Abstract
Ladle slag (LF slag) is a by-product of secondary steelmaking that presents unique valorization challenges compared to BOF or EAF slags due to its distinctive chemical composition (high Al2O3 and CaO content) and uncontrolled hydraulic activity. While other steelmaking slags can be reused as supplementary cementitious materials or aggregates, LF slag is predominantly landfilled, with over 2 million tons discarded annually in Europe alone. This study introduces a novel pyrometallurgical valorization strategy that, unlike conventional approaches focused solely on mineral recovery, simultaneously recovers both metallic and mineral value through aluminothermic reduction. This process utilizes end-of-waste aluminum scrap rather than virgin materials to reduce Fe and Si oxides, creating a circular economy solution that addresses two waste streams simultaneously. The process generates two valuable products with low liquidus temperatures: a ferrosilicon alloy (FeSi15-50 grade) and a residual oxide rich in calcium and magnesium aluminates suitable for cementitious or ceramic applications. Through the integration of FactSage thermodynamic simulations with experimental validation, it is possible to predict and control phase evolution during equilibrium cooling, an approach not previously applied to LF slag valorization. Experimental validation using industrial slags confirms the theoretical predictions and demonstrates the process operates in a near-energy-neutral, self-sustaining mode by recovering both chemical and sensible thermal energy (50–100 kWh per ton of slag). This represents approximately 90% lower energy consumption compared to conventional ferrosilicon production. The work provides a comprehensive and scalable approach to transform a problematic waste material into valuable products, supporting circular economy principles and low-carbon metallurgy objectives.
1. Introduction
Steel production forms an indispensable cornerstone of modern industrial infrastructure, sustaining global supply chains across construction, transportation, and energy sectors. Despite its critical importance for economic development, steelmaking processes are energy-intensive and environmentally impactful, accounting for approximately 8% of global energy consumption and 7% of anthropogenic CO2 emissions [1]. As industrialized nations pivot towards low-carbon manufacturing, the metallurgical sector faces mounting pressure to implement resource-efficient and circular technologies.
Today’s steel production is dominated by two principal routes: the integrated Blast Furnace–Basic Oxygen Furnace (BF-BOF) process and the Electric Arc Furnace (EAF) process. The former enables high productivity from iron ore but is characterized by intensive fossil fuel utilization and elevated carbon intensity [2]. In contrast, EAF technology, based on scrap recycling, offers a lower-emission alternative and has achieved significant market penetration in several countries, notably Italy, where it constitutes the primary steelmaking route.
Slags are generated at different stages of steelmaking and exhibit significant compositional variations depending on their origin. BF slags are entirely recycled in cement manufacturing, while BOF and EAF slags have established reuse pathways as supplementary cementitious materials (SCM), in road construction, and as high-quality abrasion-resistant aggregates for concrete [3,4]. However, ladle furnace (LF) slags remain among the most underutilized by-products of the steel industry, with limited valorization options [5].
Unlike other steelmaking slags, LF slags present unique challenges for valorization due to their distinctive characteristics. These slags are characterized by high alumina content (15%–30% Al2O3), elevated calcium oxide levels (40%–60% CaO), high melting temperatures (1450–1550 °C), significant hydraulic reactivity, and minimal residual metal content. Their mineralogical composition, dominated by calcium aluminate phases such as C12A7, C3A, and CA, renders them unsuitable for conventional slag applications like road construction or concrete aggregates [6]. Additionally, compositional variability between different production batches further complicates the development of standardized valorization solutions.
Consequently, LF slags are routinely landfilled, contributing to environmental burden and resource inefficiency [7]. In Europe alone, more than 2 million tons of LF slag are landfilled annually, with associated environmental and economic costs [8]. The sustainable valorization of this waste stream represents both an environmental necessity and an economic opportunity for the steel industry.
Concurrent with decarbonization efforts, the steel sector is undergoing a paradigm shift towards circular economy frameworks that prioritize waste minimization and materials recovery. Slag valorization has thus emerged as a focal point of research, particularly through high-temperature processes capable of simultaneously recovering valuable elements while transforming the oxide matrix into functional materials. Recent investigations have explored the utilization of LF slags in cementitious applications or as fluxing agents, yet the potential for integrated recovery of both metallic and non-metallic fractions remains insufficiently explored [9,10].
The present study addresses this research gap by proposing an aluminothermic reduction process for the strategic upgrading of ladle slags. The approach differs from previous valorization strategies in three key aspects: (1) it enables simultaneous recovery of both metallic and mineral value from the slag, (2) it utilizes end-of-waste aluminum as a reducing agent rather than virgin materials, and (3) it approaches energy neutrality through self-sustaining exothermic reactions. The process exploits the chemical reactivity of aluminum scrap to reduce iron and silicon oxides within the slag, forming a ferrosilicon alloy suitable for reintegration into steelmaking as a deoxidizer. Simultaneously, the residual slag, dominated by calcium and magnesium aluminates, becomes amenable to secondary reuse as a fluxing material in steel refining or as a substitute for high-alumina calcium aluminate cements.
Notably, this process approaches energy neutrality, as the sensible heat of the molten slag combined with the highly exothermic reaction enthalpy of the aluminothermic reduction provides sufficient thermal input. This enables internal energy recovery on the order of 50–100 kWh per ton of slag [11]. The low-Si grade FeSi alloys produced meet ISO 5445 [12] requirements for aluminum content, while higher-grade FeSi45/FeSi50 alloys retain excess aluminum (2.2%–2.5%) above permitted limits, restricting their use to internal steelmaking applications. In this scenario, ferrosilicon production avoids competing with traditional FeSi markets, where production is optimized at an industrial scale through carbothermic reduction in pure quartz and iron [13].
To assess the feasibility and robustness of this approach, a combination of equilibrium thermodynamic modeling (FactSage 8.3) and laboratory-scale reduction trials was employed. Simulations guided the optimization of reducing agent dosages and reaction temperatures, while experiments validated the predicted phase assemblages through XRD, XRF, and SEM-EDS analysis [14,15,16,17]. Both metallic and oxide products were critically evaluated for chemical composition and potential reuse applications. A complementary Life Cycle Assessment (LCA) compared the proposed route with conventional FeSi production via submerged arc furnace, highlighting significant environmental benefits in CO2 emissions, raw material substitution, and waste diversion [18].
It is important to clarify that the primary objective of this study is not to develop a competitive alternative to conventional commercial ferrosilicon production but rather to design a sustainable valorization strategy for white ladle slag, a large-volume and problematic industrial by-product of secondary steelmaking. The process is intended as an environmentally sound and economically attractive method for reintegrating metallurgical residues into industrial value chains, reducing landfill volumes, and decreasing reliance on virgin materials, thereby supporting sustainable practices within the steel industry.
2. Materials and Methods
2.1. Materials and Theoretical Framework
2.1.1. Slag Characterization and Properties
The study utilizes a steel production slag, designated as SLAG_AV. SLAG_AV originates from a conventional Electric Arc Furnace (EAF) steel plant that primarily produces components for the automotive, transportation, and agricultural machinery industries. Ladle furnace slag compositions vary significantly depending on the steelmaking process, as reported by Fleischanderl et al. [19] and Iacobescu et al. [13]. Setién et al. [9] thoroughly investigated the distinction between LF slags and other typologies, proposing mineralogical criteria for their identification. X-ray fluorescence (XRF) analysis revealed the chemical composition shown in Table 1. This composition confirms that SLAG_AV represents typical LF/white slag from secondary steelmaking.

Table 1.
Chemical composition of SLAG_AV (wt.%) (measurement uncertainties represent ±0.5% relative standard deviation for major oxides (>5 wt.%), ±1% relative standard deviation for minor oxides (1–5 wt.%), and ±5% relative standard deviation for trace oxides (<1 wt.%) based on calibration with certified geological reference materials using a Panalytical Zetium WDXRF spectrometer).
2.1.2. Reducing Agents and Supplementary Materials
In accordance with circular economy principles, the reduction process employs post-consumer scrap materials. Aluminum that meets “end-of-waste” (EoW) criteria as formalized in the EU Waste Framework Directive (2008/98/EC) with specific implementation criteria [18] was utilized.
Approximately one-fifth of this aluminum exists in oxide form, with the remainder in a metallic state. The EoW aluminum also contains magnesium oxide (5%) and manganese oxide (1%), which contribute to the chemical interactions during treatment. Fleischanderl et al. [19] demonstrated the effectiveness of post-consumer aluminum as a reducing agent in metallurgical reactions.
Using only the stoichiometric amount of aluminum scrap to reduce the oxides (SiO2, Fe2O3, MnO, TiO2) in the starting slag creates problems. This approach shifts the reaction products outside the minimum liquidus temperature region in the MgO-CaO-Al2O3 ternary phase diagram Consequently, a higher reaction temperature becomes necessary, leading to unfavorable energy requirements and lower conversion efficiency of the aluminothermic reaction.
To reach the composition with minimum liquidus temperature, the alumina content in the final slag must be increased. This requires adding excess silica sand beyond what the starting slag contains. The aluminum reacts with SiO2 to form Al2O3, shifting the residual slag composition toward the Al2O3 vertex of the phase diagram. This compositional adjustment ensures a thermodynamically favorable liquidus temperature in the final slag, enabling proper phase separation and enhancing overall process efficiency.
Iron scrap, a staple in steelmaking, is similarly utilized with approximately one-tenth present as oxide. Iron scrap is added to achieve the correct ferroalloy composition. This addition ensures that the final ferrosilicon alloy achieves the requisite Fe:Si ratio comparable with the FeSi25 (initial hypothetical target) classification. By calibrating the Fe content, the process yields a standardized FeSi25 ferroalloy containing approximately 20%–23% Si, aligning with industrial specifications and ensuring compatibility with downstream steelmaking applications.
The silicon oxide source consisted primarily of silica sand, characterized by SiO2 (≈85%) and Al2O3 (≈9%) with minor quantities of other oxides. The addition of silica sand serves a critical thermodynamic function in the SLAG_AV valorization process. This strategic component allows sufficient aluminum reductant to be transformed into alumina in the reaction products, obtaining the correct composition of minimal liquidus temperature for the final Ca aluminate product.
The silica sand may be effectively substituted by spent foundry sand with similar composition, plus carbonaceous material from the pyrolysis of phenolic thermosetting resins used in the forming process. This recycling-based material strategy minimizes reliance on virgin resources while supporting sustainability objectives.
2.2. Experimental Methods and Analysis
Thermodynamic simulations were conducted using FactSage 8.3 to assess the feasibility and equilibrium behavior of the proposed aluminothermic reduction process. The modeling is based on the CALPHAD (CALculation of PHAse Diagrams) method, as described by Bale et al. [14], which allows accurate prediction of phase changes in multicomponent systems.
Experimental XRF measurements of input composition guide the stoichiometric addition of reducing agents. For product optimization, the primary criterion is achieving low liquidus temperature in both the metallic and oxide phases. This approach allows the reaction to proceed while the temperature decreases from the initial slagging temperature, without requiring additional heating. Such thermal management is critical for process efficiency and energy conservation during the aluminothermic reduction.
Jung et al. [15] have demonstrated the effectiveness of computational thermodynamic modeling in predicting phase formation during cooling of complex oxide–metal systems.
The calculations target the production of FeSi25, as this represents the most challenging case: it requires additional iron, which may exceed the amount carried over during slagging while still producing a marketable ferroalloy. The quantity of reductant is primarily dictated by the silica content in the initial slag, not by the target ferroalloy grade.
Any ferrosilicon alloy for commercial use must comply with ISO 5445 standards, particularly regarding maximum Al content. This content directly relates to reaction advancement in this process. FeSi15-25 ferroalloy production satisfies these requirements. However, higher Si activity in FeSi45 or FeSi50 decreases the aluminothermic reaction’s conversion efficiency. This results in residual Al concentrations of 2.2%–2.5%, which exceeds the allowable limits (2.0% for FeSi45 and 1.5% for FeSi50). Consequently, FeSi45 or FeSi50 ferroalloys produced via this process would be suitable only for internal reuse in secondary steel refining.
Thermodynamic simulations enable precise determination of stoichiometric reactant proportions required to achieve the target phase equilibria and optimize product composition within specified thermochemical constraints. These calculations provide a quantitative framework for predicting reaction pathways and final product distributions as a function of reactant composition and temperature.
Empirical testing, serving as a “proof of concept,” employs an induction furnace operating on electromagnetic induction. Bermúdez et al. [20] optimized the heating process by induction in high-temperature metallurgical processes, defining operational parameters to maximize energy efficiency.
This process generates heat through the Joule effect, where an alternating magnetic field heats conductive material. It provides precise control over the heating area with energy efficiency ranging from 75% to 95%.
The induction system used in the experimental setup (Figure 1) operates with two distinct frequencies: a heating frequency coupled with the reaction crucible and materials to optimize power transfer, and a stirring frequency that ensures effective mixing of the reactants. This dual-frequency arrangement facilitates homogeneous heating of the molten bath while providing controlled stirring through frequency adjustment.


Figure 1.
Photograph of the graphite crucible (dark) supported by an alumina structure (light). The crucible is inside the coil of the induction furnace with the refractory block with a hole at the top. The furnace is in operation with Mg vapors evolving and violently oxidizing in contact with atmospheric air. The image shows a glimpse of the melt inside the crucible while cooling.
The setup consists of a graphite crucible inserted into a refractory alumina container, functioning as a susceptor to heat the contained materials; a solenoidal induction coil surrounding the crucible, carrying an alternating current; an optical pyrometer (AMETEK Land Cyclops 055L-2F (AMETEK Land, Dronfield, UK)) with a measurement range of 1000 to 2000 °C and an accuracy of <0.5% (K) of reading; and a refractory cover to enclose and protect the system. The pyrometer was positioned to take readings through the central hole in the refractory lid, targeting the surface of the melt.
The temperature at which aluminothermic reactions occur directly influences the reaction equilibria, which in turn determine the composition and microstructure of the final products.
All materials are weighed according to the ratios defined by the thermodynamic simulations and proportioned to the crucible volume capacity of 10 L.
The crucible is charged with 2100 g of slag, 1090 g of iron (plus 109 g of Fe2O3), 480 g of aluminum (plus 96 g of Al2O3), 24 g of MgO, 4.8 g of MnO, 250 g of SiO2, with 23.2 g of additional Al2O3, 6.37 g of K2O, 3.93 g of Na2O, and 3.7 g of Fe2O3.
A refractory block with a central hole is used as a lid to limit heat loss while allowing pyrometric temperature measurement at the surface of the melt, as thermocouple insertion is impractical. Upon complete cooling, the crucibles are sectioned to facilitate the extraction of reacted materials, revealing distinct phase separation. The experiment using SLAG_AV as the initial material shows clear separation between the residual oxide slag and reduced ferroalloy (Figure 2).

Figure 2.
Separation between the residual oxide slag and the reduced ferroalloy.
Notable observations include white deposits of pure MgO forming on the graphite crucible walls above the melt level. These deposits result from the violent oxidation of metallic magnesium gas evolving at temperatures exceeding 1800 °C. This gaseous metallic magnesium phase, which becomes predominant in the gas phase in the temperature range of 1800–2000 °C, reacts with atmospheric oxygen.
This reaction creates a visible flame above the crucible and deposits MgO on the crucible walls. Significant crucible corrosion was also observed. Samples of both ferroalloy and slag are extracted via mechanical fragmentation for subsequent mineralogical and compositional analyses. Selected fragments are also prepared for microstructural examination through resin encapsulation, polishing, and SEM-EDS analysis.
Multiple analytical techniques are employed for comprehensive characterization:
X-ray Fluorescence (XRF): XRF quantifies the bulk chemical composition of the input oxide fraction using a Panalytical Zetium WDXRF spectrometer equipped with an Rh-anode X-ray tube and multiple detectors. Wavelength-dispersive optics allows precise differentiation of overlapping spectral lines with enhanced detection limits. Calibrations utilize over 70 certified geological reference materials, with detection limits ranging from 0.005% to 0.2% for major oxides and 3–10 ppm for trace elements.
X-ray Diffraction (XRD): XRD for slag analyses employs a Philips X’Pert PRO diffractometer with a cobalt (Co) anode source (λ = 1.78897 Å) and Bragg–Brentano HD geometry. This radiation source proves particularly suitable for iron-rich samples by minimizing fluorescence. Patterns are collected from 3° to 85° 2θ, with a virtual step size of 0.0017° (using a linear detector) and a counting time of 100 s/step. The application of XRD analysis with Rietveld refinement for quantification of crystalline phases in slags has been validated by De la Torre et al. [16], demonstrating accuracy exceeding 95% for major components. The resulting high-resolution diffractograms enabled phase identification and semi-quantitative analysis via Rietveld refinement using PDF-4+ databases. The ferroalloy, instead, is analyzed using a Siemens D5000 diffractometer with Ni-filtered Cu-Kα radiation.
Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy (SEM-EDS): Microstructural and elemental characterization for slag and ferroalloy utilizes a JEOL JSM-6490LV SEM equipped with an IXRF Systems 500 EDS detector. Goldstein et al. [17] described advanced techniques of SEM-EDS microanalysis for multiphase systems, with particular emphasis on optimizing operational parameters for metallurgical samples.
To ensure analytical consistency and eliminate bias, the slag sample was finely ground and homogenized prior to analysis, except for specimens designated for SEM/EDS microstructural examination. Sample preparation followed a systematic protocol differentiated by analytical technique:
For bulk analysis, coarse fragments were initially reduced using a Gutenberg press and a Retsch BB50 jaw crusher. Subsequently, fine grinding was performed in a Retsch RM0 mortar grinder. For XRD analysis, powders were sieved to <20 μm, while for XRF analysis, slag powders were pressed into pellets using a wax–boric acid base (1:3 ratio) under 6 tons of uniaxial load.
For microstructural characterization via SEM-EDS, a meticulous resin embedding procedure was implemented. This process began with careful selection of representative material fragments precisely cut using a water-lubricated diamond cutting machine. After drying, these fragments were positioned in a Teflon holder and encased in a two-component epoxy resin (Araldite 2020) mixed with hardener in a specific ratio. The resulting capsules were left to solidify overnight in a controlled environment at 40 °C. The solidified capsules then underwent progressive polishing using increasingly fine sandpaper (from coarse 60 grit to ultra-fine 4000 grit), followed by a two-stage treatment with polycrystalline diamond suspension. This procedure created the perfectly smooth surface required for high-quality imaging and accurate elemental analysis. The final critical step involved ensuring proper electrical conductivity by placing a graphite strip beneath the capsules, preventing charge accumulation that would otherwise distort the analytical signals.
This comprehensive sample preparation protocol enabled reliable multi-technique characterization while maintaining consistent sample properties across different analytical methods.
3. Results
The Results section is organized into two main parts. First, the thermodynamic simulation results are presented, showing the optimal conditions for FeSi recovery with complete reduction, including simulated inputs and outputs of the valorization process. Then, the experimental test results are discussed, including detailed analysis of the process products and environmental performance assessment. This structure allows for comparison between theoretical predictions and actual experimental outcomes, highlighting both the agreements and discrepancies between simulation and practical implementation.
3.1. SLAG_AV: Optimal FeSi Recovery—Thermodynamic Simulation
Table 2 presents the mass balance for SLAG_AV valorization, showing both the input materials used and the output materials obtained during the thermodynamic valorization process. The table also includes the normalized composition of the final products based on FactSage equilibrium calculations.

Table 2.
Mass balance for SLAG_AV valorization and normalized final product composition (FactSage mass balance averaged).
Equilibrium calculations were performed from 2000 °C down to 1000 °C at 100 °C intervals to determine phase compositions and transformation temperatures. This approach identifies the liquidus temperatures of both the metallic and oxide phases along with their equilibrium compositions at various temperatures. At high temperatures (2000–1400 °C), the simulations predict the formation of a gas phase primarily composed of Mg, K, and Na, which constitutes an important feature in the system’s mass balance.
The thermodynamic optimization of the reaction yields 76.80 g of FeSi alloy (76.63% Fe, 21.72% Si) and 125.69 g of residual slag (41.11% CaO, 50.41% Al2O3, 1.09% SiO2). The minimal silica content in the residual slag indicates efficient reduction at the calculated equilibrium conditions and supports potential secondary applications for this co-product.
The CaO-MgO-SiO2-Al2O3 diagram (Figure 3) illustrates the equilibrium pathway for the slag transformation. The initial slag composition (red point) is located in the calcium silicate region (Ca2SiO4) with a liquidus temperature of approximately 1600 °C. After valorization, the final slag composition (green point) shifts to the calcium magnesium aluminate domain with a lower liquidus temperature of about 1400 °C. This compositional transition from silicate-dominant to aluminate-rich phases crosses multiple isothermal contours in the phase diagram, reflecting the changing liquidus temperatures.
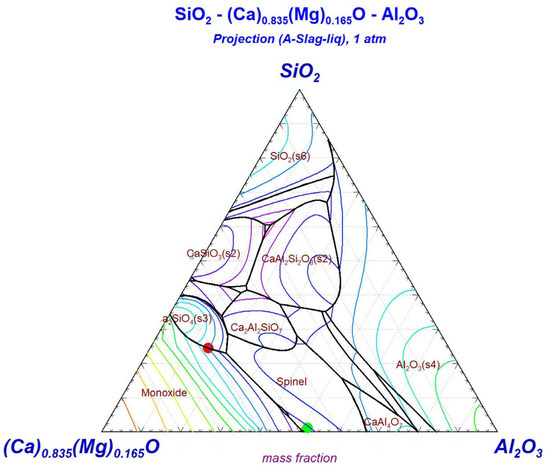
Figure 3.
SiO2-Ca0.835Mg0.165O-Al2O3 projection diagram.
According to thermodynamic simulation, at the optimized final slag composition, the first solid phase to form upon cooling from the melt is spinel MgAl2O4 at approximately 1410 °C, followed by CaAl2O4 and Ca3MgAl4O10 at around 1350 °C. The solidification sequence concludes at approximately 1270 °C when the liquid slag phase is completely consumed.
To maintain the slag in the ternary eutectic zone (with a liquidus temperature of approximately 1400 °C), the equilibrium calculations indicate that the alumina content in the residual slag must range between 30 and 35 wt%. The black dashed line trajectory in Figure 4 effectively illustrates this compositional constraint, demonstrating the amount of Al2O3 that must be added to the initial silica-free composition to reach eutectic regions with minimum liquidus temperatures. This compositional requirement is directly linked to the concurrent formation of the ferrosilicon phase in the metallic portion of the system.
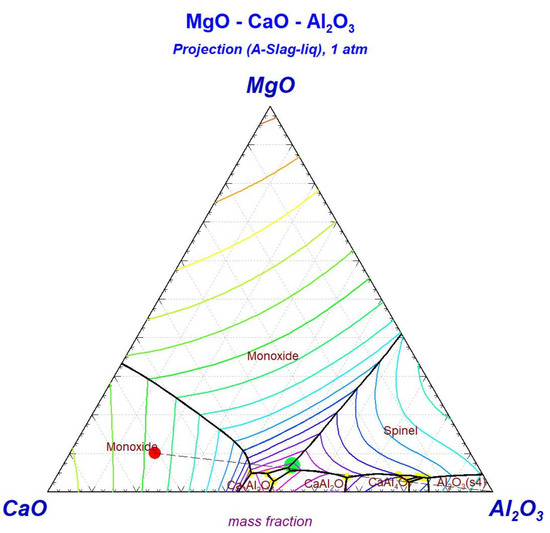
Figure 4.
MgO-CaO-Al2O3 projection diagram.
For the metallic phase, the thermodynamic calculations utilize the binary Fe-Si phase diagram, which exhibits two eutectic points: one at 18–22 wt% Si and another at 50–60 wt% Si, both with melting points of approximately 1200–1220 °C. The process targets FeSi25 production to maintain proximity to the first eutectic composition, minimizing energy requirements and limiting unreacted metallic aluminum in the final ferroalloy to comply with UNI-ISO 5445 specifications.
According to the equilibrium calculations, the liquidus temperature of the iron–silicon alloy system is approximately 1230 °C, with Fe1Si1 as the first phase to form. Complete solidification occurs at 1160 °C with the formation of ordered body-centered cubic structures (BCC_B2 phase) characteristic of Fe3Si-type intermetallics. The overall silicon content across all metallic phases averages approximately 22.25%, close to the targeted FeSi composition.
From an energetic perspective, the equilibrium calculations consider the initial slag in its molten state at 1550 °C with additions of iron scrap, aluminum, and silica sand at ambient temperature. The final process temperature is fixed at 1410 °C, corresponding to the liquidus temperature of the newly valorized slag. Thermodynamic calculations reveal an enthalpy change of −2.492365 × 104 J for 100 g of processed material, which translates to −69.23 kWh/ton of slag.
This negative value demonstrates that the valorization process is exothermic according to thermodynamic principles, providing significant advantages for industrial implementation, including reduced energy consumption and potential for heat recovery.
3.2. SLAG_AV: Optimal FeSi Recovery—Experimental Test
The experimental analysis of the SLAG_AV valorization process products is presented in two distinct parts. First, a comprehensive characterization of the residual slag phase is conducted through various analytical techniques, including XRF, XRD, and SEM-EDS to determine its chemical composition, mineralogical structure, and microstructural features. Then, the metallic phase (ferroalloy) is analyzed to evaluate its composition and properties. This approach allows for a systematic comparison between the thermodynamic simulation predictions and the actual experimental outcomes for both product streams, highlighting the key differences and their underlying causes.
3.2.1. Residual SLAG Analysis
Elemental Composition (XRF Analysis)
Comparing the experimental data with thermodynamic simulation results (Table 2) reveals several significant discrepancies. The simulation predicts higher Al2O3 values (50.41 wt.% vs. 43.53 wt.% experimental). The silicon distribution in the residual slag (4.72 wt.% SiO2) indicates more extensive silica reduction than predicted by thermodynamic simulations (1.09 wt.% SiO2). Substantial crucible corrosion was observed during the experiments, with evidence that graphite from the crucible dissolved into the liquid phases during the process. These observations demonstrate enhanced reducing conditions achieved during the experiments, likely due to the combined effects of aluminothermic reduction and the carbothermic contribution from dissolved graphite resulting from crucible interaction.
Furthermore, the simulations predict a substantially higher MgO content (7.39 wt.%) compared to experimental findings (4.20 wt.%). This difference can be attributed to the partial volatilization of magnesium at elevated process temperatures (>1800 °C), which forms a gaseous phase that oxidizes upon contact with the atmosphere and deposits as MgO on the crucible walls [21].
Both the flames observed in the furnace fumes and the lower MgO content in the residual slag suggest that higher temperatures were reached during the experimental reaction than initially measured. This indicates an inaccurate pyrometer reading, which likely measured the evolving gas and flame temperature rather than the temperature of the reacting mass.
Trace oxides in the residual slag include Fe2O3 (0.06 wt.%), while Na2O, TiO2, K2O, and P2O5 each constitute approximately 0.02 wt.%. MnO was detected at the lowest concentration (0.005 wt.%).
Mineralogical Composition (XRD Analysis)
The crystallographic characterization via XRD analysis (Figure 5) reveals a predominant presence of calcium aluminate in the form of mayenite (12CaO·7Al2O3), constituting 86% of the crystalline phases. When compared to equilibrium calculations, this mayenite phase is not predicted by the thermodynamic model, which instead favors the formation of Ca2MgAl4O10 and CaAl2O4. Calcium aluminosilicate present as gehlenite (Ca2Al2SiO7) represents 9% of the sample according to the XRD analysis. This significant discrepancy between experimental results and thermodynamic predictions can be primarily attributed to kinetic factors dominating over thermodynamic equilibrium behavior. Thermodynamic models calculate the equilibrium phase composition, assuming the system has sufficient time to reach the most energetically stable state. However, under actual experimental conditions, the formation of mayenite (12CaO·7Al2O3) is kinetically favored over the phases predicted by the model.
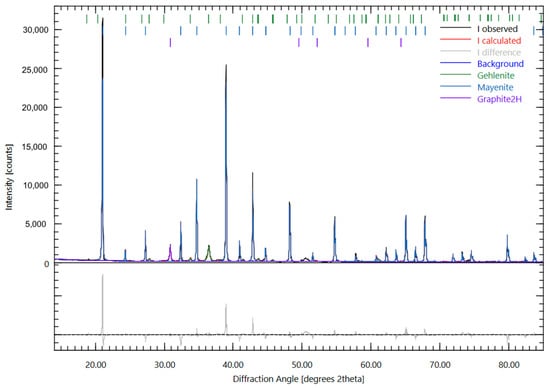
Figure 5.
Residual slag XRD analysis.
Mayenite is known for its rapid crystallization under relatively fast cooling conditions, while Ca2MgAl4O10 and CaAl2O4 phases might require longer atomic diffusion times or more specific nucleation conditions. Additionally, the presence of impurities or minor elements in the real system, not fully considered in the thermodynamic model, could selectively catalyze the formation of mayenite.
Graphitic carbon content measures 5%, originating from the crucible dissolution phenomenon not accounted for and thus not predicted by the equilibrium thermodynamic modeling. Notably, the detection of graphitic carbon in the oxide phase indicates partial dissolution of the graphite crucible at high temperatures. However, the preservation of this carbon in its graphitic form suggests it did not fully participate in carbothermic reduction reactions during the process, despite being available in the system.
Microstructural Analysis (SEM-EDS)
The comprehensive EDS mapping (Figure 6) of the residual oxide slag reveals calcium and aluminum as the predominant elemental components, constituting 30.5 wt.% and 23.6 wt.%, respectively. The substantial oxygen content, measured at 34.1 wt.%, strongly indicates the formation of calcium/magnesium aluminate phases as the primary constituents. When converting these elemental compositions to their oxide equivalents (Ca to CaO, Al to Al2O3, etc.) and normalizing after excluding carbon, the results show good alignment with the previously reported XRF data (Table 3). The normalization of elemental compositions was performed to establish a direct comparison with the XRF data, which inherently represents oxide compositions rather than elemental values. Since EDS provides elemental analysis while XRF reports oxide compositions, normalization after excluding carbon content (which is considered an experimental artifact) was necessary to enable a meaningful comparison between these complementary analytical techniques.
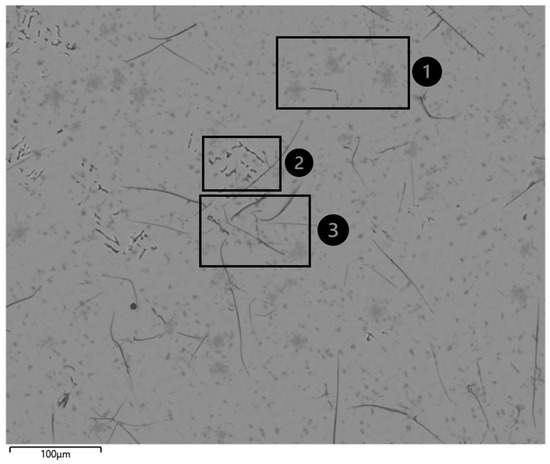
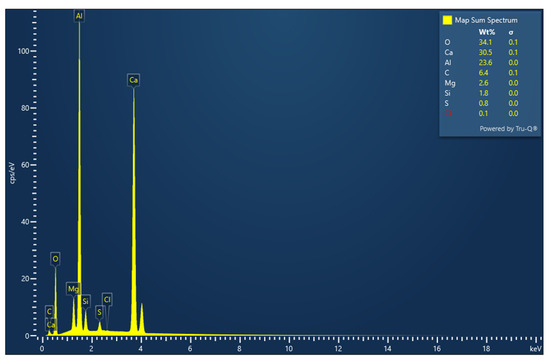
Figure 6.
Microstructure and EDS global image of residual slag. Dark-gray regions (1): Silicon accumulations. Agglomerated black particulates (2): Identified as carbon intrusions, explicitly attributed to crucible reactivity phenomena and not representative of the original slag composition. Randomly distributed linear formations (3): Also identified as carbon intrusions from crucible reactions, representing experimental artifacts rather than inherent microstructural features.

Table 3.
Chemical composition (XRF) of residual slag (measurement uncertainties represent ±0.5% relative standard deviation for major oxides (>5 wt.%), ±1% relative standard deviation for minor oxides (1–5 wt.%), and ±5% relative standard deviation for trace oxides (<1 wt.%) based on calibration with certified geological reference materials using a Panalytical Zetium WDXRF spectrometer).
The oxide equivalents were calculated by converting the elemental weight percentages obtained from EDS to their corresponding oxide forms using stoichiometric conversion factors. These converted values were then normalized to 100% after excluding the carbon content to obtain comparable compositional data.
The calculated CaO equivalent (approximately 42.7 wt.% normalized) compares well with the XRF value of 46.49 wt.%, while the calculated Al2O3 (approximately 44.6 wt.% normalized) closely matches the XRF measurement of 43.53 wt.%. Similarly, the calculated SiO2 (approximately 3.8 wt.% normalized) and MgO (approximately 4.3 wt.% normalized) values correspond reasonably well with the XRF values of 4.72 wt.% and 4.20 wt.%, respectively.
The carbon content of 6.4 wt.% is an artifact, as previously mentioned, resulting from the reactivity with the graphite crucible used during the experiments and does not represent an inherent component of the slag. Minor elements include magnesium (2.6 wt.%), silicon (1.8 wt.%), sulfur (0.8 wt.%) and chlorine (0.1 wt.%). The consistency between these two analytical techniques reinforces the reliability of compositional characterization.
The microstructural examination of the residual oxide phase distinctly illustrates elemental maps of Ca (top-left), O (top-right), Si (center-left), C (center-right), C (center-right), Mg (bottom-left) and S (bottom-right (Figure 7):
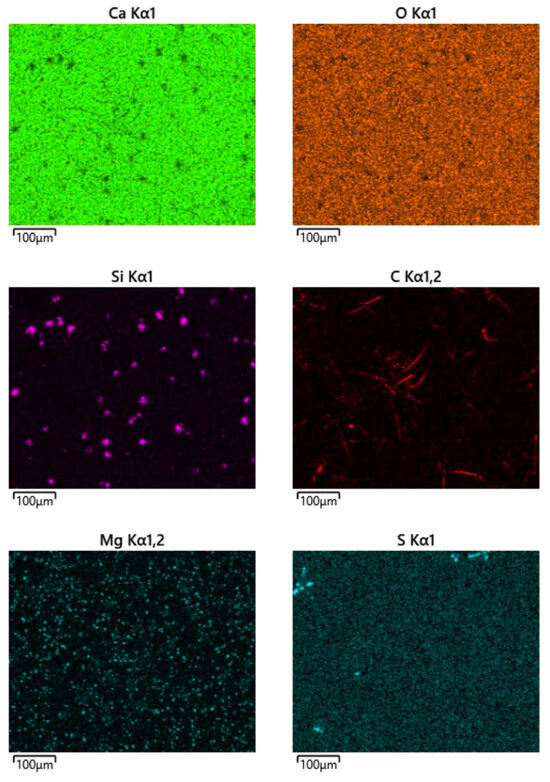
Figure 7.
Elemental maps Ca (top-left), O (top-right), Si (center-left), C (center-right), C (center-right), Mg (bottom-left) and S (bottom-right).
3.2.2. Ferroalloy Analysis
Mineralogical Composition (XRD Analysis) (Figure 8).
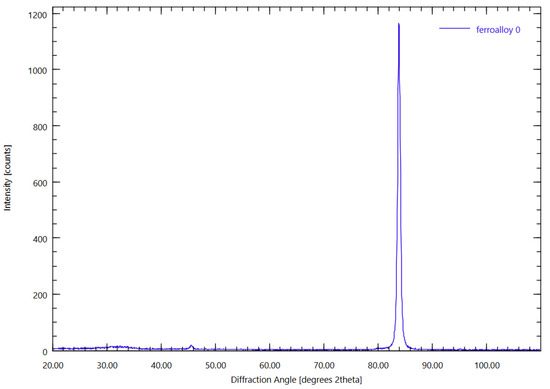
Figure 8.
Ferroalloy XRD analysis.
XRD analysis reveals only one distinct peak in the position of iron silicide, confirming the formation of the ferrosilicon alloy as predicted by thermodynamic simulations.
Elemental Composition (SEM-EDS Analysis)
The elemental composition of the SLAG_AV ferroalloy includes Fe at 74.83 wt.%, Si at 19.46 wt.%, Al at 4.21 wt.%, Mn at 0.93 wt.%, Cr at 0.22 wt.%, and Ti at 0.36 wt.% (Figure 9, Figure 10 and Table 4). While these findings align with thermodynamic simulations predicting a ferrosilicon alloy formation, the silicon content (19.46 wt.%) is slightly lower than would be expected for a standard FeSi25 alloy.

Figure 9.
Characteristic morphological patterns (1-2-3) on ferroalloy analyzed using SEM-EDS.
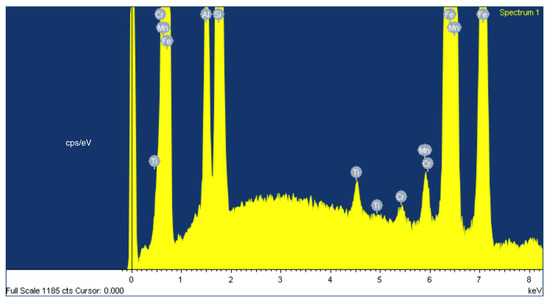
Figure 10.
EDS global image of ferroalloy.

Table 4.
Map Sum Spectrum for Figure 10.
Three types of microstructural patterns are recognized in the ferroalloy (Figure 10):
- Vermiculate structure (1): an enrichment in Si at 33.59 wt.% and the appearance of C at 13.84 wt.%, which is not detected among the main elements in the total map scan. This carbon presence confirms the artifact from reactivity with the graphite crucible during cooling and is highlighted by the phase diagram in the graphite region.
- Cluster of small dots (2): these dots confirm the C intrusion with a higher concentration at 44.90 wt.%. In this case, the microstructure appears spheroidal rather than linear. The elemental composition of the dotted structure reveals Fe at 50.62 wt.%, Si at 2.74 wt.%, C at 44.90 wt.%, Al at 0.51 wt.%, Mn at 0.72 wt.%, and Ca at 0.51 wt.%.
- Larger, featureless spots (3): EDS punctual analysis reveals a Ti intrusion at 80.49 wt.%. Indeed, Ti is detected in the total map scan at 0.36 wt.% and foreseen by the simulation, and its concentration in this spot confirms its presence in the form of this intrusion. Moreover, apart from the repeated presence of C at 11.20 wt.% (another crucible artifact), another relevant minor element identified is Cr at 3.32 wt.%. The elemental composition of the featureless spot intrusion includes Ti at 80.49 wt.%, C at 11.20 wt.%, V at 2.71 wt.%, Cr at 3.32 wt.%, Fe at 1.94 wt.%, and S at 0.33 wt.%.
A final global comprehensive comparison between thermodynamic simulations and experimental results reveals several significant discrepancies that warrant systematic analysis (Table 5 and Table 6). These differences provide valuable insights into the process dynamics and limitations of equilibrium modeling for such complex reaction systems.

Table 5.
Comparison between simulation and experimental results for residual slag obtained through the valorization process.

Table 6.
Comparison between simulation and experimental results for ferroalloy obtained through the valorization process.
The higher CaO concentration in the experimental residual slag (46.49 wt.% vs. 41.11 wt.% simulated, representing a +13.1% relative deviation), coupled with lower Al2O3 content (43.53 wt.% vs. 50.41 wt.% simulated, a -13.6% relative deviation), suggests that the aluminothermic reduction reaction did not achieve the extent predicted by equilibrium calculations. Quantitatively, the experimental Al2O3 formation reached only 86.4% of the predicted value, with a stoichiometric correlation coefficient of 0.78 between CaO increase and Al2O3 decrease. This deviation can be attributed to two primary factors: reaction kinetics limitations and non-uniform temperature distribution within the reaction volume. The formation of a solid layer at the reaction interface likely impeded mass transfer, creating localized non-equilibrium conditions that affected the overall conversion efficiency.
The considerably higher SiO2 content in the experimental residual slag (4.72 wt.% vs. 1.09 wt.% simulated) represents the most significant deviation from thermodynamic predictions. This phenomenon can be analyzed through reaction thermodynamics: the standard Gibbs free energy (ΔG°) for the reduction of SiO2 by aluminum becomes more negative with increasing temperature, but the reaction is still less thermodynamically favorable than the reduction in other oxides present in the system. Quantitative analysis shows that a temperature deviation of +50–75 °C above the measured values would predict the observed SiO2 retention in the slag, consistent with our observations of temperature measurement limitations discussed previously.
The lower MgO content in the experimental slag (4.20 wt.% vs. 7.39 wt.% predicted) provides compelling evidence of magnesium volatilization at elevated process temperatures. At temperatures exceeding 1800 °C, magnesium partial pressure increases significantly (reaching ~0.1 atm at 1850 °C), promoting volatilization. Mass balance calculations indicate that approximately 40%–45% of the initial magnesium content was volatilized during processing, consistent with the white MgO deposits observed on the crucible walls. This phenomenon serves as an indirect temperature indicator, providing strong evidence that reaction temperatures substantially exceeded those used in the thermodynamic simulations.
The presence of carbon in the experimental slag (~5.00 wt.%, estimated by XRD) from crucible dissolution creates a more complex redox environment than modeled in the simulations. Carbon content affects the oxygen potential of the system, potentially creating localized reducing conditions that differ from the bulk environment. However, the preservation of carbon in graphitic form rather than its participation in carbothermic reduction suggests complex kinetic limitations at the reaction interface.
In the ferroalloy phase, the significantly higher Al content (4.21 wt.% vs. 0.32 wt.% simulated) indicates incomplete aluminum consumption, likely due to reaction kinetics limitations rather than thermodynamic constraints. This residual aluminum directly impacts silicon recovery, as evidenced by the lower Si content (19.46 wt.% vs. 21.72 wt.% simulated). The relationship between these deviations confirms that silicon reduction from SiO2 was partially limited by mass transfer phenomena and localized temperature variations rather than by the availability of reducing agent.
These systematic deviations between simulation and experimental results highlight the limitations of equilibrium-based models for complex high-temperature metallurgical processes. While thermodynamic simulations provide valuable guidance for process design, the influence of reaction kinetics, mass transfer limitations, and temperature gradients must be considered for accurate prediction of experimental outcomes in industrial-scale implementations.
3.3. Environmental Performance Assessment: Life Cycle Assessment Results
The environmental implications of the valorization process demonstrated in this work for obtaining FeSi25 ferroalloy through the alternative ladle slag utilization treated in this work reveal substantial sustainability benefits compared to conventional manufacturing pathways (the traditional method employs quartz as a silicon source and petroleum coke as a reducing agent within high-temperature electric arc furnaces).
A comprehensive Life Cycle Assessment conducted using GaBi software v10.6.2.9 according to ISO 14040 [22] and ISO 14044 [23] standards documents remarkable improvements across all environmental impact categories when utilizing secondary materials rather than primary resources. These considerations presented here are an extract from a complete LCA study. The assessment adopts a cradle-to-gate approach spanning from resource extraction to factory output, with a clearly defined functional unit of 1 ton of FeSi25 ferroalloy, quantifying these advantages through the CML 2001 methodology (release: August 2016) with a diverse range of midpoint indicators including Global Warming Potential, Acidification Potential, and Abiotic Resource Depletion.
The valorization approach examined in this research focuses on the AV-based process (treated in this article), which incorporates post-consumer aluminum chips (347 kg/ton FeSi25) as reducing agents, iron scrap (635 kg/ton FeSi25) as an alloying element, and quartz (381 kg/ton FeSi25) as a silica source, eliminating the need for petroleum coke that dominates the environmental footprint of conventional production (Figure 11). The specific inventory data reveals that the AV process requires 253 kWh of electricity per ton of FeSi25 produced, compared to 2700 kWh in conventional manufacturing—a reduction of over 90% in energy demand—despite relying on the Italian electricity grid mix, which is approximately 63% fossil-fuel-based.
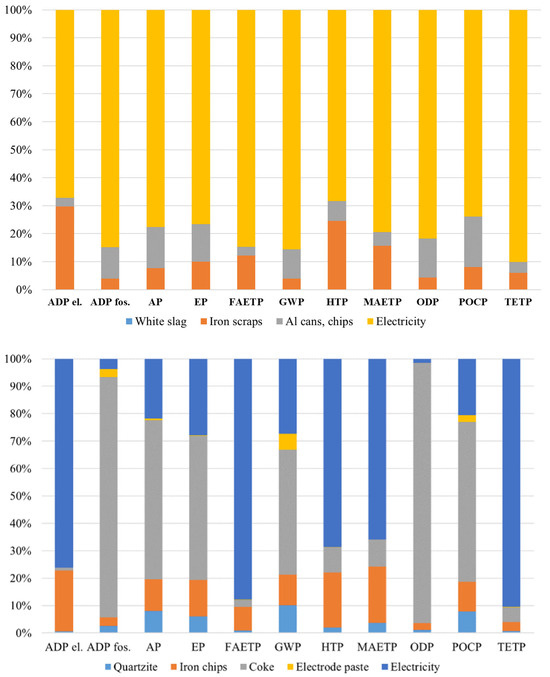
Figure 11.
Midpoint indicator results of AV process-based ferroalloy production (above) and the traditional ferroalloy production process (below).
The AV-based process yields substantial improvements ranging from 73% to 95% reduction in environmental burden across all impact categories when compared to conventional ferroalloy manufacturing. These dramatic improvements stem from several synergistic factors: the application of the cut-off approach to white slag inputs (1140 kg/ton FeSi25), which allows them to enter the system burden-free; the complete elimination of carbon-intensive coke (which contributes up to 88% of Abiotic Depletion of fossil resources and 95% of Ozone Depletion Potential in conventional production); the strategic utilization of post-consumer aluminum as the primary reducing agent; and significantly reduced electricity requirements.
The contribution analysis reveals that in the AV-based route, iron scraps constitute the primary source of environmental burden in several categories, most notably Abiotic Depletion Potential—elements (around 65%) and Human Toxicity Potential (approximately 55%–60%). Electricity consumption also remains a significant environmental driver, particularly influencing Global Warming Potential (40%–70%), Abiotic Depletion of fossil resources, and various toxicity-related indicators. Quartz, used as an additional input in the AV route, contributes modestly but consistently, especially in categories such as Abiotic Depletion (fossil) and Ozone Depletion Potential.
This finding underscores the importance of electrical grid composition, as the assessment models production within the Italian context, where approximately 63% of electricity derives from fossil fuels. Despite this relatively carbon-intensive energy supply, the AV-based pathway still maintains dramatic environmental advantages over conventional production due to significantly lower absolute electricity requirements (253 kWh vs. 2700 kWh per ton) and elimination of coke as a reducing agent. The conventional FeSi25 production process, by contrast, reveals an environmentally intensive profile dominated by two principal contributors: coke and electricity. Coke emerges as the most significant environmental burden, contributing up to 88% of impacts in categories such as Abiotic Depletion of fossil resources and 95% in Ozone Depletion Potential. This carbon-intensive reducing agent alone accounts for nearly half of the Global Warming Potential in conventional production.
From a broader sustainability perspective, the slag valorization approach demonstrated in this research aligns with multiple Sustainable Development Goals by promoting industrial innovation, responsible resource consumption, and climate action. The production of valuable co-products such as calcium aluminate (1560 kg/ton FeSi25) creates additional opportunities for cross-sectoral synergies with cement and ceramic industries, further enhancing the circular economic potential of this technological approach. Life cycle modeling employs mass-based allocation between the ferroalloy product and residual oxide phases, ensuring consistency with physical outputs while adhering to established methodological frameworks for waste valorization.
Certain limitations must be acknowledged, including the exclusion of use and end-of-life phases (the study employs a cradle-to-gate analysis, focusing solely on raw material extraction, production, and transportation while omitting the product usage and disposal stages). This common LCA limitation is acceptable when comparing production alternatives where post-production phases would have equivalent environmental impacts, as in the comparison between steel slag valorization and conventional FeSi25 production and the application of generic transport modeling. The assessment conclusively demonstrates that high-temperature valorization of steel slags represents an environmentally superior alternative to conventional FeSi25 production. This environmental validation complements the technical and microstructural analyses previously discussed, providing a comprehensive sustainability case for the proposed valorization process. The dramatic reductions in environmental impact across all categories, coupled with the technical feasibility demonstrated through detailed characterization, establish this valorization pathway as a promising approach for developing more sustainable and resource-efficient metallurgical value chains.
4. Discussion
4.1. Residual Oxide Slag Composition and Structure
The SLAG_AV residual slag exhibits predominant calcium aluminate phases, specifically mayenite (12CaO·7Al2O3), constituting 86% of the crystalline phases (XRD analysis). This mineralogical composition represents a significant transformation from the original white slag composition, creating a material with potential applications in cementitious systems and ceramic industries.
The unexpected carbon content of approximately 5 wt.% experimentally observed in the residual slag and the more extensive silica reduction than the predicted one by thermodynamic simulations represents a significant finding with implications for experimental setup.
4.2. Ferroalloy Composition and Microstructure
The SLAG_AV ferroalloy does not closely approach the hypothetical target FeSi25 composition obtained during the simulations. Even if the technical feasibility of the proposed valorization process has been demonstrated, the FeSi obtained is more like FeSi15.
In fact, according to ISO 5445 standards, FeSi25 typically requires silicon content between 20 and 30 wt.%, with 25 wt.% being the nominal value. The achieved 19.46 wt.% Si content falls slightly below the lower limit of the specification but remains within a usable range for many steelmaking applications. The similarity between simulated (76.63 wt.% Fe, 21.72 wt.% Si) and experimental results validates the thermodynamic modeling approach for predicting phase evolution in simpler slag systems. The minor deviation from target composition, particularly the presence of 4.21 wt.% unoxidized Al, likely results from a combination of higher-than-expected process temperatures that enhanced the aluminothermic reduction efficiency rather than from carbothermic contributions (as evidenced by the presence of unreacted graphite in the slag phase). This indicates areas for process refinement but does not fundamentally compromise the material’s utility as a ferroalloy addition in steelmaking.
4.3. Experimental Limitations
The laboratory-scale setup employed in this research introduced several important limitations that must be acknowledged and addressed in future studies:
Graphite Crucible Interaction: The use of graphite crucibles for induction heating resulted in significant carbon intrusion into both the slag and ferroalloy phases. It is categorically out of the question to use graphite crucibles in industrial implementation, and alternative heating and containment systems must be developed.
Temperature Control Challenges: The evidence of temperatures exceeding the intended 1650 °C process ceiling provides critical insights into the reaction dynamics of the process. The white deposits found on crucible tops, identified as predominantly MgO in SLAG_AV through SEM-EDS analysis, confirm the volatilization of magnesium at temperatures above 1800 °C. This phenomenon aligns with established thermodynamic data showing significant magnesium vapor pressure at these temperatures. The gaseous transport mechanism explains the observed magnesium depletion in the residual slag (4.20 wt.% experimental vs. 7.39 wt.% predicted) and offers a reliable indicator of temperature excursions during processing.
Reduction Mechanism Assessment: The graphite content (5%) detected in the residual slag through XRD analysis, along with its preservation in graphitic form, suggests a possible minimal contribution of carbothermic reduction to the overall process. This observation is further supported by the carbon content in the ferroalloy remaining within expected levels for the target FeSi composition. The presence of unoxidized aluminum in the alloy (4.21 wt.%) can be attributed to thermodynamic equilibrium limitations. The concentration of metallic aluminum in the metal phase represents the main driving force for silica reduction in the slag: higher Al concentration provides a greater driving force for reduction, resulting in lower silica concentration at equilibrium. This equilibrium is strongly influenced by temperature: higher temperatures favor a greater presence of metallic aluminum in the metal phase and modify the silica content in the slag at equilibrium.
4.4. Environmental Performance Assessment
The Life Cycle Assessment conducted within this research provides a comprehensive quantification of the environmental advantages offered by the proposed valorization approach. The dramatic reductions in environmental impact of 73%–95% for the AV-based process across all impact categories establish this technology as not merely an incremental improvement but a transformative approach to ferroalloy production.
The contribution analysis reveals that electricity consumption dominates the environmental profile of the AV-based production route, accounting for a significant portion of impacts in several categories. This finding highlights the importance of clean energy sourcing in determining the ultimate environmental performance of technology. Despite using the relatively carbon-intensive Italian electricity mix (approximately 63% fossil-based), the valorization process still achieves remarkable environmental improvements due to its significantly lower absolute energy requirements compared to conventional production.
The complete elimination of metallurgical coke represents perhaps the most environmentally significant aspect of the valorization approach. In conventional ferroalloy production, coke contributes up to 88% of Abiotic Depletion Potential for fossil resources, 95% of Ozone Depletion Potential, and 46% of Global Warming Potential. The substitution of this carbon-intensive reducing agent with post-consumer aluminum effectively decouples ferroalloy production from fossil carbon, aligning with decarbonization imperatives in hard-to-abate industrial sectors.
4.5. Broader Implications and Future Directions
The demonstrated valorization process aligns with several key sustainability transitions occurring in industrial sectors. By transforming a currently landfilled waste stream into valuable products (ferroalloy and calcium aluminate-based materials), the process exemplifies circular economy principles in action. The approach closes material loops within the metallurgical sector and creates cross-sectoral material flows through the potential use of calcium aluminates in cement and ceramic applications.
In addition, the production of calcium aluminate-rich residual slag creates opportunities for industrial symbiosis with cement manufacturers and ceramic producers. These high-value products (calcium aluminate phases) could serve as supplementary cementitious materials or raw materials for specialized ceramics, creating economic and environmental synergies across industrial sectors.
Moreover, by eliminating coke as a reducing agent and significantly reducing energy requirements, the valorization process offers a concrete decarbonization pathway for metallurgical production. This aligns with broader industrial strategies to reduce carbon intensity in hard-to-abate sectors through process innovation and material substitution.
And last, but not least, the recovery of valuable elements (silicon, iron, and potentially others) from waste streams contributes to resource security objectives, reducing dependence on primary raw materials and their associated environmental and geopolitical challenges.
Obviously, further optimizations could guarantee better results. For example, the possibility to adopt the best temperature control strategies, alternative heating systems, and separation techniques to enhance product quality and consistency.
5. Conclusions
This research has successfully demonstrated an innovative valorization approach for white ladle slag through pyrometallurgical treatment, offering a technologically viable and environmentally advantageous pathway for producing FeSi with low solidus temperature ferroalloy while generating potentially valuable calcium aluminate co-products. Despite challenges in process control and consistency, the fundamental viability of the approach has been established through detailed characterization and life cycle assessment.
The process achieves environmental impact reductions of 73%–95% compared to conventional ferroalloy production, primarily through the elimination of coke as a reducing agent, utilization of secondary materials, and significantly lower energy requirements. These substantial environmental benefits, combined with the potential economic value of recovered materials and avoided waste management costs, establish the technology as a promising contribution to sustainability in metallurgical industries.
The research findings provide a strong foundation for future development and implementation of this valorization approach, offering a pathway to transform an underutilized waste stream into valuable resources while significantly reducing environmental impacts associated with both waste management and conventional ferroalloy production. Technology thus represents not merely an end-of-pipe solution but a fundamental rethinking of material flows in metallurgical value chains, aligned with circular economy principles and industrial decarbonization imperatives.
Author Contributions
Conceptualization, G.A. and M.B. and K.B.; methodology, M.B. and F.D. and K.B.; software, F.D.; validation, F.D. and M.B.; formal analysis, F.D. and R.F. and M.A.; investigation, M.B. and F.D.; resources, M.B.; data curation, F.D. and R.F.; writing—original draft preparation, F.D.; writing—review and editing, F.D. and M.B.; visualization, M.B. and G.A. and K.B.; supervision, M.B.; project administration, G.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Maurizio Bellotto is employees of Opigeo S.R.L., 36040 Grisignano di Zocco (VI), Italy. The paper reflects the views of the scientists and not the company.
References
- Hasanbeigi, A. An International Benchmarking of Energy and CO2 Intensities; Global Efficiency Intelligence: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Pardo, N.; Moya, J.A. Prospective scenarios on energy efficiency and CO2 emissions in the European Iron & Steel industry. Energy 2013, 54, 113–128. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.-C.; Shi, C.; Pan, S.-Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Luxán, M.P.; Sotolongo, R.; Dorrego, F.; Herrero, E. Characteristics of the slags produced in the fusion of scrap steel by electric arc furnace. Cem. Concr. Res. 2000, 30, 517–519. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Menad, N.; Kanari, N.; Save, M. Recovery of high grade iron compounds from LD slag by enhanced magnetic separation techniques. Int. J. Miner. Process. 2014, 126, 1–9. [Google Scholar] [CrossRef]
- Setién, J.; Hernández, D.; González, J.J. Characterization of ladle furnace basic slag for use as a construction material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Barati, M.; Esfahani, S.; Utigard, T.A. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Gasik, M.M. Introduction. In Handbook of Ferroalloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–7. [Google Scholar] [CrossRef][Green Version]
- ISO 5445:1980; Ferrosilicon—Specification and Conditions of Delivery. International Organization for Standardization: Geneva, Switzerland, 1980.[Green Version]
- Iacobescu, R.I.; Koumpouri, D.; Pontikes, Y.; Saban, R.; Angelopoulos, G.N. Valorisation of electric arc furnace steel slag as raw material for low energy belite cements. J. Hazard. Mater. 2011, 196, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Jung, I.-H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimization of the FeO-Fe2O3-MgO-SiO2 system. Metall. Mater. Trans. B 2004, 35, 877–889. [Google Scholar] [CrossRef]
- De La Torre, A.G.; Bruque, S.; Aranda, M.A.G. Rietveld quantitative amorphous content analysis. J. Appl. Crystallogr. 2001, 34, 196–202. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2018; p. 550. ISBN 978-1-4939-6674-5. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Fleischanderl, A.; Gennari, U.; Ilie, A. ZEWA—Metallurgical process for treatment of residues from steel industry and other industrial sectors to generate valuable products. Ironmak. Steelmak. 2004, 31, 444–449. [Google Scholar] [CrossRef]
- Bermúdez, A.; Gómez, D.; Muñiz, M.C.; Salgado, P.; Vázquez, R. Numerical simulation of a thermo-electromagneto-hydrodynamic problem in an induction heating furnace. Appl. Numer. Math. 2009, 59, 2082–2104. [Google Scholar] [CrossRef]
- Han, J.; Fu, D.; Guo, J.; Ji, Z.; Zhang, T. Volatilization and condensation behavior of magnesium vapor during magnesium production via a silicothermic process with magnesite. Vacuum 2021, 189, 110227. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).