Abstract
Clay/natural rubber (Clay–NR) nanocomposites are a sustainable material class with a wide range of applications. The type and amount of the filler added to the rubber matrix promote significant changes in the matrix properties. Montmorillonitic clays (MMT) are mineral, natural fillers. This study investigates the effect of a modified Brazilian polycationic MMT on the nanocomposite rheology and mechanical properties. The MMT was incorporated into a NR matrix in its natural state, and after modification by cation exchange (MMTNa) and organophilization (MMTORG), at concentrations of 2.5 and 5 phr (parts per hundred parts of rubber). The natural and modified clays were characterized by XRD, FTIR, BET, and SEM/EDS. Mechanical tests (tensile strength, elongation at break, and modulus at 100%) indicated that the use of MMTNa led to increased strength and modulus, whereas a minor decrease in the elongation was observed. However, the use of MMTORG yielded the most significant improvements in the mechanical properties. The rheology tests indicated that the Payne effect was not observed, and the strain-dependent behavior arises from matrix-dominated mechanisms, rather than disruption of a filler network. Vulcanization curves showed that the NR-MMTORG composites exhibited higher torque values, corroborated by higher crosslink densities. These findings highlight the critical role of cation exchange modification in optimizing MMT dispersion and interfacial interactions within NR matrices, providing design principles for high-performance sustainable nanocomposites.
1. Introduction
The development of sustainable materials is a scientific response to mitigate humanity’s impact on the planet [1,2], and their use could represent the earth’s preservation for future generations. In this way, the clay–natural rubber (Clay–NR) nanocomposites are good examples of sustainable materials. NR maintains a strong market share due to its inherent qualities and ongoing research and development efforts that have consistently improved its quality. Commercially, NR (cis-isoprene) is almost entirely sourced from the latex of the Hevea brasiliensis tree, native to the Amazon [3,4]. NR exhibits enhanced stiffness under high strain conditions, due to the extension of polymer chains between crosslinks as they approach their limiting extensibility, as well as to strain-induced crystallization. This behavior confers upon natural rubber its characteristic high tensile strength, long fatigue life, and excellent tear resistance properties [5]. Furthermore, NR exhibits superior performance compared to synthetic rubbers with regard to several critical properties, such as abrasion resistance, resistance to crack propagation, resilience, and elasticity, which confers a highly advantageous position across a wide range of applications [6]. NR is primarily utilized in the production of tires and automotive components, accounting for over 70% of its consumption. Significant alternative applications involve latex-based products, footwear, adhesives, and even engineering and industrial products [7,8]. Clays are abundant in the Earth’s crust and were probably among the first materials handled by humankind, with several uses even to the present day. They can be defined as hydrated aluminosilicates, essentially formed by clay minerals, and present plasticity when a determined amount of water is added. The crystalline structure is lamellar, composed of layers of tetrahedral (Si-O) and octahedral (Al, Mg, or Fe-O/OH) sheets. The stacking arrangement of these sheets (1:1 or 2:1) determines the type of clay mineral and its properties, such as swelling capacity or plasticity [9]. Crystals of colloidal size exhibit a high specific surface area, typically with particle dimensions less than 2 µm. A fraction of these crystals can also present nanometric dimensions, ranging from 1 to 100 nm [10,11]. They are eco-friendly rubber fillers due to low production energy. According to the particle size, clays could be used as fillers or reinforcements. Particles greater than 5 µm are mostly used to reduce cost due to their poor performance as a reinforcement. On the other hand, particles smaller than 2 µm promote wear resistance and higher modulus. It is possible to use up to 300 phr clay in rubber compounds. However, both types, due to intrinsic characteristics, tend to slow down the curing by absorbing accelerators [12].
1.1. Montmorillonite and Its Modification
Montmorillonite (MMT) is an important industrial clay mineral, widely used in numerous industrial processes. After its extraction, beneficiation, and industrial processing, it presents significant improvement in properties such as specific area, adsorption capacity, chemical stability, and even rheological properties. MMT is a well-known eco-friendly filler, belonging to the group of smectite clay minerals [13,14], which have a layered structure (2:1) consisting of two silica tetrahedral sheets and one alumina octahedral sheet, united by shared oxygen atoms. Isomorphic substitutions can occur in the tetrahedral sheets, Al3+ for Si4+, and in the octahedral sheets, Mg2+ for Al3+, thereby promoting a negative charge, which is balanced by exchangeable cations between the layers. This gives smectites their cation exchange capacity [15,16]. It can also be divided into swelling and non-swelling types. Swelling type (sodium montmorillonite—MMTNa) expands up to 20 times its dry volume in water, forming a thixotropic gel, a unique clay property. Non-swelling type (calcium montmorillonite—MMTCa) presents, in its structure, a high content of Fe2+ and Mg2+ (octahedral sheet) or Ca2+ and Mg2+ instead of Na+ as exchangeable cations; however, reducing the content of these elements can allow some swelling [9,17]. Natural MMTNa presents crystals in the form of small, very fine subhedral lamellae, similar to corn flakes, which confer a high specific surface area ranging from 150 to 200 m2/g, and a high cation exchange capacity between 80 and 130 meq/100 g. On the other hand, MMTCa typically displays larger particle sizes and a correspondingly lower specific surface area, generally between 50 and 80 m2/g along with a cation exchange capacity between 40 and 70 meq/100 g [13,18]. In order to promote the compatibility between clay and polymer, the MMT must be modified, using different methods and reagents, into organophilic montmorillonite (MMTORG), thereby inducing hydrophobicity [19]. However, to achieve better results, the MMTCa can be transformed into MMTNa. To achieve this condition, the sodification process (alkaline activation) is carried out, based on the cationic exchange of Ca2+ and Mg2+ for Na+ using Na2CO3, a route that has remained a technological paradigm since the 1930s (German patents DE 613037, and DE 644315), with only optimization adjustments for different applications [19,20,21,22,23,24]. Generally, there are two routes to obtain organophilic clays: solid-state interaction and cation exchange in solution. The latter is the most common route, where polar organic cations (typically quaternary ammonium salts) substitute the exchangeable cations that exist in the clay’s interlayer. This process makes the clay surface hydrophobic, allowing it to interact with other organic compounds. Neutral organic compounds, on the other hand, do not undergo cation exchange; instead, they are adsorbed onto the clay’s surface or within the expanded interlayers through various interactions, such as van der Waals forces, ion–dipole interactions, acid-base reactions, charge transfer, hydrogen bonds, and coordination bonds [13,25,26]. Finally, there is a wide range of organic cations that could be exchanged with the interlayer cations: hexadecyltrimethylammonium bromide (CTAB) [27,28,29,30,31,32], tetramethylammonium bromide (TMABr), and trimethylphenylammonium bromide (TPAB) [33], dodecylpyridinium chloride (C12pyCl) and hexadecylpyridinium chloride (C16pyCl) [34], octadecylammonium (ODA) [35], dimethyldioctadecyl-ammonium chloride (DDAC) [36], and finally, hexadecyltrimethylammonium chloride (CTAC) [37,38]. As a result of this process, an organophilic montmorillonite (MMTORG) emerges, which offers high-value-added applications.
1.2. Clay/Natural Rubber Nanocomposites
The use of composite materials has increased considerably in the last decades. Polymer matrix composites (PMCs), in particular, are of interest, primarily due to low density, high mechanical properties, and the possibility of customizing properties with relatively simple processing, thus establishing them as a promising field in materials development [39]. Nanotechnology has enabled the study of nanomaterials and the creation of nanocomposites with superior properties compared to pure polymers or traditional composites [40]. Therefore, polymeric nanocomposites are hybrid materials consisting of an organic polymer matrix with a small percentage of dispersed inorganic nanoparticles [41].
Various nanofillers, such as graphite, clay, silica, and carbon nanotubes, have been utilized in their preparation [42]. Those reinforcements are in the nanometer scale, and approximately 104 times finer than human hair. When incorporated into the polymer matrix, they could promote enhanced properties without compromising their inherent processability, mechanical integrity, or significantly increasing their mass. Furthermore, they offer benefits beyond merely enhancing mechanical performance or replacing existing filler methods [43].
Polymer nanocomposites with layered silicates, given their structure and morphology, have gained significant interest due to their potential to enhance material properties [44]. They can be classified into immiscible, intercalated, and exfoliated. In the intercalated system, the polymeric material permeates and expands the interlayer galleries of the silicate; however, the fundamental layered integrity is preserved. In contrast, an exfoliated system is characterized by the complete separation and uniform dispersion of individual silicate layers within the polymer matrix [45].
Clay minerals can enhance polymer matrices by improving several properties, including barrier efficacy, thermal stability, flame retardancy, mechanical strength, degradation resistance, and rheological properties. Furthermore, they positively impact the heat distortion temperature, corresponding with an increase in the polymer’s glass transition temperature (Tg) [46].
In this way, there is also growing demand for the development of soft nanocomposites, with a focus on nanofiller-loaded rubber, presenting elevated elastic elongation and recovery capacity after cyclic stress [47].
According to Ohrdorf and Flachberger [19], a notable absence of industrial-scale use of MMTCa for the production of MMTORG exists within the current literature. The authors attribute that to the consistent inability of alkaline activation processes to fully convert the MMTCa into a sufficiently homoionic form through sodium intercalation. Consequently, the resulting product, when compared to natural MMTNa, fails to achieve the technical performance required for viability in industrial applications.
Regarding the research using commercial sodium montmorillonite in the synthesis of nanocomposites, among the available studies, López-Manchado et al. [48] evaluated the effects of unmodified and organically modified Na-bentonite in natural rubber (NR) vulcanization kinetics. They concluded that clay has an important role in the crosslinking formation, improved even more after clay’s organophilization, based on vulcanizing results. Arroyo et al. [49] investigated the use of MMTNa, and MMTNa modified with octadecan-1-amine (octadecylamine) as a substitute to carbon black. They concluded that MMTNa promoted the lowest reinforcement effect to natural rubber compounds, while the organophilized MMT was an effective reinforce, better than carbon black. Masa et al. [50] studied the effect of MMTNa addition to prepare natural rubber (NR) nanocomposites by latex compounding method, and phenolic resin (PhOH) to crosslink NR. They attributed the crystallization of crosslinked NR at low strain to clay dispersion. Nevertheless, at high strain, the enhanced crystallization was credited to the clay and the crosslink points.
Finally, among the innumerous investigations in the literature employing organophilic clay, it is possible to mention the most recent: Sattayanurak et al. [51] investigated the influence of a clay modifier on the characteristics of natural rubber tire compounds reinforced with silica and silica/nanoclay. They concluded that the modifying agent, regardless of its form (i.e., included in organoclay or added separately), holds substantial potential to further improve NR/silica-reinforced tire technology. Kausar et al. [52] performed a review about the nanoclay reinforcement effect in green polymer matrices. Among other topics, they highlighted the importance of ensuring the nanoclay’s dispersion, intercalation, and exfoliation strategies to attain the full potential reinforcement properties of the filler. They also highlight the need for the conversion to nanoclay-based salts, and the subsequent formation of the organomodified nanoclay. Archibong et al. [53] presented a review of recent advancements in montmorillonite (MMT)-reinforced rubber and polymer nanocomposites (RCNs/PCNs). They emphasized that MMT modification and processing are key to creating novel materials with enhanced properties. The authors noted that organoclay and compatibilizers are crucial for better adhesion and exfoliation, and that combining covalent bonding, graft polymerization, and MMT surface treatment significantly boosts RCN/PCN properties. Furthermore, adding MMT and quaternary ammonium salts (QAS) to biopolymers improves tensile strength, thermal stability, and other properties. Sasikumar et al. [54] bring an overview of nanoclays, their modification procedures, and different methodologies for natural rubber/nanoclay composites synthesis. They also discussed some nanocomposites properties, e.g., mechanical, gas barrier, and thermal. In their understanding, for engineering applications, in tire technology particularly, the natural rubber/nanoclay composites are more promising than other composites, which use different nanofillers.
The above discussed works aim to enhance compatibility and promote desirable properties in clay/elastomers nanocomposites. However, most of them use, as the starting material (filler), either natural or commercially available sodic MMTs, and few employ natural MMTCa. The latter kind of MMT has lower prices and, in many countries, like Brazil, MMTCa is abundant, whereas MMTNa is unavailable in its natural form. The strategic use of natural rubber and natural montmorillonite holds the potential to advance the development of ecological nanocomposites, thereby promoting greater environmental sustainability. This could be particularly relevant for applications in the footwear and tire industries.
The aim of this study was to determine the effects of the modification of a Brazilian montmorillonite on the rheological and mechanical properties of clay/natural rubber nanocomposites. The modification process involved (i) alkaline activation, to obtain sodium-modified montmorillonite (MMTNa), based on the cation exchange of Ca2+ and Mg2+ for Na+; and (ii) organophilization, based on cation exchange with quaternary ammonium salts to obtain organophilic montmorillonite (MMTORG). The clays, in the proportion of 2.5 and 5 phr (parts per hundred rubber), were mechanically incorporated into a Brazilian natural rubber matrix from the Amazon region, followed by vulcanizing additives.
2. Materials and Methods
2.1. Materials
The natural MMTCa was obtained from a supplier in Boa Vista, Paraiba State, Northeastern region of Brazil. The reagents utilized in this study included sodium carbonate (Na2CO3), P.A. grade, provided by Casa Americana (São Paulo, SP, Brazil); hexadecyltrimethylammonium chloride (CTAC) supplied as a 50% w/w solution (Sunquart© CT 50) by AQIA Innovative Chemical Ltd. (São Paulo, SP, Brazil); and deionized water. The natural rubber utilized was pale crepe, sourced from Hevea brasiliensis (Brazil) and supplied by DLP Ind. Com. Ltd. DLP Rubber Industry, Trade, and Products Ltd. (Monte Aprazivel, SP, Brazil), with a Mooney viscosity of 85.9 (1′ + 4′ − 100 °C), 0.29% of ash content, 0.12% of volatile materials, and 0.02% of grime. Furthermore, the formulations for the rubber compounds were composed of specific percentages of stearic acid (octadecanoic acid, C18H36O2), zinc oxide (ZnO), sulfur (S8), and accelerator MBTS (dibenzothiazyl disulfide) from the brand Basile Chemical Ltd. (São Paulo, SP, Brazil).
2.2. Methods
2.2.1. Montmorillonite Modification (Sodification and Organophilization)
The as-received natural MMTCa sodification process was conducted considering an average cation exchange capacity (CEC) for Brazilian MMTs, according to Santos [9], of approximately 100 meq/100 g.
The montmorillonite sodification process [55] started with the preparation of a sodium carbonate aqueous solution, using the proportion of 5 wt.% of Na2CO3/100 g clay, assisted by magnetic stirrer. Subsequently, 6 wt.% of MMTCa (#200 mesh, dried at 60 °C for 24 h) was slowly added to this solution. The mixture was then mechanically mixed for 4 h at 40 °C, followed by a 72 h resting period, after which the water was removed by siphoning. The wet clay was then dried in a circulating air oven, followed by manual grinding and sieving (#200 mesh).
The organophilization process [56] was conducted by adding 38 mL of an aqueous solution of the quaternary ammonium salt (50 wt.%) to the suspension (3 L of distilled water containing 60 g of sodium-modified montmorillonite—MMTNa) and was mechanically mixed for 30 min at room temperature. The mixture then settled for 24 h, after which the water containing excess salt was removed by siphoning. The MMTORG obtained was washed with distilled water, dried in an oven at 50 °C for 72 h, followed by manual grinding and sieving (#200 mesh).
2.2.2. Nanocomposite
To investigate the effect of MMT (both natural and modified) on the rheology and mechanical properties of nanocomposites, different clay concentrations (2.5 and 5 phr, parts per hundred rubber) were incorporated into the natural rubber (NR) matrix.
Three distinct conditions of MMT were evaluated as follows: as-received natural calcium montmorillonite (MMTCa), sodium-modified clay (MMTNa), and organophilic clay (MMTORG). A natural rubber (NR) compound was also prepared and utilized as a control sample.
The pre-mixing process was conducted in a 1 L Banbury rubber mixer (Taida Machinery Industry Co., model M2-58, Taichung, Taiwan) preheated to 30 °C. Initially, 700 g of NR was masticated for 1 min. Subsequently, the specified clay concentration (2.5 or 5 phr) was introduced, followed by an additional 5 min of mixing. Following this, the additives were incorporated into the premix using an open two-roll mill. To ensure homogeneity, the premix was subjected to ten passes through the mill, with each additive being sequentially introduced and mixed for a duration of 5 min.
The rubber compounds were vulcanized into 2 mm thick plates utilizing a hydraulic press, SIRMA Public Limited Company Machinery Industry and Trade (Boituva, SP, Brazil) operating at 150 °C. This temperature was selected based on the empirically determined optimal vulcanization time (t90). Subsequently, test specimens for mechanical characterization were prepared from these vulcanized plates. The nomenclature and detailed composition are presented in Table 1.

Table 1.
Nomenclature and composition, quantities expressed in phr, of natural rubber compounds and nanocomposites NR–clay; where NR = natural rubber compound, CA25 = 2.5 phr of MMTCa, NA25 = 2.5 phr of MMTNa, ORG25 = 2.5 phr of MMTORG, NA50 = 5 phr of MMTNa, ORG50 = 5 phr of MMTORG, and S = single accelerator.
2.2.3. Montmorillonite, Natural Rubber Compounds, and Nanocomposite Characterization
The characterizations of both natural and modified Brazilian montmorillonite clays were performed by X-ray fluorescence (XRF), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), specific surface area (SSA/BET) analysis, and scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS).
The presented contents were determined on a sample fused with lithium tetraborate, in the QZF-1 (quartz and feldspar) calibration, relative to quantitative analysis by comparison with certified reference materials, in an X-ray fluorescence spectrometer (XFR), Malvern Panalytical (Worcestershire, UK), Zetium model. The loss on ignition (LOI) was performed at 1020 °C for 2 h.
The mineral phases were identified using X-ray diffraction (XRD) by the powder method, performed on an Empyrean X-ray diffractometer (Malvern Panalytical, Worcestershire, UK). The diffractometer operated at 40 mA and 40 kV, utilizing Cu Kα radiation, with a scan from 3° to 70° (2θ), step of 0.02° for one second.
The clay mineral structure was studied by Fourier-transform infrared (FTIR) spectroscopy with attenuated total reflectance (ATR), performed using a Nicolet iS50 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Spectra were recorded in the 4000 to 500 cm−1 range, with a resolution of 4 cm−1 and 64 scans.
The specific surface area (SSA) was determined using a surface area analyzer Gemini III 2375 (Micromeritics Instrument Corporation, Norcross, GA, USA) employing nitrogen (N2) adsorption based on the Brunauer–Emmett–Teller (BET) method. Prior to analysis, the MMT clays underwent pretreatment at 140 °C for 24 h under vacuum (~1 millibar).
Micrographic images were obtained using an Inspect F50 Field Emission Gun Scanning Electron Microscope (FEG-SEM) from FEI Company (Hillsboro, OR, USA). Coupled with an energy-dispersive X-ray analyzer (EDS), this instrument also allowed the determination of semi-quantitative chemical composition of MMTs and scanning electron microscopy (SEM) of unvulcanized natural rubber and MMTs.
Rheology and mechanical tests on natural rubber compounds and nanocomposites were conducted. The vulcanization profiles were determined using an oscillatory disc rheometer (Rubber Process Analyzer—RPA, model Elite, TA Instruments Inc., New Castle, DE, EUA). Measurements were conducted at 150 °C for 60 min, following the ASTM D2084 standard [57]. To evaluate the Payne effect, based on the ASTM D8059 standard [58], strain sweep analysis of unvulcanized NR compounds was performed using an RPA Elite (TA Instruments), angular strains ranging from 0.1% to 70% at 60 °C and a constant frequency of 1.7 Hz, with data acquired at three points per decade. The crosslink density was quantified via swelling tests, based on ASTM D2765 standard [59]. Initially, specimens were weighed and then immersed in toluene for 72 h. Post-immersion, the specimens were reweighed before being placed in an oven at 60 °C for an additional 72 h. Following oven drying, a final weight measurement was recorded. The resultant data were subsequently employed in the Flory–Rehner equation to calculate the crosslink density [60].
Mechanical characterization, including the determination of tensile strength, elongation at break, and modulus at 100%, in accordance with ASTM D412-06 [61], took place. These tests were performed using a KE-3000 MP universal testing machine (Kratos Industrial Equipment, Cotia, SP, Brazil). The mechanical tests were performed on a minimum of ten specimens. Hardness tests were also performed, based on ASTM D2240-15 [62], employing a portable analog Shore A durometer (Bareiss North America Inc., Stouffville, ON, Canada), model D-89610). Five measurements were taken for each specimen with a 1 s delay time following the application of the load.
3. Results
3.1. MMT Characterization Results
3.1.1. MMTCa Physical Characterization
The results of the specific surface area (SSA) by BET method, moisture content, density, and Foster swelling tests conducted with the as-received natural MMTCa, presented in Table 2, are in accordance with the values found in the literature [13,15].

Table 2.
Tests results of MMTCa physical characterization results.
3.1.2. MMTCa XRF and XRD
The as-received natural MMTCa sample chemical composition obtained by XRF with loss ignition is shown in Table 3.

Table 3.
Chemical composition (wt.%) of MMTCa by XRF.
The elements identified are compatible to a natural calcium montmorillonite [63,64,65]. It should be noted that clays contain iron ions in their structure, or adsorbed on the clay mineral particles. The presence of these ions (Fe3+) in the clay structure can reduce its exchange capacity which may be detrimental to the sodification and, hence, organophilization processes.
Analyzing the diffraction patterns of MMTCa, Figure 1, it is possible to observe the presence of the phases montmorillonite (M), kaolinite (K), and quartz (Q). The characteristic peaks of MMT are identified as follows: the peak at 2θ = 5.54°, associated to the basal plane spacing (d001) of 1.59 nm, and the peaks at 2θ = 19.83° and 27.48. The kaolinite peak at 2θ = 12.31° is associated to d(001) = 0.72 nm, and the quartz peak observed at 2θ = 26.62° may be associated to d(001) = 0.33 nm [66,67].
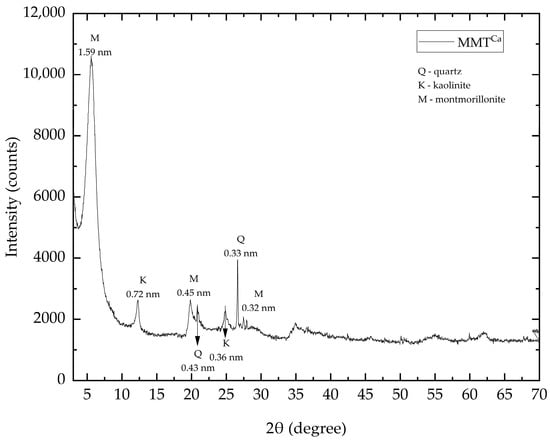
Figure 1.
XRD patterns of as-received natural MMTCa.
3.1.3. MMTCa FTIR-ATR
Figure 2 shows the FTIR spectrum of as-received natural MMTCa. The characteristic bands of O–H stretching vibration bands were observed in the range of 3695 to 3620 cm−1, originating from OH groups coordinated to various octahedral cations, primarily Al2OH, Al-Mg-OH, or Al-Fe-OH [23]. The deformation vibration band of montmorillonite’s interlayer water was observed at 1632 cm−1 [68]. The strongest absorption band, characteristic of clay minerals and corresponding to Si–O stretching vibration, was observed at 1000 cm−1 [69]. Finally, bending vibrations of Al2OH and Al–OH can be observed at 910 and 522 cm−1, respectively [70].
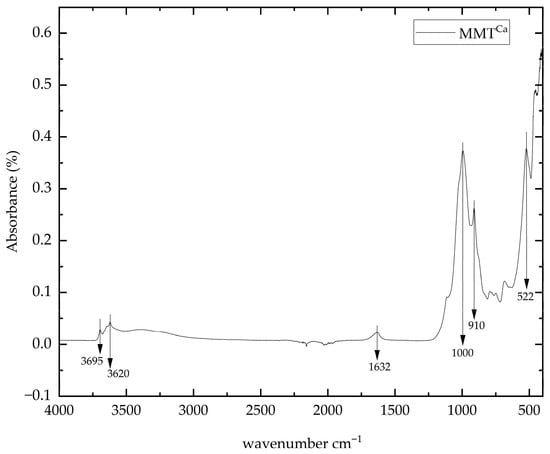
Figure 2.
FTIR-ATR spectrum of as-received natural MMTCa.
3.1.4. MMTCa SEM/EDS
Scanning electron microscopy (SEM) images of the as-received natural MMTCa were acquired using an Everhart–Thornley (ETD) detector to collect secondary electrons (SE). These images, obtained at magnifications of 1000, 10,000, and 35,000 times, are presented in Figure 3a–c. It is possible to observe their typical lamellar structure, with agglomerates of small size clay particles (5 to 60 µm), and the typical irregular forms as “corn flakes” [13,18]. Table 4 presents the semi-quantitative chemical analysis of the as-received natural MMTCa acquired by energy-dispersive X-ray spectroscopy (EDS) (full area Figure 3a). Based on the MMT theoretical formula IV(Si4) VI(Al2−y Mgy) O10 (OH)2, yM+.nH2O [71], and the result from semi-quantitative chemical analysis obtained by EDS, it is possible to identify the sample as montmorillonite. The elemental composition, predominantly silicon, aluminum, and iron, is consistent with this classification. The presence of magnesium, calcium, and potassium further suggests isomorphic substitutions in both the tetrahedral and octahedral sheets, a characteristic feature of MMT. Titanium was also identified as an impurity. These findings align with Brazilian montmorillonite [9].
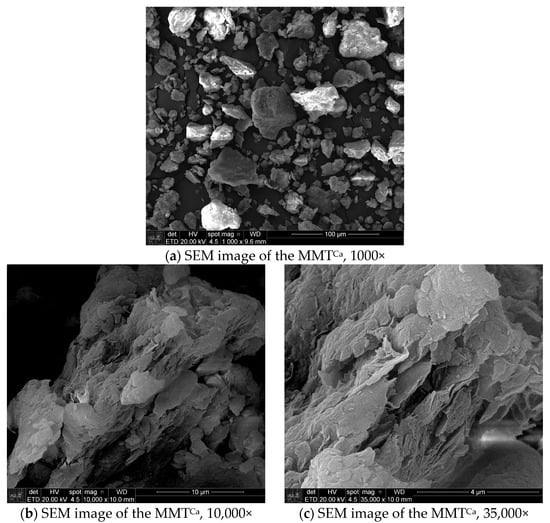
Figure 3.
Scanning electron microscopy images of as-received natural MMTCa.

Table 4.
MMTCa semi-quantitative chemical analysis (wt.%) acquired by EDS.
3.1.5. Modified MMTs Physical Characterization
Table 5 presents the results of physical characterization of modified clays (MMTNa, MMTORG), that included density, moisture content, specific surface area (SSA), and Foster swelling tests. For comparison, the results for MMTCa are also included.

Table 5.
Physical characterization of the MMTs, as-received (MMTCa) and modified (MMTNa, MMTORG).
Both sodification and organophilization processes promote a decrease in the apparent density and moisture content of MMT, by different mechanisms. Sodification increases hydration and electrostatic repulsion by cation exchange (Ca2+ for Na+), which also facilitates the entry of water molecules between the lamellae. These molecules bind to the structure, leaving little free water, whereas organophilization involves the intercalation of bulky, low-density organic molecules, i.e., quaternary ammonium salts, which are hydrophobic and, in this way, prevent water from entering the interlayer space but also repel water molecules from the MMT’s surface. MMT modifications resulted in a decrease in specific surface area (SSA). The reduction in the specific surface area of MMTNa is primarily caused by the reduction in the interlamellar space, impeding the entrance of the larger N2 molecules. For MMTORG, the reduction is directly caused by the intercalation of bulky CTAC cations that physically block the available surface area, and is a clear indicator of successful clay organophilization [23,72,73].
In relation to Foster swelling of modified MMTs, sodium exchange primarily enhances its hydrophilic swelling capacity by facilitating the ingress of water molecules into the interlayer space, a phenomenon driven by reduced electrostatic forces and increased osmotic pressure. During the organophilization process, the substitution of interlamellar cations by quaternary ammonium salts, such as CTAC, promotes the physical intercalation of these bulky organic cations between the clay lamellae. This leads to an observable increase in the lamellar distances, thereby facilitating further intercalation and confirming successful modification [15]. The enhanced swelling observed in the modified clays, consistent with the expectations, serves as a robust indicator of the successful execution of the modification procedures.
3.1.6. Modified MMTs XRD
XRD patterns of MMTs (MMTCa, MMTNa, and MMTORG) are shown in Figure 4. It is possible to observe the displacement of MMT principal peak in modified clays.
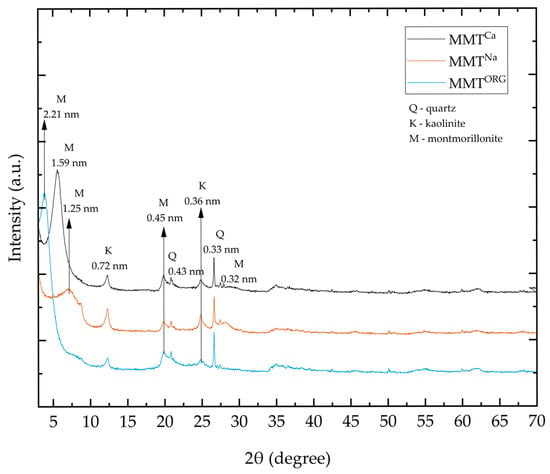
Figure 4.
XRD patterns of MMTCa, MMTNa, and MMTORG.
The performed clay modifications, sodium exchange and organophilization, promoted structural alterations in the montmorillonites crystalline structure. Basal distances were determined by Bragg’s law, using the first diffraction peak. The presence of Na+ ion promoted a decrease in d(001), characteristic to MMTNa, to 1.25 nm at 2θ = 7.05°, associated with a less intense and wider peak, indicative of a more disorganized crystal structure. On the other hand, such basal distance is typical of the sodium montmorillonite, with the presence of some water in the interlayer [65,66,74]. In the opposite way, an increase in the basal distance was observed with MMTORG, 2.21 nm at 2θ = 3.98°, due to the intercalation of the quaternary ammonium salt (CTAC) [22,56,75]. These outcomes confirmed the efficacy of both modification processes.
3.1.7. Modified MMTs FTIR-ATR
Figure 5 shows the FTIR spectra of all MMTs. The analysis revealed the similarities and differences between as-received natural MMTCa and modified clays (MMTNa, and MMTORG).
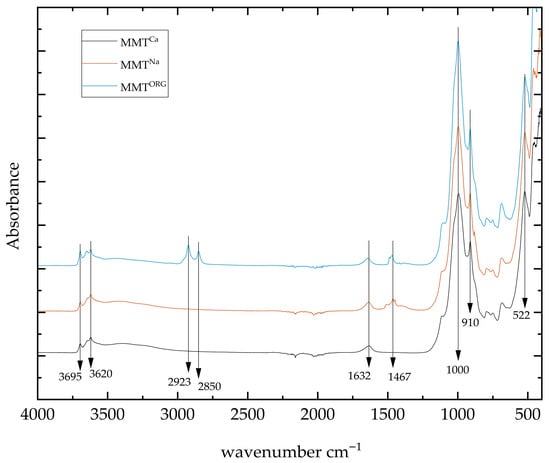
Figure 5.
FTIR spectra of MMTCa, MMTNa, and MMTORG.
The characteristic bands of O–H stretching vibration bands were observed in the range of 3695 to 3620 cm−1, originating from OH groups coordinated to various octahedral cations, primarily Al2OH, Al-Mg-OH, or Al-Fe-OH [23]. The CTAC presence could be confirmed observing the –CH2 peaks at 29,238, and 2850 cm−1 [24]. The deformation vibration band of montmorillonite’s interlayer water was observed at 1632 cm−1 [68]. Carbonate groups absorption bands are typically observed near 1400 cm−1, with a specific band at 1467 cm−1, and asymmetric stretching of (CO3)−2 [49,76]. The strongest absorption band, characteristic of clay minerals and corresponding to Si–O stretching vibration, was observed at 1000 cm−1 [69]. Finally, bending vibrations of Al2OH and Al–OH can be observed at 910 and 522 cm−1, respectively [70]. Thus, these results could indicate the successful clay modification process, i.e., the clay sodification via cation exchange (Ca2+/Na+), and the subsequent CTAC intercalation which produced MMTNa and MMTORG, respectively.
3.1.8. Modified MMTs SEM/EDS
The SEM images of modified MMTs (MMTNa and MMTORG) were obtained using secondary electrons detector, at magnifications of 1000, 10,000, and 35,000 times. These are shown in Figure 6 and Figure 7, respectively. These electron micrographs show the changes induced by both modification processes.
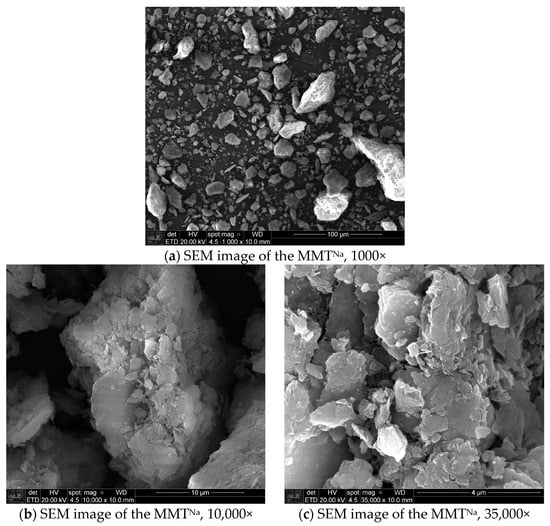
Figure 6.
Scanning electron microscopy images of MMTNa.
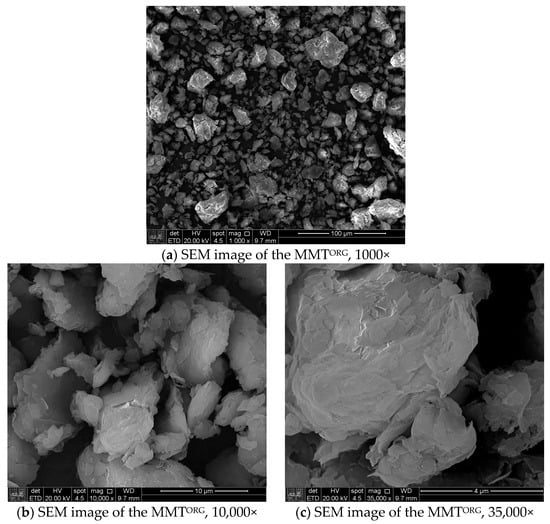
Figure 7.
Scanning electron microscopy images of MMTORG.
The cation exchange (Ca2+ for Na+) inherent to the sodification process induces a reduction in the forces of interparticle attraction, which results in the disaggregation, size diminution, and dispersion of the clay particles. Additionally, the presence of hydrated sodium ions facilitates a structural reorganization of the clay’s layered network, leading to a more compact arrangement. Consequently, a reduction in particle sizes, predominantly within the 10 to 20 µm range, is observed, alongside dispersed aggregates, smaller stacking sizes, and tactoids with more compacted lamellae in the MMTNa. However, some particles with sizes similar to those of the as-received MMTCa are still identifiable.
A significant difference was observed after organophilization, which introduced organic molecules and resulted in morphological alterations. The modification induces a reduction in the average particle size as evidenced by smaller particles (5–10 µm), although not entirely uniform, with presence of some larger ones (up to 40 µm). The presence of the organic chains also induces new attractive forces, such as van der Waals forces, which draw the aggregates closer together, showing moderate stacking dimensions. The most significant effect of organophilization, however, is exfoliation, which consists of the separation of the clay’s lamellae. This phenomenon is crucial for optimizing the clay’s performance as a reinforcing agent in polymer matrices.
Table 6 and Table 7 present the semi-quantitative chemical analysis of the modified clays (MMTNa and MMTORG), acquired by EDS (full area Figure 6a and Figure 7a). The characteristic MMT oxide percentages remain consistent with those of the unmodified clay, except for the introduction of sodium and trace amounts of chlorine. These elements are a direct result of the sodification and organophilization processes, respectively, thereby confirming the effectiveness of the modifications.

Table 6.
MMTNa semi-quantitative chemical analysis (wt.%) acquired by EDS.

Table 7.
MMTORG semi-quantitative chemical analysis (wt.%) acquired by EDS.
3.2. Clay/NR Nanocomposite Characterization Results
3.2.1. MMTs/NR-SEM
In order to preliminarily evaluate the affinity of the clay particles with the NR matrix, pre-mixtures of NR (unvulcanized) with 2.5 and 5 phr of clay were prepared in Banbury mixer, pressed for 5 min at 140 °C, and observed by SEM. Figure 8 shows the micrographs of (a) natural rubber (NR) without additives and clay; (b–d) NR containing 2.5 phr of as-received MMTCa, MMTNa, and MMTORG; and (e–g) NR with 5 phr of as-received MMTCa, MMTNa, and MMTORG, respectively.
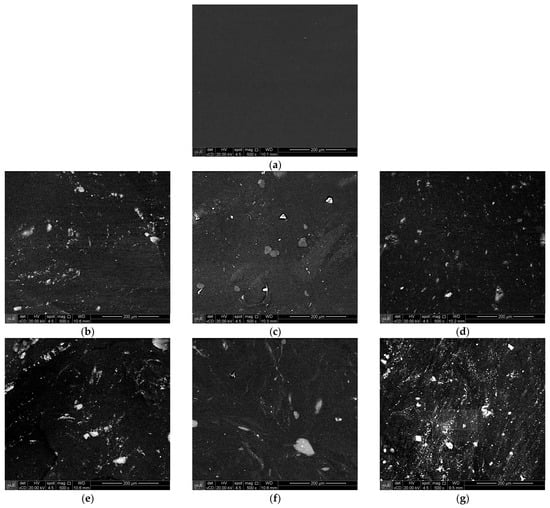
Figure 8.
SEM images (backscattered electrons detector), magnification of 500×, of: (a) NR without additives and filler; (b–d) NR plus 2.5 phr of MMTCa, MMTNa, and MMTORG; and (e–g) NR plus 5 phr of MMTCa, MMTNa, and MMTORG.
The image of pure NR revealed an essentially smooth surface. The micrographs of the pre-mixtures reveal the presence of irregularly shaped particles, on the order of 5 to 30 µm, throughout the surface. Hrachová et al. [77], studying organoclay/natural rubber nanocomposites, also observed the presence of particles on the surface although achieving a good dispersion of the nanoparticles within the natural rubber matrix (demonstrated by transmission electron microscopy images).
3.2.2. Rheology (RPA and Swelling Test)
Figure 9 and Figure 10 show the vulcanization curves acquired by Rubber Process Analyzer (RPA) over 60 min and the main parameters derived from these curves for the studied formulations. The evaluated parameters include the scorch time (tS1), optimum vulcanization time (t90), cure rate index (CRI), maximum torque (MH), and torque difference (ΔM). A comparative analysis of these parameters enables the assessment of changes in rheological behavior and vulcanization efficiency as a function of clay type and content in the clay/NR nanocomposites.
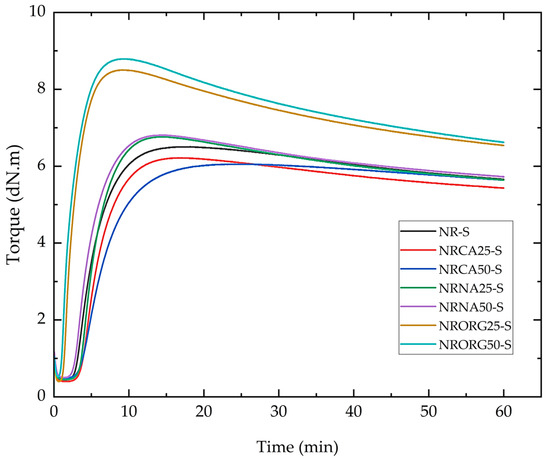
Figure 9.
Vulcanization curves of natural rubber compound and nanocomposites prepared with one accelerator (MBTS) and containing 2.5 and 5 phr of clays (MMTCa, MMTNa, and MMTORG), acquired by RPA.
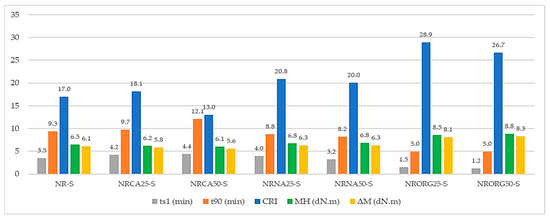
Figure 10.
Vulcanization parameters (ts1, t90, CRI, MH, and ΔM) of NR compound and nanocomposites prepared with 2.5 and 5 phr of clays (MMTCa, MMTNa and MMTORG), obtained by RPA.
The effect of different types of MMT on the rheometric properties revealed distinct curing behaviors. MMTCa acted as a vulcanization retarder, extending both ts1 and t90 while reducing MH and ΔM. These effects became more pronounced with increasing clay content, particularly reflected in the extended t90 and reduced CRI, although no significant variations were observed in the other parameters. This behavior indicates a lower degree of crosslinking.
In contrast, MMTNa promoted cure efficiency. At 2.5 phr, ts1 increased while t90 decreased, and at 5 phr, both times were shortened. The distinct effects on scorch time at different loadings suggest that MMTNa may act as an additive carrier, influencing accelerator or activator availability during vulcanization. In both cases, increases in MH, ΔM, and CRI were observed. The most pronounced changes were observed with MMTORG, which markedly accelerated vulcanization, leading to sharp reductions in ts1 and t90. Simultaneously, MH and ΔM increased proportionally to clay concentration. Interestingly, although CRI values were higher than in the other compounds, they displayed an inverse dependence on organoclay content. This behavior can be attributed to the characteristics of the surfactant used for organophilization (hexadecyltrimethylammonium chloride), whose quaternary ammonium groups (–N+(CH3)3) can interact electrostatically with polar vulcanization additives such as the accelerator or zinc complexes, leading to partial adsorption or restricted mobility of these species within the clay galleries. At higher MMTORG contents, this effect may delay the later stages of vulcanization, broadening the interval between ts1 and t90 and consequently decreasing the CRI. This interpretation suggests a balance between improved early activation and diffusion-limited crosslinking at higher organoclay loadings [68,78].
The modest increase in torque values in MMTNa nanocomposites suggests poor matrix–filler interaction, most likely due to its hydrophilic nature, which is incompatible with the hydrophobic rubber matrix [79]. This incompatibility hinders exfoliation and proper dispersion, limiting its effectiveness as a reinforcing agent [80]. However, the concurrent increase in CRI suggests a different role: the MMTNa may act primarily as a carrier for vulcanization additives. By absorbing and gradually releasing the curing agents, it facilitates a more efficient and faster crosslinking reaction without providing significant mechanical reinforcement [81,82].
In sharp contrast, the MMTORG appears to function through a dual mechanism. First, the amine groups from the organic modifier can catalyze the vulcanization reaction, accounting for the drastic reduction in ts1 and the increase in CRI [83]. Second, the alkyl chains of the surfactant enhance the organophilic character of the clay, improving its compatibility with the non-polar rubber chains. This promotes intercalation and exfoliation, increasing the interfacial area and improving stress transfer from the matrix to the filler [48]. The stronger torque increments (MH and ΔM) provide direct evidence of this effect, reflecting a higher crosslink density and superior mechanical reinforcement [74,84].
Figure 11 shows the crosslink density for natural rubber compound and nanocomposite with 2.5 and 5 phr of MMTCa and modified clays (MMTNa and MMTORG). A proportional decrease in crosslink density was observed with MMTCa content, which can be attributed to its retardant effect on the vulcanization process, as previously discussed. In contrast, the incorporation of 2.5 phr of modified clay (MMTNa and MMTORG) increased crosslink formation, particularly in the case of MMTORG, reflecting enhanced filler–matrix interactions. At a higher incorporation of modified clay (5 phr), however, divergent behavior was observed as follows: a reduction in crosslink density with MMTNa and an increase with MMTORG, relative to the neat natural rubber compound.
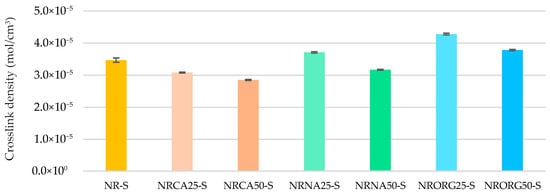
Figure 11.
Crosslink density of NR compound and nanocomposites containing 2.5 and 5 phr of clays (MMTCa, MMTNa, and MMTORG).
At 5 phr, the results strongly suggest that the saturation point of this formulation was reached. At lower contents, particularly at the optimum concentration of 2.5 phr, the modified montmorillonites facilitated crosslink formation, an effect likely associated with chemical interactions between the zinc oxide and the clay’s structural components, more pronounced in the organoclay.
Figure 12 shows the storage modulus (G’) obtained from strain sweep tests for the NR compound and nanocomposites, performed in the Rubber Process Analyzer (RPA). As expected for elastomeric matrices, G’ decreased with increasing strain due to strain-induced softening mechanisms, such as rubber-bond slippage and partial crosslink rupture. Although this behavior is commonly associated with the Payne effect in filled rubbers, the G’-strain curves of the clay/NR nanocomposites closely followed that of the neat NR compound, and no systematic increase in the modulus drop (ΔG’) with clay content was observed. Therefore, while G’ decreased with strain for all samples, no additional Payne-type contribution attributable to a filler–filler network was detected within the explored strain range. The observed softening is thus ascribed mainly to matrix-related mechanisms rather than to the breakdown of clay–clay structures [85,86,87,88,89].
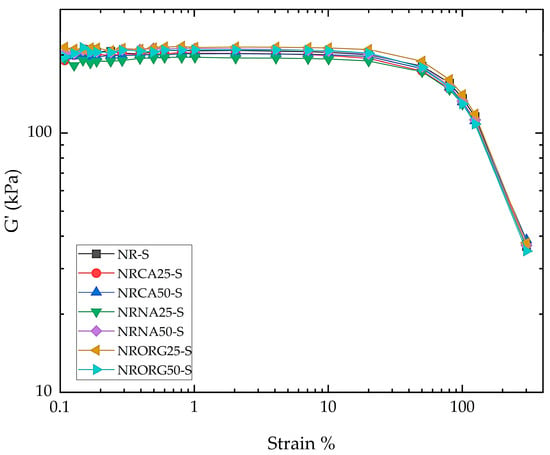
Figure 12.
Storage modulus (G’) obtained from strain sweep tests of NR compound and all nanocomposites, performed in RPA.
The quantity of crosslinks is fundamental in determining the properties of rubber compounds. A lower crosslink density yields softer, more flexible, and elastic materials, whereas a higher density produces a more rigid network, resulting in harder and more resistant compounds. Thus, controlling the crosslink density enables precise tuning of the mechanical performance of rubber for specific applications.
In the studied clay/NR formulations, nanocomposites containing 5 phr of filler showed a tendency toward reduced crosslink density, suggesting softer materials suitable for applications requiring cushioning and flexibility, such as running shoes or soft tire treads, where grip and traction are prioritized over durability. Conversely, formulations with 2.5 phr of clay, particularly those containing MMTORG, exhibited the highest crosslink density and are more appropriate for applications demanding greater stiffness and resistance.
Overall, understanding the rheological behavior of rubber compounds is essential for the rational selection of fillers, additives, and rubbers (natural or synthetic) in blends, enabling the design of tailored materials for targeted industrial applications.
3.2.3. Mechanical Tests
To evaluate the influence of montmorillonite modifications on the mechanical properties of clay/NR nanocomposites, 2.5 and 5 phr of MMTCa, MMTNa, and MMTORG were incorporated into natural rubber compounds.
Figure 13 shows the mean values and standard deviation of the mechanical tests results: (a) hardness, (b) tensile strength, (c) elongation at break, and (d) modulus at 100%.
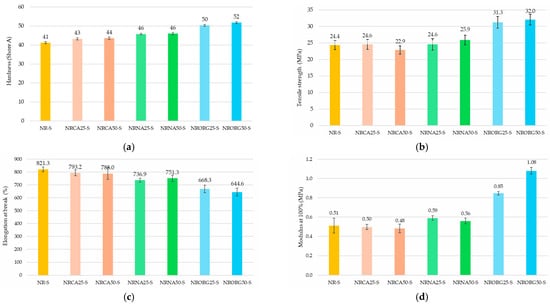
Figure 13.
Hardness (a), tensile strength (b), elongation at break (c), and modulus at 100% (d), of NR compound and nanocomposites prepared with 2.5 and 5 phr of clays (MMTCa, MMTNa, and MMTORG).
An overall increase in hardness was observed across all nanocomposites after clay incorporation. This enhancement was proportional to clay content and similar for nanocomposites containing the same clay type. MMTNa increased hardness by approximately 12%, while MMTORG produced the largest effect, with increases of up to 27% at 5 phr.
Tensile strength generally improved with clay addition. Comparable values were achieved with 2.5 phr of MMTCa or MMTNa, while 5 phr of MMTNa further enhanced strength. The highest tensile strength values were obtained with both 2.5 and 5 phr of MMTORG. The only exception was the 5 phr of MMTCa nanocomposite, which showed a decrease relative to neat NR compound, likely due to poor dispersion in the rubber matrix. As expected, elongation at break followed the opposite trend, with progressive reductions as filler content increased. For modulus at 100%, both MMTNa and MMTORG contributed to stiffness enhancement, with the effect being most pronounced in the 5 phr MMTORG nanocomposite.
Although neither MMTNa nor MMTORG formed a strong percolated network in the NR matrix, particularly evident for MMTNa, their dispersion and chemical affinity were sufficient to act as effective reinforcing fillers, contributing to increased stiffness and tensile strength. The reinforcement achieved with MMTORG was more pronounced due to its superior compatibility with rubber matrix, resulting in marked improvements in hardness, modulus, and tensile strength, compared to neat NR compound. These outcomes can also be associated with the nature of the crosslinking process, including specific network structures and the higher proportion of polysulfidic crosslinks [78,90,91].
As previously discussed, the rheological results highlight the potential of clay/NR nanocomposites for applications in tire and footwear formulations. These industries require careful selection of raw materials and formulations to balance performance and durability. Mechanical properties such as hardness, tensile strength, elongation at break, and modulus are particularly critical: hardness governs the trade-off between grip and longevity, tensile strength and elongation ensure resistance to deformation without fracture, and modulus determines stiffness, a key factor for handling in tires and for comfort or protection in footwear. In this context, the mechanical results presented here reinforce the relevance of clay modifications in tailoring NR compounds to the specific demands of tire treads and shoe soles.
4. Conclusions
This study demonstrated that an as-received polycationic (mostly calcium) Brazilian montmorillonite from Paraiba State can be successfully used to produce sodium-modified, and subsequently organophilized, MMTs, able to be used as filler and reinforcement material in natural rubber compounds. This route is particularly relevant in regions where natural MMTNa is not available.
The different montmorillonite types distinctly influence the vulcanization and mechanical performance of natural rubber compounds. MMTCa acted as a vulcanization retarder and, at higher contents, reduced tensile strength and elongation, despite modest increases in hardness. Sodium-modified montmorillonite (MMTNa) exhibited complex behavior, promoting cure efficiency through reduced t90, increased MH, ΔM, and CRI, while also enhancing hardness, tensile strength, and 100% modulus, although with reduced elongation. Organophilized montmorillonite (MMTORG) markedly accelerated curing and exhibited superior dispersion within the NR matrix, leading to the most significant enhancements in hardness, tensile strength, and modulus.
Importantly, none of the clay-filled formulations exhibited a Payne effect, indicating that reinforcement was governed mainly by increased crosslink density and matrix–filler interactions rather than filler–filler networking. Taken together, these findings highlight the potential of MMTCa and MMTNa as cost-effective natural fillers, while MMTORG stands out as an effective reinforcing agent.
Ultimately, this study demonstrates that montmorillonite selection enables the tailoring of NR nanocomposites for specific applications. Such strategic combinations of natural rubber and clay promote the development of more sustainable, high-performance materials, with direct relevance for footwear and tire formulations.
Author Contributions
C.G.B.A.: Conceptualization, methodology, investigation, data analysis, writing, and supervision. G.A.M.d.S.: Investigation, and data analysis. M.C.C.: Conceptualization, methodology, investigation, data analysis, and writing. A.C.G.N.: Conceptualization, methodology, investigation, data analysis, and writing. T.S.V.: Conceptualization, methodology, data analysis, review, and supervision. S.M.T.: Conceptualization, methodology, data analysis, review, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the following laboratories of Polytechnic School, University of São Paulo, for their valuable support: Laboratory of Microstructure and Eco-efficiency – LME (Department of Civil Construction Engineering), and Laboratory of Ceramic Processing – LPC and Polymer Laboratory – PolLab (Department of Metallurgical and Materials Engineering).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BET | Brunauer–Emmett–Teller |
| CTAC | hexadecyltrimethylammonium chloride |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| MBTS | Dibenzothiazyl disulfide |
| MMT | Montmorillonite |
| MMTCa | Calcium montmorillonite |
| MMTNa | Sodium-modified montmorillonite |
| MMTORG | Organophilized montmorillonite |
| NR | Natural rubber |
| SEM | Scanning Electron Microscopy |
| SSA | Specific Surface Area |
| XRF | X-ray Fluorescence |
| XRD | X-ray Diffraction |
References
- Tazmeen, T.; Mir, F.Q. Sustainability through Materials: A Review of Green Options in Construction. Results Surf. Interfaces 2024, 14, 100206. [Google Scholar] [CrossRef]
- Mutlu, H.; Barner, L. Getting the Terms Right: Green, Sustainable, or Circular Chemistry? Macromol. Chem. Phys. 2022, 223, 2200111. [Google Scholar] [CrossRef]
- Feldman, D. Natural Rubber Nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 629–634. [Google Scholar] [CrossRef]
- Rippel, M.M.; Bragança, F.D.C. Natural rubber and clay nanocomposites. Quim. Nova 2009, 32, 818–826. [Google Scholar] [CrossRef]
- Davies, B. Natural Rubber—Its Engineering Characteristics. Mater. Des. 1986, 7, 68–74. [Google Scholar] [CrossRef]
- Junkong, P.; Ikeda, Y. Properties of Natural Rubbers from Guayule and Rubber Dandelion. In Chemistry, Manufacture, and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 177–201. ISBN 9780128188439. [Google Scholar]
- Ong, E.-L.; Eng, A.-H. NATURAL RUBBER. In The Vanderbilt Rubber Handbook; Sheridan, M.F., Ed.; R.T. Vanderbilt Company, Inc.: Norwalk, CT, USA, 2010; pp. 23–56. [Google Scholar]
- Kohjiya, S. Sustainable Development of Natural Rubber in the 21st Century. In Chemistry, Manufacture, and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 463–479. ISBN 9780128188439. [Google Scholar]
- Santos, P.D.S. Ciência e Tecnologia de Argilas, Aplicada Às Argilas Brasileiras e Aplicações; v. 1-2.; Edgar Blücher: São Paulo, Brazil, 1975. [Google Scholar]
- Murray, H.H. Clays in. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH Verlag GmBH: Weinheim, Germany, 2000. [Google Scholar]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Mouri, H. Fillers. In Rubber Technologist’s Handbook; Rapra Technology Limited: Shropshire, UK, 2001; pp. 131–165. [Google Scholar]
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2, ISBN 9780444517012. [Google Scholar]
- Hosseini, S.M.S.; Mirzaei, M. Assessment of the Colloidal Montmorillonite Dispersion as a Low-Cost and Eco-Friendly Nanofluid for Improving Thermal Performance of Plate Heat Exchanger. SN Appl. Sci. 2020, 2, 1719. [Google Scholar] [CrossRef]
- Diaz, F.R.V.; Santos, P.D. Studies on the Acid Activation of Brazilian Smectitic Clays. Quim. Nova 2001, 24, 345–353. [Google Scholar]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 5, ISBN 9780080982588. [Google Scholar]
- Bechtner, P. Bentonite. In Industrial Minerals and Rocks; Dolbear, S.H., Bowles, O., Bain, H.F., Ball, S.H., Bradley, W.W., Gillson, J.L., Joslin, G.A., Meagher, E.C., Rockwood, N.C., Smith, H.I., Eds.; American Institute of Mining, Metallurgical and Petroleum Engineers: New York, NY, USA, 1949; Volume 1, pp. 119–126. [Google Scholar]
- Keller, W.D. Scanning Electron Micrographs of Claystone Altering to Flint Clay. Clays Clay Miner. 1982, 30, 150–152. [Google Scholar] [CrossRef]
- Ohrdorf, K.H.; Flachberger, H. Processing of Calcium Montmorillonites for Use in Polymers. In Polymer Nanoclay Composites; Laske, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–25. ISBN 9780323312721. [Google Scholar]
- Magzoub, M.I.; Nasser, M.S.; Hussein, I.A.; Benamor, A.; Onaizi, S.A.; Sultan, A.S.; Mahmoud, M.A. Effects of Sodium Carbonate Addition, Heat and Agitation on Swelling and Rheological Behavior of Ca-Bentonite Colloidal Dispersions. Appl. Clay Sci. 2017, 147, 176–183. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Russell, S.J. Cellulose Acetate/Sodium-Activated Natural Bentonite Clay Nanofibres Produced by Free Surface Electrospinning. J. Mater. Sci. 2018, 53, 10891–10909. [Google Scholar] [CrossRef]
- Hayakawa, T.; Oya, M.; Minase, M.; Fujita, K. Applied Clay Science Preparation of Sodium-Type Bentonite with Useful Swelling Property by a Mechanochemical Reaction from a Weathered Bentonite. Appl. Clay Sci. 2019, 175, 124–129. [Google Scholar] [CrossRef]
- Silva Filho, E.A.; D’Agostini Vazzoler, F.S.; Vazzoler, H.; Uliana, F.; Valenzuela Diaz, F.R. Organophilic Clays and Their Application in Atrazine Adsorption. Ceramica 2021, 67, 158–163. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, Z.; Zhou, G.; Chen, C.; Sang, Y.; Yu, Z.; Jiang, L.; Mei, Y.; Wei, Y. Preparation and Properties of Organically Modified Na-Montmorillonite. Materials 2023, 16, 3184. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, Preparation and Applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Silva, A.R.V.; Ferreira, H.C. Esmectitas Organofílicas: Conceitos, Estruturas, Propriedades, Síntese, Usos Industriais e Produtores/Fornecedores Nacionais. Rev. Eletrônica Mater. Process. 2008, 3.3, 1–11. [Google Scholar]
- Pereira, K.A.B.; Aguiar, K.L.N.P.; Oliveira, P.F.; Vicente, B.M.; Pedroni, L.G.; Mansur, C.R.E. Synthesis of Hydrogel Nanocomposites Based on Partially Hydrolyzed Polyacrylamide, Polyethyleneimine, and Modified Clay. ACS Omega 2020, 5, 4759–4769. [Google Scholar] [CrossRef]
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of Emerging Pollutants in Aqueous Phase by Heterogeneous Fenton and Photo-Fenton with Fe2O3-TiO2-Clay Heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434–38445. [Google Scholar] [CrossRef]
- Resende, R.F.; Silva, T.F.B.; Santos, N.A.D.V.; Papini, R.M.; Magriotis, Z.M. Anionic Collector Adsorption onto Bentonites and Potential Applications in the Treatment of Mining Wastewater. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 629, 127401. [Google Scholar] [CrossRef]
- de Souza, F.M.; dos Santos, O.A.A. Assessment of Fixed Bed Adsorption of 2,4-D Herbicide onto Modified Bentonite Clay. Water. Air. Soil Pollut. 2022, 233, 158. [Google Scholar] [CrossRef]
- Oliveira, L.H.; de Lima, I.S.; Enedina, E.R.; de Lima, S.G.; Trigueiro, P.; Osajima, J.A.; da Silva-Filho, E.C.; Jaber, M.; Fonseca, M.G. Essential Oil in Bentonite: Effect of Organofunctionalization on Antibacterial Activities. Appl. Clay Sci. 2023, 245, 107158. [Google Scholar] [CrossRef]
- Taibi, Z.; Bentaleb, K.; Bouberka, Z.; Pierlot, C.; Vandewalle, M.; Volkringer, C.; Supiot, P.; Maschke, U. Adsorption of Orange G Dye on Hydrophobic Activated Bentonite from Aqueous Solution. Crystals 2023, 13, 211. [Google Scholar] [CrossRef]
- Aguiar, K.L.N.P.; Pereira, K.A.B.; Mendes, M.S.L.; Pedroni, L.G.; Oliveira, P.F.; Mansur, C.R.E. Study of the Modification of Bentonite for the Formation of Nanocomposite Hydrogels with Potential Applicability in Conformance Control. J. Pet. Sci. Eng. 2020, 195, 107600. [Google Scholar] [CrossRef]
- França, D.B.; Trigueiro, P.; Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Monitoring Diclofenac Adsorption by Organophilic Alkylpyridinium Bentonites. Chemosphere 2020, 242, 125109. [Google Scholar] [CrossRef] [PubMed]
- Capelezzo, A.P.; Celuppi, L.C.M.; Macuvele, D.L.P.; Zeferino, R.C.F.; Zanetti, M.; Bender, J.P.; de Mello, J.M.M.; Fiori, M.A.; Riella, H.G. Obtaining and Characterization of Bentonite Organophilic Incorporated with Geranyl Acetate and Its Application as Mycotoxins’ Binder in Simulated Gastrointestinal Fluids. Appl. Clay Sci. 2023, 237, 106915. [Google Scholar] [CrossRef]
- Nag, A.; Hayakawa, T.; Minase, M.; Ogawa, M. Organophilic Clay with Useful Whiteness. Langmuir 2022, 38, 2979–2985. [Google Scholar] [CrossRef]
- Guo, H.; Jing, X.; Zhang, L.; Wang, J. Preparation of Inorganic-Organic Pillared Montmorillonite Using Ultrasonic Treatment. J. Mater. Sci. 2007, 42, 6951–6955. [Google Scholar] [CrossRef]
- Nafees, M.; Waseem, A.; Khan, A.R. Comparative Study of Laterite and Bentonite Based Organoclays: Implications of Hydrophobic Compounds Remediation from Aqueous Solutions. Sci. World J. 2013, 2013, 681769. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Bhandari, R.; Sharma, C.; Krishna Dhakad, S.; Pinca-Bretotean, C. Polymer Matrix Composites: A State of Art Review. Mater. Today Proc. 2022, 57, 2330–2333. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A Comprehensive Review on the Recent Advancements in Natural Rubber Nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer-Layered Silicate Nanocomposites: Synthesis, Properties and Applications. Appl. Organomet. Chem. 1998, 12, 675–680. [Google Scholar] [CrossRef]
- Dick, J.S. How to Improve Rubber Compounds. In How to Improve Rubber Compounds; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2014; pp. I–XIII. ISBN 9781569905333. [Google Scholar]
- Vaia, R.A.; Wagner, H.D. Framework for Nanocomposites. Mater. Today 2004, 7, 32–37. [Google Scholar] [CrossRef]
- Witschnigg, A. Characterization of Polymer Nanocomposites Based on Layered Silicates. In Polymer Nanoclay Composites; Laske, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 93–126. ISBN 9780323312721. [Google Scholar]
- Krishnamoorti, R.; Yurekli, K. Rheology of Polymer Layered Silicate Nanocomposites. Curr. Opin. Colloid Interface Sci. 2001, 6, 464–470. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, V.R.; Coombs, M. Applications of Clay–Polymer Nanocomposites. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5, pp. 539–586. ISBN 9780080982595. [Google Scholar]
- Kohjiya, S.; Kato, A.; Ikeda, Y. Rubbery Materials and Soft Nanocomposites. In Reinforcement of Rubber: Visualization of Nanofiller and the Reinforcing Mechanism; Kohjiya, S., Kato, A., Ikeda, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–12. ISBN 9789811537899. [Google Scholar]
- López-Manchado, M.A.; Arroyo, M.; Herrero, B.; Biagiotti, J. Vulcanization Kinetics of Natural Rubber-Organoclay Nanocomposites. J. Appl. Polym. Sci. 2003, 89, 1–15. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.A.; Herrero, B. Organo-Montmorillonite as Substitute of Carbon Black in Natural Rubber Compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Masa, A.; Iimori, S.; Saito, R.; Saito, H.; Sakai, T.; Kaesaman, A.; Lopattananon, N. Strain-induced Crystallization Behavior of Phenolic Resin Crosslinked Natural Rubber/Clay Nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42580. [Google Scholar] [CrossRef]
- Sattayanurak, S.; Sahakaro, K.; Kaewsakul, W.; Dierkes, W.K.; Reuvekamp, L.A.E.M.; Blume, A.; Noordermeer, J.W.M. Elucidating the Role of Clay-Modifier on the Properties of Silica-and Silica/Nanoclay-Reinforced Natural Rubber Tire Compounds. Express Polym. Lett. 2021, 15, 666–684. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H. State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities. Minerals 2022, 12, 1495. [Google Scholar] [CrossRef]
- Archibong, F.N.; Orakwe, L.C.; Ogah, O.A.; Mbam, S.O.; Ajah, S.A.; Okechukwu, M.E.; Igberi, C.O.; Okafor, K.J.; Chima, M.O.; Ikelle, I.I. Emerging Progress in Montmorillonite Rubber/Polymer Nanocomposites: A Review. J. Mater. Sci. 2023, 58, 2396–2429. [Google Scholar] [CrossRef]
- Sasikumar, S.; Kishore Sivaram, S.; Yadav, P.K.; Murugesan, S. Review on the Development of Natural Rubber/Nanoclay Nanocomposites; INC: New York, NY, USA, 2024; ISBN 9780443133909. [Google Scholar]
- Valenzuela-Diaz, F.R.; de Abreu, L.D.V.; Santos, P.D.S. Hot Sodium Cation Exchange in Light-Green Smectitic Clay from Campina Grande, Paraiba. Ceramica 1997, 42, 290–293. [Google Scholar]
- Valenzuela-Díaz, F.R. Preparation of Organophilic Clays from a Brazilian Smectitic Clay. Key Eng. Mater. 2001, 189–191, 203–207. [Google Scholar] [CrossRef]
- ASTM D2084; Standard Test Method for Rubber Property—Vulcanization Using Oscillating Disk Cure Meter. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D8059; Standard Test Method for Rubber Compounds—Measurement of Unvulcanized Dynamic Strain Sof-Tening (Payne Effect) Using Sealed Cavity Rotorless Shear Rheometers. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D2765; Standard Test Methods for Determination of Gel Content and Swell Ratio of Crosslinked Ethylene Plastics. ASTM International: West Conshohocken, PA, USA, 2024.
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- ASTM D412-06; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers-Tension. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2017.
- Souza Santos, P. Ciência e Tecnologia de Argilas, 2nd ed.; Edgard Blücher: São Paulo, Brazil, 1989; Volume 1. [Google Scholar]
- Yaghmaeiyan, N.; Mirzaei, M.; Delghavi, R. Montmorillonite Clay: Introduction and Evaluation of Its Applications in Different Organic Syntheses as Catalyst: A Review. Results Chem. 2022, 4, 100549. [Google Scholar] [CrossRef]
- Delbem, M.F.; Valera, T.S.; Valenzuela-Diaz, F.R.; Demarquette, N.R. Modification of a Brazilian Smectite Clay with Different Quaternary Ammonium Salts. Quim. Nova 2010, 33, 309–315. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. ISBN 9780080982588. [Google Scholar]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification. In Mineralogical Society Monograph No. 5; Mineralogical Society of Great Britain and Ireland: London, UK, 1980; pp. 305–360. [Google Scholar]
- Sun, Y.; Luo, Y.; Jia, D. Preparation and Properties of Natural Rubber Nanocomposites with Solid-state Organomodified Montmorillonite. J. Appl. Polym. Sci. 2008, 107, 2786–2792. [Google Scholar] [CrossRef]
- Farmer, V.C.; Palmieri, F. The Characterization of Adsorption Bonds in Clays by Infrared Spectroscopy. In Soil Science; Gieseking, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 573–670. [Google Scholar]
- Yu, Y.; Chen, Z.; Zhang, Q.; Jiang, M.; Zhong, Z.; Chen, T.; Jiang, J. Modified Montmorillonite Combined with Intumescent Flame Retardants on the Flame Retardancy and Thermal Stability Properties of Unsaturated Polyester Resins. Polym. Adv. Technol. 2019, 30, 998–1009. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Introduction to Clay Science. In Developments in Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5, pp. 1–7. ISBN 9780080982595. [Google Scholar]
- Hrachová, J.; Komadel, P.; Chodák, I. Natural Rubber Nanocomposites with Organo-Modified Bentonite. Clays Clay Miner. 2009, 57, 444–451. [Google Scholar] [CrossRef]
- Michot, L.J.; Villiéras, F. Surface Area and Porosity. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 319–332. [Google Scholar]
- Chakraborty, S.; Kar, S.; Dasgupta, S.; Mukhopadhyay, R.; Bandyopadhyay, S.; Joshi, M.; Ameta, S.C. Study of the Properties of In-Situ Sodium Activated and Organomodified Bentonite Clay—SBR Rubber Nanocomposites—Part I: Characterization and Rheometric Properties. Polym. Test. 2010, 29, 181–187. [Google Scholar] [CrossRef]
- De Paiva, L.B.; Morales, A.R.; Díaz, F.R.V. Organophilic Clays: Characteristics, Preparation Methods, Intercalation Compounds and Characterization Techniques. Ceramica 2008, 54, 213–226. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds—Part B, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470405888. [Google Scholar]
- Hrachová, J.; Komadel, P.; Jochec-Mošková, D.; Krajči, J.; Janigová, I.; Šlouf, M.; Chodák, I. Properties of Organo-Clay/Natural Rubber Nanocomposites: Effects of Organophilic Modifiers. J. Appl. Polym. Sci. 2013, 127, 3447–3455. [Google Scholar] [CrossRef]
- George, S.C.; Rajan, R.; Aprem, A.S.; Thomas, S.; Kim, S.S. The Fabrication and Properties of Natural Rubber-Clay Nanocomposites. Polym. Test. 2016, 51, 165–173. [Google Scholar] [CrossRef]
- Avalos, F.; Ortiz, J.C.; Zitzumbo, R.; López-Manchado, M.A.; Verdejo, R.; Arroyo, M. Effect of Montmorillonite Intercalant Structure on the Cure Parameters of Natural Rubber. Eur. Polym. J. 2008, 44, 3108–3115. [Google Scholar] [CrossRef]
- Rajab-Qurchi, M.; Toiserkani, H. Fabrication and Evaluation of Organoclay-Reinforced Epoxidized Natural Rubber Nanocomposites: A Comprehensive Study. Polymer 2024, 299, 126976. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Zinc Loaded Clay as Activator in Sulfur Vulcanization: A New Route for Zinc Oxide Reduction in Rubber Compounds. Rubber Chem. Technol. 2004, 77, 336–355. [Google Scholar] [CrossRef]
- Weiss, Z.; Klika, Z.; Čapková, P.; Janeba, D.; Kozubová, S. Sodium-Cadmium and Sodium-Zinc Exchangeability in Montmorillonite. Phys. Chem. Miner. 1998, 25, 534–540. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, V.; Kumar, V. CHAPTER 2. Nanofillers in Natural Rubber. In Natural Rubber Materials, Volume 2: Composites and Nanocomposites; Thomas, S., Maria, H.J., Joy, J., Chan, C.H., Pothen, L.A., Eds.; Royal Society of Chemistry: London, UK, 2013; Volume 2, pp. 34–72. ISBN 9781849737654. [Google Scholar]
- Varghese, S.; Karger-Kocsis, J. Melt-compounded Natural Rubber Nanocomposites with Pristine and Organophilic Layered Silicates of Natural and Synthetic Origin. J. Appl. Polym. Sci. 2004, 91, 813–819. [Google Scholar] [CrossRef]
- Payne, A.R. Strainwork Dependence of Filler-loaded Vulcanizates. J. Appl. Polym. Sci. 1964, 8, 2661–2686. [Google Scholar] [CrossRef]
- Payne, A.R. A Note on the Conductivity and Modulus of Carbon Black-loaded Rubbers. J. Appl. Polym. Sci. 1965, 9, 1073–1082. [Google Scholar] [CrossRef]
- Payne, A.R.; Whittaker, R.E. Low Strain Dynamic Properties of Filled Rubbers. Rubber Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Vita, S.; Ricotti, R.; Dodero, A.; Vicini, S.; Borchardt, P.; Pinori, E.; Castellano, M. Rheological, Mechanical and Morphological Characterization of Fillers in the Nautical Field: The Role of Dispersing Agents on Composite Materials. Polymers 2020, 12, 1339. [Google Scholar] [CrossRef]
- Shi, X.; Sun, S.; Zhao, A.; Zhang, H.; Zuo, M.; Song, Y.; Zheng, Q. Influence of Carbon Black on the Payne Effect of Filled Natural Rubber Compounds. Compos. Sci. Technol. 2021, 203, 108586. [Google Scholar] [CrossRef]
- Mathew, N.M. Natural Rubber. In Rubber Technologist’s Handbook; De, S.K., White, J.R., Eds.; iSmithers Rapra Publishing: Shropshire, UK, 2001; pp. 11–45. ISBN 978-1859572627. [Google Scholar]
- Yang, X.; Song, G.; Li, P.; Gu, Z.; Wang, L.; Sun, L.; Gao, L. The Structure, Properties and Application of NR/BR/OMMT Nanocomposites. High Perform. Polym. 2010, 22, 649–665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).