C14-HSL Quorum Sensing Signal Molecules: Promoting Role in Chalcopyrite Bioleaching Efficiency
Abstract
1. Introduction
2. Materials and Methods
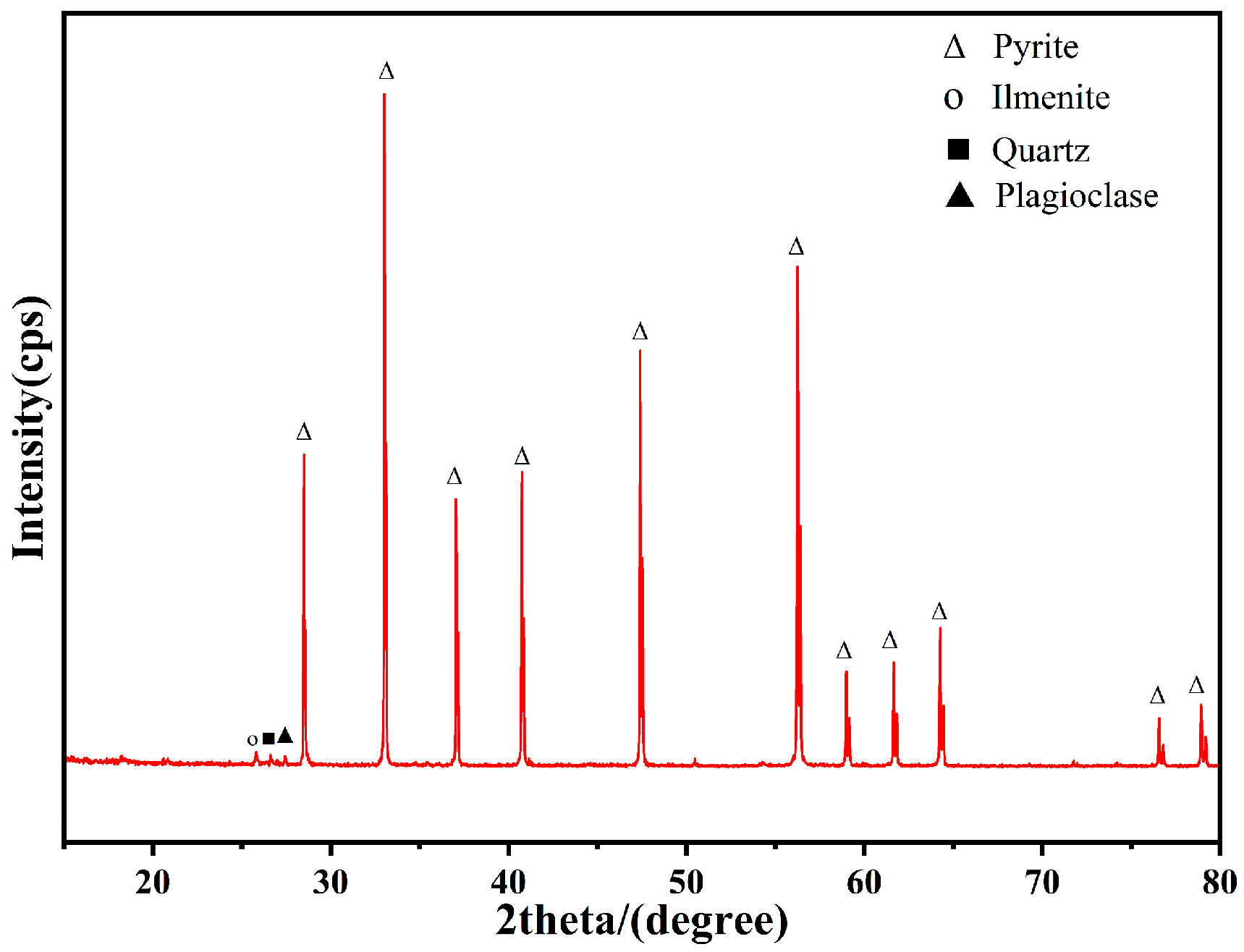
2.1. Preparation of Minerals
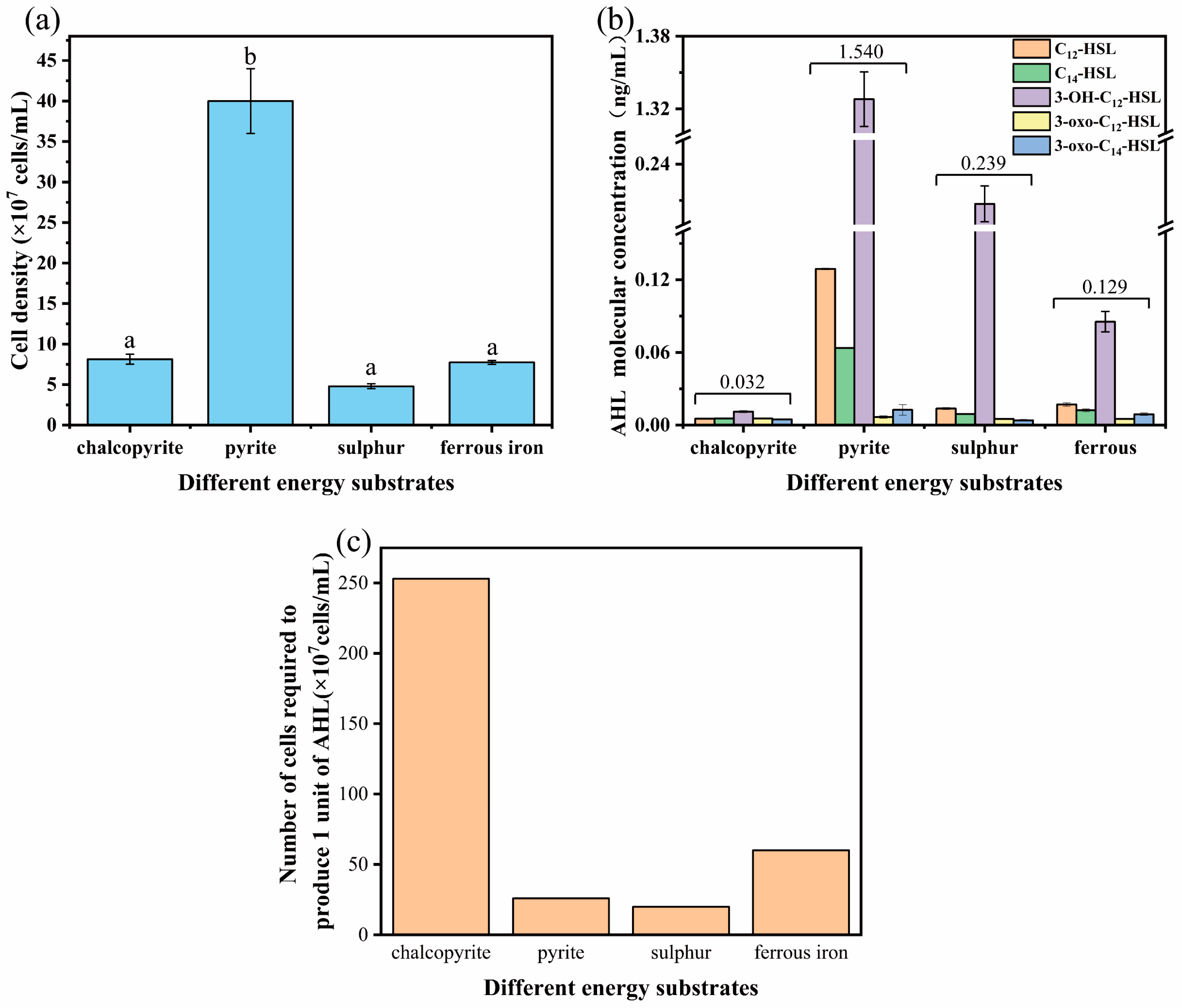
2.2. Strain and Cultivation of Strains with Different Energy Substrates
2.3. Quantification of AHL via High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS)
2.4. Bioleaching Experiments with Different Signal Molecules
2.5. Analysis of Main Physicochemical Properties in Solution
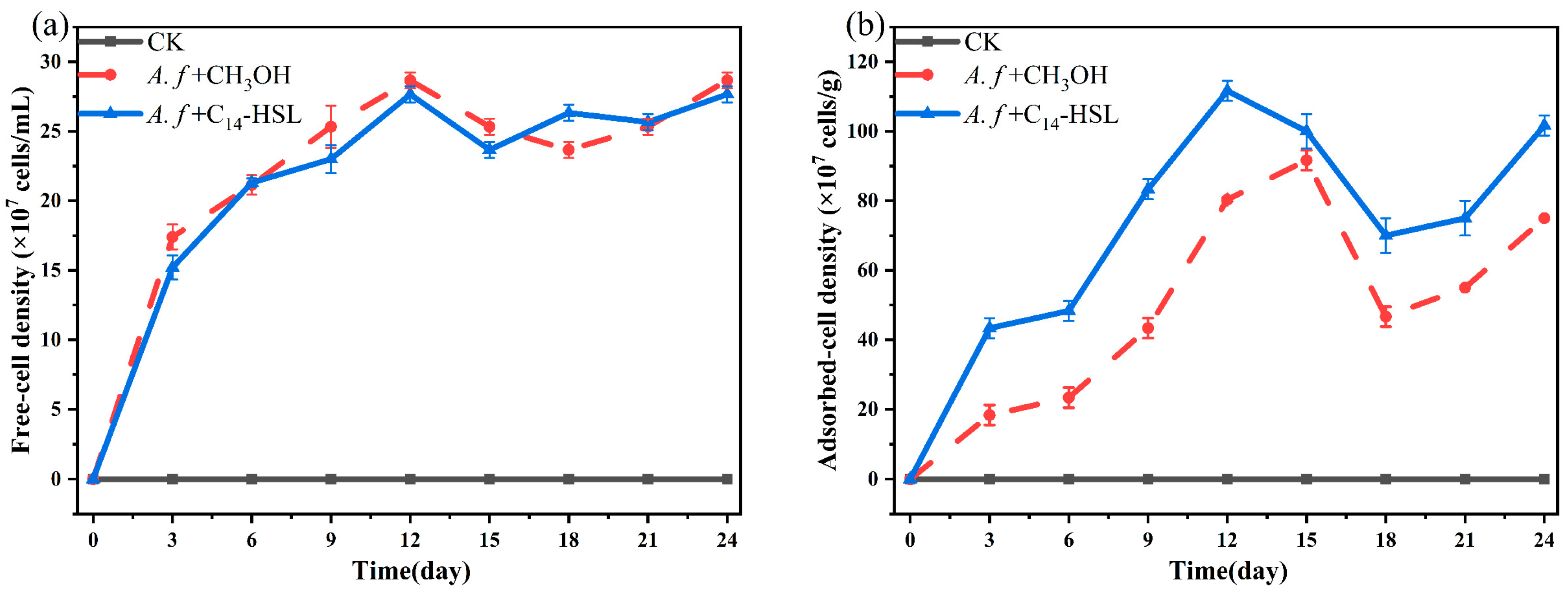
2.6. Counting of Free and Adsorbed Microorganisms
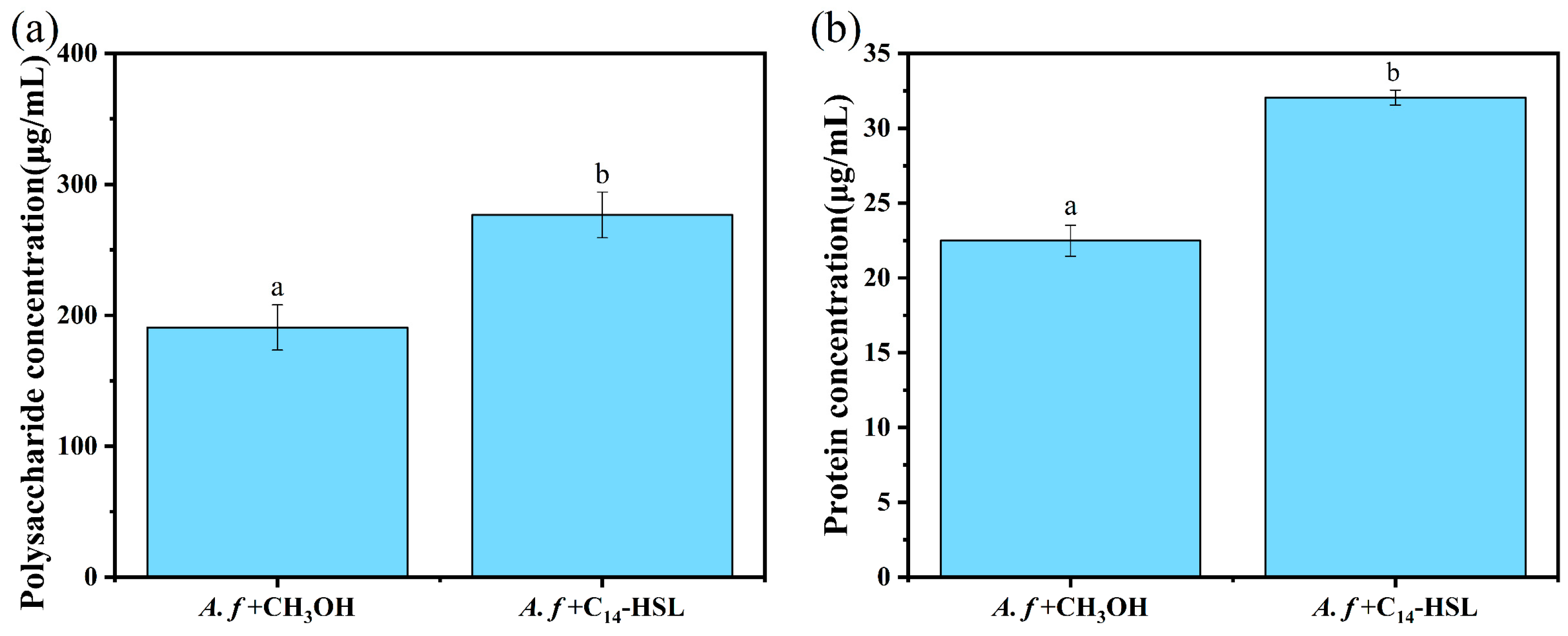
2.7. Extraction and Determination of Extracellular Polymeric Substances
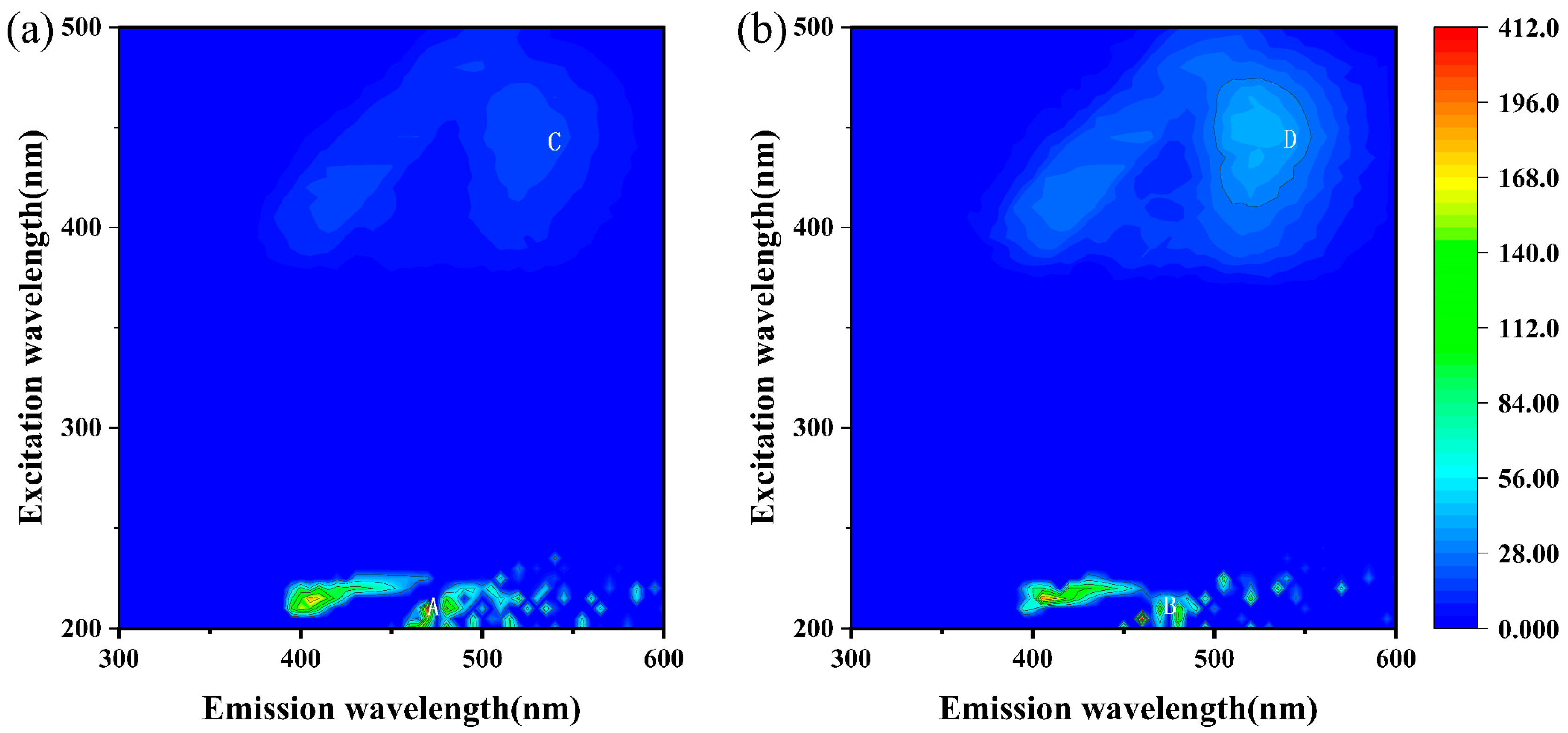
2.8. Three-Dimensional Fluorescence Spectral Analysis of Extracellular Polymeric Substances
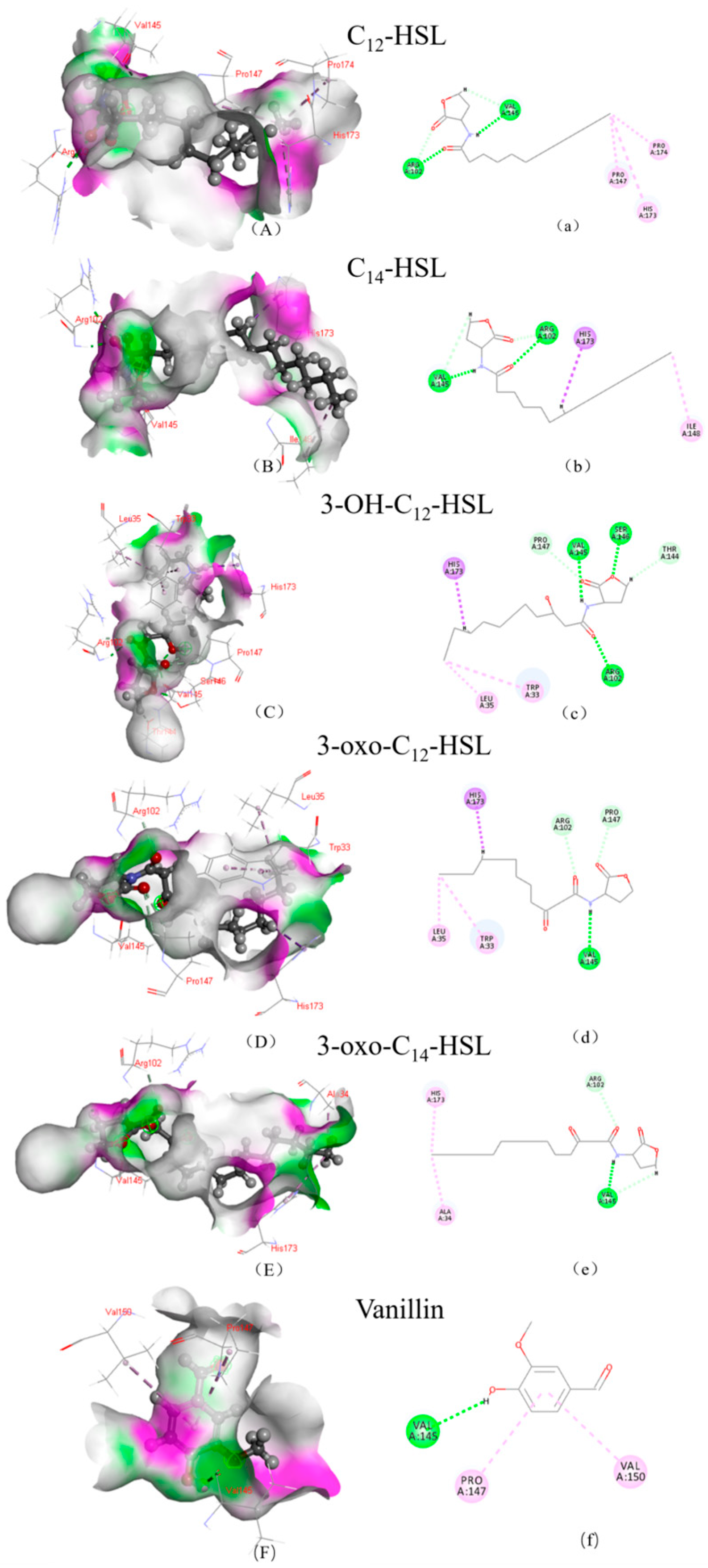
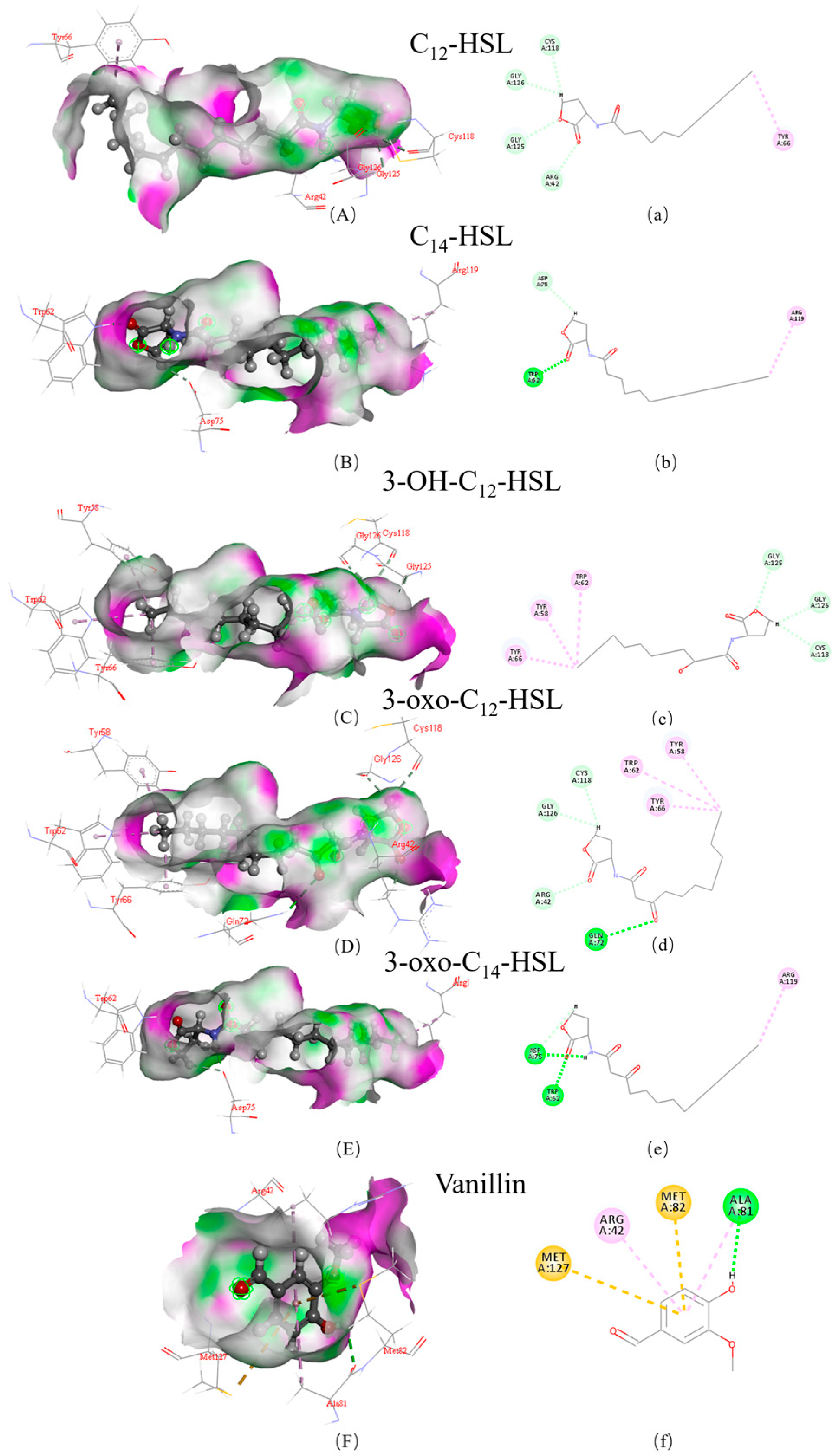
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
3.1. Generation and Identification of AHL in A. ferrooxidans
3.2. Structural Insights into the Mechanisms of AfeI/AfeR-AHL Interaction
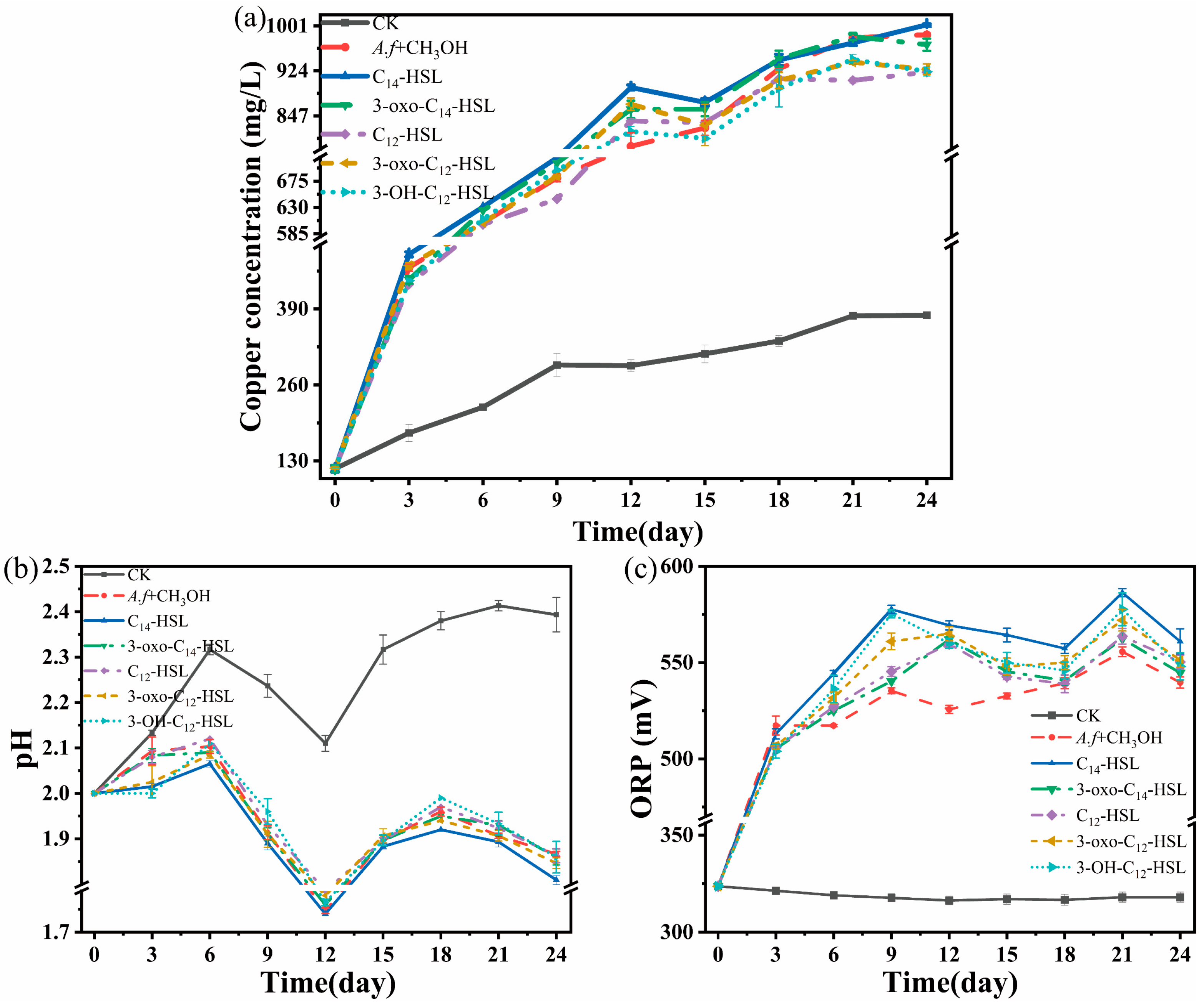
3.3. Effect of Additional Signal Molecules on Chalcopyrite Leaching by A. ferrooxidans
3.4. The Influence of C14-HSL on EPS Production During Chalcopyrite Bioleaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C14-HSL | N-tetradecanoyl-L-homoserine lactone |
| AHLs | Acyl-homoserine lactones |
| EPS | Extracellular polymeric substances |
| QS | Quorum sensing |
| XRD | X-ray diffraction |
| HPLC-MS | High-performance liquid chromatography–mass spectrometry |
| 3-oxo-C12-HSL | N-3-oxododecanoyl-L-homoserine lactone |
| 3-OH-C12-HSL | N-3-hydroxydodecanoyl-DL-homoserine lactone |
| C12-HSL | N-dodecanoyl-L-homoserine lactone |
| 3-oxo-C14-HSL | N-3-oxotetradecanoyl-L-homoserine lactone |
| ES+ | Electrospray ionization positive |
| ORP | Oxidation-reduction potential |
| RISCs | Reducing inorganic sulfur compounds |
| SAM | S-adenosyl-L-methionine |
References
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.-M. Recent Progress in Biohydrometallurgy and Microbial Characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Bakhti, A.; Moghimi, H.; Bozorg, A.; Stankovic, S.; Manafi, Z.; Schippers, A. Comparison of Bioleaching of a Sulfidic Copper Ore (Chalcopyrite) in Column Percolators and in Stirred-tank Bioreactors Including Microbial Community Analysis. Chemosphere 2024, 349, 140945. [Google Scholar] [CrossRef] [PubMed]
- Watling, H. Microbiological Advances in Biohydrometallurgy. Minerals 2016, 6, 49. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The Dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Dunbar, W.S. Biotechnology and the Mine of Tomorrow. Trends Biotechnol. 2017, 35, 79–89. [Google Scholar] [CrossRef]
- Giese, E.C. Evidences of EPS-iron (III) Ions Interactions on Bioleaching Process Mini-review: The Key to Improve Performance. Orbital Electron. J. Chem. 2019, 11, 200–204. [Google Scholar] [CrossRef]
- Bellenberg, S.; Díaz, M.; Noël, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm Formation, Communication and Interactions of Leaching Bacteria During Colonization of Pyrite and Sulfur Surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and Promise of Bacterial Quorum Sensing Research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Williams, P.; Winzer, K.; Chan, W.C.; Cámara, M. Look Who’s Talking: Communication and Quorum Sensing in the Bacterial World. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1119–1134. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Sarmah, B.K.; Nandi, S.P. Quorum Sensing: Its Role in Microbial Social Networking. Res. Microbiol. 2020, 171, 159–164. [Google Scholar] [CrossRef]
- Hamza, F.; Zinjarde, S. Use of Marine Microorganisms in Designing Anti-infective Strategies for Sustainable Aquaculture Production. J. Appl. Microbiol. 2023, 134, lxad128. [Google Scholar] [CrossRef] [PubMed]
- Roca Subirà, I.; Espinal, P.; Vila-Farrés, X.; Vila Estapé, J. The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Front. Microbiol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Saipriya, K.; Swathi, C.H.; Ratnakar, K.S.; Sritharan, V. Quorum-sensing System in Acinetobacter baumannii: A Potential Target for New Drug Development. J. Appl. Microbiol. 2020, 128, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, C.; Greenberg, E.P. Listening in on Bacteria: Acyl-homoserine Lactone Signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 685–695. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Williams, P. Quorum Sensing, Communication and Cross-kingdom Signaling in the Bacterial World. Microbiology 2008, 153, 3923–3938. [Google Scholar] [CrossRef]
- Farah, C.; Vera, M.; Morin, D.; Haras, D.; Jerez, C.A.; Guiliani, N. Evidence for a Functional Quorum-Sensing Type AI-1 System in the Extremophilic Bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 7033–7040. [Google Scholar] [CrossRef]
- Rivas, M.; Seeger, M.; Jedlicki, E.; Holmes, D.S. Second Acyl Homoserine Lactone Production System in the Extreme Acidophile Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2007, 73, 3225–3231. [Google Scholar] [CrossRef]
- Gao, X.Y.; Fu, C.A.; Hao, L.; Gu, X.F.; Wang, R.; Lin, J.Q.; Liu, X.M.; Pang, X.; Zhang, C.J.; Lin, J.Q.; et al. The Substrate-dependent Regulatory Effects of the AfeI/R System in Acidithiobacillus ferrooxidans Reveals the Novel Regulation Strategy of Quorum Sensing in Acidophiles. Environ. Microbiol. 2021, 23, 757–773. [Google Scholar] [CrossRef]
- González, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverría, A.; Soulère, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL Signaling Molecules with a Large Acyl Chain Enhance Biofilm Formation on Sulfur and Metal Sulfides by the Bioleaching Bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef]
- Mamani, S.; Moinier, D.; Denis, Y.; Soulère, L.; Queneau, Y.; Talla, E.; Bonnefoy, V.; Guiliani, N. Insights into the Quorum Sensing Regulon of the Acidophilic Acidithiobacillus ferrooxidans Revealed by Transcriptomic in the Presence of an Acyl Homoserine Lactone Superagonist Analog. Front. Microbiol. 2016, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y. Regulatory Function and Molecular Mechanism of the Quorum Sensing System in Acidithiobacillus ferroxidans ATCC 23270; Wanfang Data: Beijing, China, 2020. [Google Scholar]
- Galloway, W.R.J.D.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum Sensing in Gram-Negative Bacteria: Small-Molecule Modulation of AHL and AI-2 Quorum Sensing Pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Grasland, B.; Vallée-Réhel, K.; Dufau, C.; Haras, D. On-line High-performance Liquid Chromatography–mass Spectrometric Detection and Quantification of N-acylhomoserine Lactones, Quorum Sensing Signal Molecules, in the Presence of Biological Matrices. J. Chromatogr. A 2003, 1002, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Sousa, B.C.; Colombo, S.; Afonso, C.B.; Melo, T.; Pitt, A.R.; Spickett, C.M.; Domingues, P.; Domingues, M.R.; Fedorova, M.; et al. Evaluation of Air Oxidized PAPC: A Multi Laboratory Study by LC-MS/MS. Free Radic. Biol. Med. 2019, 144, 156–166. [Google Scholar] [CrossRef]
- Esparza, M.; Cárdenas, J.P.; Bowien, B.; Jedlicki, E.; Holmes, D.S. Genes and Pathways for CO2 Fixation in the Obligate, Chemolithoautotrophic Acidophile, Acidithiobacillus ferrooxidans, Carbon fixation in A. ferrooxidans. BMC Microbiol. 2010, 10, 229. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, M.; Zhang, S.; Zhao, D.; Duan, J.; Wang, W.; Yan, L. Iron and Sulfur Oxidation Pathways of Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2019, 35, 60. [Google Scholar] [CrossRef]
- Parsek, M.R.; Val, D.L.; Hanzelka, B.L.; Cronan, J.E.; Greenberg, E.P. Acyl Homoserine-lactone Quorum-Sensing Signal Generation. Proc. Natl. Acad. Sci. USA 1999, 96, 4360–4365. [Google Scholar] [CrossRef]
- Ahlgren, N.A.; Harwood, C.S.; Schaefer, A.L.; Giraud, E.; Greenberg, E.P. Aryl-homoserine Lactone Quorum Sensing in Stem-nodulating Photosynthetic Bradyrhizobia. Proc. Natl. Acad. Sci. USA 2011, 108, 7183–7188. [Google Scholar] [CrossRef]
- Rivas, M.; Seeger, M.; Holmes, D.S.; Jedlicki, E. A Lux-like Quorum Sensing System in the Extreme Acidophile Acidithiobacillus ferrooxidans. Biol. Res. 2005, 38, 283–297. [Google Scholar] [CrossRef]
- Valdés, J.; Veloso, F.; Jedlicki, E.; Holmes, D. Metabolic Reconstruction of Sulfur Assimilation in the Extremophile Acidithiobacillus ferrooxidans Based on Genome Analysis. BMC Genom. 2003, 4, 51. [Google Scholar] [CrossRef]
- Kharpan, B.; Shyam, A.; Nandi, R.; Paul, S.; Paul, P.C.; Mondal, P.; Kumar, D.; Choudhury, S.; Ray, S. Antiparasitic Activity, DNA/BSA Binding Interaction, Molecular Docking and DFT Studies of Mesogenic l-leucine Based Schiff Base and Its Derivatize Cu(II) and Zn(II) Complexes. J. Mol. Struct. 2024, 1304, 137633. [Google Scholar] [CrossRef]
- Soulère, L.; Guiliani, N.; Queneau, Y.; Jerez, C.A.; Doutheau, A. Molecular Insights into Quorum Sensing in Acidithiobacillus ferrooxidans bacteria Via Molecular Modelling of The Transcriptional Regulator AfeR and of the Binding Mode of Long-chain Acyl Homoserine Lactones. J. Mol. Model. 2008, 14, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, A. In silico analysis of quorum sensing modulators: Insights into Molecular Docking and Dynamics and Potential Therapeutic Applications. PLoS ONE 2025, 20, e0325830. [Google Scholar] [CrossRef]
- Luo, B.; Wu, S.; Liu, W.; Zhang, D.; Liu, R.; Liu, T.; Sun, Z.; Wei, Z.; Liu, M.; Shi, Z.; et al. Mechanistic Insights into the Orthogonal Functionality of an AHL-mediated Quorum-sensing Circuit in Yersinia pseudotuberculosis. Synth. Syst. Biotechnol. 2025, 10, 174–184. [Google Scholar] [CrossRef]
- Banderas, A.; Guiliani, N. Bioinformatic Prediction of Gene Functions Regulated by Quorum Sensing in the Bioleaching Bacterium Acidithiobacillus ferrooxidans. Int. J. Mol. Sci. 2013, 14, 16901–16916. [Google Scholar] [CrossRef]
- Malik, S.; Akram, F.; Ali, M.; Javed, M.; Mateen, R.M.; Zidan, A.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Farouk, A.-E.; et al. Unveiling Quorum Sensing Mechanisms: Computational Docking and Dynamics of Bacterial Receptors and Ligands. J. Mol. Struct. 2025, 1323, 140730. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Han, P.; Dong, H.; Liang, X.; Yin, G.; Wu, D.; Yang, Y.; Liu, S.; Liu, M.; et al. N-acyl-homoserine lactones (AHLs) in Intertidal Marsh: Diversity and Potential Role in Nitrogen Cycling. Plant Soil. 2020, 454, 103–119. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Greenberg, E.P.; Oliver, C.M.; Oda, Y.; Huang, J.J.; Bittan-Banin, G.; Peres, C.M.; Schmidt, S.; Juhaszova, K.; Sufrin, J.R.; et al. A New Class of Homoserine Lactone Quorum-sensing Signals. Nature 2008, 454, 595–599. [Google Scholar] [CrossRef]
- Wu, A.; Yin, S.; Qin, W.; Liu, J.; Qiu, G. The Effect of Preferential Flow on Extraction and Surface Morphology of Copper Sulphides During Heap Leaching. Hydrometallurgy 2009, 95, 76–81. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of Chalcopyrite with Ferric Ion. Part I General. Aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Luo, X.; Teng, T.; Jiang, C.; Drewniak, L.; Yang, Z.; Yin, H. An Integrated Insight into bioleaching Performance of Chalcopyrite Mediated by Microbial Factors: Functional Types and Biodiversity. Bioresour. Technol. 2021, 319, 124219. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Y.; Liu, X.-J.; Fu, C.-A.; Gu, X.-F.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Lin, J.-Q.; Chen, L.-X. Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System. Minerals 2020, 10, 222. [Google Scholar] [CrossRef]
- Vardanyan, A.; Vardanyan, N.; Khachatryan, A.; Zhang, R.; Sand, W. Adhesion to Mineral Surfaces by Cells of Leptospirillum, Acidithiobacillus and Sulfobacillus from Armenian Sulfide Ores. Minerals 2019, 9, 69. [Google Scholar] [CrossRef]
- Jacquin, C.; Lesage, G.; Traber, J.; Pronk, W.; Heran, M. Three-dimensional Excitation and Emission Matrix Fluorescence (3DEEM) for Quick and Pseudo-quantitative Determination of Protein- and Humic-like Substances in Full-scale Membrane Bioreactor (MBR). Water Res. 2017, 118, 82–92. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2004, 37, 5701–5710. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Zhou, W.; Cheng, H.; Zhou, H. Insight to the Early-stage Adsorption Mechanism of Moderately Thermophilic Consortia and Intensified Bioleaching of Chalcopyrite. Biochem. Eng. J. 2019, 144, 40–47. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Zhu, M.; Takeichi, Y.; Ohigashi, T.; Suga, H.; Jinno, M.; Makita, H.; Sakata, M.; Ono, K.; Mase, K.; et al. Direct Detection of Fe(II) in Extracellular Polymeric Substances (EPS) at the Mineral-Microbe Interface in Bacterial Pyrite Leaching. Microbes Environ. 2016, 31, 63–69. [Google Scholar] [CrossRef]
- La Vars, S.M.; Newton, K.; Quinton, J.S.; Cheng, P.-Y.; Wei, D.-H.; Chan, Y.-L.; Harmer, S.L. Surface Chemical Characterisation of Pyrite Exposed to Acidithiobacillus ferrooxidans and Associated Extracellular Polymeric Substances. Minerals 2018, 8, 132. [Google Scholar] [CrossRef]

| AHL | Chemical Formula | Detection of [M + H]+ Ion | Structured Formula |
|---|---|---|---|
| C12-HSL | C16H29O3N | 284.3 | 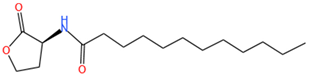 |
| 3-OH-C12-HSL | C16H29O4N | 300.3 | 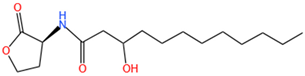 |
| 3-oxo-C12-HSL | C16H27O4N | 298.3 | 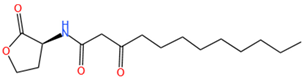 |
| C14-HSL | C18H33O3N | 312.2 | 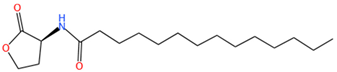 |
| 3-oxo-C14-HSL | C18H31O4N | 326.3 | 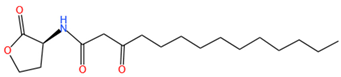 |
| AHL | LibDock Score | Hydrogen Bond Binding Site | Hydrophobic Bond Binding Site | |||
|---|---|---|---|---|---|---|
| AfeI | AfeR | AfeI | AfeR | AfeI | AfeR | |
| 3-oxo-C14-HSL | 128.87 | 143.914 | A/VAL:145 A/ARG:102 | A/ASP:75 A/TRP:62 | A/HIS:173 A/ALA:34 | A/ARG:119 |
| C14-HSL | 123.159 | 139.272 | A/ARG:102 VAL:145 | A/TRP:62 A/ASP:75 | A/HIS:173 A/ILE:148 | A/ARG:119 |
| 3-OH-C12-HSL | 123.039 | 133.245 | A/VAL:145 A/SER:146 A/ARG:102 A/THR:144 A/PRO:147 | A/GLY:125 A/GLY:126 A/CYS:118 | A/HIS:173 A/TRP:33 A/LEU:35 | A/TRP:62 A/TYR:58 A/TYR:66 |
| 3-oxo-C12-HSL | 122.509 | 130.135 | A/VAL:145 A/PRO:147 A/ARG:102 | A/GLN:72 A/CYS:118 A/GLY:126 A/ARG:42 | A/HIS:173 A/TRP:33 A/LEU:35 | A/TYR:66 A/TRP:62 A/TYR:58 |
| C12-HSL | 120.501 | 127.537 | A/ARG:102 A/VAL:145 | A/CYS:118 A/GLY:126 A/CLY:125 A/ARG:42 | A/PRO:174 A/PRO:147 A/HIS:173 | A/TYR:66 |
| Vanillin | 77.377 | 78.012 | A/VAL:145 | A/ALA:81 | A/PRO:147 A/VAL:150 | A/ARG:42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Luo, W.; Yao, Z.; Li, Y.; Wu, X.; Ibrahim, N.; Begum, S.; Liang, Y. C14-HSL Quorum Sensing Signal Molecules: Promoting Role in Chalcopyrite Bioleaching Efficiency. Minerals 2025, 15, 1248. https://doi.org/10.3390/min15121248
Chen S, Luo W, Yao Z, Li Y, Wu X, Ibrahim N, Begum S, Liang Y. C14-HSL Quorum Sensing Signal Molecules: Promoting Role in Chalcopyrite Bioleaching Efficiency. Minerals. 2025; 15(12):1248. https://doi.org/10.3390/min15121248
Chicago/Turabian StyleChen, Shiqi, Wang Luo, Zexing Yao, Yiran Li, Xinhong Wu, Nazidi Ibrahim, Shadab Begum, and Yili Liang. 2025. "C14-HSL Quorum Sensing Signal Molecules: Promoting Role in Chalcopyrite Bioleaching Efficiency" Minerals 15, no. 12: 1248. https://doi.org/10.3390/min15121248
APA StyleChen, S., Luo, W., Yao, Z., Li, Y., Wu, X., Ibrahim, N., Begum, S., & Liang, Y. (2025). C14-HSL Quorum Sensing Signal Molecules: Promoting Role in Chalcopyrite Bioleaching Efficiency. Minerals, 15(12), 1248. https://doi.org/10.3390/min15121248







