Research on the Purification Technology of Quartz from a Mining Area in Jiangxi by Acid Leaching
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Raw Materials
2.2. Acid-Leaching Experiments
2.3. Materials Characterization
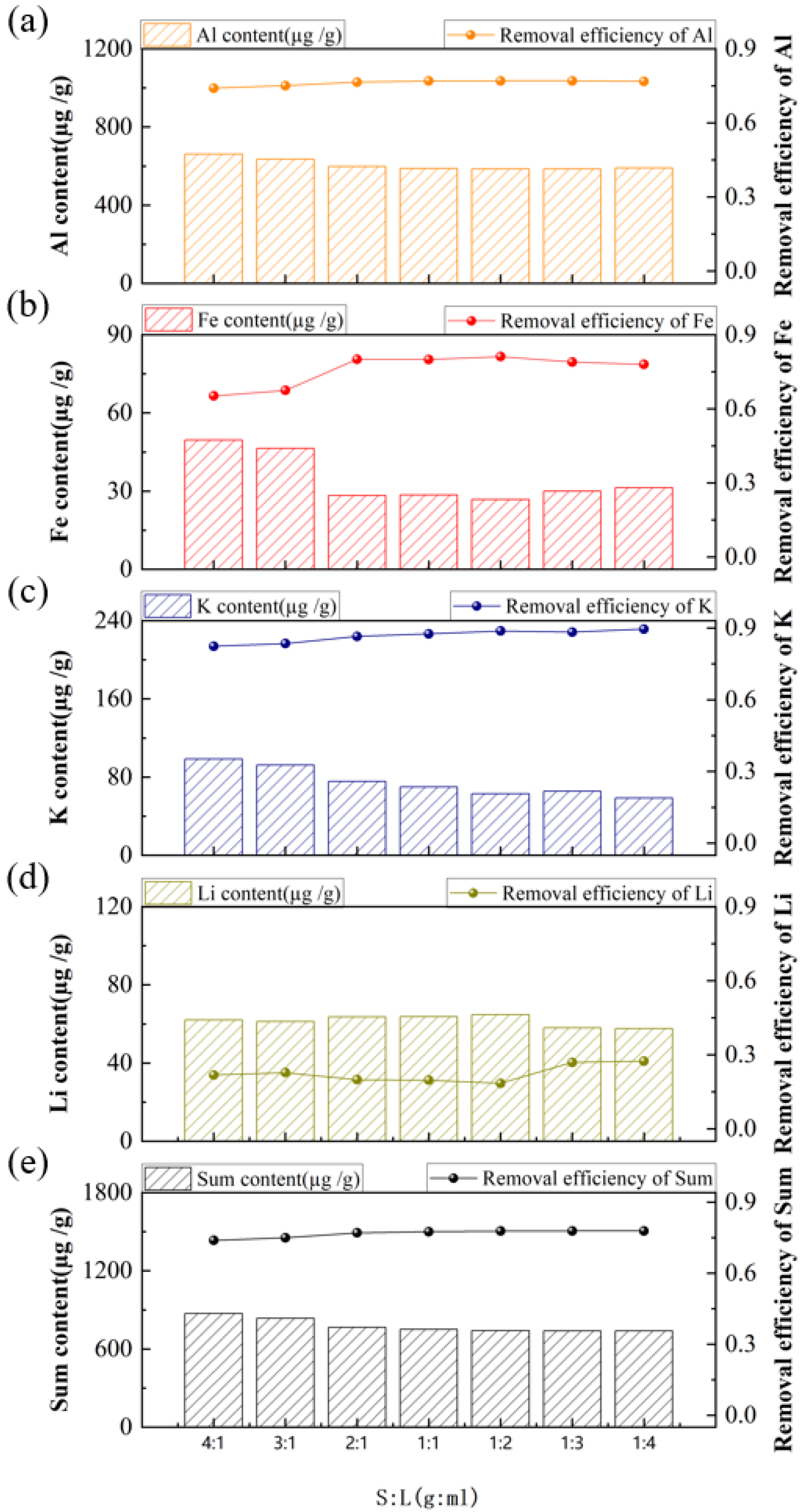
3. Results and Discussion
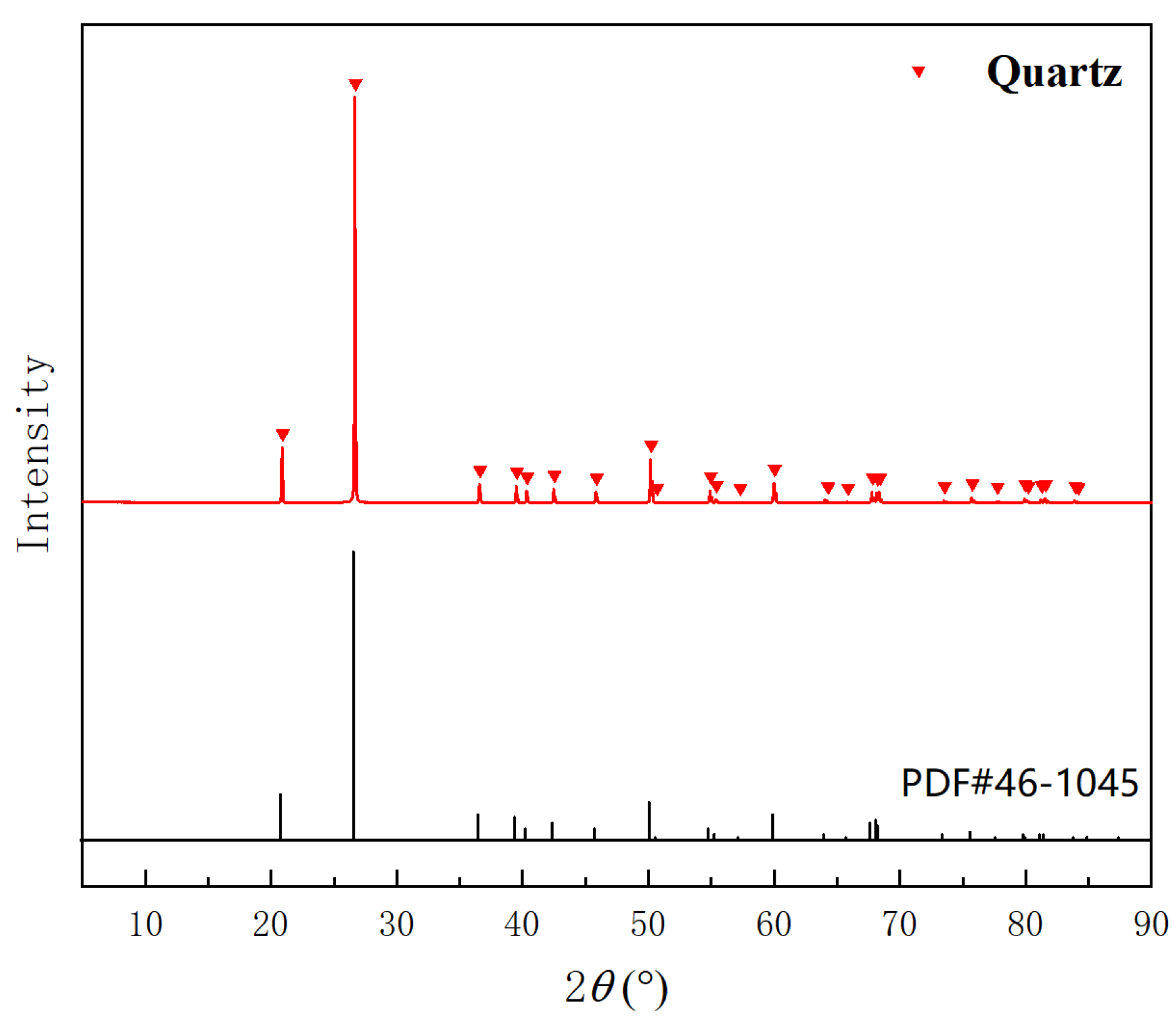
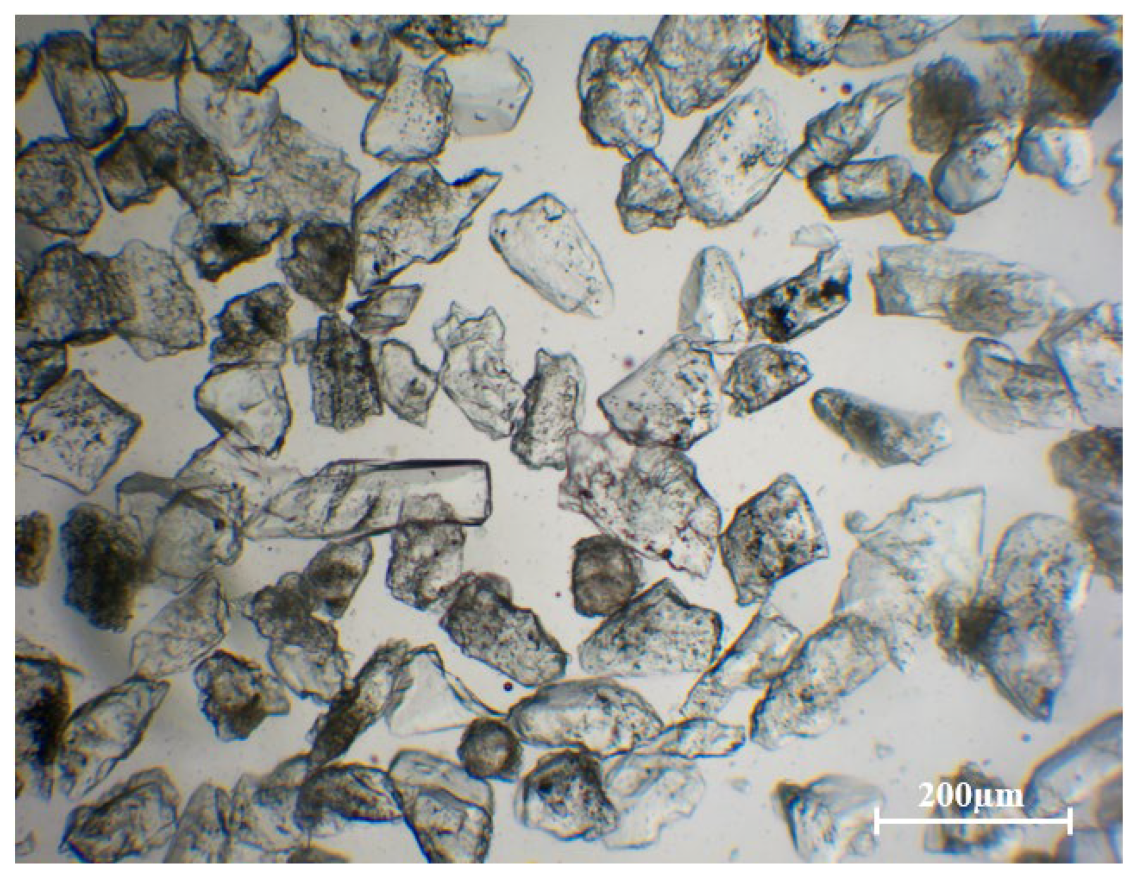
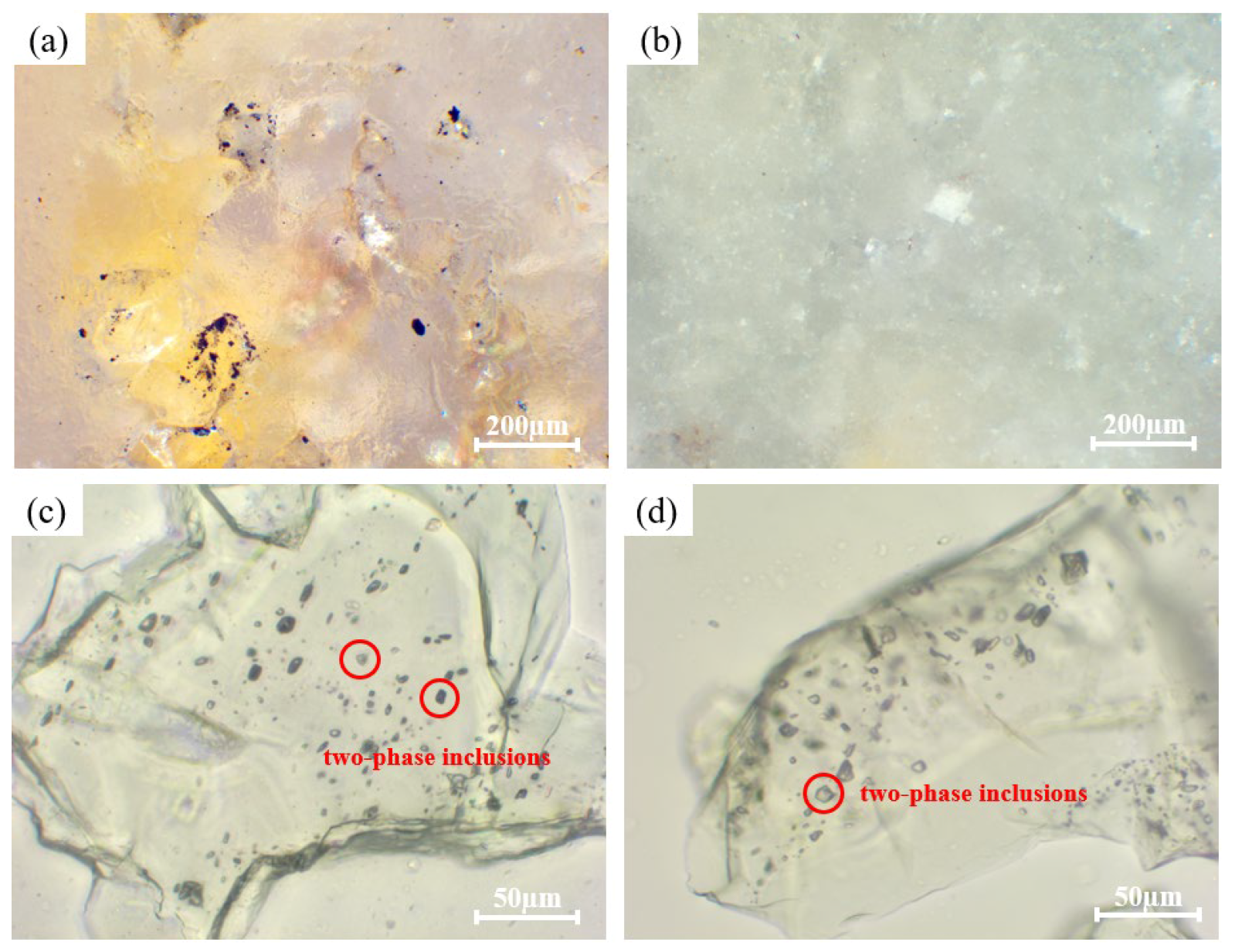
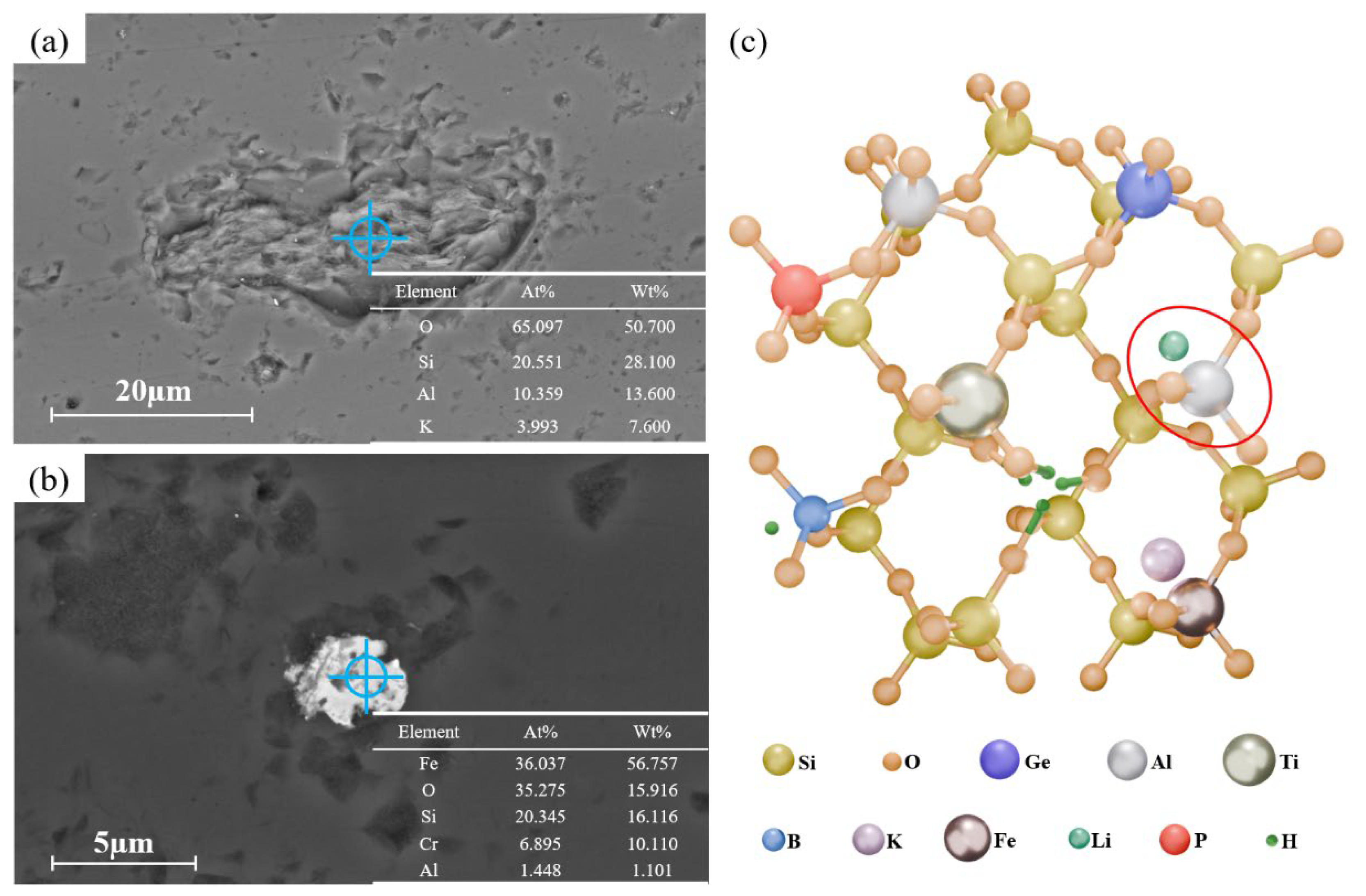
3.1. Impurities in Quartz Sand
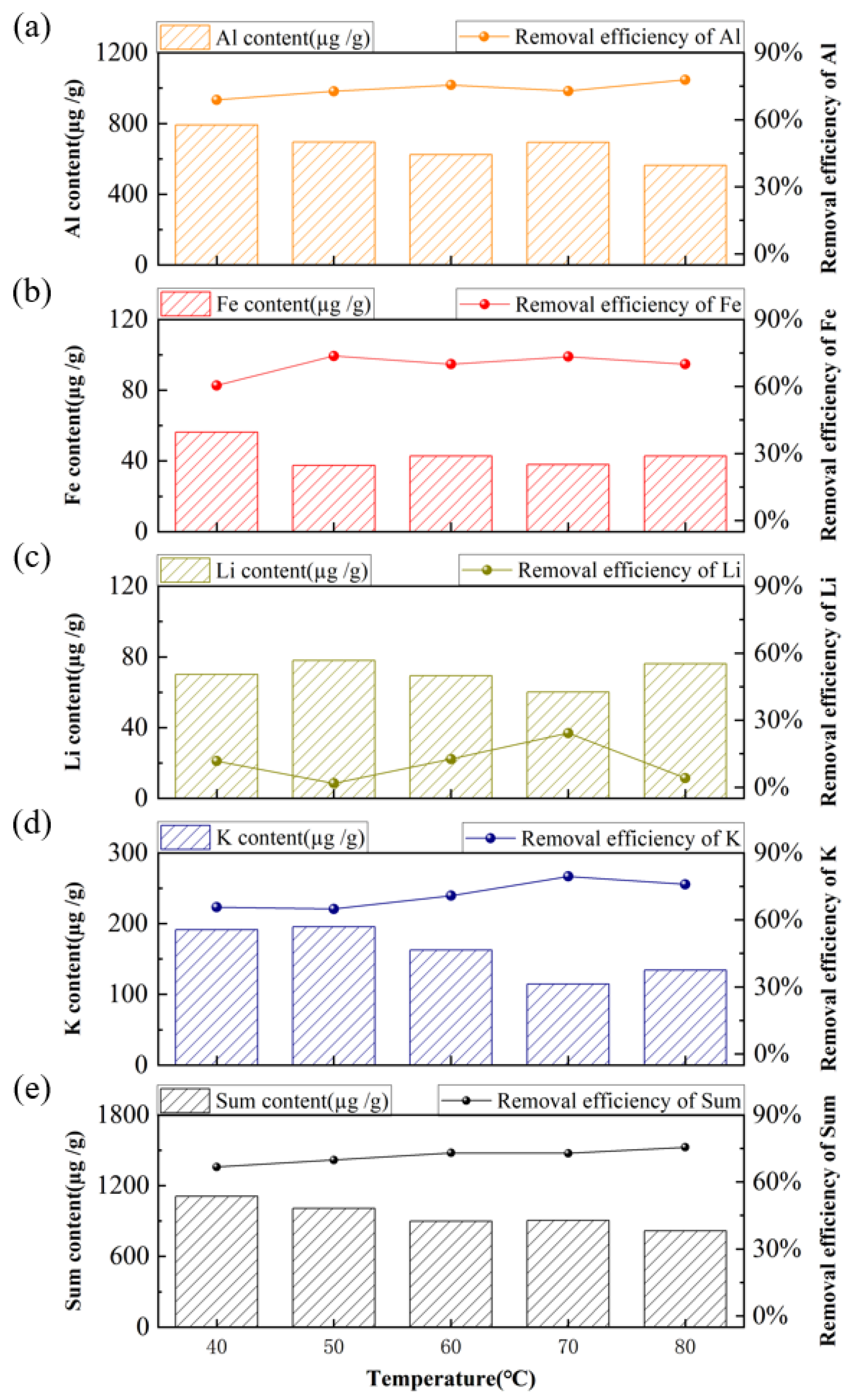
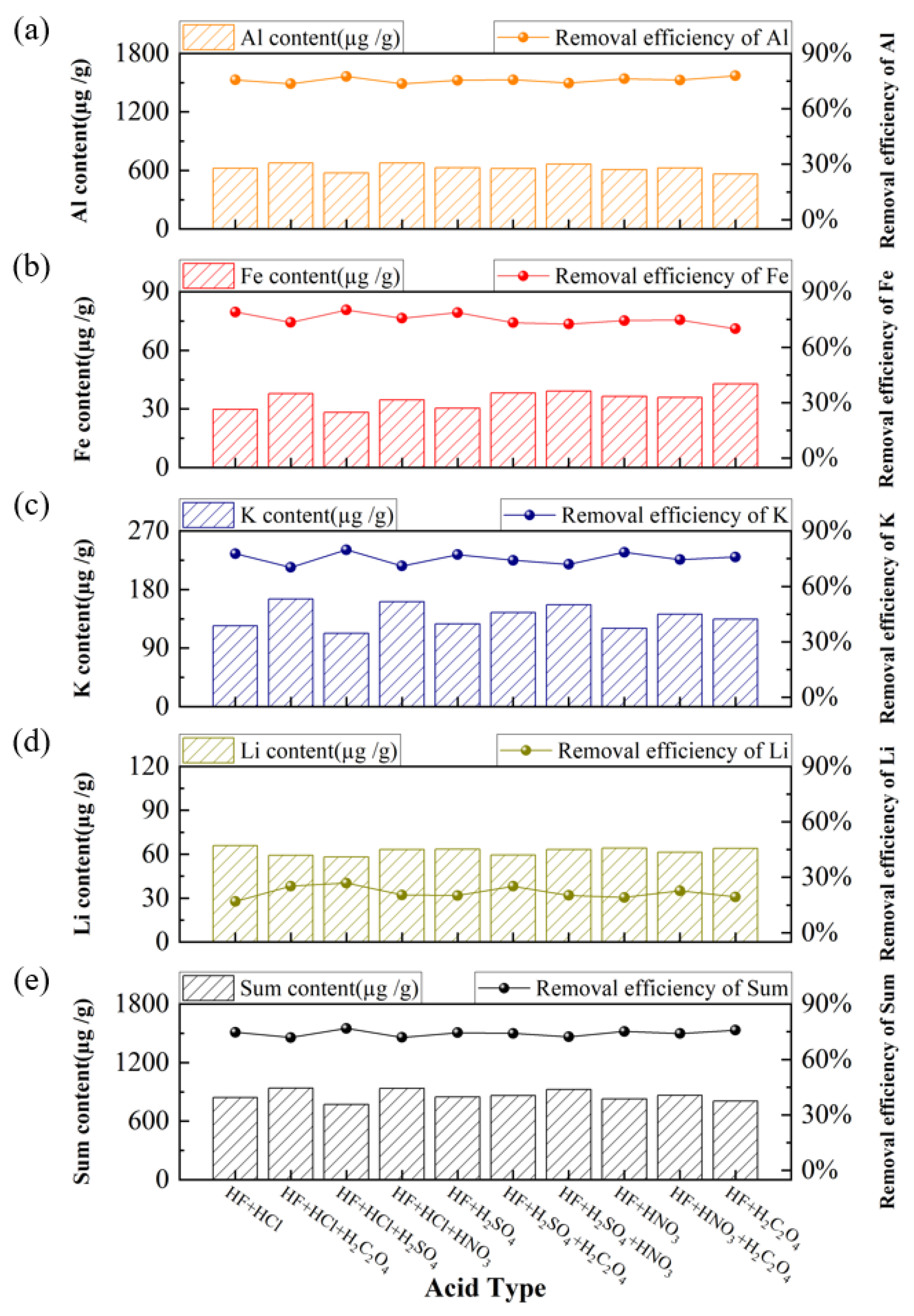
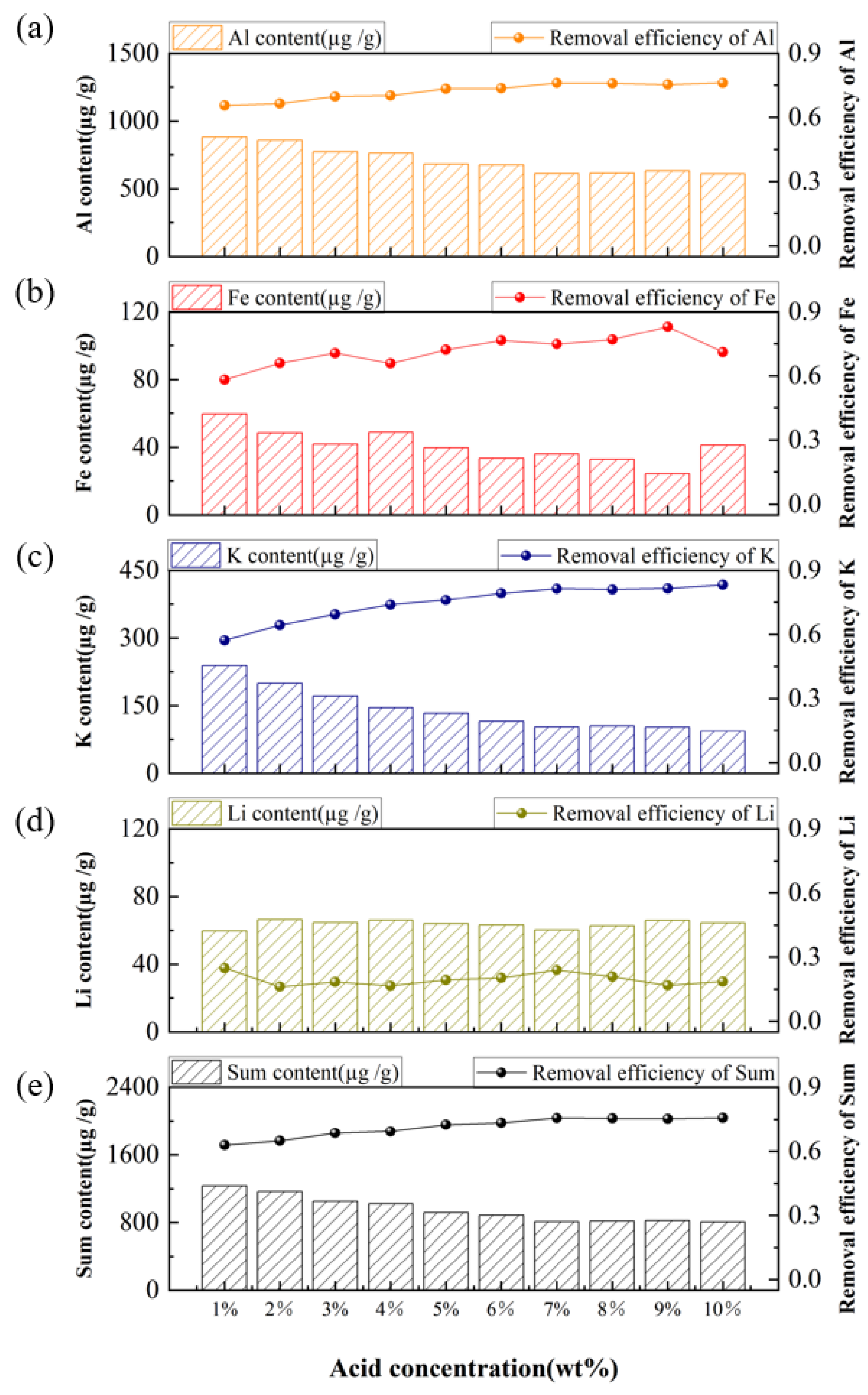
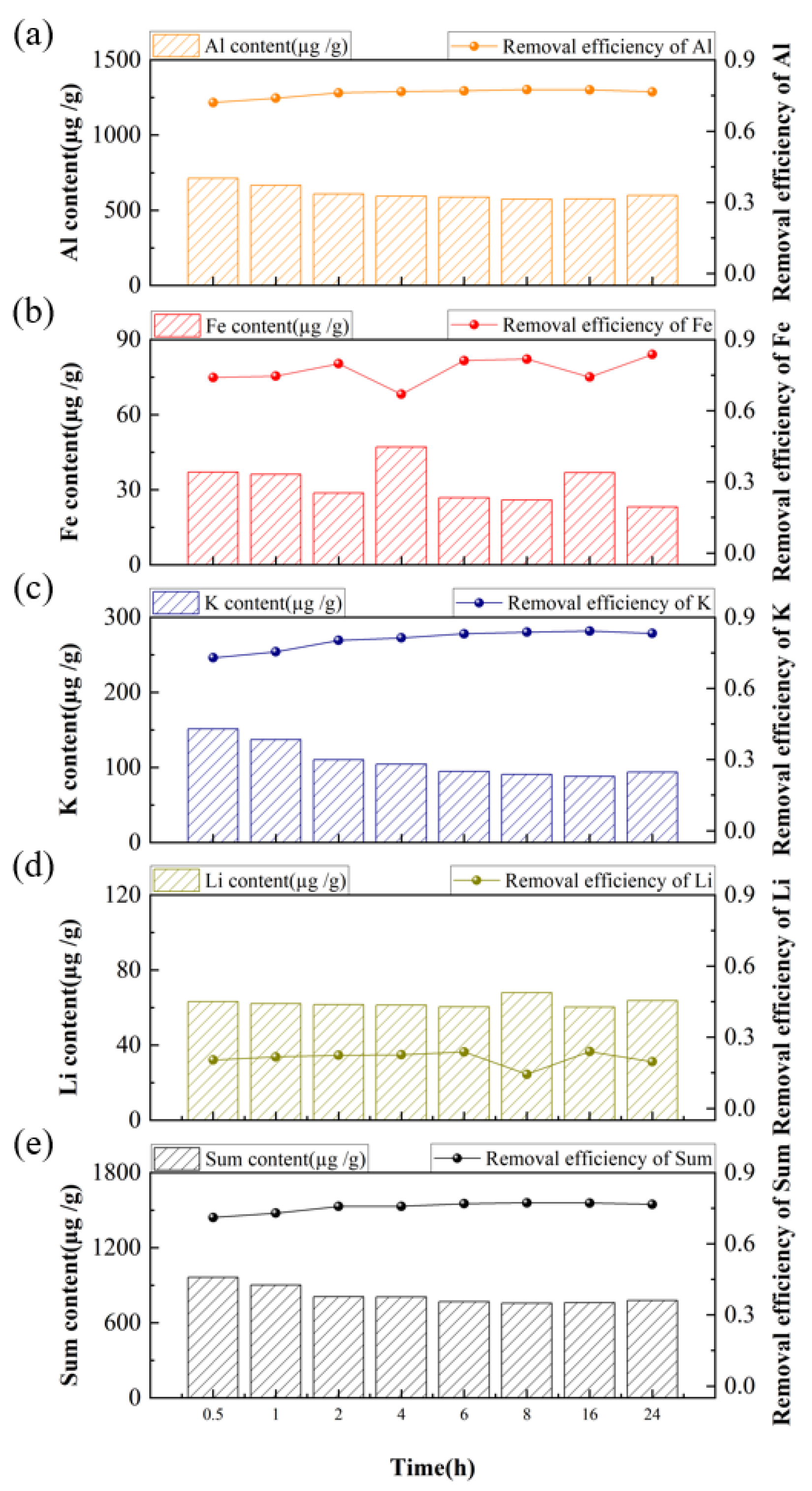
3.2. Purification of Quartz Sand by Acid-Leaching Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heynke, U.; Leeder, O.; Schulz, H. On distinguishing quartz of hydrothermal or metamorphogenic origin in different monomineralic veins in the eastern part of Germany. Mineral. Petrol. 1992, 46, 315–329. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Ai, G.; Guo, S.; Zhao, J.; Deng, X.; Wei, K.; Ma, W. Hot-pressure acid leaching changes grain boundaries to deeply remove impurities in quartz sand. J. Mater. Res. Technol. 2024, 30, 3705–3713. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, X.; Huang, H.; Zhou, L.; Xiong, T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process. 2012, 114, 30–34. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Wu, J.; Wu, D.; Wei, K.; Ma, W. Effect of quartz crystal structure transformations on the removal of iron impurities. Hydrometallurgy 2021, 204, 105715. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Pagliari, L.; Pavese, A.; Sciascia, L.; Merli, M.; Francescon, F. The formation of silica high temperature polymorphs from quartz: Influence of grain size and mineralising agents. J. Eur. Ceram. Soc. 2015, 35, 4547–4555. [Google Scholar] [CrossRef]
- Qu, J.; Chen, Z.; Wu, D.; Ma, W. Study on the Purification Mechanism of Low-Grade Silicon Ore through a Combination of Direct Roasting and Pressure Leaching. Silicon 2024, 16, 5257–5271. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.; Lei, S. Mineralogy and processing of hydrothermal vein quartz from Hengche, Hubei Province (China). Minerals 2017, 7, 161. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Z.; Liu, Y.; Gao, H.; Wu, F.; Song, Y. The effects of calcination-water quenching on quartz purification and its mechanism. Min. Metall. Explor. 2023, 40, 2519–2527. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Xia, Z.; Li, W.; Lei, S. Influences of Na2CO3 roasting and H3PO4 hot-pressure leaching on the purification of vein quartz to achieve high-purity quartz. Hydrometallurgy 2023, 218, 106065. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P.; Zhao, Z. Recovery and preparation of high-grade silica from iron ore tailings by S-HGMS coupling with acid leaching technology: Description of separation mechanism and leaching kinetics. Powder Technol. 2023, 424, 118523. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the mechanism of the dissolution of quartz and silica in aqueous solutions. ACS Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.D.; Li, X.L.; Song, Y.S.; Zhou, G.Y. Experimental Research on preparation technics of high-purity quartz material. Key Eng. Mater. 2017, 748, 17–21. [Google Scholar] [CrossRef]
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Mineral. Mag. 2009, 73, 569–583. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P.; Badanina, E. Water-and boron-rich melt inclusions in quartz from the Malkhan pegmatite, Transbaikalia, Russia. Minerals 2012, 2, 435–458. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Ma, C.; Wei, F.; Zhang, H. A new insight into the influence of fluid inclusions in high-purity quartz sand on the bubble defects in quartz glass: A case study from vein quartz in the dabie mountain. Minerals 2024, 14, 794. [Google Scholar] [CrossRef]
- Mifsud, C.; Fujioka, T.; Fink, D. Extraction and purification of quartz in rock using hot phosphoric acid for in situ cosmogenic exposure dating. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 294, 203–207. [Google Scholar] [CrossRef]
- Ambikadevi, V.R.; Lalithambika, M. Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 2000, 16, 133–145. [Google Scholar] [CrossRef]
- Pan, X.; Li, S.; Li, Y.; Guo, P.; Zhao, X.; Cai, Y. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wang, H.; Zhu, C.; Ren, B. A review on removal of iron impurities from quartz mineral. Minerals 2023, 13, 1128. [Google Scholar] [CrossRef]
- Mowla, D.; Karimi, G.; Ostadnezhad, K. Removal of hematite from silica sand ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Martínez-Luévanos, A.; Rodríguez-Delgado, M.G.; Uribe-Salas, A.; Carrillo-Pedroza, F.R.; Osuna-Alarcón, J.G. Leaching kinetics of iron from low grade kaolin by oxalic acid solutions. Appl. Clay Sci. 2011, 51, 473–477. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Stegger, P.; Lehmann, G. Dynamic effects in a new substitutional center of trivalent iron in quartz. Phys. Status Solidi (B) 1989, 151, 463–467. [Google Scholar] [CrossRef]
- Miyoshi, N.; Yamaguchi, Y.; Makino, K. Successive zoning of Al and H in hydrothermal vein quartz. Am. Mineral. 2005, 90, 310–315. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Li, X.; Chang, Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid. Ultrason. Sonochem. 2011, 18, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Kline, W.E.; Fogler, H.S. Dissolution of silicate minerals by hydrofluoric acid. Ind. Eng. Chem. Fundam. 1981, 20, 155–161. [Google Scholar] [CrossRef]
- Knauss, K.G. Muscovite dissolution kinetics as a function of pH and time at 70 °C. Geochim. Cosmochim. Acta 1989, 53, 1493–1501. [Google Scholar] [CrossRef]
- Brantley, S.L. Kinetics of mineral dissolution. In Kinetics of Water-Rock Interaction; Springer: New York, NY, USA, 2008; pp. 151–210. [Google Scholar]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.-Z.; Wang, H.-D. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.Y.; Lei, S.M.; Liu, Y.Y.; Xia, Z.J.; Xie, F.X. Trace muscovite dissolution separation from vein quartz by elevated temperature and pressure acid leaching using sulphuric acid and ammonia chloride solutions. Physicochem. Probl. Miner. Process. 2018, 54, 448–458. [Google Scholar]
- Yang, C.; Li, S.; Bai, J.; Liu, Y.; Wang, G. Advanced Purification of Industrial Quartz Using Calcination Pretreatment Combined with Ultrasound-Assisted Leaching. Acta Geodyn. Geomater. 2018, 15, 187–195. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Shen, Q.; Zhang, Y.; Hu, Y. Further Purification of Industrial Quartz by Much Milder Conditions and a Harmless Method. Environ. Sci. Technol. 2010, 44, 7673–7677. [Google Scholar] [CrossRef] [PubMed]
| Element | Al | Fe | B | Ca | Cr | K | Li |
|---|---|---|---|---|---|---|---|
| Content | 2551.88 | 142.48 | 5.33 | 23.38 | 5.38 | 557.79 | 79.34 |
| Mg | Mn | Na | Ni | Ti | Total | Sum | SiO2 |
| 48.44 | 2.62 | 18.16 | 7.84 | 41.04 | 3483.68 | 3331.49 | 99.65% |
| Element | Al | Fe | B | Ca | Cr | K | Li |
|---|---|---|---|---|---|---|---|
| Content | 587.65 | 28.53 | 0.34 | 13.63 | 1.81 | 69.71 | 63.68 |
| Mg | Mn | Na | Ni | Ti | Total | Sum | SiO2 |
| 7.83 | 1.42 | 4.63 | 1.23 | 15.29 | 795.75 | 749.57 | 99.92% |
| Sample | Correlation Coefficient | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Acid-leaching temperature | Al, K | 0.941 | (0.347, 0.996) | 0.017 |
| Al, Fe | −0.515 | (−0.961, 0.673) | 0.374 | |
| Al, Li | 0.575 | (−0.623, 0.967) | 0.31 | |
| K, Fe | −0.557 | (−0.965, 0.640) | 0.33 | |
| K, Li | 0.583 | (−0.616, 0.968) | 0.302 | |
| Fe, Li | −0.912 | (−0.994, −0.154) | 0.031 | |
| Acid type | Al, K | 0.078 | (−0.580, 0.675) | 0.83 |
| Al, Fe | 0.81 | (0.369, 0.953) | 0.004 | |
| Al, Li | 0.031 | (−0.611, 0.648) | 0.932 | |
| K, Fe | 0.54 | (−0.136, 0.873) | 0.107 | |
| K, Li | 0.065 | (−0.588, 0.668) | 0.858 | |
| Fe, Li | −0.139 | (−0.707, 0.538) | 0.702 | |
| Acid concentration | Al, K | 0.974 | (0.892, 0.994) | 0 |
| Al, Fe | 0.828 | (0.415, 0.958) | 0.003 | |
| Al, Li | 0.026 | (−0.613, 0.645) | 0.943 | |
| K, Fe | 0.838 | (0.440, 0.961) | 0.002 | |
| K, Li | −0.151 | (−0.713, 0.529) | 0.677 | |
| Fe, Li | −0.218 | (−0.745, 0.477) | 0.546 | |
| Leaching time | Al, K | 0.982 | (0.903, 0.997) | 0 |
| Al, Fe | 0.284 | (−0.526, 0.824) | 0.495 | |
| Al, Li | −0.036 | (−0.722, 0.686) | 0.933 | |
| K, Fe | 0.363 | (−0.459, 0.850) | 0.376 | |
| K, Li | −0.055 | (−0.731, 0.676) | 0.897 | |
| Fe, Li | −0.409 | (−0.865, 0.415) | 0.314 | |
| Solid-to-liquid ratio | Al, K | 0.929 | (0.585, 0.990) | 0.002 |
| Al, Fe | 0.337 | (−0.558, 0.869) | 0.46 | |
| Al, Li | 0.034 | (−0.738, 0.767) | 0.942 | |
| K, Fe | 0.04 | (−0.735, 0.770) | 0.932 | |
| K, Li | 0.345 | (−0.552, 0.872) | 0.449 | |
| Fe, Li | −0.828 | (−0.974, −0.200) | 0.021 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, G.; Lin, X.; Gao, T.; Pang, Z.; Sun, C.; Gao, W.; Zhang, R.; Xiao, H.; Xu, Q.; et al. Research on the Purification Technology of Quartz from a Mining Area in Jiangxi by Acid Leaching. Minerals 2025, 15, 1200. https://doi.org/10.3390/min15111200
Wang C, Li G, Lin X, Gao T, Pang Z, Sun C, Gao W, Zhang R, Xiao H, Xu Q, et al. Research on the Purification Technology of Quartz from a Mining Area in Jiangxi by Acid Leaching. Minerals. 2025; 15(11):1200. https://doi.org/10.3390/min15111200
Chicago/Turabian StyleWang, Chali, Guangshi Li, Xing Lin, Tianle Gao, Zhongya Pang, Chenteng Sun, Weifan Gao, Ronghua Zhang, Helin Xiao, Qian Xu, and et al. 2025. "Research on the Purification Technology of Quartz from a Mining Area in Jiangxi by Acid Leaching" Minerals 15, no. 11: 1200. https://doi.org/10.3390/min15111200
APA StyleWang, C., Li, G., Lin, X., Gao, T., Pang, Z., Sun, C., Gao, W., Zhang, R., Xiao, H., Xu, Q., Zou, X., & Lu, X. (2025). Research on the Purification Technology of Quartz from a Mining Area in Jiangxi by Acid Leaching. Minerals, 15(11), 1200. https://doi.org/10.3390/min15111200









