Enhanced Antibiotic Removal via Adsorption–Photocatalysis Using a ZnO–TiO2–Halloysite Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
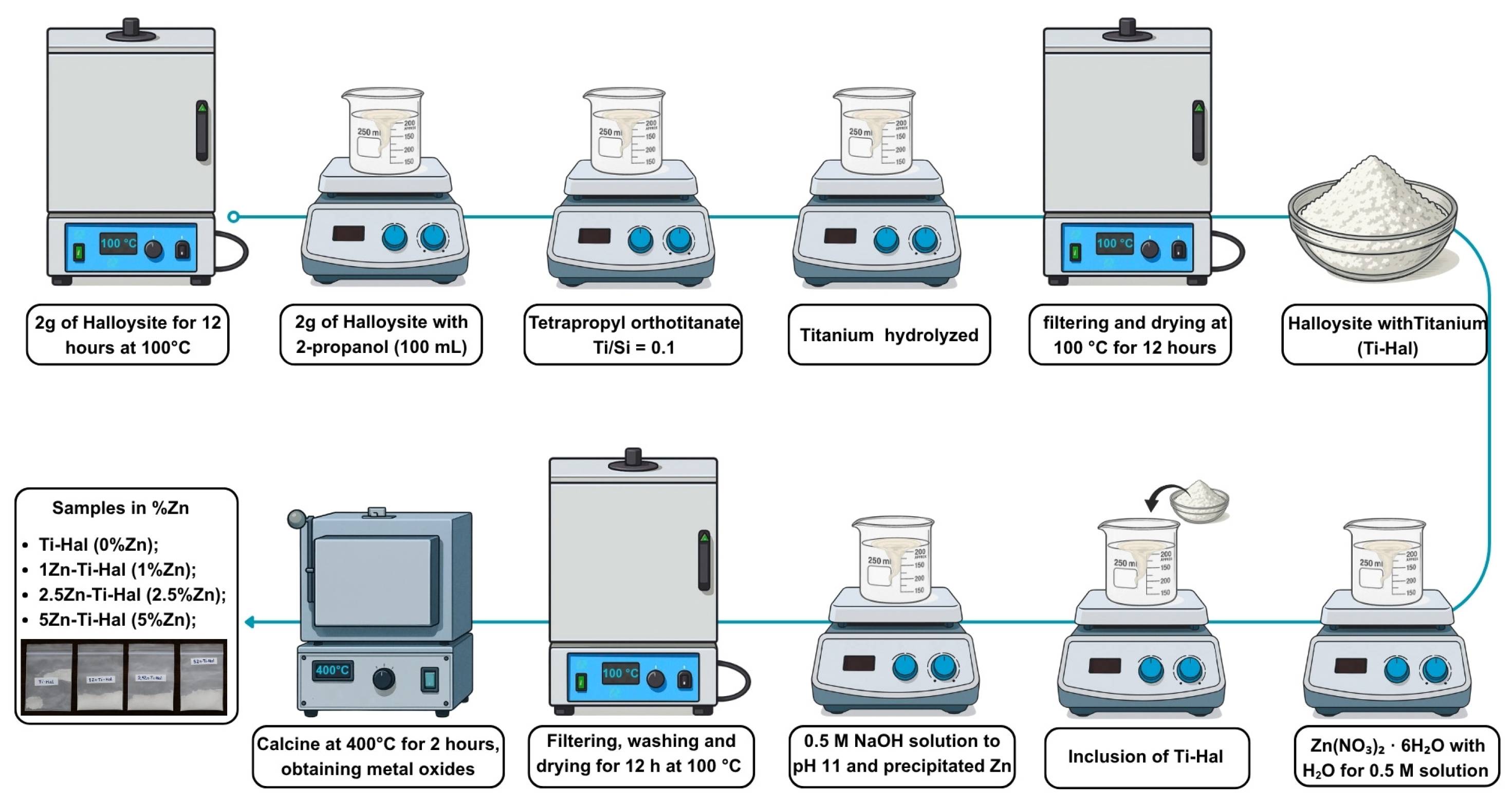
2.2. Photocatalyst Synthesis
2.3. Characterization of the Nanocomposite
2.4. Ciprofloxacin Removal Test
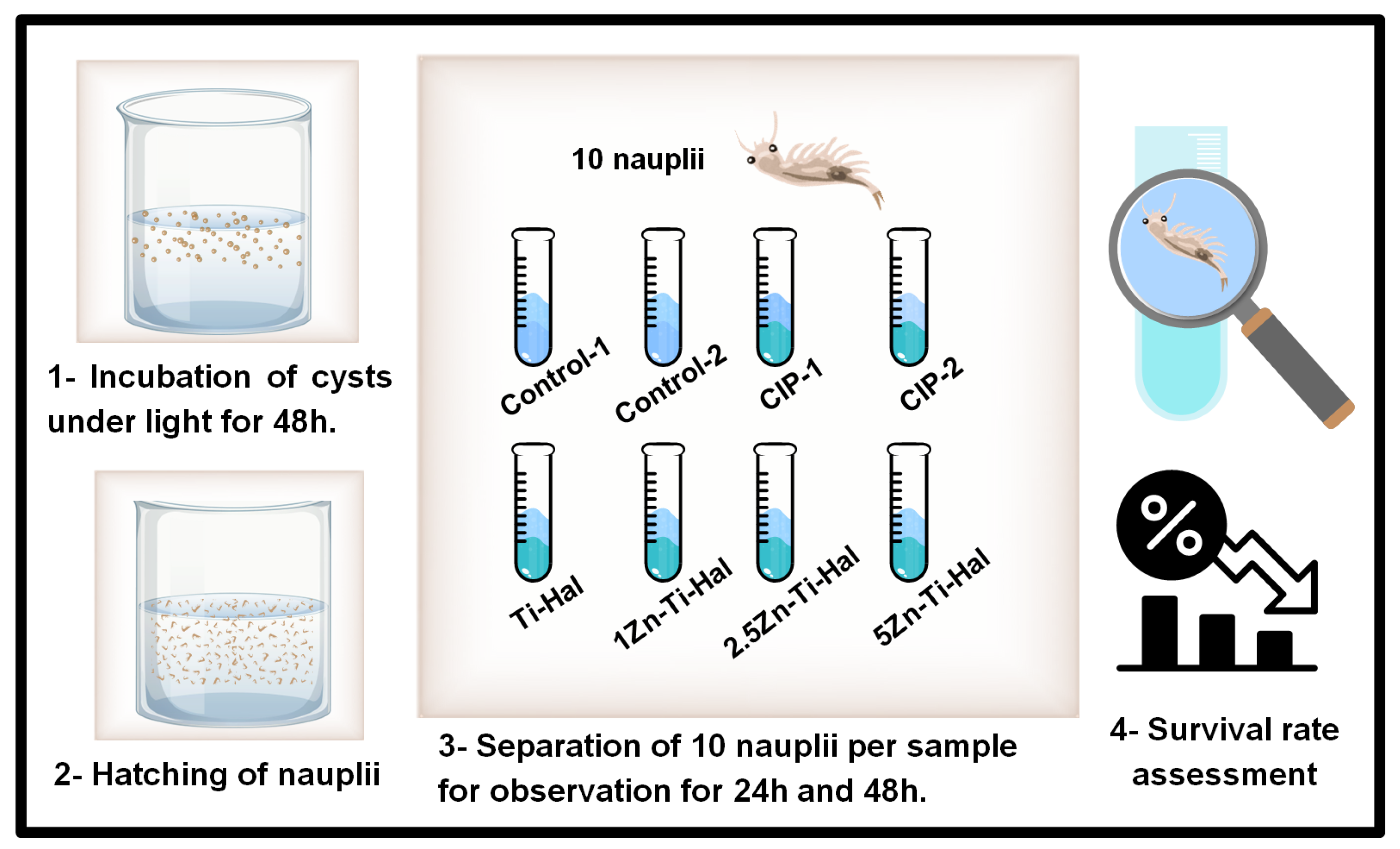
2.5. Ecotoxicity Test
2.6. Antibiotic Inactivation Test
3. Results and Discussion
3.1. Elemental and Structural Properties
3.1.1. XRF Analysis
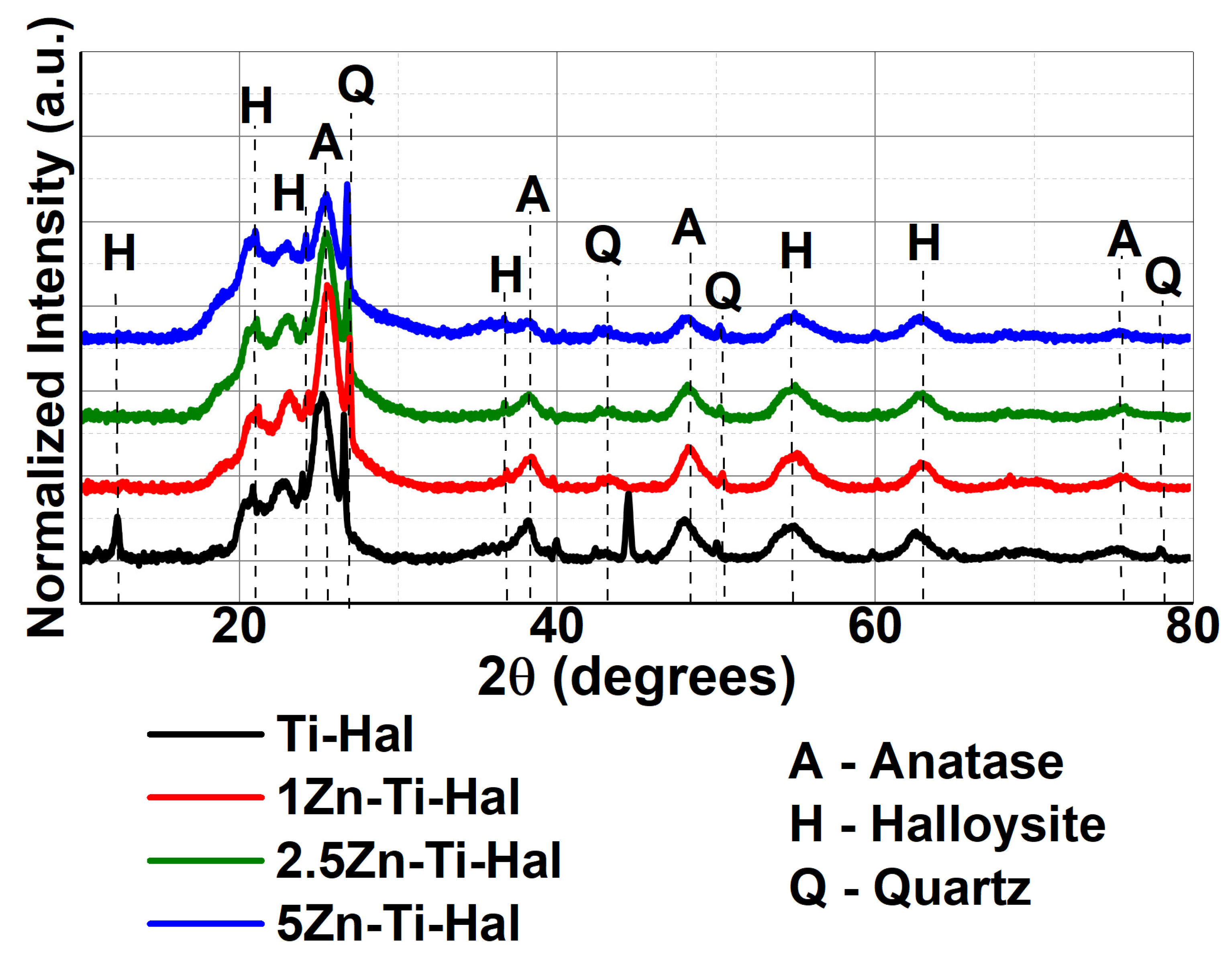
3.1.2. XRD Analysis
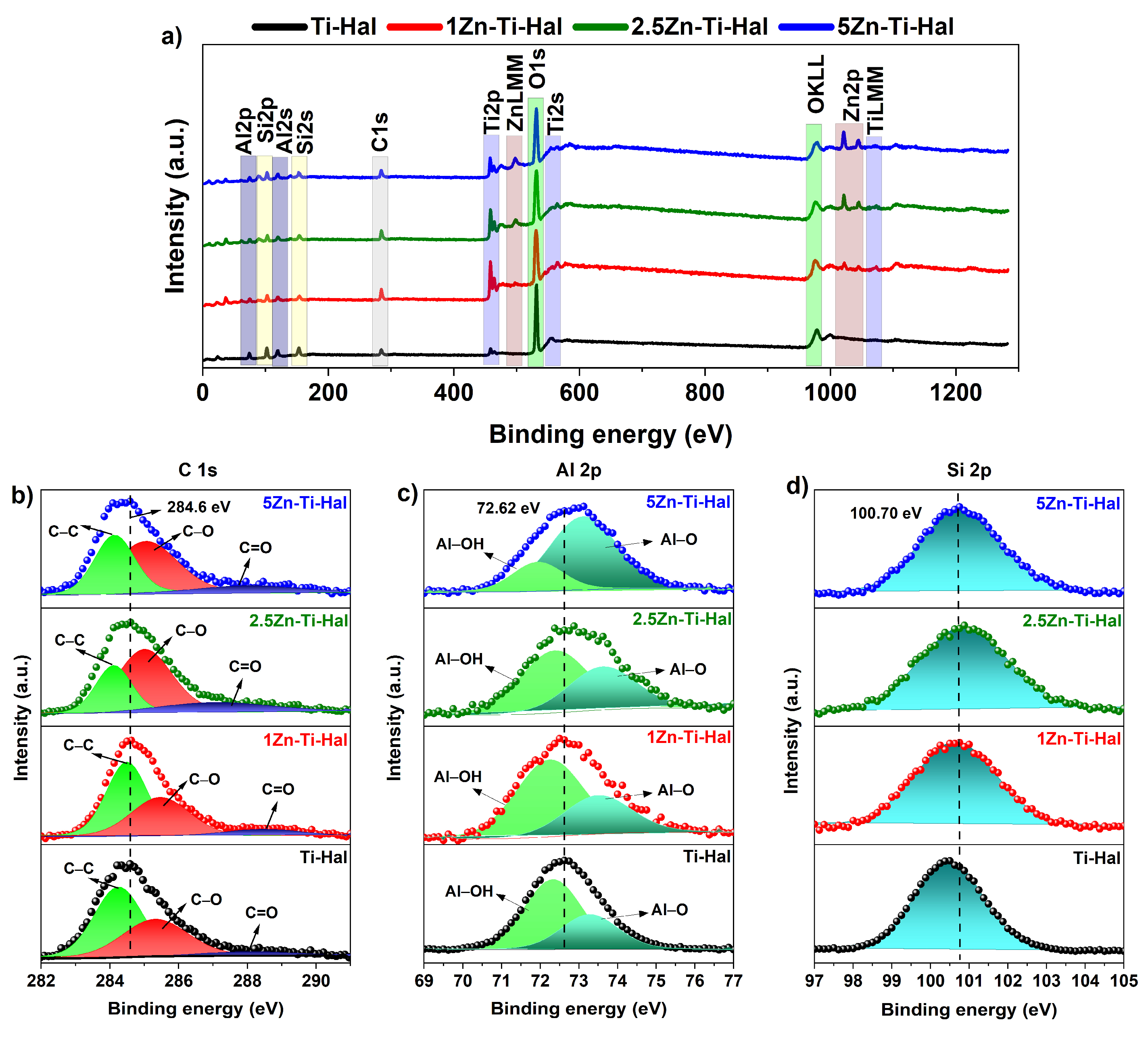
3.1.3. XPS Analysis
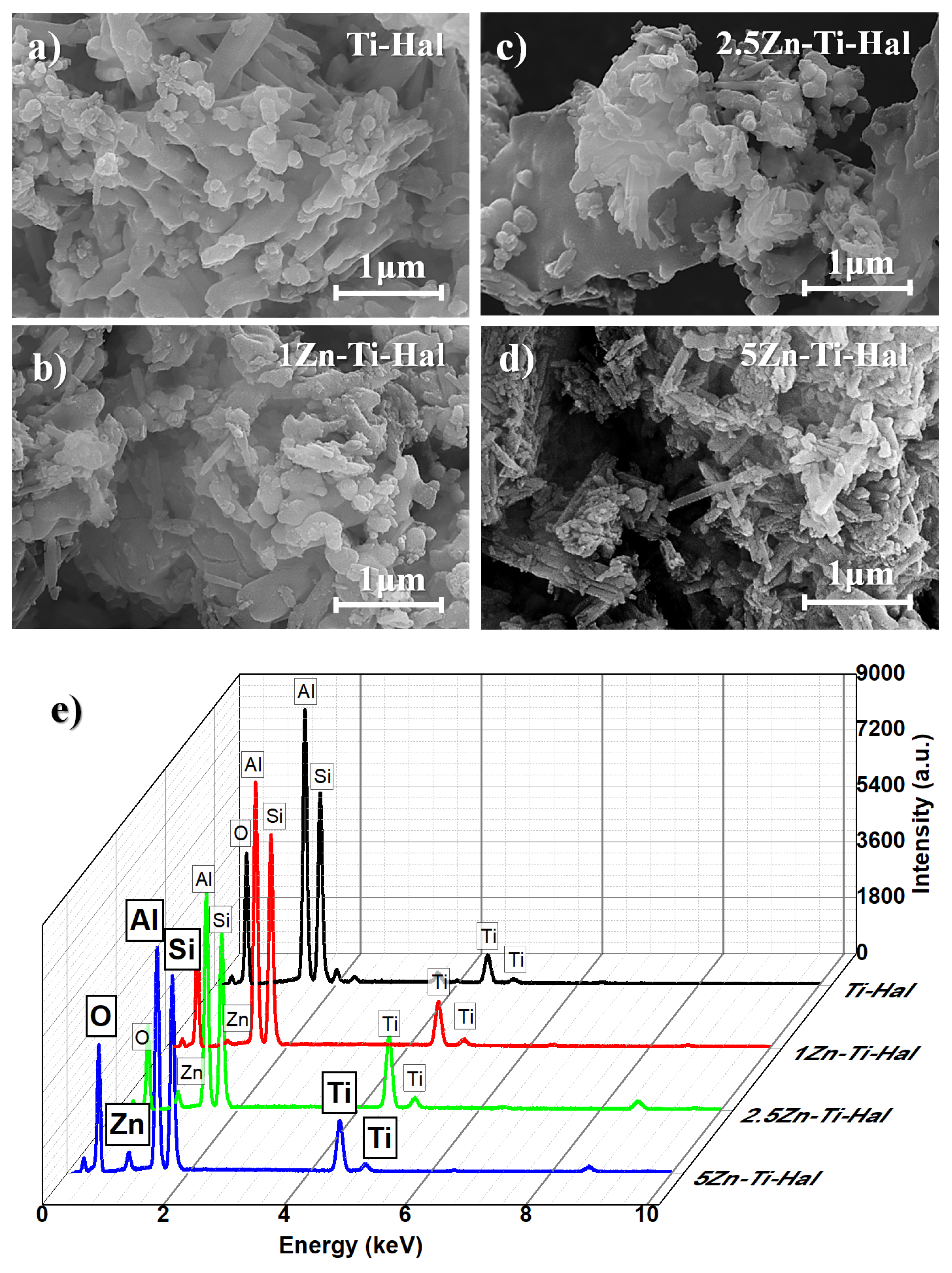
3.2. Morphological Analysis
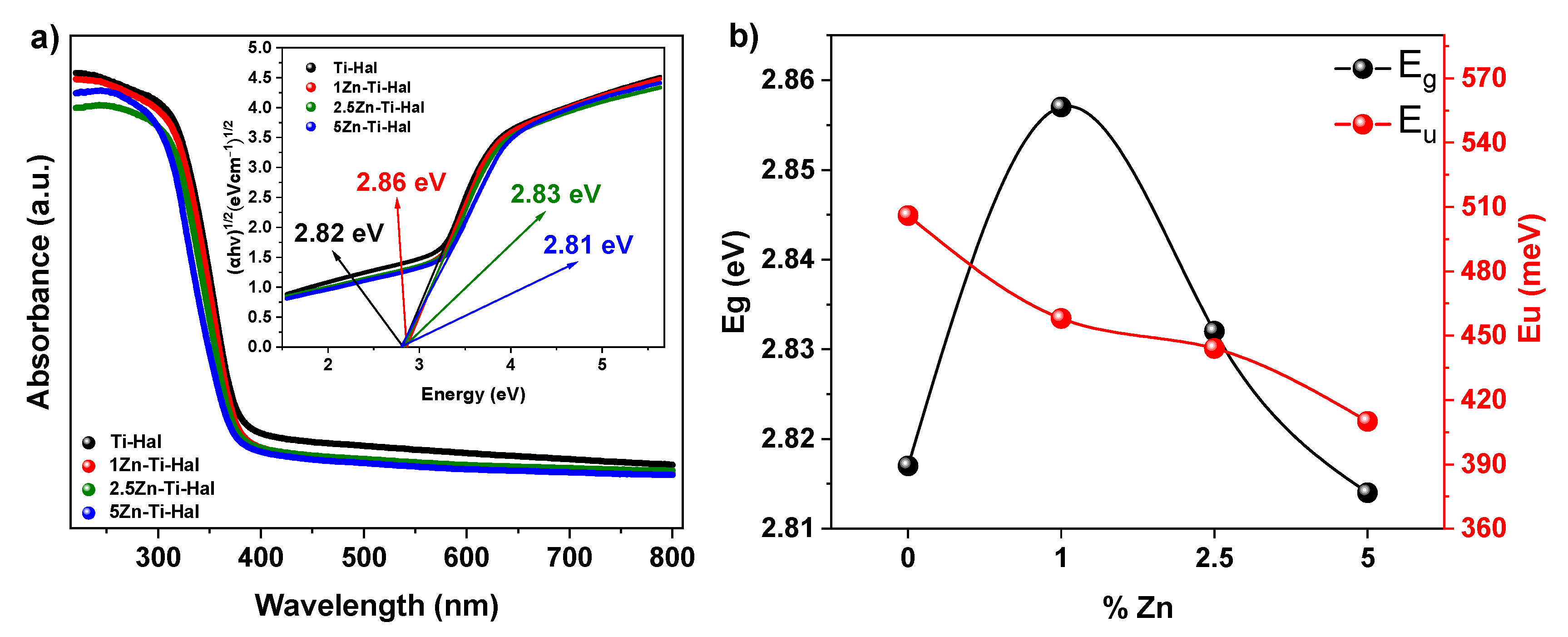
3.3. Analysis of Optical Properties
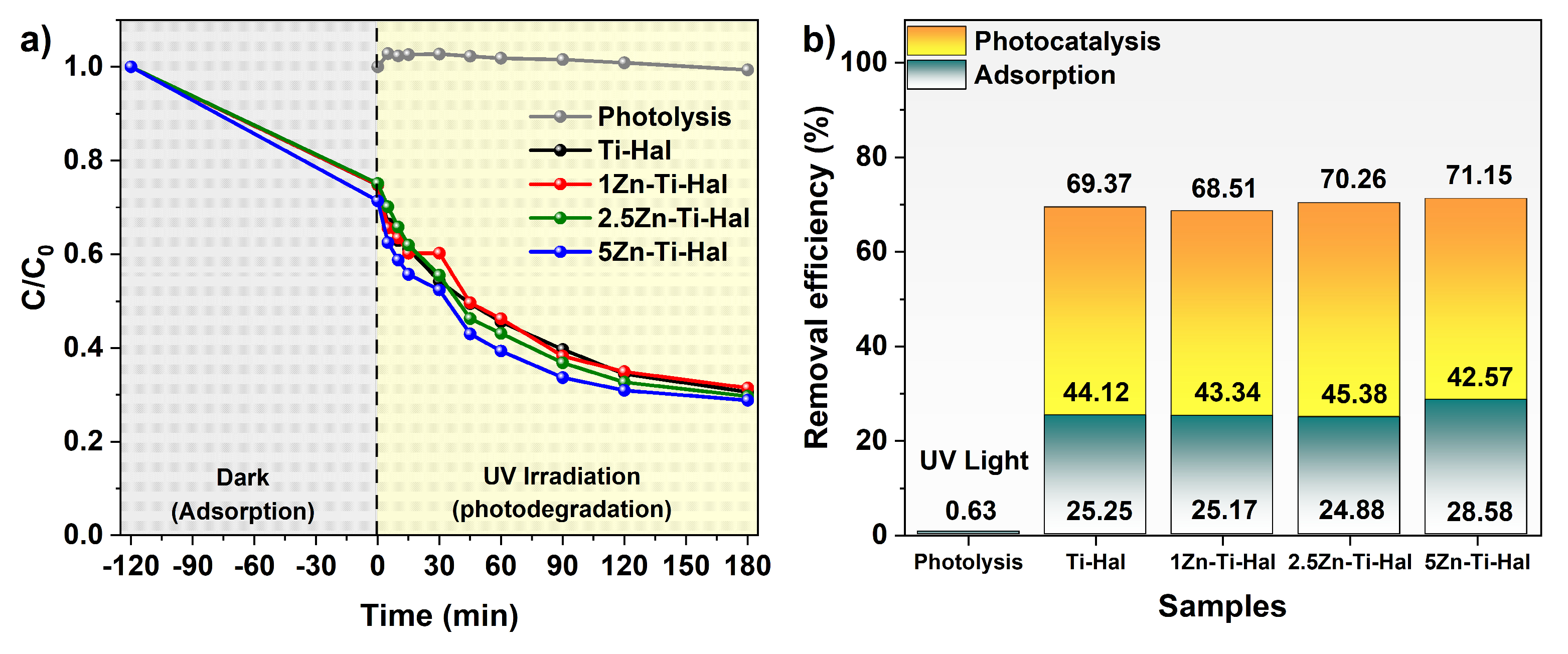
4. Removal Test
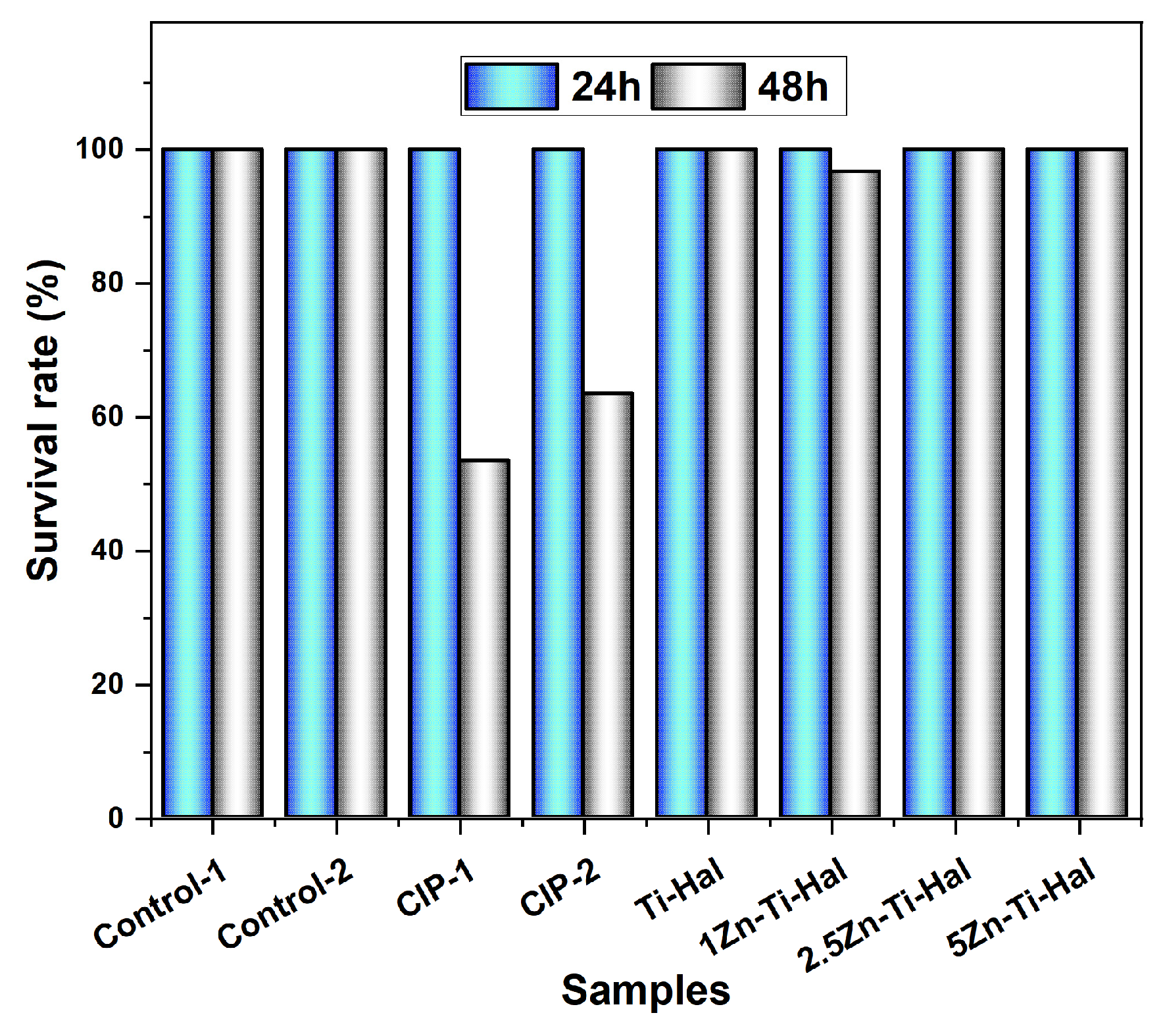
4.1. Bioassays
4.2. Pharmacological Inactivation Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.C.; Petit, C.; Apostolopoulou, A.; Stathatos, E.; Komarneni, S.; Koukouvelas, I. Halloysite and Sepiolite–TiO2 Nanocomposites: Synthesis Characterization and Photocatalytic Activity in Three Aquatic Wastes. Mater. Sci. Semicond. Process. 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Lee, J.; Seong, S.; Jin, S.; Jeong, Y.; Noh, J. Synergetic Photocatalytic-Activity Enhancement of Lanthanum Doped TiO2 on Halloysite Nanocomposites for Degradation of Organic Dye. J. Ind. Eng. Chem. 2021, 100, 126–133. [Google Scholar] [CrossRef]
- Aghababaei, N.; Abdouss, M.; Hosseini-Monfared, H.; Ghanbari, F. Photocatalytic Degradation of Diclofenac Using a Novel Double Z-Scheme Catalyst (O-g-C3N4/ZnO/TiO2@halloysite Nanotubes): Degradation Mechanism, Identification of by-Products and Environmental Implementation. J. Water Process Eng. 2023, 53, 103702. [Google Scholar] [CrossRef]
- Pereira, G.; Espínola, D.L.; Pinto, G.F.; Silva, V.; Lima, D.L.D.; Calisto, V. Photodegradation of Ciprofloxacin in Water Using Silver Nanostructures. Case Stud. Chem. Environ. Eng. 2024, 9, 100699. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms Underlying the Photocatalytic Degradation Pathway of Ciprofloxacin with Heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Albuquerque, W.; Trigueiro, P.; Silva, B.V.; Neves, L.; Almeida, L.C.; Peña-Garcia, R.R. A Novel RuO2@ZnO-Alginate-Halloysite Composite for the Effective Degradation of Eosin Yellow Dye and Ciprofloxacin Drug. Mater. Res. Bull. 2025, 182, 113178. [Google Scholar] [CrossRef]
- Zarzzeka, C.; Goldoni, J.; de Paula de Oliveira, J.d.R.; Lenzi, G.G.; Bagatini, M.D.; Colpini, L.M.S. Photocatalytic Action of Ag/TiO2 Nanoparticles to Emerging Pollutants Degradation: A Comprehensive Review. Sustain. Chem. Environ. 2024, 8, 100177. [Google Scholar] [CrossRef]
- Xu, K.; Shen, J.; Zhang, S.; Xu, D.; Chen, X. Efficient Interfacial Charge Transfer of BiOCl-In2O3 Step-Scheme Heterojunction for Boosted Photocatalytic Degradation of Ciprofloxacin. J. Mater. Sci. Technol. 2022, 121, 236–244. [Google Scholar] [CrossRef]
- Soares, A.S.; Araujo, F.P.; Osajima, J.A.; Guerra, Y.; Viana, B.C.; Peña-Garcia, R. Nanotubes/Nanorods-like Structures of La-Doped ZnO for Degradation of Methylene Blue and Ciprofloxacin. J. Photochem. Photobiol. A Chem. 2024, 447, 115235. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Tailoring Interfacial Charge Separation in Z-Scheme CuO@TiO2@halloysite Heterostructure for Efficient Photocatalytic Removal of Congo Red. J. Taiwan. Inst. Chem. Eng. 2025, 166, 105752. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chen, S.; Jiang, A.; Chen, X.; Zhu, X.; Di, Y. Activation of Peroxymonosulfate by Halloysite Nanotube-Supported TiO2 Composite for Efficient Photocatalytic Degradation of Rhodamine B. Opt. Mater. 2024, 147, 114669. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Janani Archana, K.; Christy Preetha, A.; Balasubramanian, K. Influence of Urbach Energy in Enhanced Photocatalytic Activity of Cu Doped ZnO Nanoparticles. Opt. Mater. 2022, 127, 112245. [Google Scholar] [CrossRef]
- Feitosa, R.P.; de Lima, I.S.; Guerra, Y.; da Silva-Filho, E.C.; Furtini, M.B.; Almeida, L.; Peña-Garcia, R.R.; Martín, I.B.; Cecília, J.A.; Osajima, J.A. Cerium-Doped TiO2 and Sepiolite Nanocomposites for Tetracycline Inactivation in Water Treatment. ACS Appl. Nano Mater. 2025, 8, 4324–4338. [Google Scholar] [CrossRef]
- Marana, N.L.; Sambrano, J.R.; de Souza, A.R. Propriedades Eletrônicas, Estruturais e Constantes Elásticas Do ZnO. Quim. Nova 2010, 33, 810–815. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Hao, T.; Yuan, Y.; Zhang, B.; Wang, H.; Shao, G.; Fan, B.; Lu, H. Facile Synthesis of ZnO/Halloysite Nanotube Composite with Greatly Enhanced Photocatalytic Performance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133633. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, J.; Lu, J.; Li, Y.; Ding, J. Preparation of Biodiesel by Transesterification of Castor Oil Catalyzed by Flaky Halloysite Supported ZnO/SnO2 Heterojunction Photocatalyst. Renew. Energy 2024, 227, 120516. [Google Scholar] [CrossRef]
- Rosa, D.; Remmani, R.; Bavasso, I.; Bracciale, M.P.; Di Palma, L. Biochar Supported Fe–TiO2 Composite for Wastewater Treatment: Solid-State Synthesis and Mechanistic Insights. Chem. Eng. Sci. 2025, 317, 122076. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite for Controllable Loading and Release. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 554–605. [Google Scholar]
- Ruiz-Hitzky, E.; Aranda, P.; Akkari, M.; Khaorapapong, N.; Ogawa, M. Photoactive Nanoarchitectures Based on Clays Incorporating TiO2 and ZnO Nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1140–1156. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, H.; Li, H.; Fang, C.; Xie, W.; Li, M.; Zhang, B.; Shao, G.; Lu, H.; Wang, H. Construction of CdS/Halloysite Nanotube Composite for High Efficient Photocatalytic Degradation toward Tetracycline Hydrochloride. J. Alloys Compd. 2025, 1036, 181646. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Y.; Liu, T. Enhanced Visible-Light Photocatalytic Performance of Electrospun Carbon-Doped TiO2/Halloysite Nanotube Hybrid Nanofibers. J. Colloid Interface Sci. 2015, 439, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, W.A.; Filho, A.J.N.; Romaguera-Barcelay, Y.; Medina-Carrasco, S.; Orta, M.d.M.; Trigueiro, P.; Peña-Garcia, R.R. Synergistic ZnO–CuO/Halloysite Nanocomposite for Photocatalytic Degradation of Ciprofloxacin with High Stability and Reusability. Minerals 2025, 15, 977. [Google Scholar] [CrossRef]
- Banti, C.N.; Papachristodoulou, C.; Chrysouli, M.P.; Douvalis, A.; Hadjikakou, S.K. Controlled Dual Activity of an Organometallic Antibiotic through Micelle Formulation. J. Organomet. Chem. 2024, 1012, 123130. [Google Scholar] [CrossRef]
- Nannou, C.; Maroulas, K.N.; Tsamtzidou, C.; Ladomenou, K.; Kyzas, G.Z. Photocatalytic Degradation of Veterinary Antibiotics in Wastewaters: A Review. Sci. Total Environ. 2025, 966, 178765. [Google Scholar] [CrossRef]
- Santos, G.O.; Goulart, L.A.; Cordeiro-Junior, P.J.; Sanchez-Montes, I.; Lanza, M.R. Pharmaceutical Contaminants: Ecotoxicological Aspects and Recent Advances in Oxidation Technologies for Their Removal in Aqueous Matrices. J. Environ. Chem. Eng. 2022, 10, 108932. [Google Scholar] [CrossRef]
- de Lima, I.S.; Silva, A.S.; Nascimento, A.M.S.S.; de Oliveira, L.H.; Morais, A.Í.S.; Barreto, H.M.; Peña-Garcia, R.; Cuevas, M.D.M.O.; Argôlo Neto, N.M.; Osajima, J.A.; et al. Synthesis and Characterization of Cassava Gum Hydrogel Associated with Chlorhexidine and Evaluation of Release and Antimicrobial Activity. Macromol. Biosci. 2024, 24, 2300507. [Google Scholar] [CrossRef]
- Banti, C.; Hadjikakou, S. Evaluation of Toxicity with Brine Shrimp Assay. Bio-protocol 2021, 11, e3895. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, H.; Zhou, H.; Hao, L.; Xu, H.; Zhou, X. Mt-Supported ZnO/TiO2 Nanocomposite for Agricultural Antibacterial Agent Involving Enhanced Antibacterial Activity and Increased Wettability. Appl. Clay Sci. 2021, 214, 106296. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Zakaria, Y.; Gladich, I.; Kochkodan, V.; Lawler, J. XPS, Structural and Antimicrobial Studies of Novel Functionalized Halloysite Nanotubes. Sci. Rep. 2022, 12, 21633. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Yu, T.; Ding, N.; Wang, M.; Chen, Y. Photocatalytic Performance of Biochar-Modified TiO2 (C/TiO2) for Ammonia–Nitrogen Removal. RSC Adv. 2023, 13, 24237–24249. [Google Scholar] [CrossRef]
- Qu, K.; Huang, L.; Hu, S.; Liu, C.; Yang, Q.; Liu, L.; Li, K.; Zhao, Z.; Wang, Z. TiO2 Supported on Rice Straw Biochar as an Adsorptive and Photocatalytic Composite for the Efficient Removal of Ciprofloxacin in Aqueous Matrices. J. Environ. Chem. Eng. 2023, 11, 109430. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Peng, R.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Preparation of TiO2-SiO2 Aperiodic Mesoporous Materials with Controllable Formation of Tetrahedrally Coordinated Ti4+ Ions and Their Performance for Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 127–136. [Google Scholar] [CrossRef]
- Rajkumar, N.; Ramachandran, K. Oxygen Deficiency and Room Temperature Ferromagnetism in Undoped and Cobalt-Doped TiO2 Nanoparticles. IEEE Trans. Nanotechnol. 2011, 10, 513–519. [Google Scholar] [CrossRef]
- Akter, N.; Ahmed, T.; Haque, I.; Hossain, M.K.; Ray, G.; Hossain, M.M.; Islam, M.S.; Ali shaikh, M.A.; Akhtar, U.S. XPS Valence Band Observable Light-Responsive System for Photocatalytic Acid Red114 Dye Decomposition Using a ZnO–Cu2O Heterojunction. Heliyon 2024, 10, e30802. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite Based Nanocomposites and Photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Pereira da Silva, C.A.; Santos Araújo, N.J.; Morais Oliveira-Tintino, C.D.; Barbosa Filho, J.M.; Alencar, G.G.; de Araújo-Neto, J.B.; dos Santos, J.S.; Soares, J.B.; Domiciano, C.B.; Silva, D.A.; et al. Effect of Betulinic Acid on MepA Efflux Pump Inhibition in Staphylococcus aureus: Antibacterial and Molecular Study. Steroids 2025, 215, 109572. [Google Scholar] [CrossRef]
- Verbel-Olarte, M.I.; Serna-Galvis, E.A.; Jimenez-Lopez, D.M.; Jojoa-Sierra, S.D.; Porras, J.; Pulgarin, C.; Torres-Palma, R.A. First Evidence That Antibiotic-Resistant Bacteria Are Inactivated by Chemical Species Produced through the Solar Photosensitization of Ciprofloxacin in Water. Sci. Total Environ. 2025, 963, 178442. [Google Scholar] [CrossRef]
- Park, K.-H.; Kim, D.; Jung, M.; Kim, D.Y.; Lee, Y.-M.; Lee, M.S.; Hong, K.-W.; Bae, I.-G.; Hong, S.I.; Cho, O.-H. Effects of Sub-Inhibitory Concentrations of Nafcillin, Vancomycin, Ciprofloxacin, and Rifampin on Biofilm Formation of Clinical Methicillin-Resistant Staphylococcus aureus. Microbiol. Spectr. 2024, 12, e03412-23. [Google Scholar] [CrossRef]
- Kergaravat, S.V.; Hernández, S.R.; Gagneten, A.M. Second-, Third- and Fourth-Generation Quinolones: Ecotoxicity Effects on Daphnia and Ceriodaphnia Species. Chemosphere 2021, 262, 127823. [Google Scholar] [CrossRef]
- Sreekanth, K.; Sarath Josh, M.K.; Sethulakshmi, K.H.; Joseph, J.; Joseph, B.J.; Mohammed Hashim, K.K.; Manoj, E.; Aravindakumar, C.T.; Radhakrishnan, E.K. Diclofenac-Mediated Resensitisation of Methicillin-Resistant Staphylococcus aureus (MRSA) to Ciprofloxacin via NorA Efflux Pump Inhibition. Chem. Eng. J. 2025, 523, 167983. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Synergistic synthesis of Carrisa edulis fruit extract capped heterogeneous CuO-ZnO-HNT composite for photocatalytic removal of organic pollutants. Inorganica Chim Acta 2023, 551, 121457. [Google Scholar] [CrossRef]


| Composition in Percent (%) | |||||||
|---|---|---|---|---|---|---|---|
| Samples | Al2O3 | SiO2 | TiO2 | ZnO | Fe2O3 | CaO | P2O5 |
| Ti-Hal | 35.87 | 36.09 | 26.62 | - | 0.64 | 0.29 | 0.48 |
| 1Zn-Ti-Hal | 35.42 | 35.44 | 25.76 | 2.14 | 0.60 | 0.28 | 0.44 |
| 2.5Zn-Ti-Hal | 35.14 | 34.76 | 24.09 | 4.69 | 0.58 | 0.27 | 0.46 |
| 5Zn-Ti-Hal | 33.77 | 33.14 | 22.62 | 9.19 | 0.54 | 0.27 | 0.45 |
| Sample | Absorption Band (nm) | Band Gap (eV) | Urbach Energy (meV) |
|---|---|---|---|
| Ti-Hal | 439.71 | 2.82 | 506 |
| 1Zn-Ti-Hal | 433.56 | 2.86 | 458 |
| 2.5Zn-Ti-Hal | 438.16 | 2.83 | 444 |
| 5Zn-Ti-Hal | 441.28 | 2.81 | 410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, J.R.; Feitosa, R.P.; de Lima, I.S.; Oliveira, L.H.; Silva-Filho, E.C.; Franco, F.; Cecilia, J.A.; Osajima, J.A. Enhanced Antibiotic Removal via Adsorption–Photocatalysis Using a ZnO–TiO2–Halloysite Nanocomposite. Minerals 2025, 15, 1253. https://doi.org/10.3390/min15121253
Marques JR, Feitosa RP, de Lima IS, Oliveira LH, Silva-Filho EC, Franco F, Cecilia JA, Osajima JA. Enhanced Antibiotic Removal via Adsorption–Photocatalysis Using a ZnO–TiO2–Halloysite Nanocomposite. Minerals. 2025; 15(12):1253. https://doi.org/10.3390/min15121253
Chicago/Turabian StyleMarques, Jairo R., Rodrigo P. Feitosa, Idglan S. de Lima, Luis H. Oliveira, Edson C. Silva-Filho, Francisco Franco, Juan A. Cecilia, and Josy A. Osajima. 2025. "Enhanced Antibiotic Removal via Adsorption–Photocatalysis Using a ZnO–TiO2–Halloysite Nanocomposite" Minerals 15, no. 12: 1253. https://doi.org/10.3390/min15121253
APA StyleMarques, J. R., Feitosa, R. P., de Lima, I. S., Oliveira, L. H., Silva-Filho, E. C., Franco, F., Cecilia, J. A., & Osajima, J. A. (2025). Enhanced Antibiotic Removal via Adsorption–Photocatalysis Using a ZnO–TiO2–Halloysite Nanocomposite. Minerals, 15(12), 1253. https://doi.org/10.3390/min15121253










