Influence and Mechanism of 1-Dodecyl-3-methylimidazolium Bromide on the Flotation Behavior of Quartz and Feldspar in a Neutral System
Abstract
1. Introduction
2. Experiments and Methods
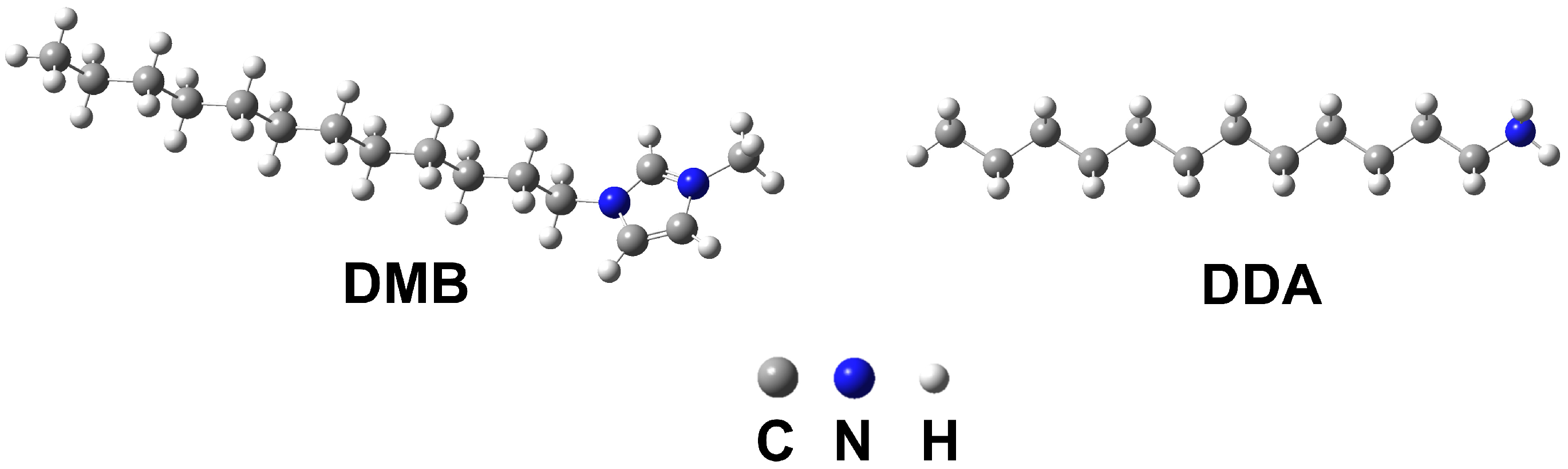
2.1. Materials and Reagents
2.2. Micro-Flotation Experiments
2.3. FTIR Analysis
2.4. Zeta Potential Measurements
2.5. DFT Calculations
3. Results and Discussion
3.1. Micro-Flotation Results
3.1.1. Effect of pH on Flotation Recovery
3.1.2. Effect of Collector Dosage on Flotation Recovery
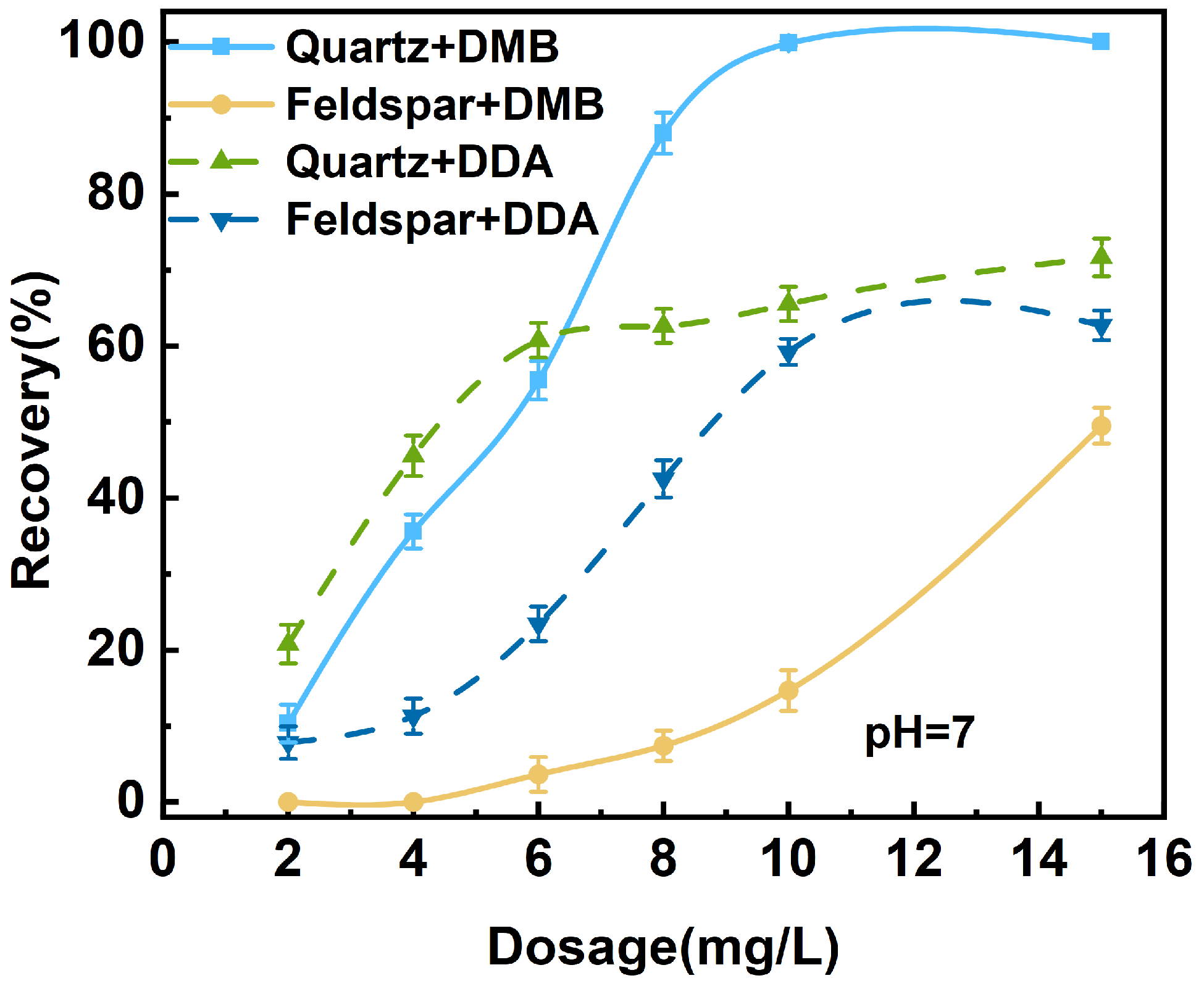
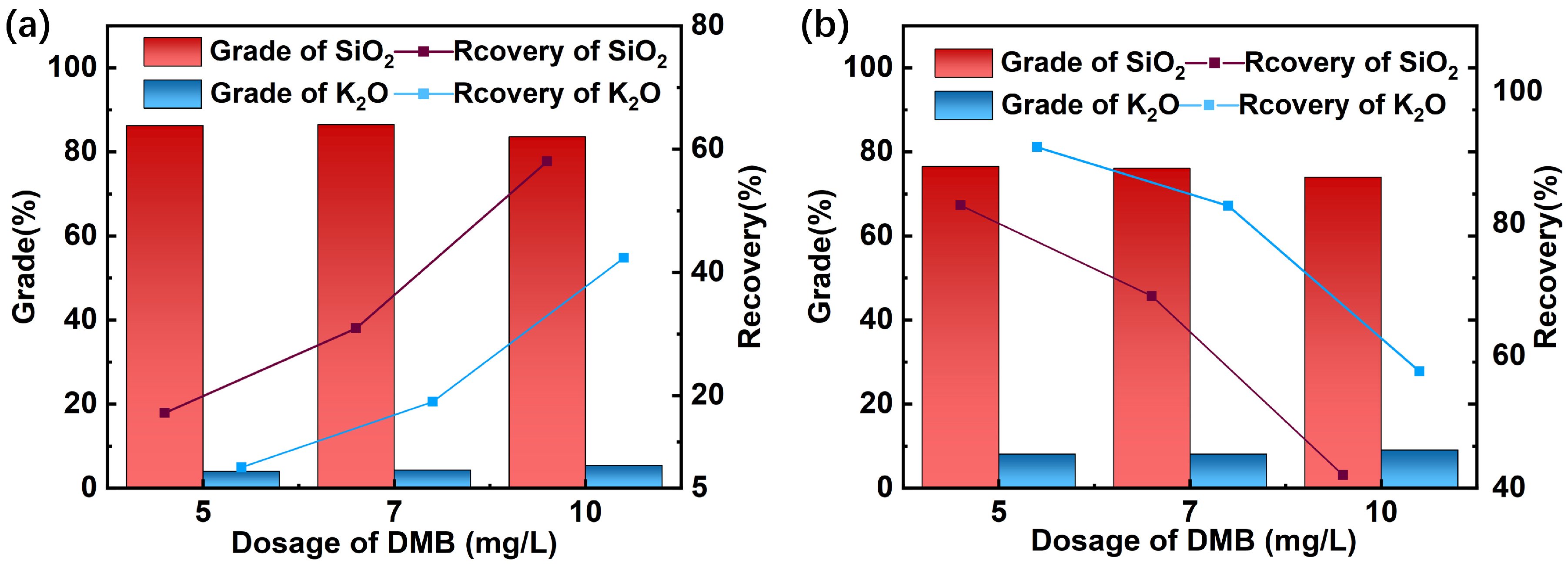
3.1.3. Mixed Mineral Flotation
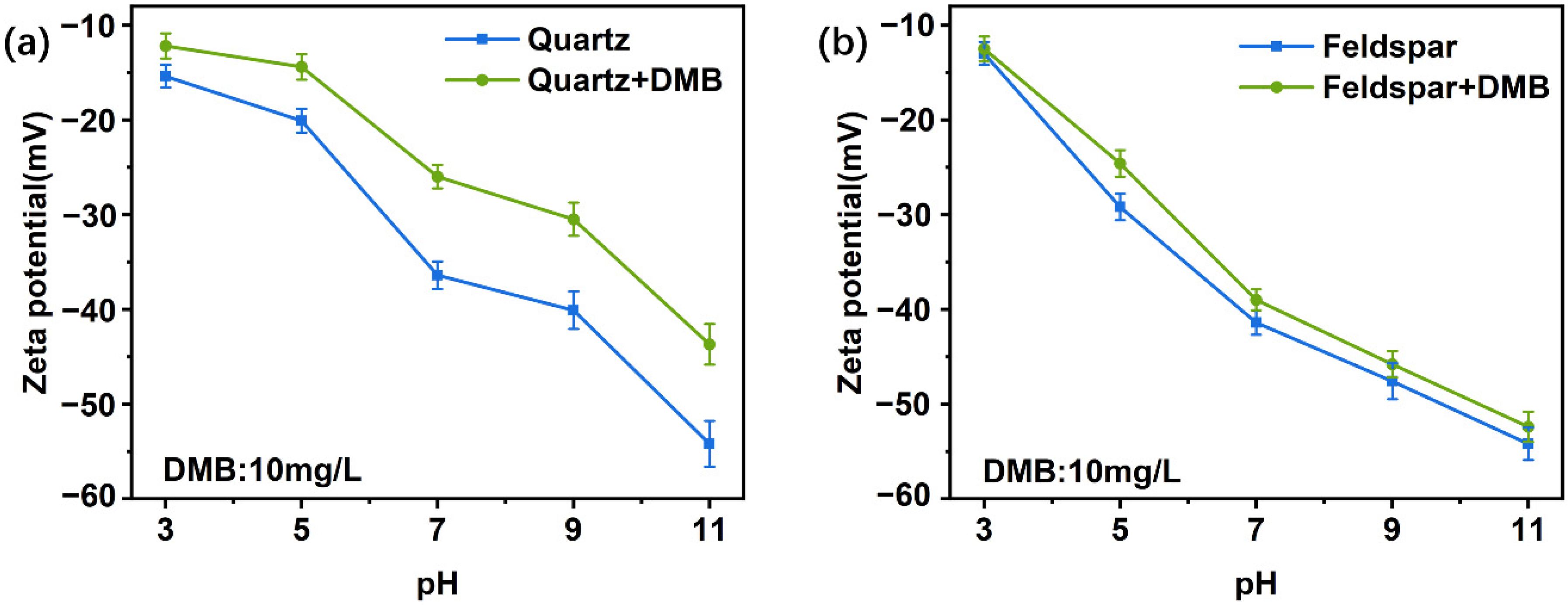
3.2. Zeta Potential Measurements
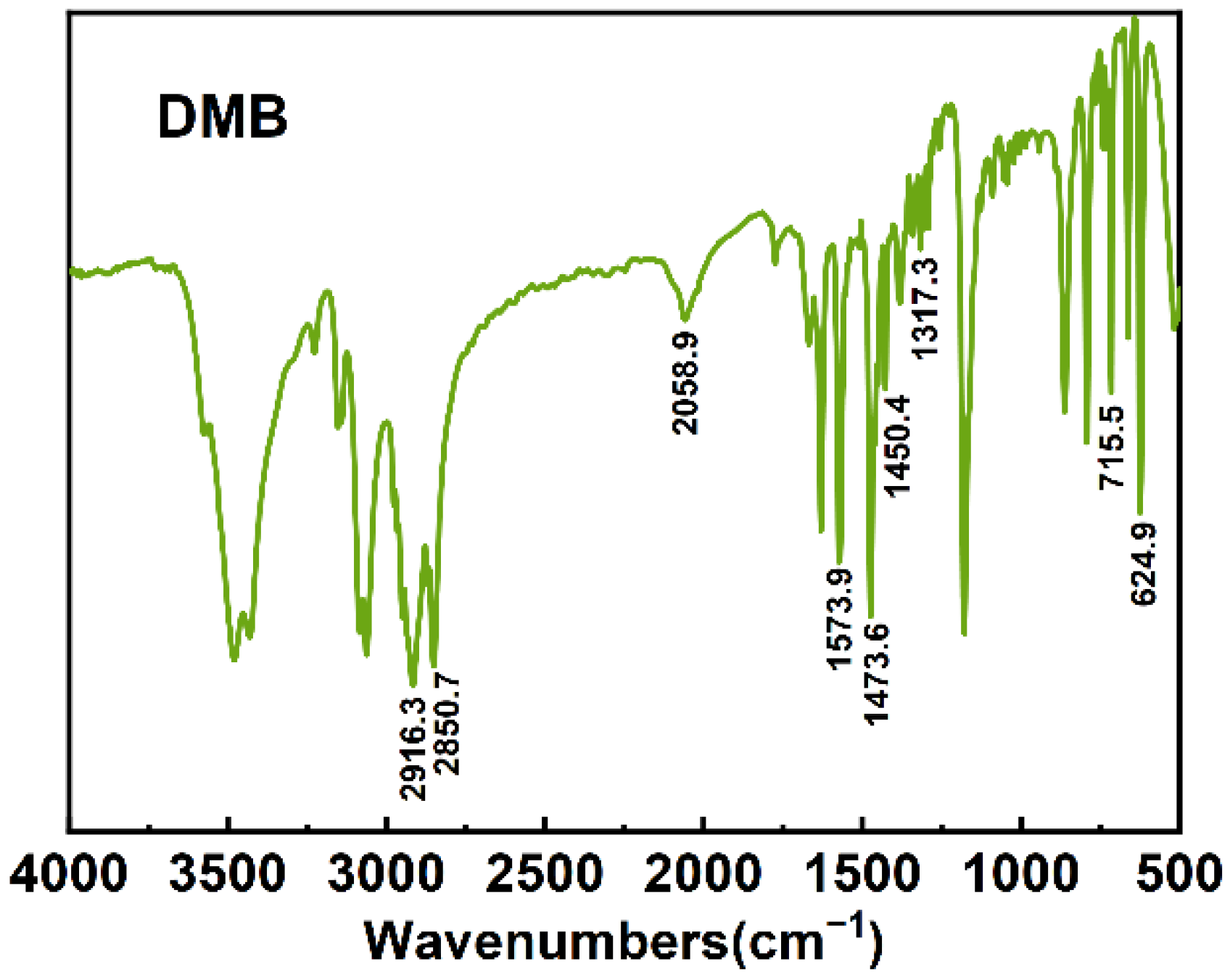
3.3. FTIR-Spectra Analysis
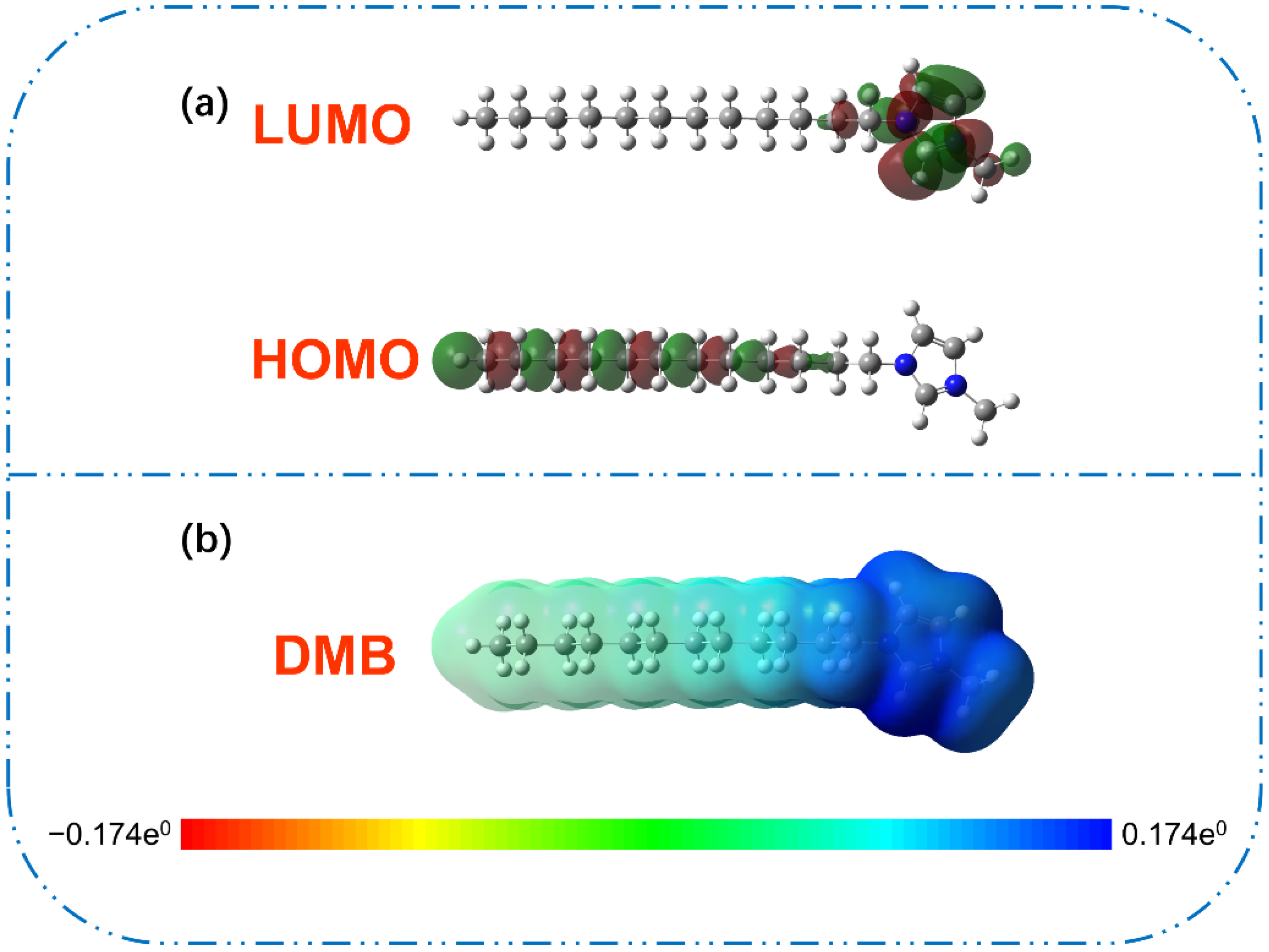
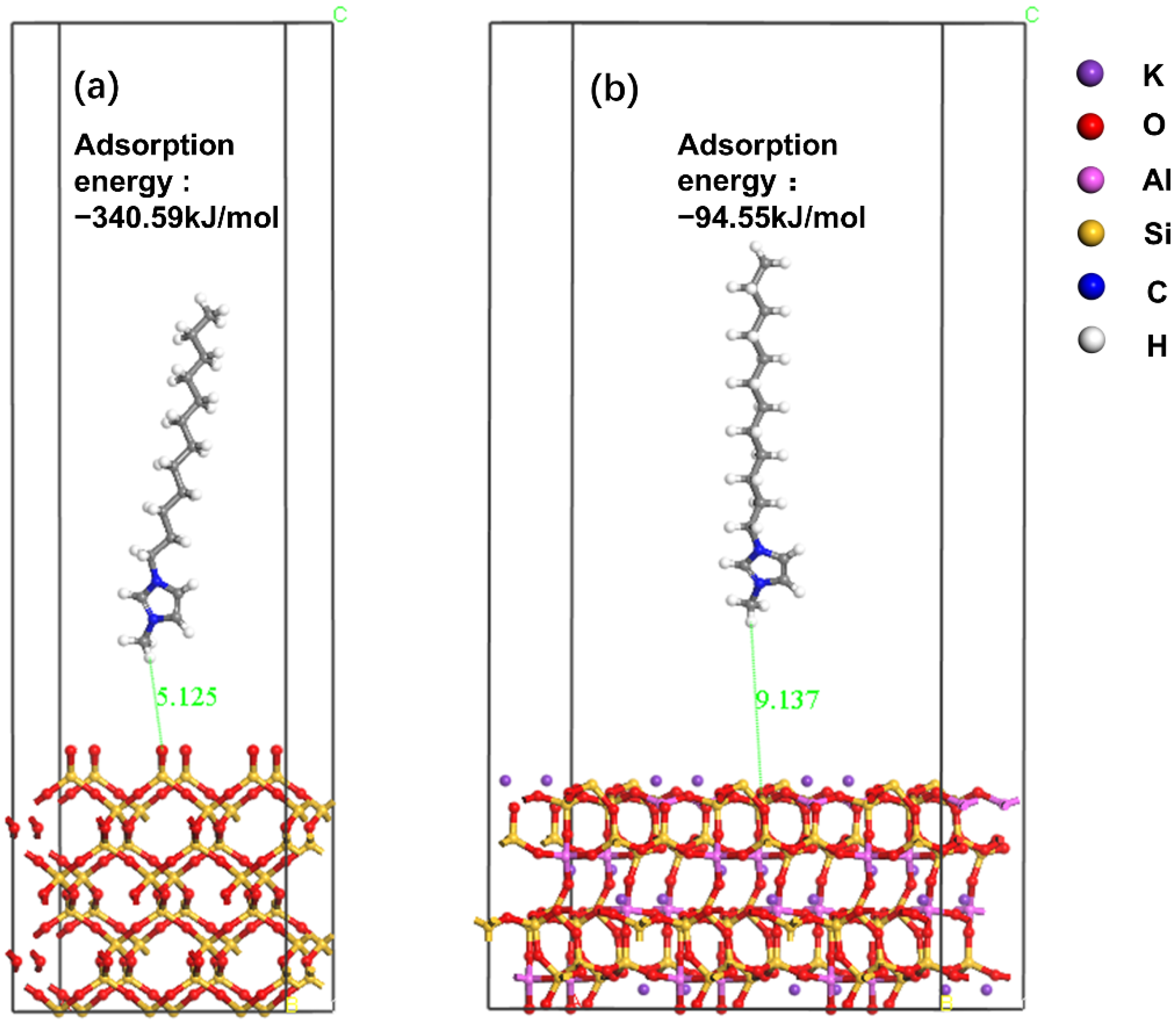
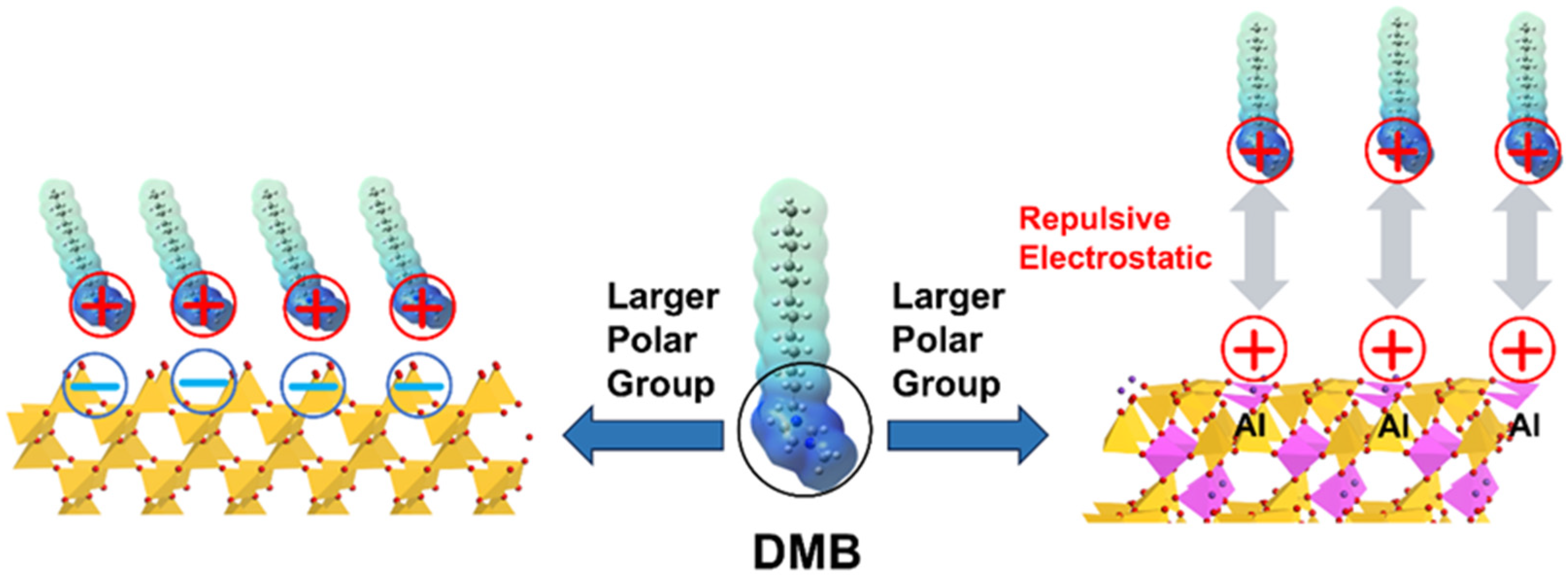
3.4. DFT Calculation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Wang, G.F.; Zhao, F.Y.; Li, W.F.; Zhu, G.; Liang, G.C.; Jian, W.; Liao, L.B.; Lv, G.C. Advances in purification technologies and applications of high-purity quartz resources. Prog. Nat. Sci. Mater. Int. 2025, 35, 51–64. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, J.G.; Wu, Z.C.; Zhang, H.Q.; Tan, X.M.; Yi, Y.J.; Tan, Q.; Liu, L. Characteristics of high-purity quartz raw materials for crucibles and exploration of key purification technologies. Miner. Eng. 2025, 231, 109446. [Google Scholar] [CrossRef]
- Vegliò, F.; Passariello, B.; Abbruzzese, C. Iron removal process for high-purity silica sands production by oxalic acid leaching. Ind. Eng. Chem. Res. 1999, 38, 4443–4448. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Q.; Ku, J.A.; Shang, H.L.; Shen, Z.C. Purification Technologies for High-Purity Quartz: From Mineralogy to Applications. Sep. Purif. Rev. 2025. [Google Scholar] [CrossRef]
- Hu, X.; Luo, X.P.; Liu, Z.S.; Zhang, Y.B.; Zhou, H.P.; Yang, Z.Z.; Tang, X.K. Flotation separation of feldspar from quartz using sodium fluosilicate as a selective depressant. Rare Met. 2024, 43, 1288–1300. [Google Scholar] [CrossRef]
- Huang, H.J.; Li, S.H.; Gou, H.R.; Zhang, N.; Liu, L.M. Efficient Recovery of Feldspar, Quartz, and Kaolin from Weathered Granite. Minerals 2024, 14, 300. [Google Scholar] [CrossRef]
- Tan, Y.P.; Li, S.K.; Xu, X.P.; Chen, P.; Gao, Z.Y.; Sun, W.; McFadzean, B.; Cao, J. Utilization of crown ether as selective collector for the flotation separation of pollucite from feldspar and quartz. Miner. Eng. 2025, 220, 109085. [Google Scholar] [CrossRef]
- Fan, X.Y.; Xiao, T.T.; Zhou, C.Y.; Wang, H.R.; Pan, Z.Q.; Wu, H.J.; Zhou, H. Mixed surfactants with solubilization behaviors: Separation of feldspar and quartz by self-assembly flotation. Miner. Eng. 2025, 221, 109130. [Google Scholar] [CrossRef]
- Gong, W.Q.; He, J.F.; Yang, B.; Xu, H.L.; Shan, Y.H.; Fu, L.X.; Wu, J.W. Interfacial synergistic mechanism of an effective combined collector sodium oleate/cetyltrimethyl ammonium chloride and its enhanced flotation separation of K-feldspar and quartz. Surf. Interfaces 2025, 60, 106017. [Google Scholar] [CrossRef]
- Lu, J.W.; Wang, N.L.; Li, S.K.; Lin, Z.Q.; Meng, Q.B.; Li, L.X. Atomic insight into the activation mechanism of feldspar by sodium oleate in flotation separation of quartz and feldspar: XPS, AFM, and molecular dynamics. Sep. Sci. Technol. 2023, 58, 2493–2504. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.D.; Ge, P.; Sun, W.; Xu, L.H.; Tang, H.H.; Wang, L. Selective flotation of quartz from feldspar using hydroxypropyl starch as depressant. Miner. Eng. 2023, 195, 108022. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Mohanty, K.; Oliva, J.; Alfonso, P.; Sampaio, C.H.; Anticoi, H. A Comparative Study of Quartz and Potassium Feldspar Flotation Process Using Different Chemical Reagents. Minerals 2024, 14, 167. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Study on Comparative Test for the Application of Purified Quartz from a Gold Ore Tailing. Compr. Util. Miner. Resour. 2021, 42, 159–162. [Google Scholar]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Li, S.; Kong, J.; Yang, C.; Bao, S. Experiment Study on Extraction of SiO2 from Gold Tailings by High Magnetic Separation Floatation Technology. Met. Mine 2018, 47, 184–188. [Google Scholar]
- Li, P.Y.; Ren, Z.J.; Xie, E.J.; Duan, S.T.; Gao, H.M.; Wu, J.X.; He, Y.H. Application of mixed collectors on quartz-feldspar by fluorine-free flotation separation and their interaction mechanism: A review. Physicochem. Probl. Miner. Process. 2021, 57, 139–156. [Google Scholar] [CrossRef]
- Jiang, X.S.; Chen, J.; Ban, B.Y.; Song, W.F.; Chen, C.; Yang, X.Y. Application of competitive adsorption of ethylenediamine and polyetheramine in direct float of quartz from quartz-feldspar mixed minerals under neutral pH conditions. Miner. Eng. 2022, 188, 107850. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, W.; Zhang, X.; Wang, R.; Zhu, G.; Cao, Y. Effects and Mechanism of a Novel Gemini Collector on Flotation Separation of Quartz from Albite. Multipurp. Util. Miner. Resour. 2025, 1–14. [Google Scholar]
- Zhang, W.D.; Ren, Q.L.; Tu, R.Y.; Liu, S.; Qiu, F.H.; Guo, Z.H.; Liu, P.; Xu, S.H.; Sun, W.; Tian, M.J. The application of a novel amine collector, 1-(dodecylamino)-2-propanol, in the reverse flotation separation of apatite and quartz. J. Mol. Liq. 2024, 399, 124377. [Google Scholar] [CrossRef]
- Aarab, I.; Amari, K.E.; He, D.; Fu, Y.H.; Boujounoui, K.; Etahiri, A. Eco-friendly apatite flotation: Unlocking the potential of spent coffee grounds as a bio-based collector. Miner. Eng. 2025, 233, 109652. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Direct flotation of low-grade Moroccan phosphate ores: A preliminary micro-flotation study to develop new beneficiation routes. Arab. J. Geosci. 2020, 13, 1252. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Maciel, R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Berezianko, I.A.; Kostjuk, S. Ionic liquids in cationic polymerization: A review. J. Mol. Liq. 2024, 397, 124037. [Google Scholar] [CrossRef]
- Yadav, S.; Baweja, K.; Kumar, C.; Sarkar, A.; Tomar, R. A Review: Applications of Ionic Liquids in Medicinal Chemistry. Chemistry Africa-A J. Tunis. Chem. Soc. 2024, 7, 2975–2988. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Liu, C.; Han, S.T.; Zhu, Y.G.; Xu, W.; Yang, S.Y. Novel Collector of a Dodecylpyridinium Chloride Ionic Liquid in the Reverse Flotation Separation of Muscovite from Apatite. Langmuir 2025, 41, 2834–2842. [Google Scholar] [CrossRef]
- Li, W.C.; Liu, W.G.; Liu, W.B.; Zhang, R.R.; Wang, S.C. Application of novel ionic liquids in flotation separation of quartz and magnesite and its mechanism. Powder Technol. 2024, 447, 120218. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Mei, G.J.; Yu, M.M.; Song, X.W. Effect and mechanism of quaternary ammonium salt ionic liquid as a collector on desulfurization and desilication from artificial mixed bauxite using flotation. Miner. Eng. 2022, 181, 107523. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, Y.L.; Guo, X.Y.; Liao, Z.H.; Huang, J.H. The role of phosphate in inhibiting the activation of quartz flotation induced by Mg2+. J. Mol. Liq. 2024, 398, 124278. [Google Scholar] [CrossRef]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental vibratnional study of imidazolium-based ionic liquids: Raman and infrared spectra of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium ethylsulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Warsi, F.; Usman, M.; Ali, M. Modulating aggregation behaviour and surface properties of cationic & anionic surfactant with surface active ionic liquid 1-decyl-3-methylimidazolium chloride C10mim Cl: Role of surfactant head group. J. Mol. Liq. 2022, 365, 120093. [Google Scholar]
- He, J.F.; Chen, H.; Zhang, M.M.; Chen, L.H.; Yao, Q.Y.; Dai, Y.P.; Zhu, L.T.; Liu, C.G. Combined inhibitors of Fe3+, Cu2+ or Al3+ and sodium silicate on the flotation of fluorite and quartz. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 643, 128702. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jiao, F.; Qin, W.Q.; Zhu, H.L.; Jia, W.H. Activation effect of lead ions on scheelite flotation: Adsorption mechanism, AFM imaging and adsorption model. Sep. Purif. Technol. 2019, 209, 955–963. [Google Scholar] [CrossRef]
- Miao, Y.Q.; He, J.Y.; Zhu, X.R.; Zhu, G.L.; Cao, S.H.; Fan, G.X.; Li, G.S.; Cao, Y.J. Hardness of surface hydroxyls and its pivotal role in the flotation of cassiterite from quartz via lead ions activation. Sep. Purif. Technol. 2024, 347, 127565. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, W.; Chen, D.X.; Lin, S.Y.; Zhang, C.Y. Effects of Interfacial Hydroxylation Microstructure on Quartz Flotation by Sodium Oleate. Langmuir 2023, 39, 2182–2191. [Google Scholar] [CrossRef]
- Ouyang, L.Y.; Huang, Z.Q.; Wang, H.L.; He, G.C.; Yu, X.Y.; Burov, V.E.; Poilov, V.Z.; Li, F.X.; Liu, R.K.; Li, W.Y.; et al. Adsorption study of 3-tetradecylamine propyl amidoxime onto rhodochrosite surface: Implications for rhodochrosite-calcite flotation separation. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 678, 132469. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.A. Recent progress of quantifying substituent effects. Sci. Sin. Chim. 2013, 43, 801–828. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Xu, X.; Liu, W. Effect of methyl substituents on flotation performance of cationic collectors. Chin. J. Eng. 2023, 45, 1247–1253. [Google Scholar]
- Cheng, T.Y.; Xing, D.Q.; Shen, Z.C.; Ma, S.; Shi, S.X.; Deng, J.Y.; Deng, J.S. Study on expanding flotation performance differentiation of quartz and magnesite by amino-trimethylphosphonic acid in dodecylamine system. J. Mol. Liq. 2024, 409, 125286. [Google Scholar] [CrossRef]
- Ma, J.; Wu, J.; Chen, Y.; Zhong, H.; Chen, X.P.; Huang, Z.Q.; Burdonov, A.E.; Vchislo, N.V.; Bavuu, C.; Ouyang, L.Y. Adsorption Mechanism of 3-Tetradecylamine Propyl Amidoxime in the Reverse Flotation Separation of Quartz from Magnetite. Langmuir 2025, 41, 21021–21031. [Google Scholar] [CrossRef]



| Sample | SiO2 | Al2O3 | Na2O | K2O | Fe2O3 |
|---|---|---|---|---|---|
| Quartz | 98.52 | 0.46 | 0.29 | 0.07 | 0.07 |
| feldspar | 64.70 | 17.97 | 2.55 | 12.16 | 0.08 |
| Mass Ratio | Product | Yield (%) | Grade (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| SiO2 | K2O | SiO2 | K2O | |||
| Quartz–Feldspar = 1:9 | Concentrate | 13.32 | 70.18 | 10.23 | 14.15 | 11.26 |
| Tailings | 86.68 | 65.39 | 12.38 | 85.85 | 88.74 | |
| Feed | 100.00 | 66.03 | 12.09 | 100.00 | 100.00 | |
| Quartz–Feldspar = 3:7 | Concentrate | 21.39 | 79.15 | 6.90 | 23.52 | 15.21 |
| Tailings | 78.61 | 70.03 | 10.47 | 76.48 | 84.79 | |
| Feed | 100.00 | 71.98 | 9.71 | 100.00 | 100.00 | |
| Quartz–Feldspar = 5:5 | Concentrate | 31.89 | 85.61 | 4.63 | 34.52 | 20.95 |
| Tailings | 68.11 | 76.04 | 8.18 | 65.48 | 79.05 | |
| Feed | 100.00 | 79.09 | 7.05 | 100.00 | 100.00 | |
| Quartz–Feldspar = 7:3 | Concentrate | 42.86 | 91.23 | 2.70 | 45.93 | 26.01 |
| Tailings | 57.14 | 80.53 | 5.76 | 54.07 | 73.99 | |
| Feed | 100.00 | 85.11 | 4.45 | 100.00 | 100.00 | |
| Quartz–Feldspar = 9:1 | Concentrate | 58.00 | 96.09 | 1.01 | 59.17 | 34.40 |
| Tailings | 42.00 | 91.56 | 2.66 | 40.83 | 65.60 | |
| Feed | 100.00 | 94.19 | 1.70 | 100.00 | 100.00 | |
| Collector | Eigenvalue/a.u. | van der Waals Volume of Polar Group (Å3) | |
|---|---|---|---|
| HOMO | LUMO | ||
| DMB | −0.346 | −0.181 | 36.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Chen, Y.; Gu, G.; Yao, X.; Hu, H. Influence and Mechanism of 1-Dodecyl-3-methylimidazolium Bromide on the Flotation Behavior of Quartz and Feldspar in a Neutral System. Minerals 2025, 15, 1235. https://doi.org/10.3390/min15121235
Chen S, Chen Y, Gu G, Yao X, Hu H. Influence and Mechanism of 1-Dodecyl-3-methylimidazolium Bromide on the Flotation Behavior of Quartz and Feldspar in a Neutral System. Minerals. 2025; 15(12):1235. https://doi.org/10.3390/min15121235
Chicago/Turabian StyleChen, Siyu, Yuan Chen, Guohua Gu, Xiang Yao, and Huanxiao Hu. 2025. "Influence and Mechanism of 1-Dodecyl-3-methylimidazolium Bromide on the Flotation Behavior of Quartz and Feldspar in a Neutral System" Minerals 15, no. 12: 1235. https://doi.org/10.3390/min15121235
APA StyleChen, S., Chen, Y., Gu, G., Yao, X., & Hu, H. (2025). Influence and Mechanism of 1-Dodecyl-3-methylimidazolium Bromide on the Flotation Behavior of Quartz and Feldspar in a Neutral System. Minerals, 15(12), 1235. https://doi.org/10.3390/min15121235





