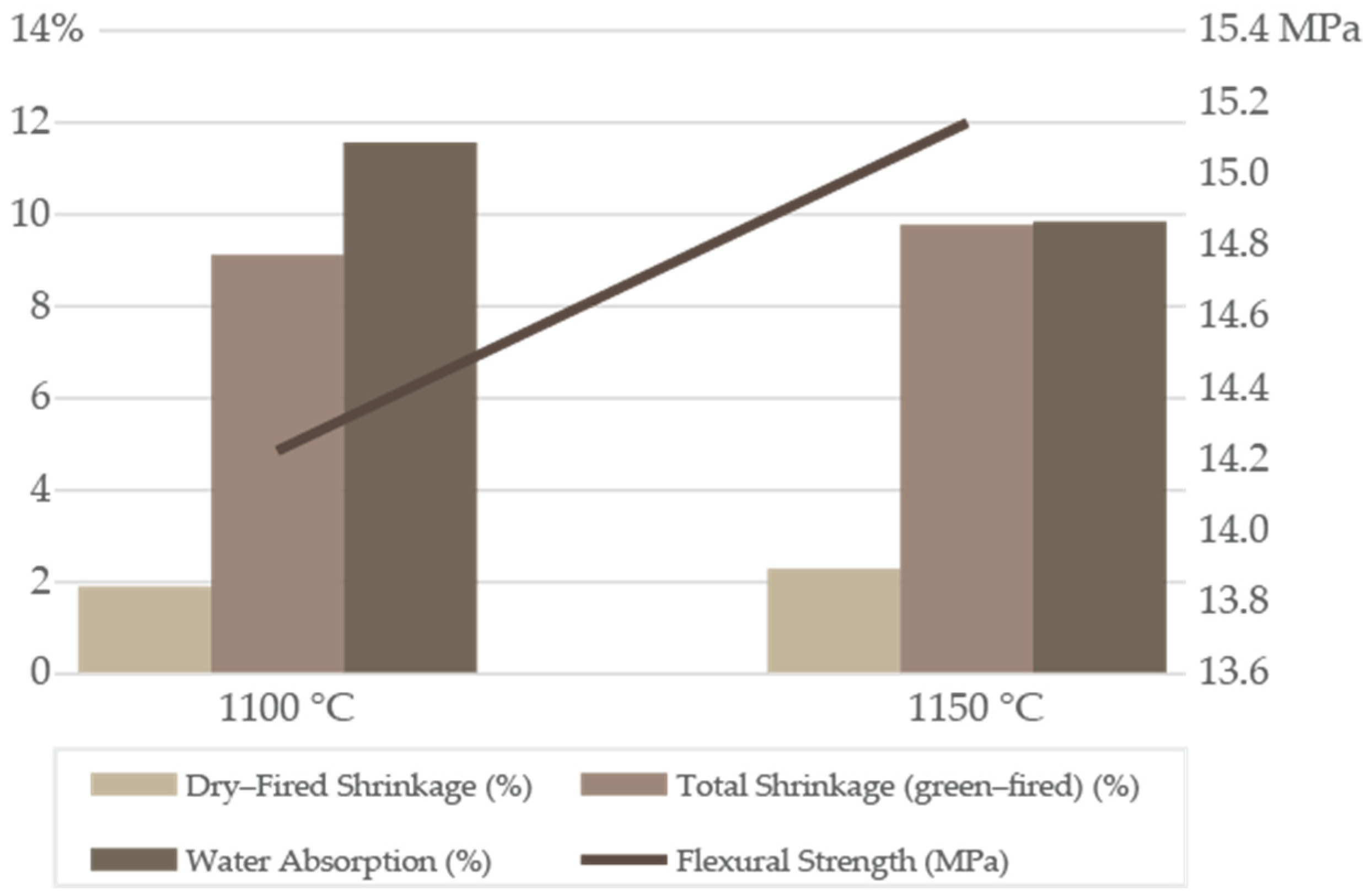
After firing at 1100 and 1150 °C, batch AD2 exhibited clear variations in physical, mechanical, and mineralogical properties, reflecting the balance between densification, porosity control, and phase transformations. At 1100 °C, it presented a total shrinkage of 9.12 ± 0.28%, FS of 13.95 ± 2.30 MPa (142.25 ± 23.49 kgf/cm
2), and WA of 11.57 ± 0.30% (
Figure 3;
Table S6). At 1150 °C, total shrinkage was 9.78 ± 0.11%, FS 14.85 ± 2.68 MPa (151.43 ± 27.36 kgf/cm
2), and WA 9.85 ± 0.27%. Results showed that increasing the firing temperature by 50 °C promoted additional densification, reflected by the reduction in WA and increase in mechanical strength. The FS increase (13.6 to 19.7 MPa) from 1100 to 1150 °C correlates with the densification indicators of dry-fired shrinkage (DFS) 1.82 ± 0.04 to 2.98 ± 0.06%; WA: 12.10 ± 0.77 to 9.91 ± 0.80%). At 1150 °C, the formation of a larger, lower-viscosity alkali–silicate liquid derived from feldspars and illite, facilitated viscous-flow sintering, particle rearrangement, and the rounding and closure of pores. Simultaneously, the mullite content and crystallinity increase, providing an interlocking crystalline skeleton within the glassy matrix. Partial quartz dissolution reduces interfacial flaws and improves glassy bonding. Together, these processes progressively reduced the proportion of open, interconnected pores, which dominate WA, and promoted the development of isolated closed pores, explaining the decrease in WA at 1150 °C. This transition in pore morphology was consistent with classical porosity–strength relations, therefore the ≈45% rise in MOR (modulus of rupture). The variation in FS between the optimized batches (AD2 vs. XB1) and between firing temperatures was primarily explained by differences in liquid-phase formation, mineralogical reactivity, and resulting bulk density. The AD2 formulation, richer in smectite and alkali-bearing phases, generated a larger amount of low-viscosity liquid at 1150 °C, promoting stronger vitrification and earlier pore closure, but with slightly lower final strength than XB1 due to its higher open-porosity retention. In contrast, XB1 contained a greater proportion of illite and feldspathic components, which produced a more effective high-temperature melt and greater mullite crystallinity, yielding a denser and mechanically stronger microstructure at 1150 °C. These mineralogical and densification differences collectively account for the observed strength range. The microstructural evolution can be interpreted from the XRD-derived phase assemblages and the technological behavior of the bodies. Batch AD2, richer in smectite and alkali-bearing minerals, generated an earlier but more diluted and less viscous liquid phase, which limited pore closure and preserved a higher proportion of open porosity after firing. In contrast, the illite–feldspar–rich XB1 blend produced a more effective high-temperature silicate melt and stronger mullite development, resulting in a denser glassy matrix with fewer interconnected pores and, consequently, higher flexural strength. The moderate WA values (≈10%–12%) indicated that open porosity still dominated the ceramic body at both 1100 and 1150 °C, as expected for red ceramics not yet fully vitrified. However, the reduction in WA at 1150 °C suggested the onset of partial conversion from open to closed porosity as glassy-phase formation becomes more pronounced.
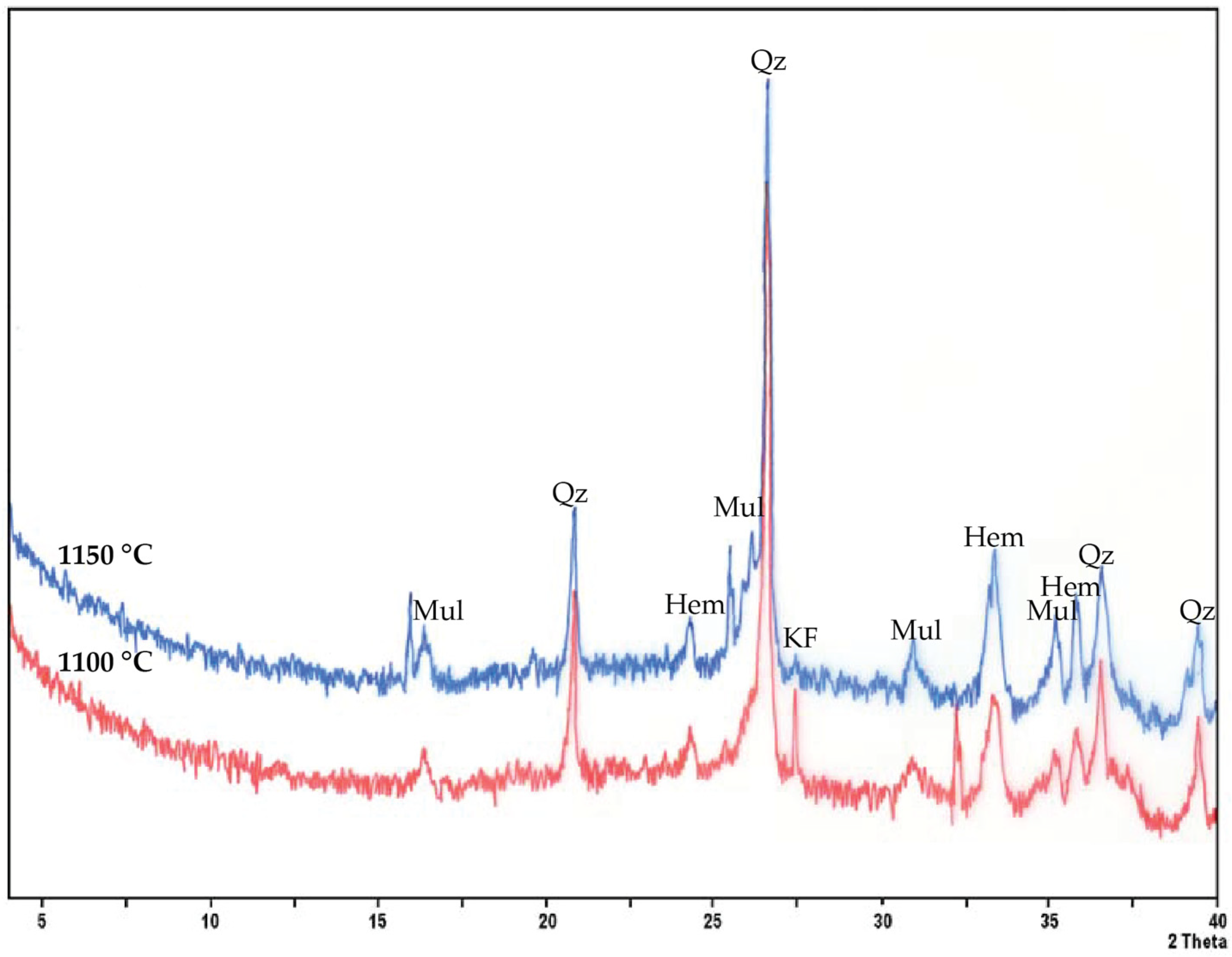
Results fell within the expected ranges for red ceramic products, where shrinkage is typically controlled <12% and WA ideally remains <15% for structural bricks and tiles [
31,
32]. The improvement in FS from 1100 to 1150 °C can be attributed to enhanced vitrification and the progressive development of mullite (3Al
2O
3·2SiO
2), a key phase that confers mechanical stability at high temperatures [
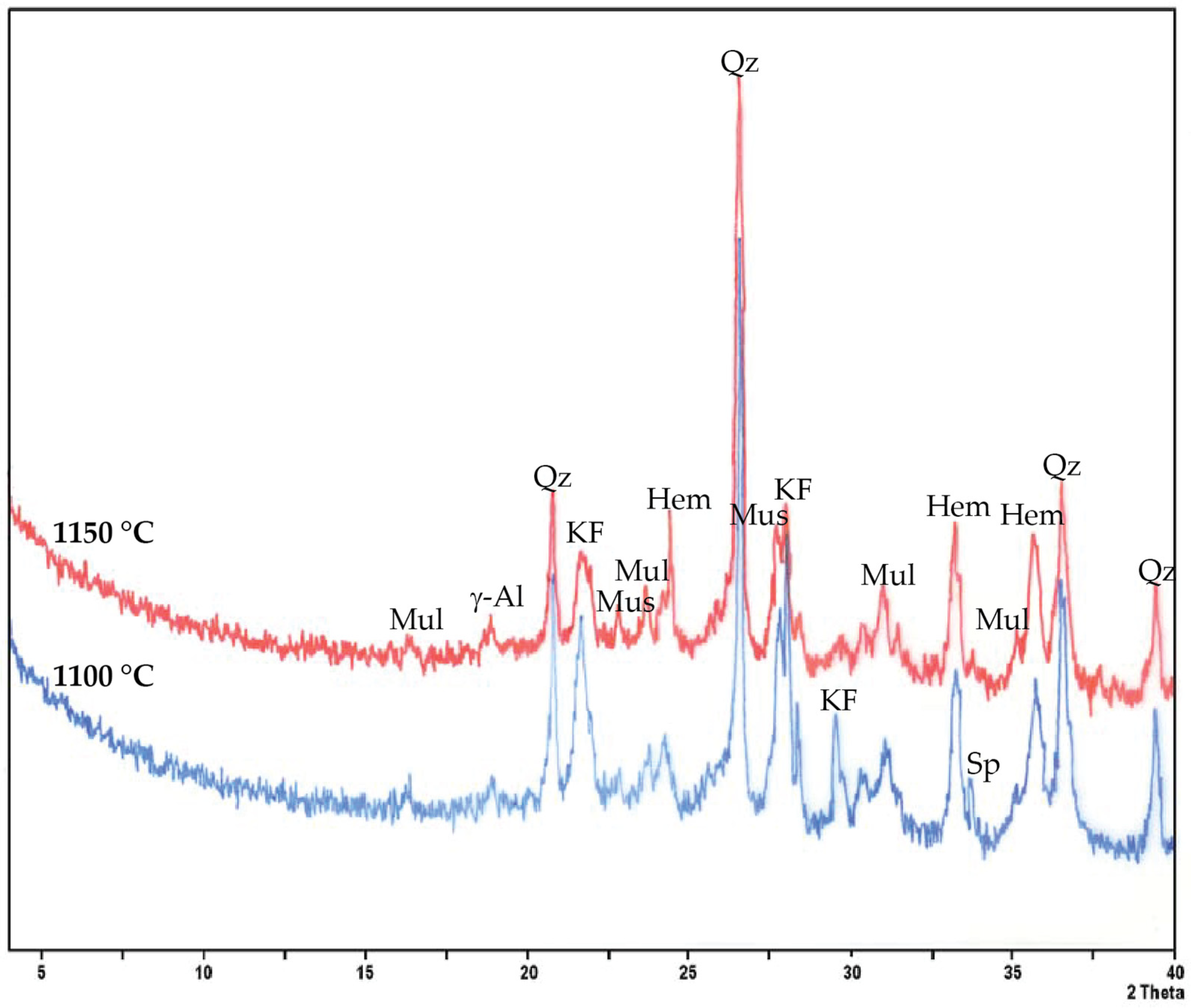
33]. The identified mineral phases (
Figure 4) showed that both firing temperatures led to the persistence of quartz (SiO
2) and K-feldspars (KAlSi
3O
8), while muscovite (K
2Al
4(Si
6Al
2)O
20(OH)
4) peaks diminished due to dehydroxylation. At 1150 °C, mullite reflections intensified in agreement with increased mechanical resistance. Hematite (Fe
2O
3), which influences the reddish coloration, remained stable at both temperatures, with the color changing from orange at 1100 °C to a darker red at 1150 °C (
Figure 5), corresponding to an enhanced Fe
3+ concentration in the ceramic matrix [
34]. At both 1100 °C and 1150 °C firing temperatures, no efflorescence was observed on AD2-fired batches. The formation of a limited spinel phase (MgAl
2O
4) at intermediate positions suggested transitional reactions of aluminosilicates. Such mineralogical evolution was coherent with the firing behavior of Fe-rich illitic-smectitic clays [
35]. The comparison with the raw samples of the AD2 batch showed that the A2 exhibited higher shrinkage and densification due to its smectite-rich composition, whereas D2 tended to buffer shrinkage due to its higher quartz content and medium plasticity. The 1:1 mixture of raw clays A2 and D2 balanced the behavior, i.e., shrinkage remained moderate, avoiding excessive warping, while sufficient vitrification ensured good mechanical strength, indicating the advantage of blending different raw materials to optimize processing properties, a practice reported in different ceramic studies (e.g., [
36,
37,
38]).
The firing temperatures (1100 and 1150 °C) yielded products with acceptable ceramic properties. For structural red ceramics, European standards [
39] specified WA values < 20% for load-bearing clay units and equivalent FS > 5 MPa (10 MPa for compressive strength). The results obtained for batch XB1 after firing at 1100 °C, preferred from an energy-saving perspective, were WA ≈ 12.1%, FS ≈ 13.7 ± 0.74 MPa, both within the regulatory limits, confirming its suitability for structural applications. However, at 1150 °C, it conferred slightly higher mechanical resistance and lower porosity, which is beneficial for durable ceramic applications, such as roof tiles subjected to environmental stress. Similar behavior, where increasing the firing temperature improved mechanical strength and densification, was reported by Karaman et al. [
40], demonstrating that higher firing temperatures resulted in increased compressive and bending strength, greater density, and reduced porosity and absorption.
Batch XB1, after the 1100 to 1150 °C step increased FS from 12.10 ± 0.77 to 9.91 ± 0.80 MPa, while lowering WA from 12.10 ± 0.77 to 9.91 ± 0.80%, with concurrent increase in dry-fired and green-fired shrinkage. The coupled decrease in WA and increase in shrinkage indicated net densification (reduced open porosity/neck growth), which quantitatively agrees with classic porosity–strength relations for ceramics [
41], where MOR increased as porosity fell. The stronger glassy bonding and higher mullite content/crystallinity at 1150 °C further supported the interpretation of a microstructure increasingly dominated by smaller, more isolated pores and a reduced interconnected pore network.
Batch XB1 (1:1 blend of residual clay B1 and Barrancos schist) [
6,
10] upon firing at 1100 °C, undergo dehydroxylation and breakdown of clay minerals, coupled with the formation of new high-temperature phases (
Figure 6). Clay minerals decompose between ≈500–700 °C, losing hydroxyl groups and long-range order. Carbonate impurities, when decomposed (≈600–800 °C), release CO
2 and yield Fe oxide, resulting in the formation of hematite. Hematite was identified at both 1100 °C and 1150 °C, suggesting the persistence of Fe
2O
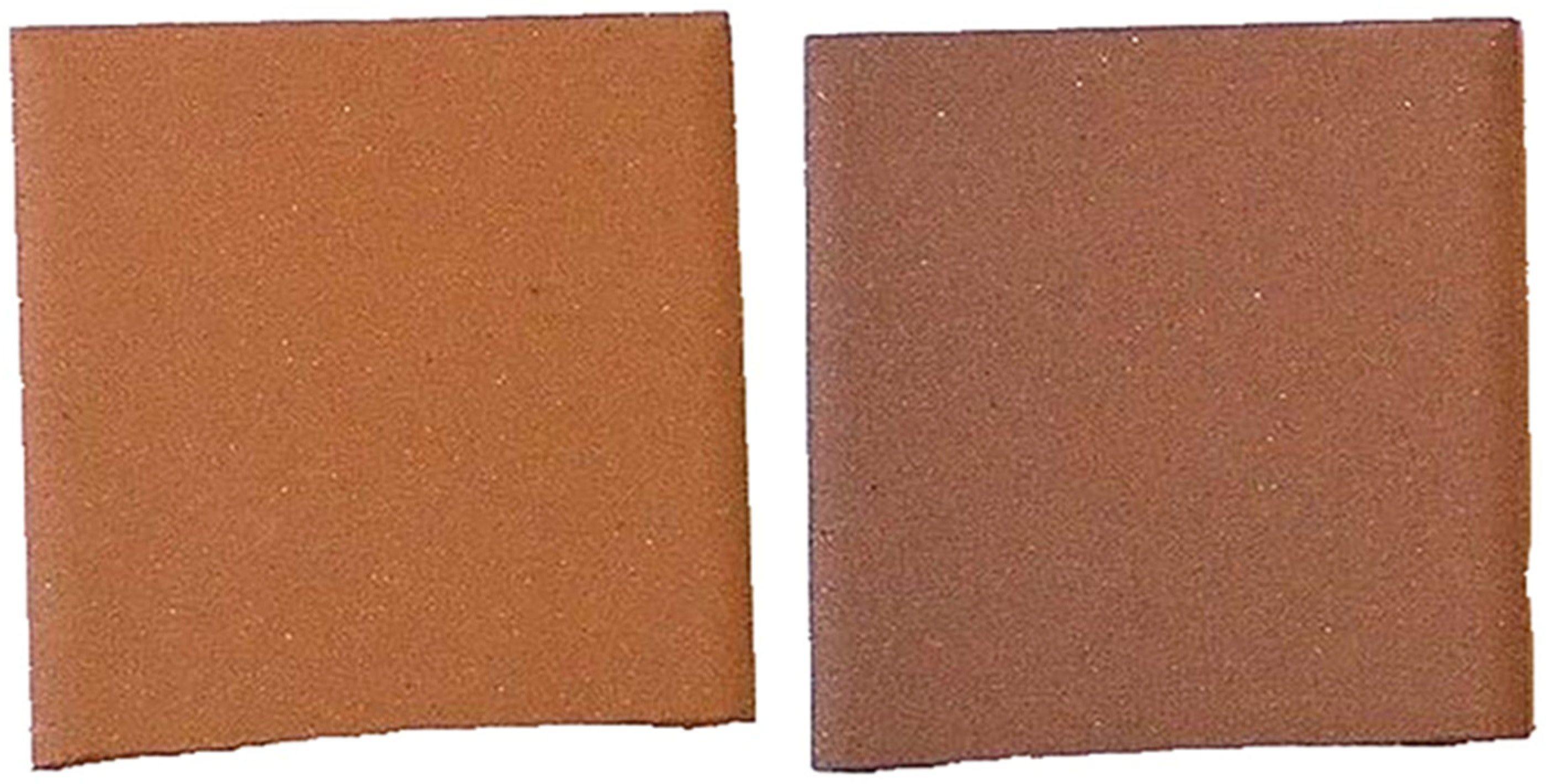
3 as a distinct phase, confirmed by the coloration of the fired batches. The dried XB1 batch showed a light orange color, related to dispersed Fe-oxides. After firing at 1100 °C, the color changed to orange ceramic, and at 1150 °C, it gained a darker orange color (
Figure 7). The darkening tone evolution with temperature could be attributed to Fe-oxidation state and concentration effects, once at higher temperature, Fe is in the Fe
3+ form, promoting slight glass phase development that intensified color saturation. Nevertheless, no undesirable color effects, such as black coring or spotting, were observed, indicating that the firing cycle allowed for the complete burn-out of any organic matter present in the dried batch and the full oxidation of Fe throughout the body. Mullite, a strengthening phase, occurred at 1100 °C and intensified at 1150 °C, reflecting its increasing crystallinity and abundance. The K-feldspar peak was present at 1100 °C and was significantly reduced at 1150 °C, due to melting into the glassy phase. Quartz persistence after firing promoted dimensional stability, with the absence of cracking after firing, suggesting that its content and grain size were not high enough to cause thermal stress or that the cooling was sufficiently controlled.
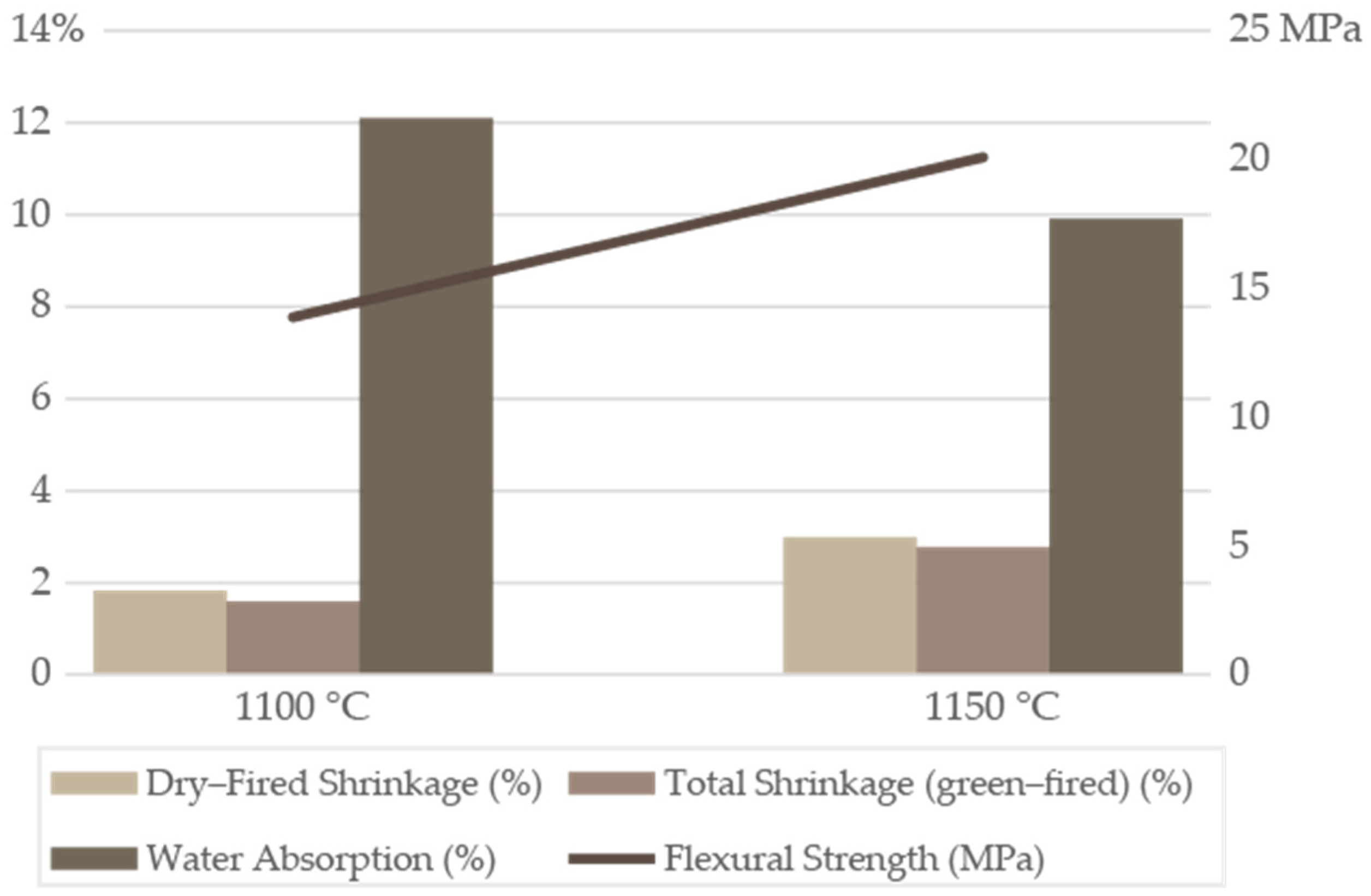
Batch XB1 ceramic properties exhibited minimal shrinkage during drying at 110 °C (
Figure 2) and only moderate shrinkage upon firing (
Figure 8;
Table S7), due to the balanced residual clay/schist formulation. Raw B1 clay, being very fine and plastic, was prone to higher drying shrinkage and cracking; however, blending with the schist, which contributed coarser particles, reduced the risk. After firing at 1100 °C, the total linear shrinkage was 1.59 ± 0.06%, a low value for a clay-based ceramic. At 1150 °C, it slightly increased to 2.77 ± 0.04%, and the shrinkage from the dry state to the fired state was 2.98 ± 0.06%. These findings were below the ≈8% upper limit cited for good structural clays [
42]. A slightly higher shrinkage at 1150 °C was expected, as the increased liquid phase formation promoted closer particle packing and pore consolidation. The low firing shrinkage will produce products that will retain the molded dimensions closely, with minimal warpage, which can be considered an advantage in manufacturing. These results aligned with observations of similar illitic–kaolinitic (XB1) blends, which reported moderate total shrinkage at 1100–1150 °C [
43]. Consistent with the shrinkage, batch XB1 retained a portion of open porosity after firing (
Figure 8). At 1100 °C, the material showed a porosity typical of structural brick bodies (WA 12.10 ± 0.77%), which decreased by 2% when the temperature was increased by 50 °C (WA 9.91 ± 0.80%). This represented a significant pore closure, a direct consequence of liquid-phase sintering and glass formation, resulting in denser and less permeable ceramic bodies. This improvement is crucial for the durability of ceramic products, as a WA ≤ 15% is considered to have robust performance, particularly in terms of moisture resistance and structural durability [
44]. Overall, XB1 batch porosity at both temperatures showed reasonable results for red structural ceramics, reflecting sufficient vitrification without over-firing.
Batch XB1 FS (
Figure 8;
Table S7) increased significantly from the dried body (2.93 ± 0.49 MPa/29.91 ± 5.03 kgf/cm
2) to 1100 °C (13.60 ± 0.74 MPa/138.69 ± 7.52 kgf/cm
2), and further at 1150 °C (19.69 ± 1.54 MPa/200.88 ± 15.69 kgf/cm
2), reflecting the microstructural bonding changes. After drying, XB1 microstructure was of a typical clay body, with clay particles packed with silt-sized quartz and feldspar particles from the schist, acting as a skeletal framework. Pores between particles were relatively large, which on drying become air-filled voids yielding the initial porosity. At 1100 °C, the microstructure evolved through sintering, with the appearance of an initial liquid phase due to the melting of feldspathic and illitic components (≈1000–1050 °C), forming a silicate melt that bonded the remaining solid particles. Although the mullite content at 1100 °C was not abundant, it provided a crystalline framework within the glass. The presence of hematite dispersed in the matrix could influence the microstructure, but do not contribute to bonding except by occupying space. The partially sintered microstructure explained the relatively high, yet not maximal, strength at 1100 °C, with a structure mechanically capable for load-bearing masonry applications, such as structural bricks and roof tiles, where FS values >5 MPa are typically required to ensure safe performance under wall or roof self-weight and service loads. At 1100 °C, batch XB1 FS exceeded the lower limit defined for structural red ceramics and consistent with studies on optimized illitic–kaolinitic clays [
45]. Therefore, at this firing temperature, XB1 presented a densified, vitrified matrix capable of resisting bending and compression stress typical of structural brickwork.
The strength gained from 1100 to 1150 °C was attributed to sintering, characterized by increased glassy-phase bonding and growth in mullite content and crystallinity, which strengthened the ceramic matrix, enabling it to resist higher stress before fracture and reducing the risk of cracking. Mullite, a refractory phase, contributed to the long-term stability of the ceramic body by enhancing its thermal shock and fire resistance. Similarly, quartz partially dissolved, promoting bonding with the matrix, which resulted in a strong, vitrified microstructure with lower porosity and higher FS. Firing at 1150 °C enhanced the sintering processes and mechanical performance of the XB1 batch, resulting in more robust products, such as bricks with higher load-bearing capacity or tiles with lower water permeability. However, a higher firing temperature implies higher energy consumption and costs, as well as a larger carbon footprint for the firing process. The darker color at 1150 °C might be considered a limitation when a lighter shade is desired. Firing at 1100 °C, the lower end of the typical firing range for structural ceramics, will reduce energy needs and emissions. The slightly higher porosity, while strong, may not meet the most stringent standards for low WA or strength for some applications such as high-performance bricks, but for, e.g., interior masonry units or traditional roof tiles, can be considered sufficient. Additionally, no efflorescence was observed on XB1 fired batches at either 1100 or 1150 °C. Raw clay B1 and schist were primarily composed of silicate minerals with low soluble sulfate or chloride content. Most alkalis (K, Na) were derived from feldspars/illite, and upon firing, they ended up in the glassy phase, rather than remaining as free salts. The absence of efflorescence suggested that XB1 bricks/tiles would not develop unsightly white deposits or suffer associated degradation.
Studies have consistently highlighted the environmental advantages of partially substituting clays with nearby materials or waste-derived materials, as this approach reduces pressure on raw material extraction and lowers the embodied carbon of ceramic products. Bonet-Martínez et al. [
46] demonstrated that replacing up to 25% of natural clay with Al-recycling residues produced fired bricks exhibiting equal or higher mechanical strength and lower thermal conductivity, thereby achieving the dual benefits of waste valorization and improved product performance. Similarly, studies incorporating up to 25% of construction and demolition waste into clay matrices have maintained mechanical behavior comparable to that of conventional clay bricks, reinforcing the sustainability potential of circular raw materials in the ceramic sector [
47]. Compared with a conventional single-clay formulation, the blended clay–schist bodies can reduce CO
2 emissions primarily by enabling earlier vitrification and thus lowering the required firing temperature by 50 °C. For typical structural-ceramic compositions, an equivalent non-flux-optimized clay would generally require ≥1150 °C to achieve similar densification and strength, meaning that the 1100 °C firing adopted for the optimized blends directly avoids the additional 60–170 MJ/t and corresponding 3–10 kg CO
2/t associated with higher-temperature processing. This showed that the strength of the blend lay not only in the use of residual resources but in tangible, quantifiable decarbonization through reduced thermal demand.
From a sustainability perspective, the use of a 1:1 clay–schist blend is significant. In the present work, this mixture not only combined complementary mineral compositions but also allowed for a reduction in firing temperature from 1150 to 1100 °C, a 50 °C decrease that directly contributes to energy and CO
2 savings. Such a reduction represents a meaningful optimization step in red-ceramic production, especially when similar physical and mechanical properties can be achieved at lower thermal input. Because fuel demand in tunnel kilns increases approximately linearly with peak temperature, even moderate reductions yield quantifiable energy benefits. For industrial brick and roof-tile production in Europe, validated energy benchmarks indicate that the specific thermal energy consumption (SEC) of modern tunnel kilns typically ranges from 2.0 to 3.6 GJ/t, depending on kiln configuration, heat recovery efficiency, and product characteristics [
48]. More detailed national datasets report average SEC values of ≈2.31 GJ/t for EU plants and, for example, ≈2.07 GJ/t for Belgian brick and roof-tile facilities, values consistent with the Intergovernmental Panel on Climate Change (IPCC) benchmarks for efficient tunnel-kiln operations [
49]. Although Belgium is cited in the IPCC datasets due to the availability of comprehensive industrial statistics, Portugal also presents relevant case studies. For instance, Almeida et al. [
50] and Carvalheira et al. [
51] reported energy audits and life-cycle assessments of Portuguese ceramic plants, confirming comparable SEC magnitudes, although nationally aggregated data remain limited.
An EU Climate Action study [
52] found that frost-resistant pavers, fired at higher peak temperatures (typically >1100 °C), consume approximately +30 Nm
3 of natural gas per 1000 wire-cut bricks, equivalent to ≈130 kWh/t (≈468 MJ/t) of additional energy relative to standard bricks. Similarly, the CER BREF [
53] indicated that higher firing temperatures, used to obtain lighter color tones or denser microstructures, can increase energy consumption by ≈47–120 kWh/t (≈170–430 MJ/t). Considering kiln-to-kiln variability (heat recovery, firing profile, and body fluxing), the temperature reduction from 1150 to 1100 °C can conservatively yield ΔE ≈ 60–170 MJ/t of thermal energy savings, with a central estimate of 100 MJ/t. Applying EU-consistent CO
2 emission factors, natural gas 56.1–56.4 kg CO
2 GJ
−1 [
54], fuel oil ≈ 77.4 kg CO
2 GJ
−1, and coal ≈ 94.6 kg CO
2 GJ
−1 [
53], produces per-ton CO
2 savings of approximately, natural gas: 3.4–9.6 kg CO
2/t (central ≈ 5.6 kg/t); fuel oil: 4.6–12.8 kg CO
2/t (central ≈ 7.7 kg/t); coal: 5.7–16.3 kg CO
2/t (central ≈ 9.6 kg/t). Although modest per ton, these reductions become operationally significant at an industrial scale. For a 100 kt/y facility, the 50 °C reduction could prevent ≈0.3–1.6 kt CO
2/y for natural-gas-fired kilns, with even greater avoidance for oil- or coal-fired systems. Beyond direct emission mitigation, lowering the peak temperature by 50 °C also extends kiln lifetime, reduces refractory wear, and lowers production costs, representing essential advantages along the European ceramic sector’s decarbonization pathway.
The 1:1 illitic–feldspathic–schist blend used in this work facilitated vitrification at lower temperatures owing to its natural alkali-flux content, in line with previous European research, where optimized clay formulations achieved 10%–20% reductions in firing energy without compromising mechanical integrity or durability [
50]. This evidence highlighted the dual role of material design and firing control as complementary strategies to enhance energy efficiency and reduce emissions in sustainable red-ceramic production. Overall, the 50 °C temperature decrease corresponds to energy savings of ≈60–170 MJ/t and CO
2 emission reductions of ≈3–10 kg/t, depending on the fuel type. When extrapolated to industrial volumes, such optimization can result in the avoidance of hundreds of tons of CO
2 annually, supporting flux-optimized formulations that lower vitrification thresholds and advance the decarbonization of the European ceramic industry.
Beyond its technical significance, this work directly contributed to global sustainability targets defined by the United Nations Sustainable Development Goals (SDGs). By enabling lower-temperature, energy-efficient ceramic processing, it supports SDG 7 (Affordable and Clean Energy) through reduced thermal energy demand and enhanced energy efficiency. The development of innovative, locally sourced clay–schist formulations aligned with SDG 9 (Industry, Innovation and Infrastructure) by fostering resilient, low-carbon industrial processes based on regional raw materials. Moreover, the valorization of residual clays and schists exemplifies SDG 12 (Responsible Consumption and Production), minimizing extraction pressures and promoting circular use of georesources. Finally, the demonstrated CO2 reduction potential of lower-temperature firing contributes directly to SDG 13 (Climate Action), reinforcing the role of sustainable materials engineering in achieving decarbonization pathways for Europe’s ceramic sector.