Research on the Combined Inhibition of Sodium Sulfide and Sodium Thioglycollate for the Flotation Separation of Chalcopyrite and Molybdenite
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Pure Mineral Flotation Test
2.3. Contact Angle Test
2.4. Sem-Eds Test
2.5. Infrared Spectroscopy Test
2.6. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Floatability of Chalcopyrite and Molybdenite
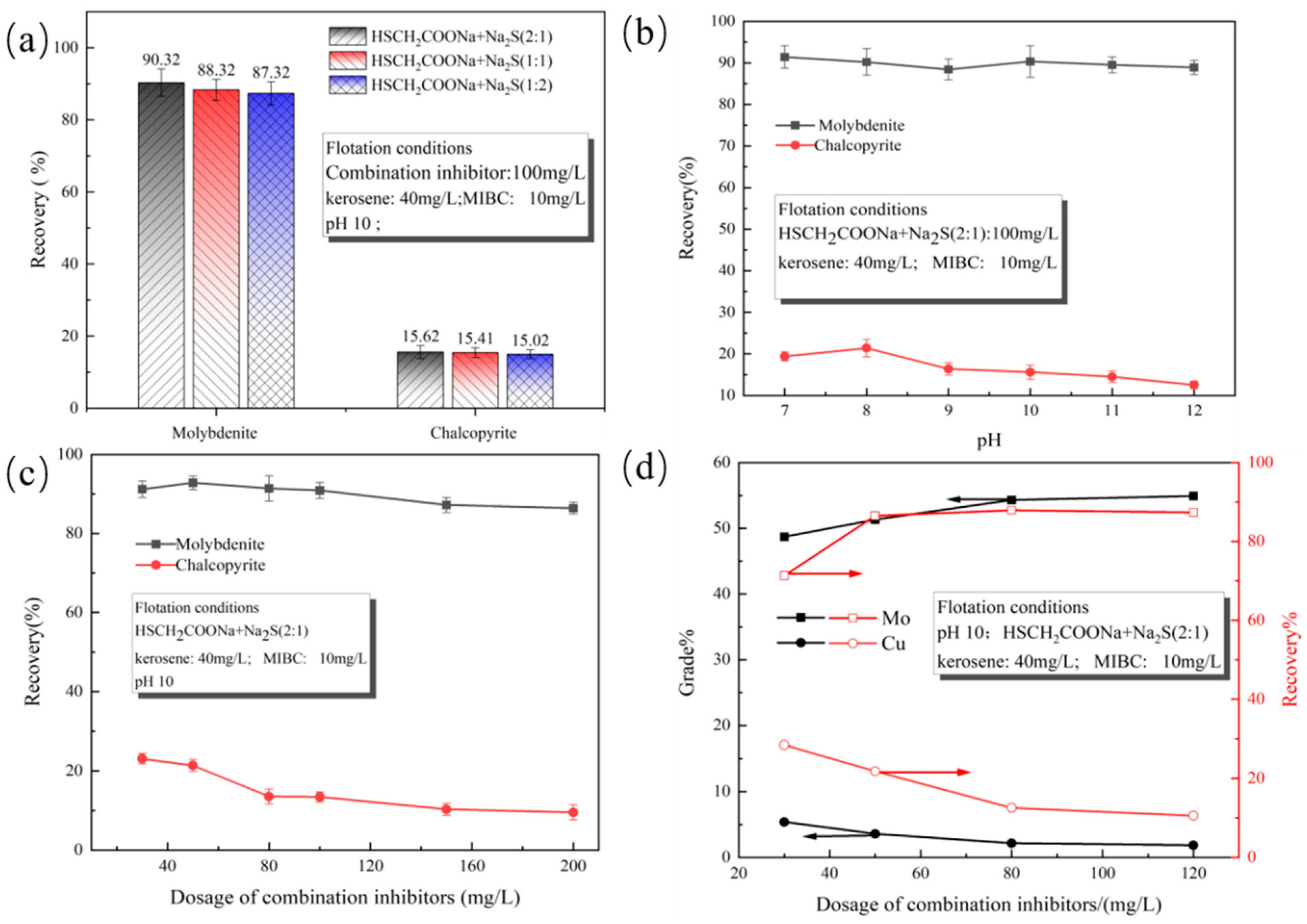
3.2. Effects of Combined Inhibitors on the Floatability of Chalcopyrite and Molybdenite
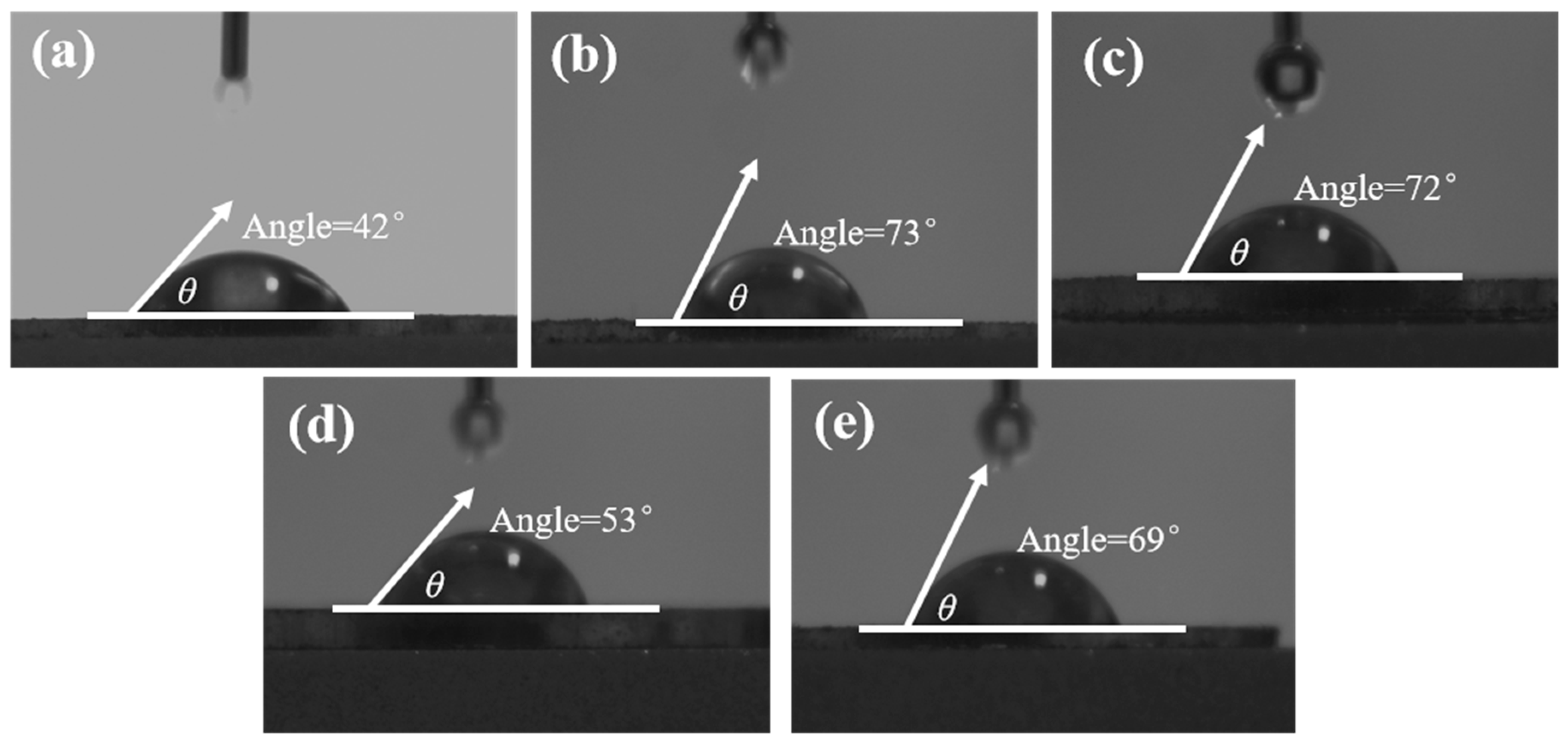
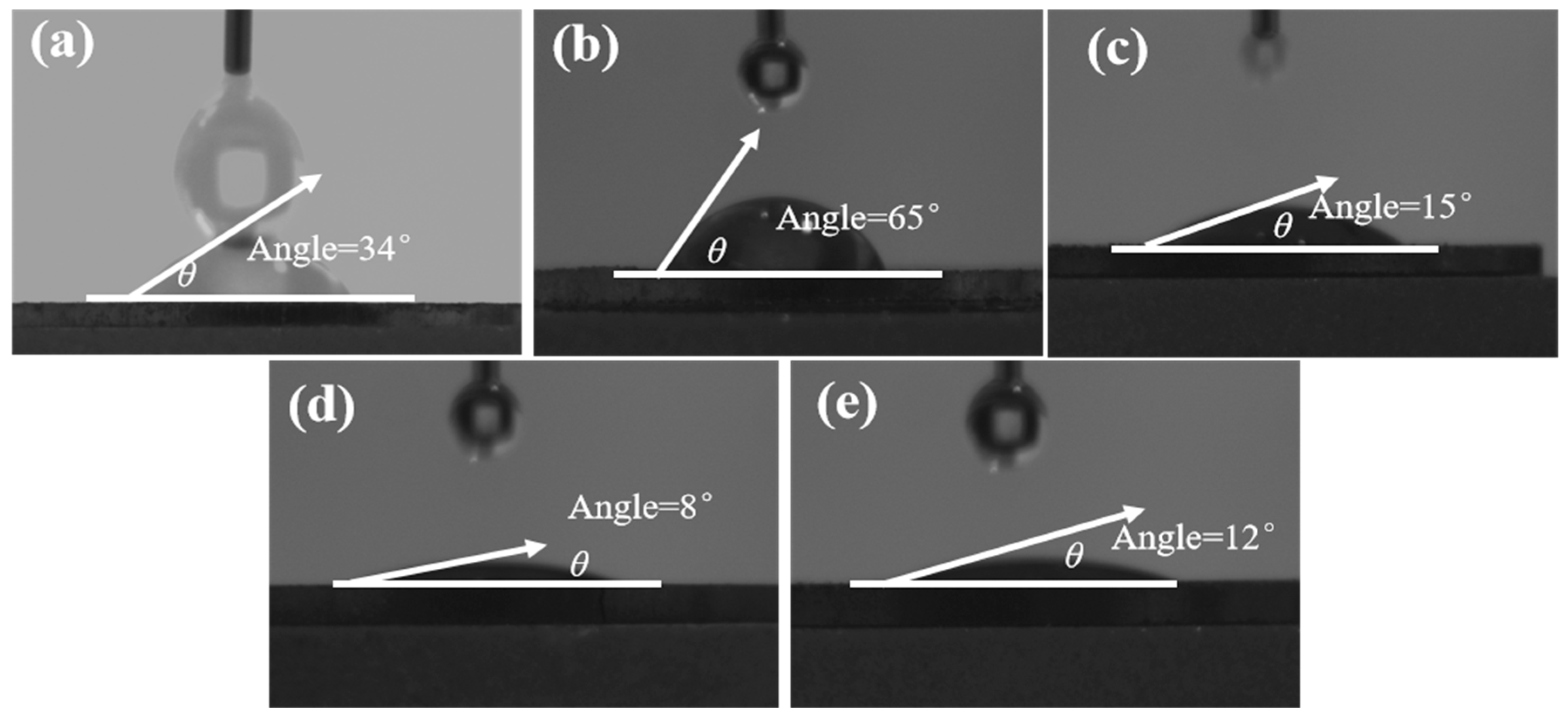
3.3. Contact Angle Measurement
3.4. Infrared Spectroscopy and Sem-Eds Analysis
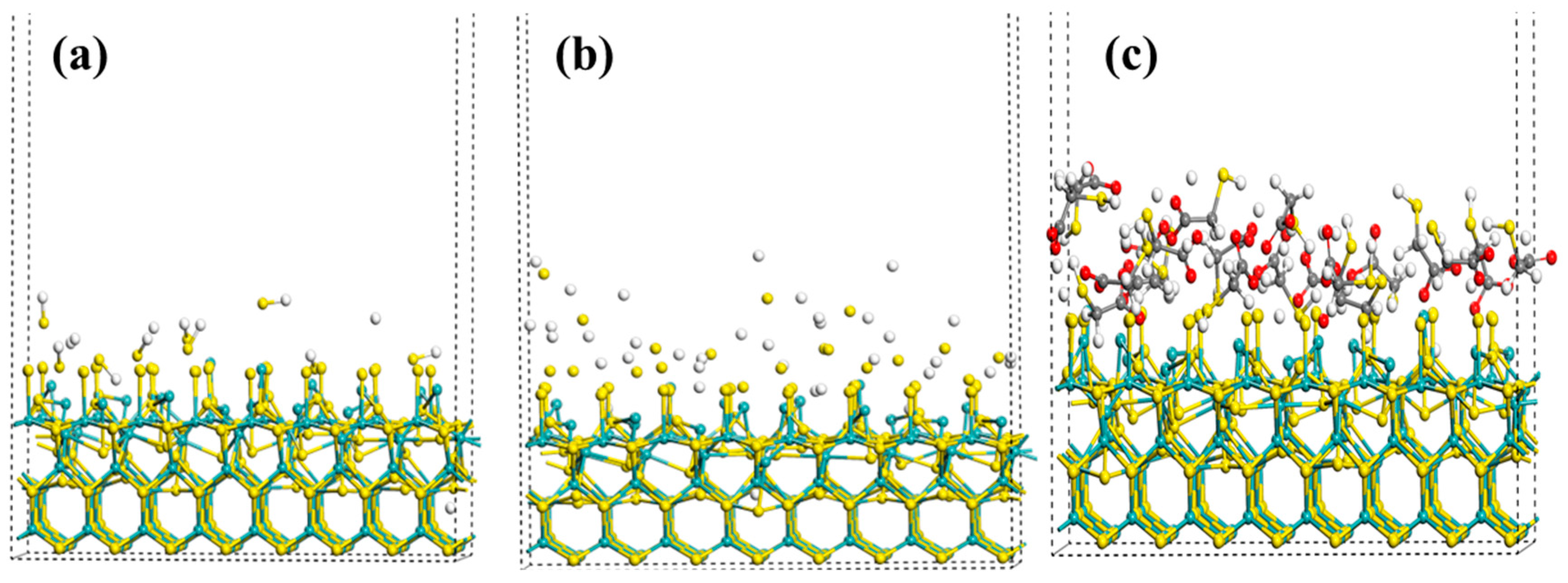
3.5. Molecular Dynamics Simulation Experiment
4. Conclusions
- (1)
- Through single-mineral flotation tests, it was concluded that the floatability of molybdenite in the kerosene system is superior to that of chalcopyrite. When the dosage of the inhibitor increases, Na2S will have a certain inhibitory effect on molybdenite, while HSCH2COONa has a relatively small influence. The two inhibitors have significant inhibitory effects on chalcopyrite, and the inhibitory ability of Na2S is stronger than that of HSCH2COONa.
- (2)
- When the dosage of the combined inhibitor of HSCH2COONa and Na2S (molar ratio 2:1) is 80 mg, its effect is better than that of using it alone. It can not only reduce the inhibition of molybdenite but also maintain a good inhibition effect on chalcopyrite. In the artificial mixed ore test, good indicators can be obtained in the molybdenum concentrate with a Mo grade of 54.34%, a recovery rate of 88.12%, and a Cu grade of 2.15%.
- (3)
- The contact angle test shows that the combined inhibitor can significantly reduce the wettability of the chalcopyrite surface while having a relatively small effect on molybdenite. Infrared spectroscopy and SEM-EDS energy spectrum indicated that the combined inhibitor C = O and S-H groups underwent chemical reactions on the surface of chalcopyrite and squeezed out kerosene on the surface of chalcopyrite. Molecular dynamics simulations indicate that the HS−, S2−, and HSCH2COO− components in the combined inhibitor are more likely to act on the surface of chalcopyrite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Yang, B.; Zhu, H.; Huang, P. Selective flotation of Cu-Mo sulfides using dithiothreitol as an environmental-friendly depressant. Miner. Eng. 2021, 168, 106929. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, X. Geology and molybdenite Re-Os age of the Dahutang granite-related veinlets-disseminated tungsten ore field in the Jiangxin Province. China. Ore Geol. Rev. 2013, 53, 422–433. [Google Scholar]
- Zhang, X.; Han, Y.; Li, Y.; Li, W.; He, J. Strengthening the flotation recovery of silver using a special ceramic-medium stirred mill. Powder Technol. 2022, 406, 117585. [Google Scholar] [CrossRef]
- Qing, W.; Wu, J.; Jiao, F. Mechanism study on flotation separation of molybdenite from chalcocite using thioglycollic acid as depressant. Int. J. Min. Sci. Technol. 2017, 27, 1043–1049. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Q.; Wen, S.; Xian, Y.; Liu, J.; Liang, G. Flotation separation of chalcopyrite from molybdenite with sodium thioglycolate: Mechanistic insights from experiments and MD simulations. Sep. Purif. Technol. 2024, 342, 126958. [Google Scholar] [CrossRef]
- Ai, G.; Xiao, G.; Feng, B. Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus. Minerals 2025, 15, 762. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Wu, J.; Yang, B.; Song, S. The efficient recovery of molybdenite fines using a novel collector: Flotation performances, adsorption mechanism and DFT calculation. Miner. Eng. 2022, 188, 107848. [Google Scholar] [CrossRef]
- Yang, B.; Zeng, M.; Yan, H. Tiopronin as a novel copper depressant for the selective flotation separation of chalcopyrite and molybdenite. Sep. Purif. Technol. 2021, 266, 118576. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Lu, Q.; Wang, X.; Zeng, H. Electrochemical investigation of the interactions of organic and inorganic depressants on basal and edge planes of molybdenite. J. Colloid Interface Sci. 2020, 570, 350–361. [Google Scholar] [CrossRef]
- Miki, H.; Tsuyoshi, H.; Yukihiro, M.; Suyantara, G. Effect of Sodium Sulfite on Floatability of Chalcopyrite and Molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Chen, G. Consumption mechanism of sodium sulfide in flotation separation of copper and molybdenum. J. Northeast. Univ. (Nat. Sci.) 2018, 39, 362–366. [Google Scholar]
- Yang, B.; Yan, H.; Zeng, M. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151, 106309. [Google Scholar] [CrossRef]
- Castro, S.; Lopez, A.; Laskowski, C. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Guo, B.; Peng, Y. Cyanide chemistry and its effect on mineral flotation. Miner. Eng. 2014, 66–68, 25–32. [Google Scholar] [CrossRef]
- Braga, P.; Chaves, A.; Luz, A. The use of dextrin in purification by flotation of molybdenite concentrates. Int. J. Miner. Process. 2014, 127, 23–27. [Google Scholar] [CrossRef]
- Fu, J.; Zhong, H.; Ou, L. Application of thioglycolic acid in molybdenite-copper sulphide separation. Min. Metall. Eng. 2002, 4, 36–38. [Google Scholar]
- Wang, X.; Zhao, B.; Liu, J. Dithiouracil, a highly efficient depressant for the selective separation of molybdenite from chalcopyrite by flotation: Applications and mechanism. Miner. Eng. 2022, 175, 107287. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Wu, M. Flotation separation of molybdenite from chalcopyrite using rhodanine-3-acetic acid as a novel and effective depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar] [CrossRef]
- Timbillah, S.; Fosu, B.; Lan, P.; Sosa-Blanco, C.; Hill, L. Towards an Understanding of the Use of Disodium Carboxymethyl Trithiocarbonate (DCMT) as an Alternative Depressant in Cu/Mo Sulfide Flotation. Min. Metall. Explor. 2021, 38, 1463–1476. [Google Scholar] [CrossRef]
- Timbillah, S.; Fosu, B.; Lan, P.; Sosa-Blanco, C.; Hill, L. Theoretical and experimental investigation of disodium carboxymethyl trithiocarbonate in Cu-Mo flotation. Miner. Eng. 2021, 169, 106943. [Google Scholar] [CrossRef]
- Suyantara, G.; Semoto, Y.; Miki, H.; Hirajima, T.; Sasaki, K.; Ochi, D. Effect of sodium metabisulfite and slaked lime on the floatability and surface properties of chalcopyrite. Powder Technol. 2022, 408, 117750. [Google Scholar] [CrossRef]
- Feng, B.; Guo, Y.; Zhang, W.; Peng, J.; Wang, H.; Huang, Z.; Zhou, X. Flotation separation behavior of chalcopyrite and sphalerite in the presence of locust bean gum. Miner. Eng. 2019, 143, 105940. [Google Scholar] [CrossRef]





| Minerals | Chemical Formula | Theoretical Content /% | Cu /% | TFe /% | S /% | Mo /% | Purity /% |
|---|---|---|---|---|---|---|---|
| molybdenite | MoS2 | 59.94 (Mo cal.) | —— | —— | 42.06 | 58.28 | 97.23 |
| chalcopyrite | CuFeS2 | 34.56 (Cu cal.) | 32.32 | 28.68 | 32.65 | —— | 93.52 |
| Crystal Surface | Components | Emineral/inhibitor | Emineral | E inhibitor | Eint (KJ/mol) |
|---|---|---|---|---|---|
| molybdenite (100) | HS− | −4523.47 | −3795.55 | 148.26 | −876.18 |
| S2− | −5376.51 | −4316.44 | −135.27 | −924.80 | |
| HSCH2COO− | −4894.29 | −3951.76 | −663.46 | −279.07 | |
| chalcopyrite (001) | HS− | −4709.63 | −3976.61 | 348.23 | −1081.25 |
| S2− | −5047.69 | −3956.42 | −35.17 | −1056.10 | |
| HSCH2COO− | −5561.57 | −4023.03 | −1196.21 | −342.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Chen, J.; He, J.; Wu, J.; Wang, D.; Xie, M.; Li, M.; Dou, K. Research on the Combined Inhibition of Sodium Sulfide and Sodium Thioglycollate for the Flotation Separation of Chalcopyrite and Molybdenite. Minerals 2025, 15, 1212. https://doi.org/10.3390/min15111212
Sun Q, Chen J, He J, Wu J, Wang D, Xie M, Li M, Dou K. Research on the Combined Inhibition of Sodium Sulfide and Sodium Thioglycollate for the Flotation Separation of Chalcopyrite and Molybdenite. Minerals. 2025; 15(11):1212. https://doi.org/10.3390/min15111212
Chicago/Turabian StyleSun, Qianyu, Jiajun Chen, Junchao He, Jiayang Wu, Dongdong Wang, Mingliang Xie, Miaomiao Li, and Kuizhou Dou. 2025. "Research on the Combined Inhibition of Sodium Sulfide and Sodium Thioglycollate for the Flotation Separation of Chalcopyrite and Molybdenite" Minerals 15, no. 11: 1212. https://doi.org/10.3390/min15111212
APA StyleSun, Q., Chen, J., He, J., Wu, J., Wang, D., Xie, M., Li, M., & Dou, K. (2025). Research on the Combined Inhibition of Sodium Sulfide and Sodium Thioglycollate for the Flotation Separation of Chalcopyrite and Molybdenite. Minerals, 15(11), 1212. https://doi.org/10.3390/min15111212




