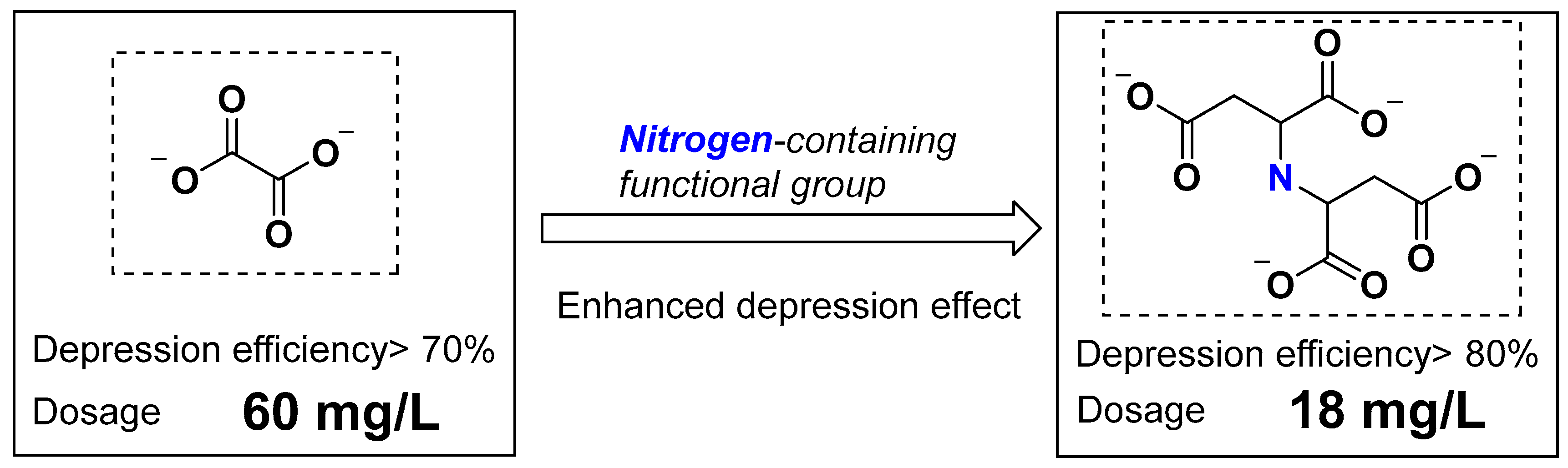
The Depression Effect of Micromolecular Depressant Containing Amino and Phosphonic Acid Group on Serpentine in the Flotation of Low-Grade Nickel Sulphide Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Synthesis and Characterization of the Dioxime Collector
2.2.1. Synthesis Procedure
- Iminodiacetic acid (IDA)
- Nitrilotriacetic acid (NTA)
- N-(phosphonomethyl)iminodiacetic acid (PMIDA)
- N-(phosphonomethyl)glycine (GLY)
2.2.2. Characterization Methods
2.3. Micro-Flotation Experiments
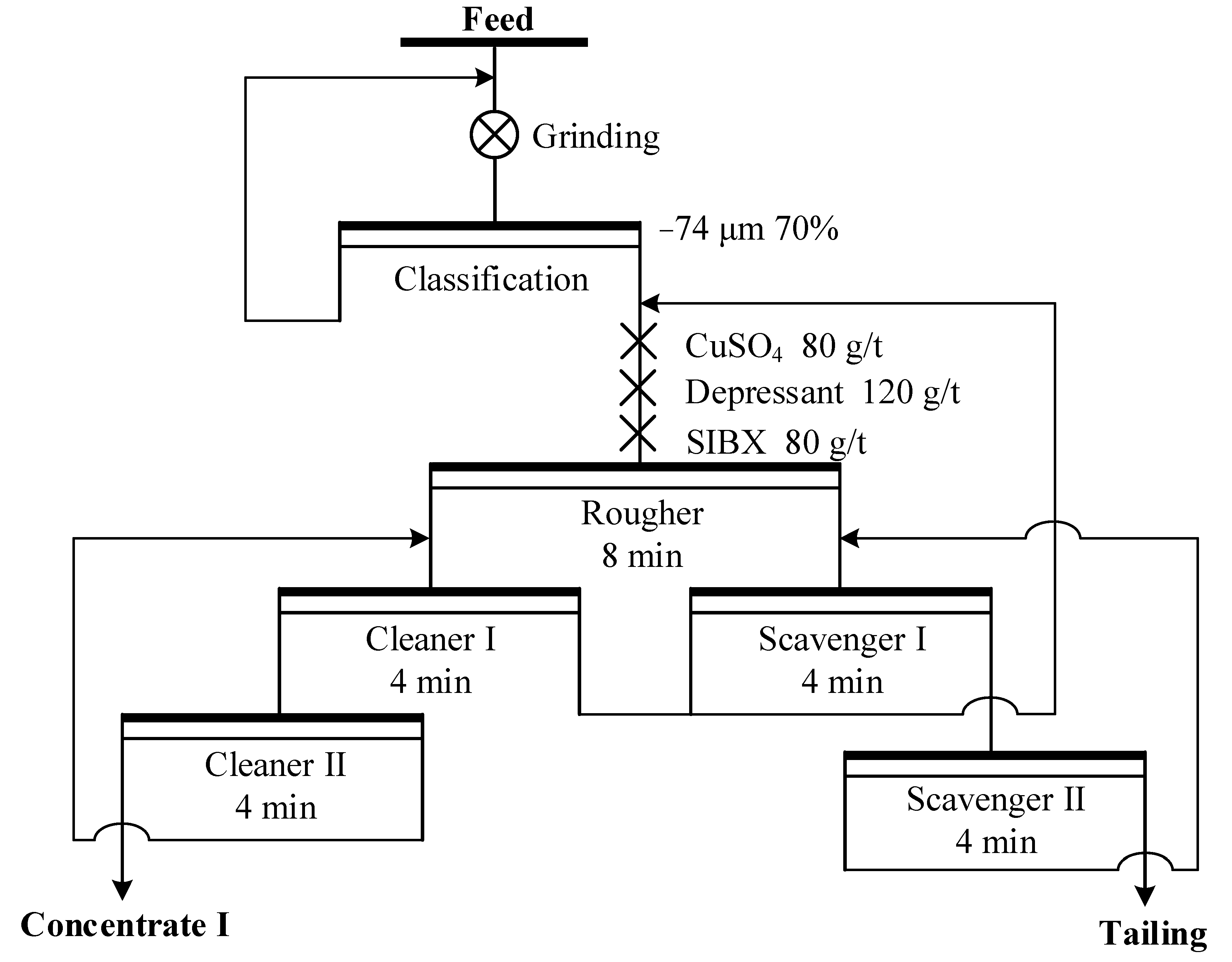
2.4. Batch Flotation Experiments
2.5. Zeta-Potential Measurements and Dlvo Calculation
2.6. Reagent Adsorption Measurements
2.7. XPS Analysis
2.8. Contact Angle Measurements
3. Results and Discussion
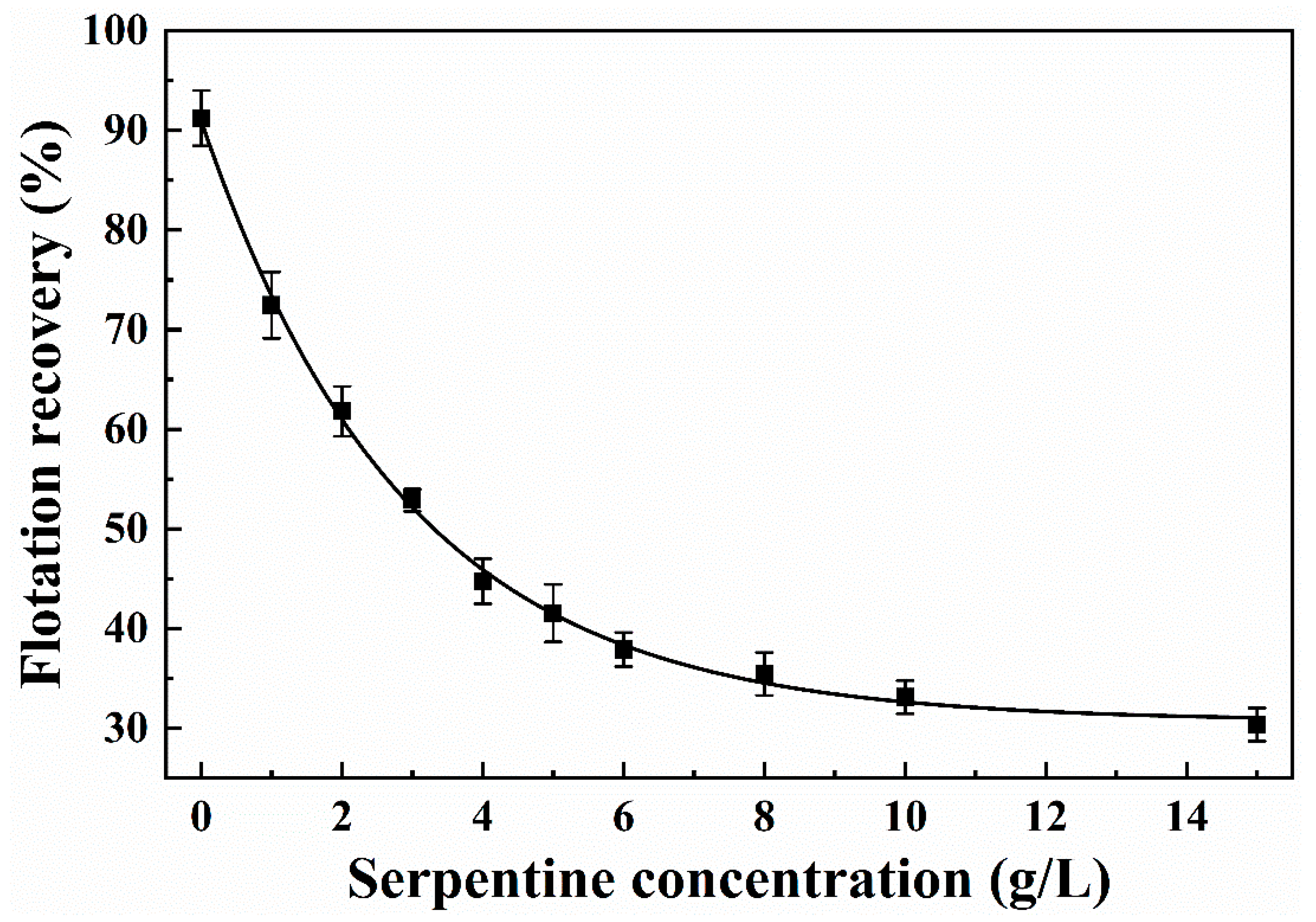
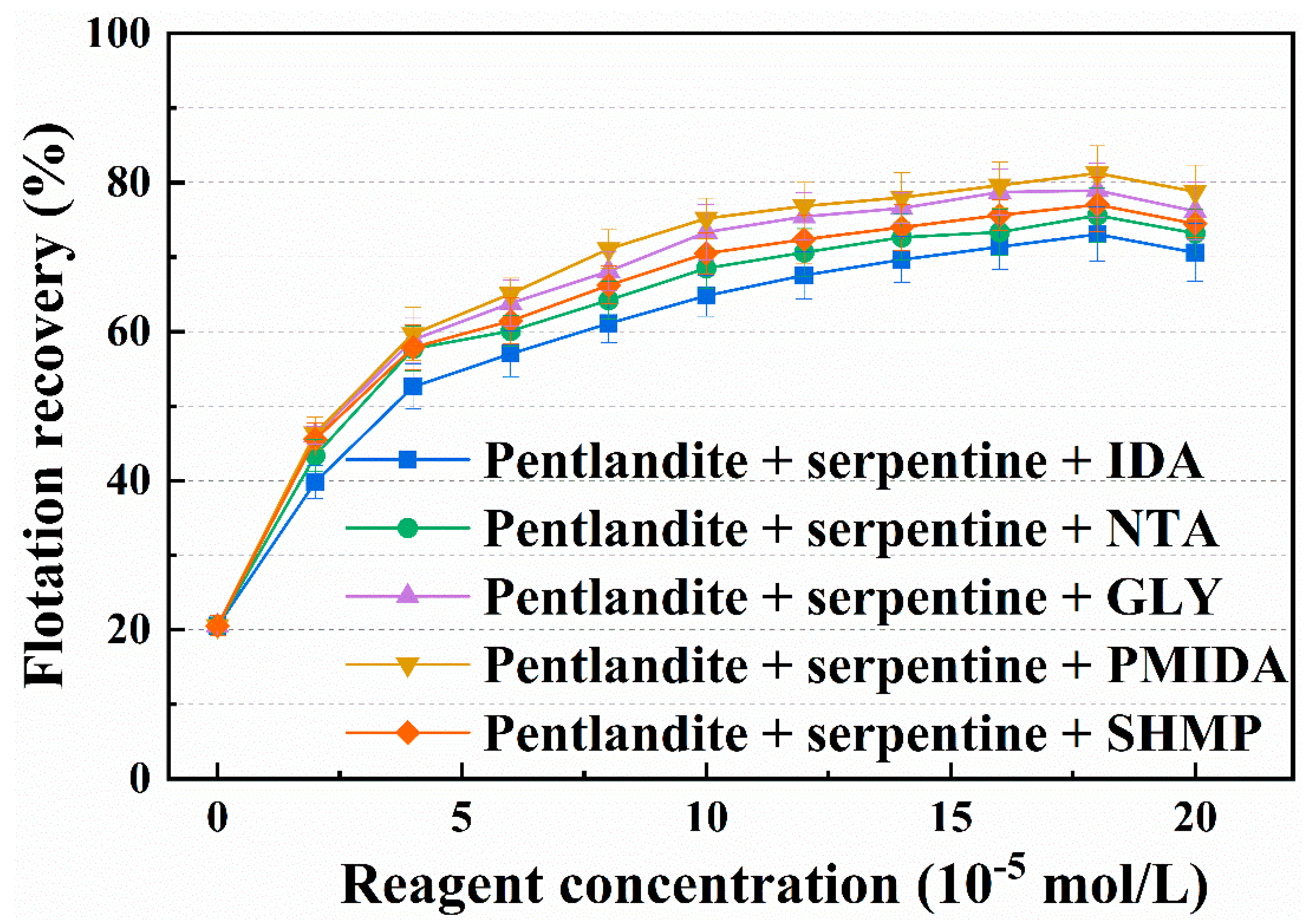
3.1. Micro-Flotation Experiments
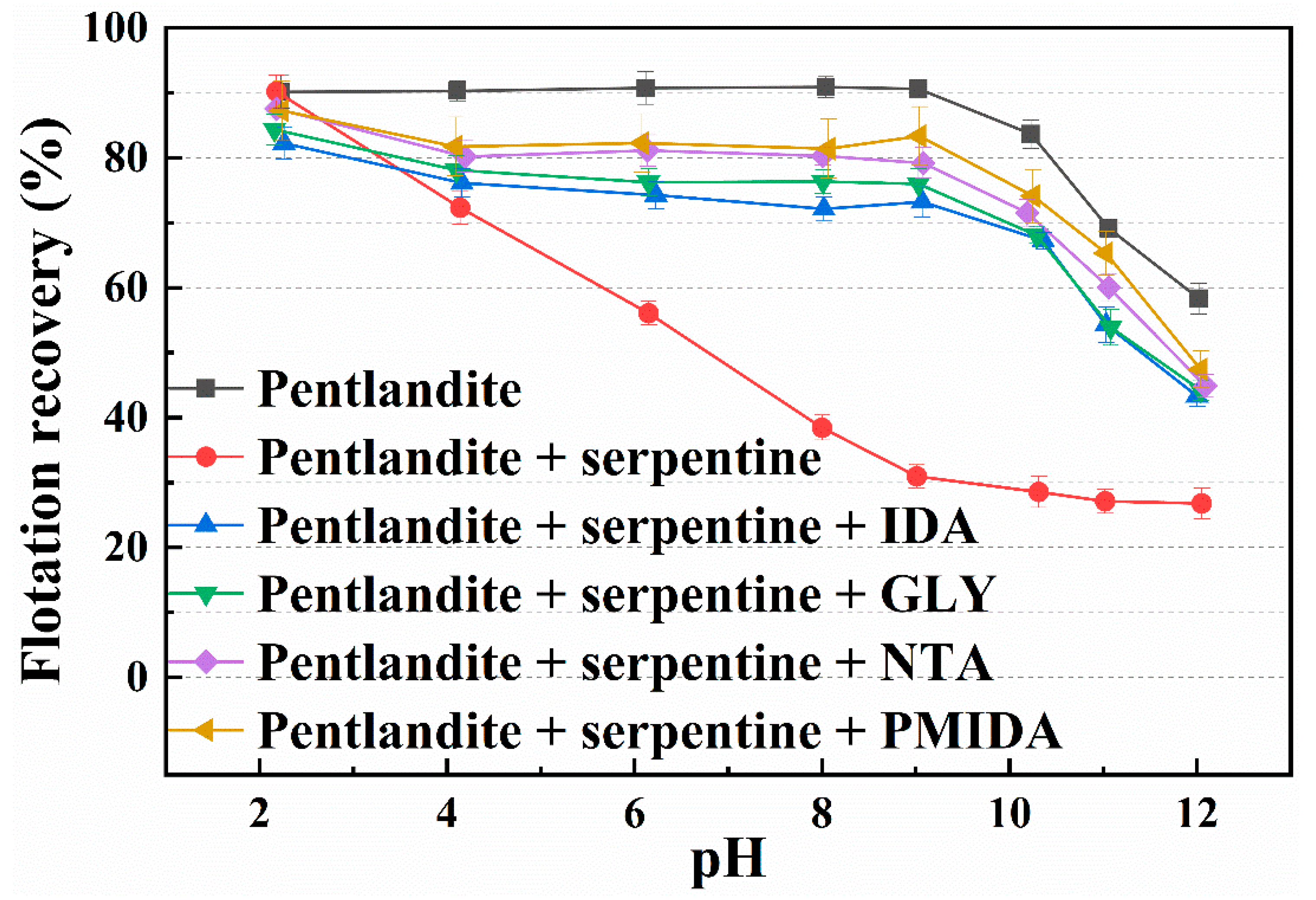
3.2. Batch Flotation Experiments
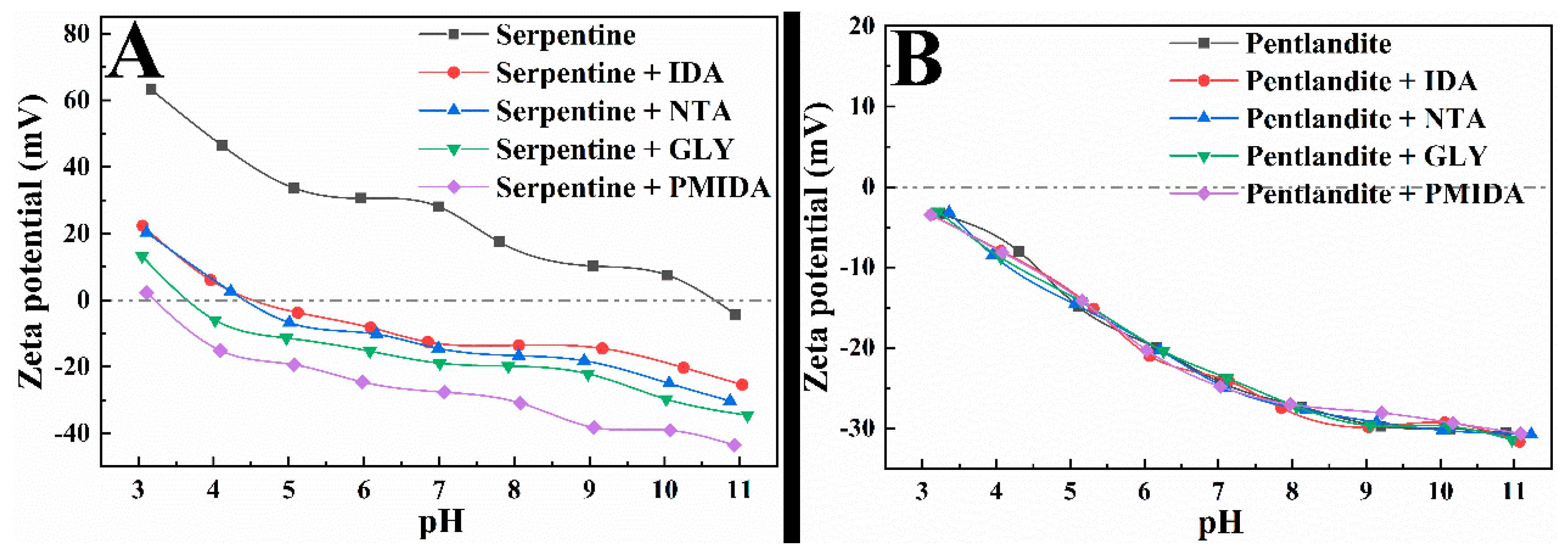
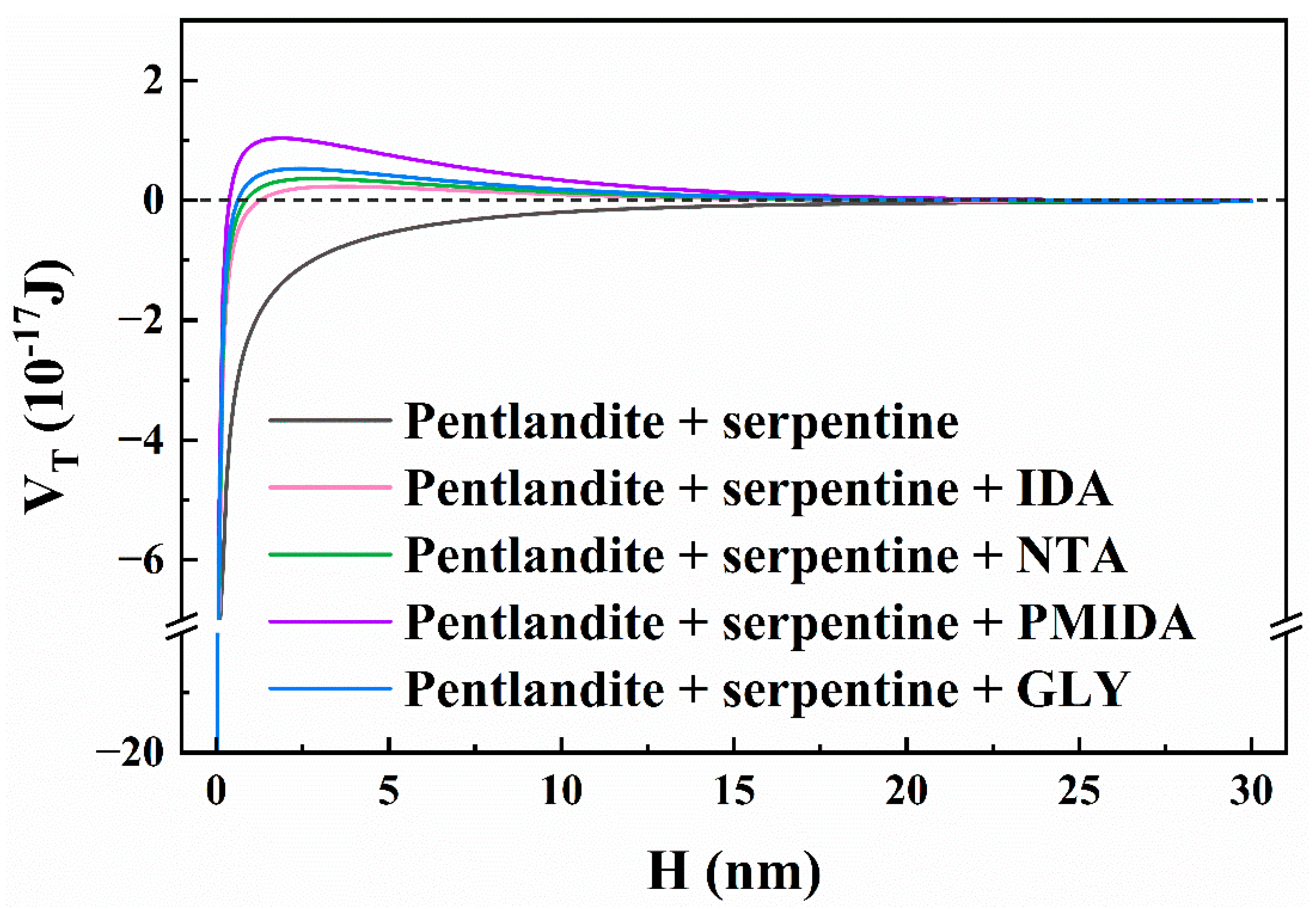
3.3. Zeta-Potential Measurements and Dlvo Calculation
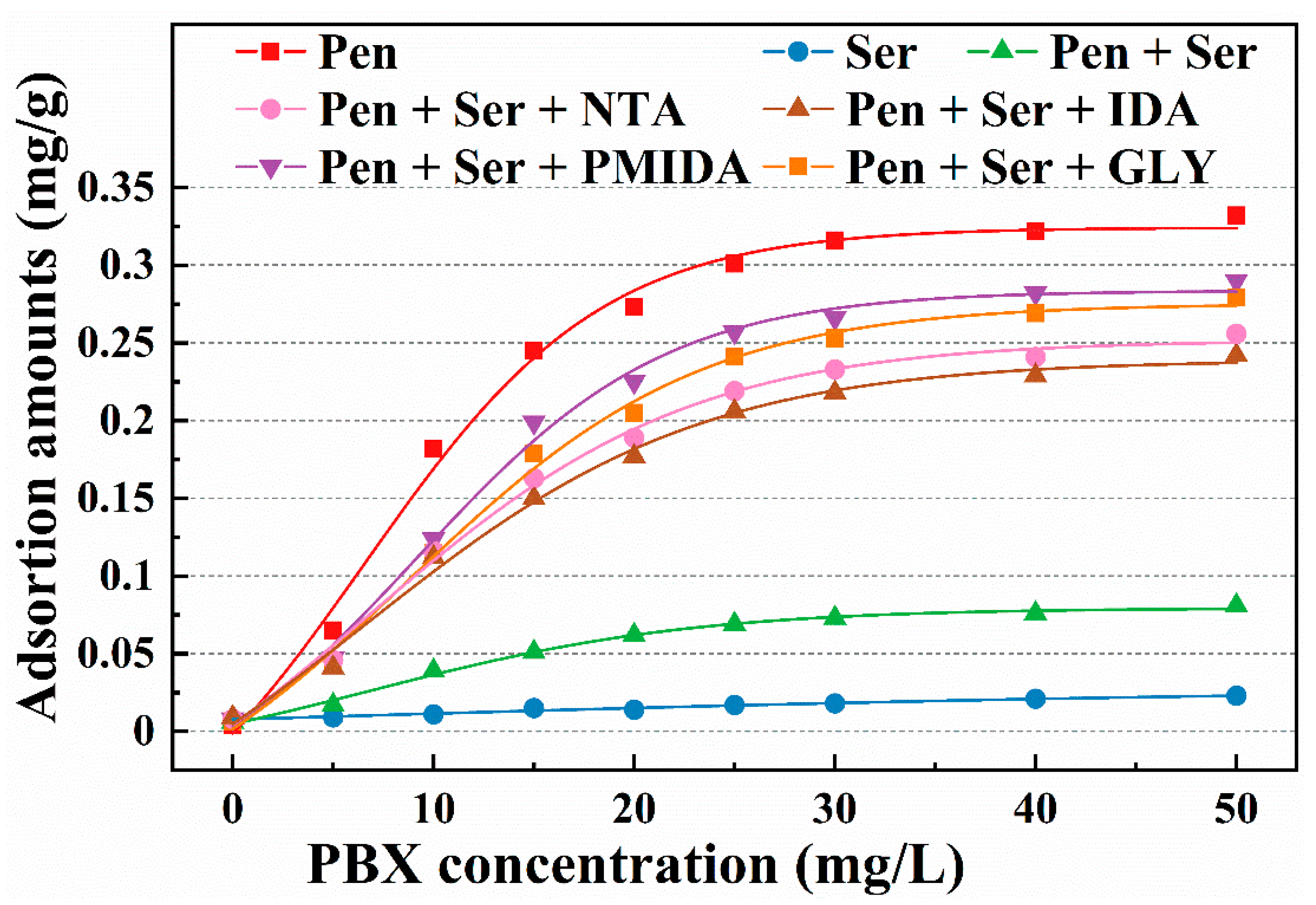
3.4. Reagent Adsorption Measurements
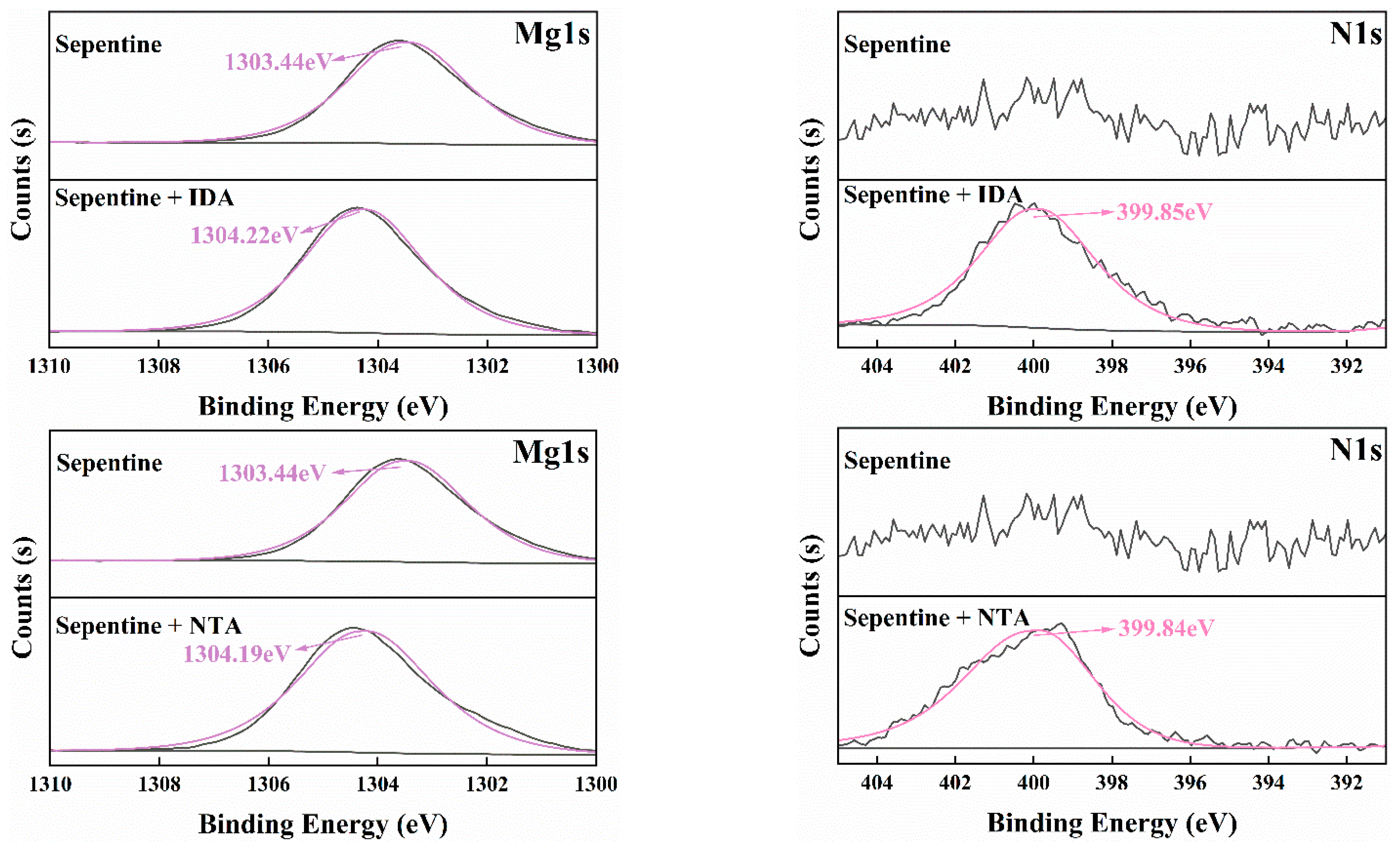
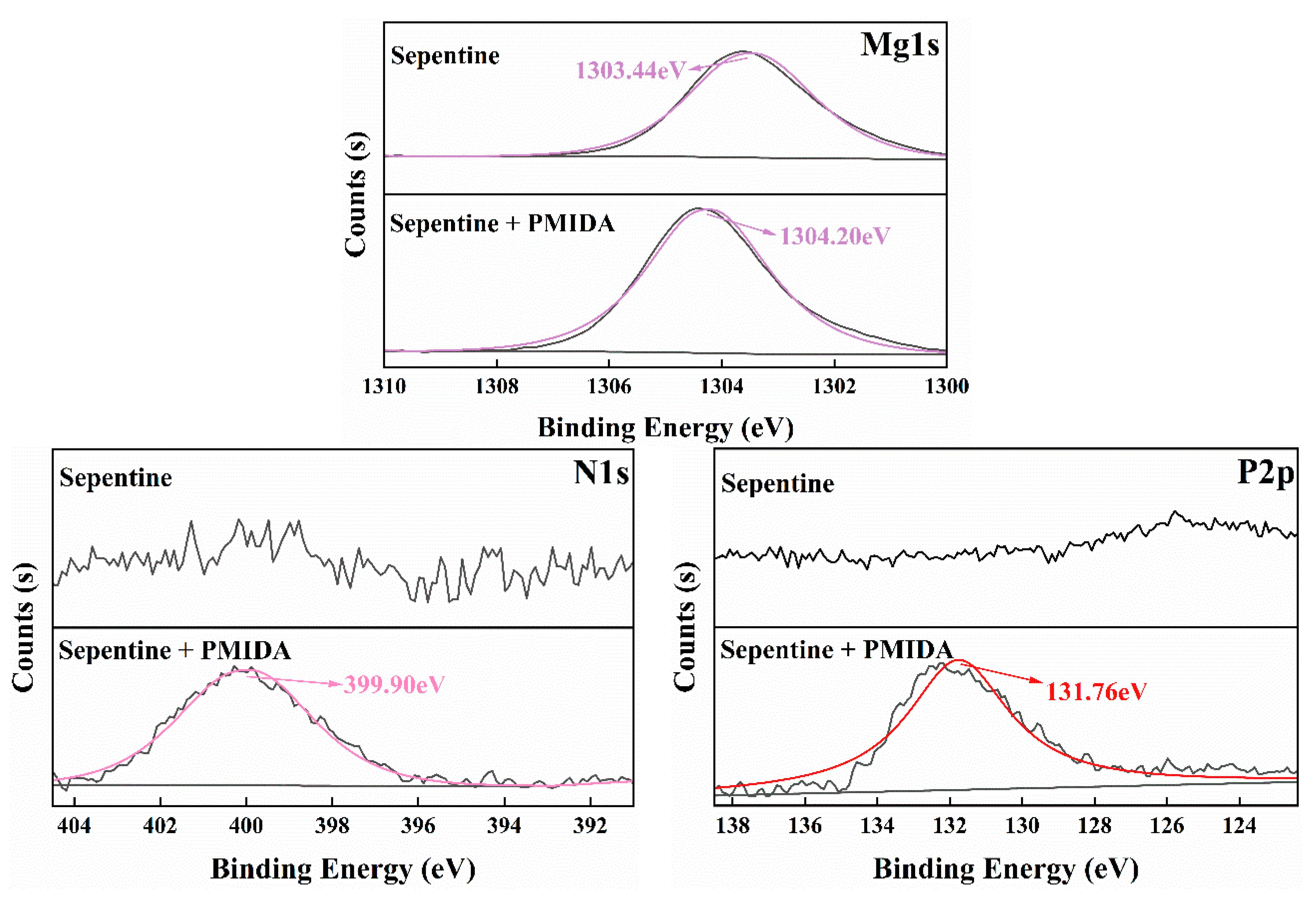
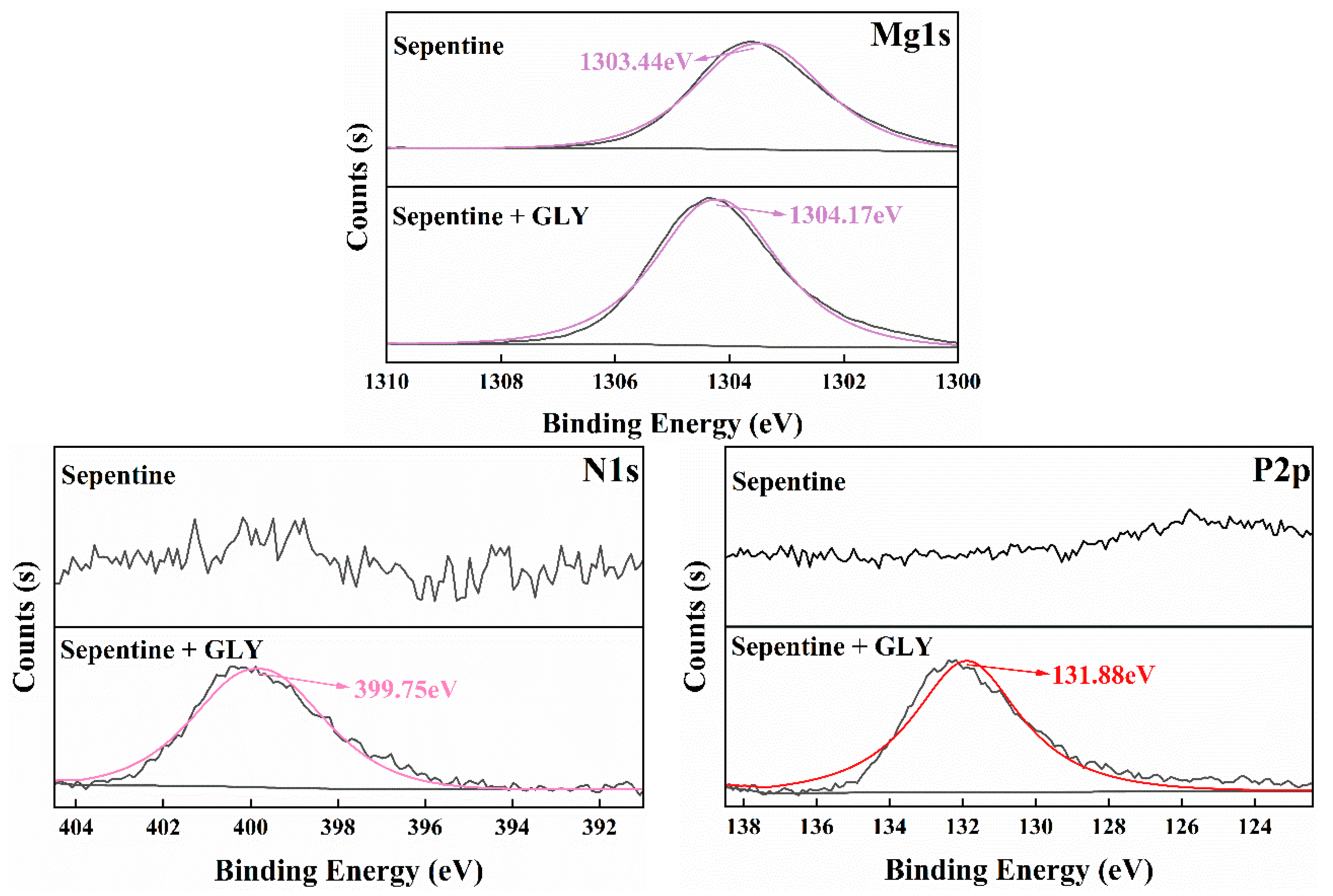
3.5. XPS Analysis
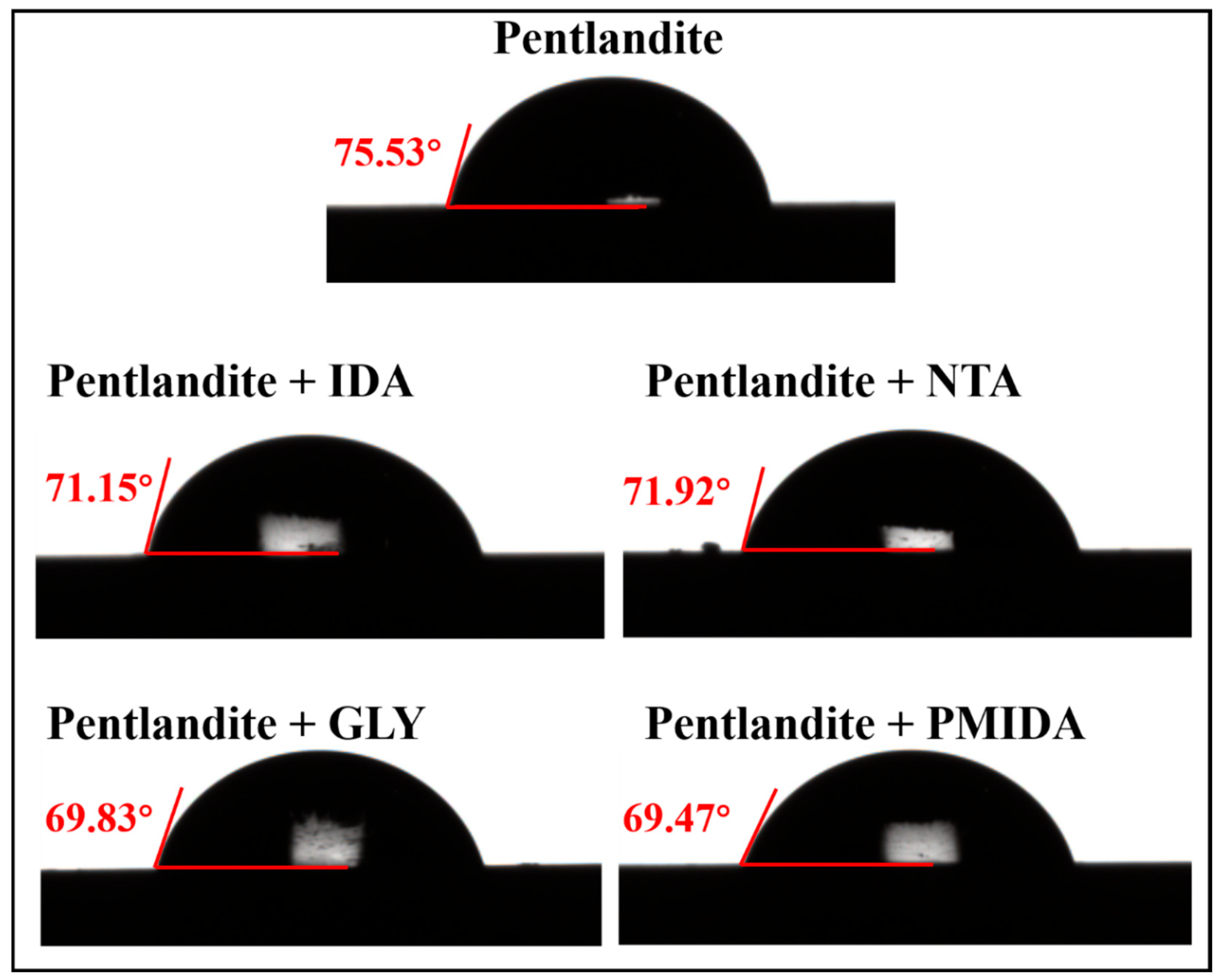
3.6. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobicki, E.R.; Liu, Q.X.; Xu, Z.H. Effect of microwave pre-treatment on ultramafic nickel ore slurry rheology. Miner. Eng. 2014, 61, 97–104. [Google Scholar] [CrossRef]
- Peek, E.; Barnes, A.; Tuzun, A. Nickeliferous pyrrhotite—”Waste or resource?”. Miner. Eng. 2011, 24, 625–637. [Google Scholar] [CrossRef]
- Senior, G.D.; Thomas, S.A. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore. Int. J. Miner. Process. 2005, 78, 49–61. [Google Scholar] [CrossRef]
- Uddin, S.; Rao, S.R.; Mirnezami, M.; Finch, J. Processing an ultramafic ore using fiber disintegration by acid attack. Int. J. Miner. Process. 2012, 102, 38–44. [Google Scholar] [CrossRef]
- Edwards, C.R.; Kipkie, W.B.; Agar, G.E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Wani, O.B.; Manzoor, S.; Molaei, N.; Shoaib, M.; Khan, S.; Zeng, H.; Bobicki, E.R. Beneficiation of Nickel from Ultramafic Ores: Using Sodium Citrate as a Green Processing Reagent. Resour. Conserv. Recycl. 2022, 186, 14. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Fornasiero, D.; Ralston, J. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids Surf. A-Physicochem. Eng. Asp. 2005, 252, 207–212. [Google Scholar] [CrossRef]
- Lu, J.W.; Yuan, Z.T.; Guo, X.F.; Tong, Z.-Y.; Li, L.-X. Magnetic separation of pentlandite from serpentine by selective magnetic coating. Int. J. Miner. Metall. Mater. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Pietrobon, M.; Grano, S.; Sobieraj, S.; Ralston, J. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from Western Australia. Miner. Eng. 1997, 10, 775–786. [Google Scholar] [CrossRef]
- Lu, J.W.; Yuan, Z.T.; Liu, J.T.; Li, L.; Wang, N.; Meng, Q. Selective agglomeration of magnetite in entlandite-serpentine system and implication for their separation. Physicochem. Probl. Miner. Process. 2017, 53, 943–955. [Google Scholar]
- Tang, X.K.; Chen, Y.F. Using oxalic acid to eliminate the slime coatings of serpentine in pyrite flotation. Miner. Eng. 2020, 149, 7. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.X.; Guo, W.; Luo, G.; Zhang, W.; Wang, H. The depression behavior and mechanism of carboxymethyl chitosan on calcite flotation. J. Mater. Res. Technol.-JMRT 2019, 8, 1036–1040. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.P.; Feng, Q.M.; Zhang, M.; Gu, Y. Talc-serpentine interactions and implications for talc depression. Miner. Eng. 2012, 32, 68–73. [Google Scholar] [CrossRef]
- Wiese, J.; Harris, P.; Bradshaw, D. The role of the reagent suite in optimising pentlandite recoveries from the Merensky reef. Miner. Eng. 2006, 19, 1290–1300. [Google Scholar] [CrossRef]
- Peng, Y.; Seaman, D. The flotation of slime-fine fractions of Mt. Keith pentlandite ore in de-ionised and saline water. Miner. Eng. 2011, 24, 479–481. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhang, G.F.; Shi, Q.; Yang, S.; Liu, D. Utilization of tetrasodium iminodisuccinate to eliminate the adverse effect of serpentine on the flotation of pyrite. Miner. Eng. 2020, 150, 6. [Google Scholar] [CrossRef]
- Liu, D.Z.; Zhang, G.F.; Chen, Y.F. Studies on the selective flotation of pyrite from fine serpentine by using citric acid as depressant. Miner. Eng. 2021, 165, 8. [Google Scholar] [CrossRef]
- Huang, J.W.; Zhang, C.Q. Inhibiting effect of citric acid on the floatability of serpentine activated by Cu(II) and Ni(II) ions. Physicochem. Probl. Miner. Process. 2019, 55, 960–968. [Google Scholar]
- Zhang, X.G.; Zhang, J.; Ye, W.L.; Pan, C.L.; Wei, X.X.; Hu, X.Q.; Luo, Y.C.; Xu, P.F. Studies on the application of N,N-bis(phosphonomethyl)-sulfamic acid in the selective flotation separation of pyrite from serpentine. Miner. Eng. 2022, 183, 9. [Google Scholar] [CrossRef]
- Gale, P.; Watts, A. Effect of bacteriorhodopsin on the orientation of the headgroup of 1,2-dimyristoyl-sn-glycero-3-phosphocholine in bilayers: A 31P- and 2H-NMR study. Biochim. Et. Biophys. Acta 1992, 1106, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, K.; Varshney, A. Free-radical polymerization of styrene with p-nitrobenzyl triphenyl phosphonium ylide as an initiator. J. Polym. Sci. Polym. Chem. 2005, 43, 6524–6533. [Google Scholar] [CrossRef]
- Dvinskikh, S.V.; Luz, Z.; Zimmermann, H.; Maliniak, A.; Sandström, D. Molecular characterization of hexaoctyloxy-rufigallol in the solid and columnar phases:: A local field NMR study. J. Phys. Chem. B 2003, 107, 1969–1976. [Google Scholar] [CrossRef]
- Jinno, A.; Ogasawara, Y.; Hashimoto, T.; Mitsumoto, M.; Chang, T.-F.M.; Sone, M.; Kurosu, H. Solid-state 13C NMR spectroscopic study of supercritical CO2 catalyzation treated polyethylene terephthalate textiles for platinum metallization. J. Supercrit. Fluids 2023, 197, 105896. [Google Scholar] [CrossRef]
- Clearfield, A.; Poojary, D.M.; Zhang, B.L.; Zhao, B.; Derecskei-Kovacs, A. Azacrown ether pillared layered zirconium phosphonates and the crystal structure of N,N′-bis(phosphonomethyl)-1,10-diaza-18-crown-6. Chem. Mat. 2000, 12, 2745–2752. [Google Scholar] [CrossRef]
- Hamdy, S.M.; Danial, A.W.; El-Rab, S.; Shoreit, A.A.M.; Hesham, A.E.-L. Production and optimization of bioplastic (Polyhydroxybutyrate) from Bacillus cereus strain SH-02 using response surface methodology. BMC Microbiol. 2022, 22, 183. [Google Scholar] [CrossRef]
- Lima, L.M.P.; Delgado, R.; Plutnar, J.; Hermann, P.; Kotek, J. A New Tris(phosphonomethyl) Monoacetic Acid Cyclam Derivative: Synthesis, Acid-Base and Metal Complexation Studies. Eur. J. Inorg. Chem. 2011, 2011, 527–538. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kawasaki, R.; Yamamoto, M.; Tsuyuki, R.; Niikura, K.; Komiya, K.-I. Molecular fluctuation of chiral and achiral smectic liquid crystals studied by 13C-NMR spectroscopy. Ferroelectrics 2016, 495, 1–16. [Google Scholar] [CrossRef]
- Dwari, R.K.; Mishra, B.K. Evaluation of flocculation characteristics of kaolinite dispersion system using guar gum: A green flocculant. Int. J. Min. Sci. Technol. 2019, 29, 745–755. [Google Scholar] [CrossRef]
- Zhang, N.N.; Pang, T.; Han, R.; Chen, S.; Li, Z.; Yu, Y.; Shi, Z.; Liu, L.; Qu, J.; Zhou, A. Interactions between bubble and particles of key minerals of diasporic bauxite through the extended DLVO theory. Int. J. Min. Sci. Technol. 2022, 32, 201–214. [Google Scholar] [CrossRef]
- Findenegg, G.H.J.N. Israelachvili: Intermolecular and Surface Forces; Academic Press: London, UK; Orlando, FL, USA; San Diego, CA, USA; New York, NY, USA; Toronto, ON, Canada; Montreal, QC, Canada; Sydney, Australia; Tokyo, Japan, 1986; Volume 90, pp. 1241–1242. [Google Scholar]
- Li, Z.H.; Han, Y.X.; Li, Y.J.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferrous Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Cui, W.Y.; Chen, J.H. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Tang, H.H.; Liu, B.J.; Li, M.S.; Zhang, Q.; Zhang, X.; Jiang, F. Cooperative effect of sodium lauryl sulfate collector and sodium pyrophosphate depressant on the flotation separation of lead oxide minerals from hematite. Int. J. Miner. Metall. Mater. 2024, 31, 1975–1984. [Google Scholar] [CrossRef]
- Wesolowski, G.; Kubala-Kukus, A.; Banas, D.; Szary, K.; Stabrawa, I.; Foks, A.; Jabłońskia, Ł.; Jagodzińskia, P.; Pajeka, M.; Stachura, R.; et al. X-ray Photoelectron Spectroscopy in the Analysis of Titanium and Palladium Nanolayers. Acta Phys. Pol. A 2024, 145, 74. [Google Scholar] [CrossRef]
- Mweene, L.; Khanal, G.P.; Etim, E. New insights into the surface chemical properties of serpentine and flotation of pyrite using xanthomonas campestris as a novel selective serpentine depressant: Experimental and DFT investigation. Miner. Eng. 2025, 222, 109141. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef]
- Kamali, K.Z.; Pandikumar, A.; Jayabal, S.; Ramaraj, R.; Lim, H.N.; Ong, B.H.; Bien, C.S.D.; Kee, Y.Y.; Huang, N.M. Amalgamation based optical and colorimetric sensing of mercury(II) ions with silver@graphene oxide nanocomposite materials. Microchim. Acta 2016, 183, 369–377. [Google Scholar] [CrossRef]
- Uhl, F.; Staemmler, V. An ab initio study of the O1s and Mg1s, Mg2s, Mg2p core electron binding energies in bulk MgO. J. Electron. Spectrosc. Relat. Phenom. 2019, 233, 90–96. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, L.H.; Zhang, C.H.; Zhou, B.X.; Zheng, S.R.; Zhang, W.G.; Cai, S.L.; Fan, J. Porous amorphous metal-organic frameworks based on heterotopic triangular ligands for iodine and high-capacity dye adsorption. Dalton Trans. 2023, 52, 12087–12097. [Google Scholar] [CrossRef]
- Thiébaut, J.M.; Belmonte, T.; Chaleix, D.; Choquet, P.; Baravian, G.; Puech, V.; Michel, H. Comparison of surface cleaning by two atmospheric pressure discharges. Surf. Coat. Technol. 2003, 169, 186–189. [Google Scholar] [CrossRef]
- Thurakkal, S.; Zhang, X.Y. Covalent functionalization of two-dimensional black phosphorus nanosheets with porphyrins and their photophysical characterization. Mater. Chem. Front. 2021, 5, 2824–2831. [Google Scholar] [CrossRef]
- Vu, N.T.; Huang, Y.B.; Weibel, J.A. High-speed visualization and infrared thermography of pool boiling on surfaces having differing dynamic wettability. Int. J. Heat Mass Transf. 2024, 221, 10. [Google Scholar] [CrossRef]
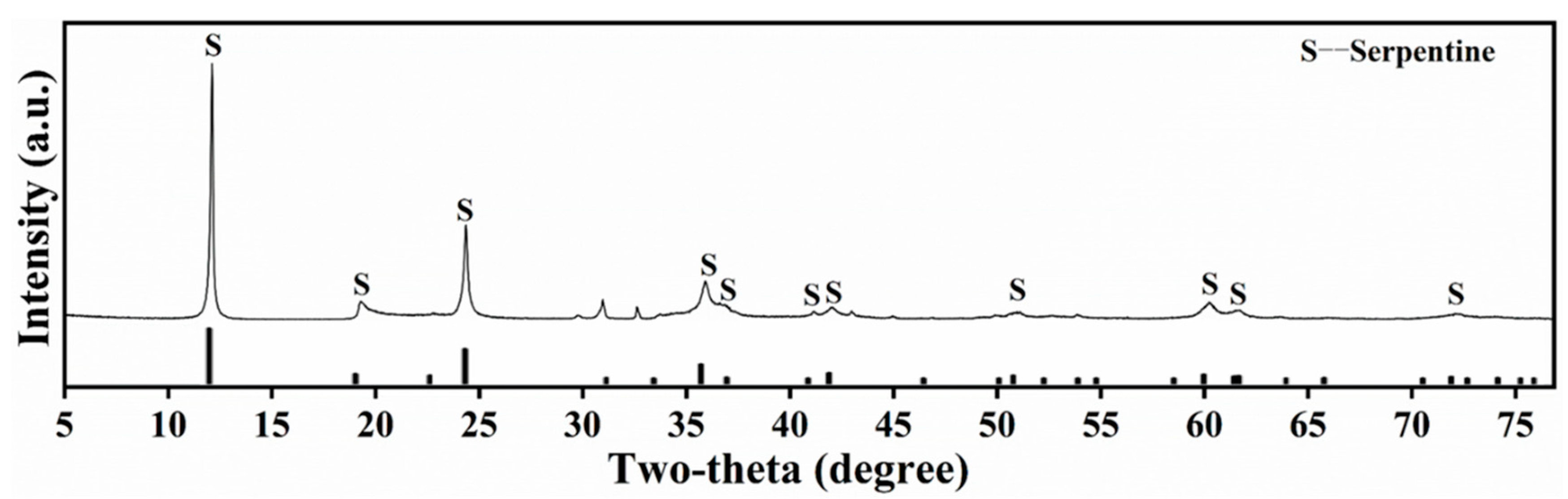
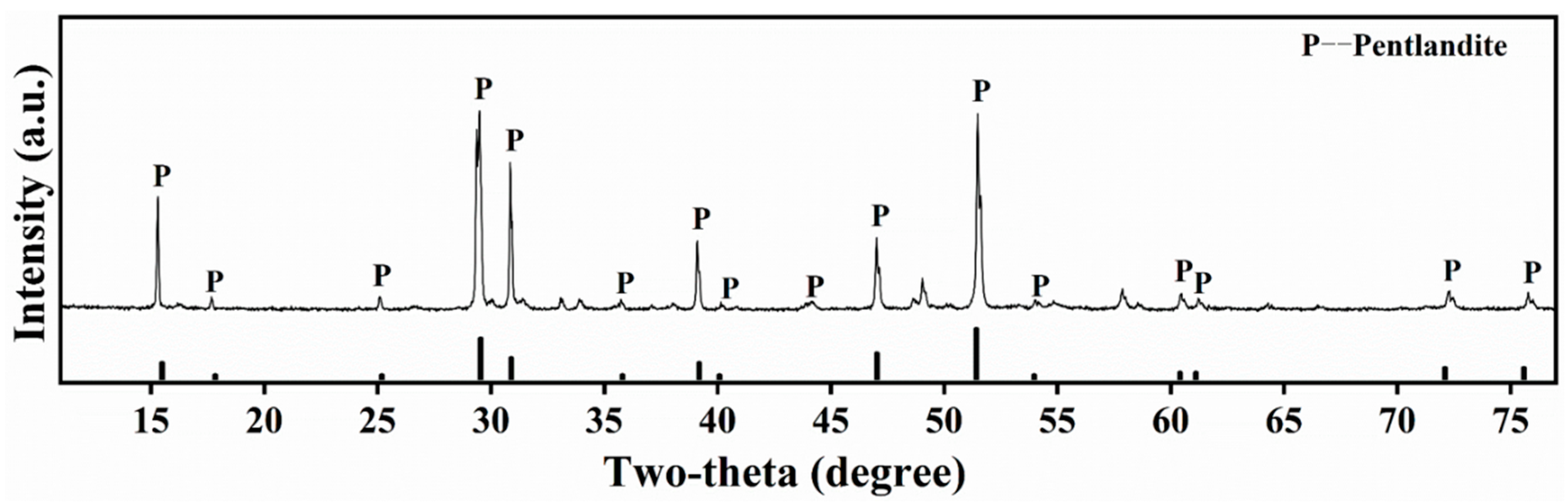
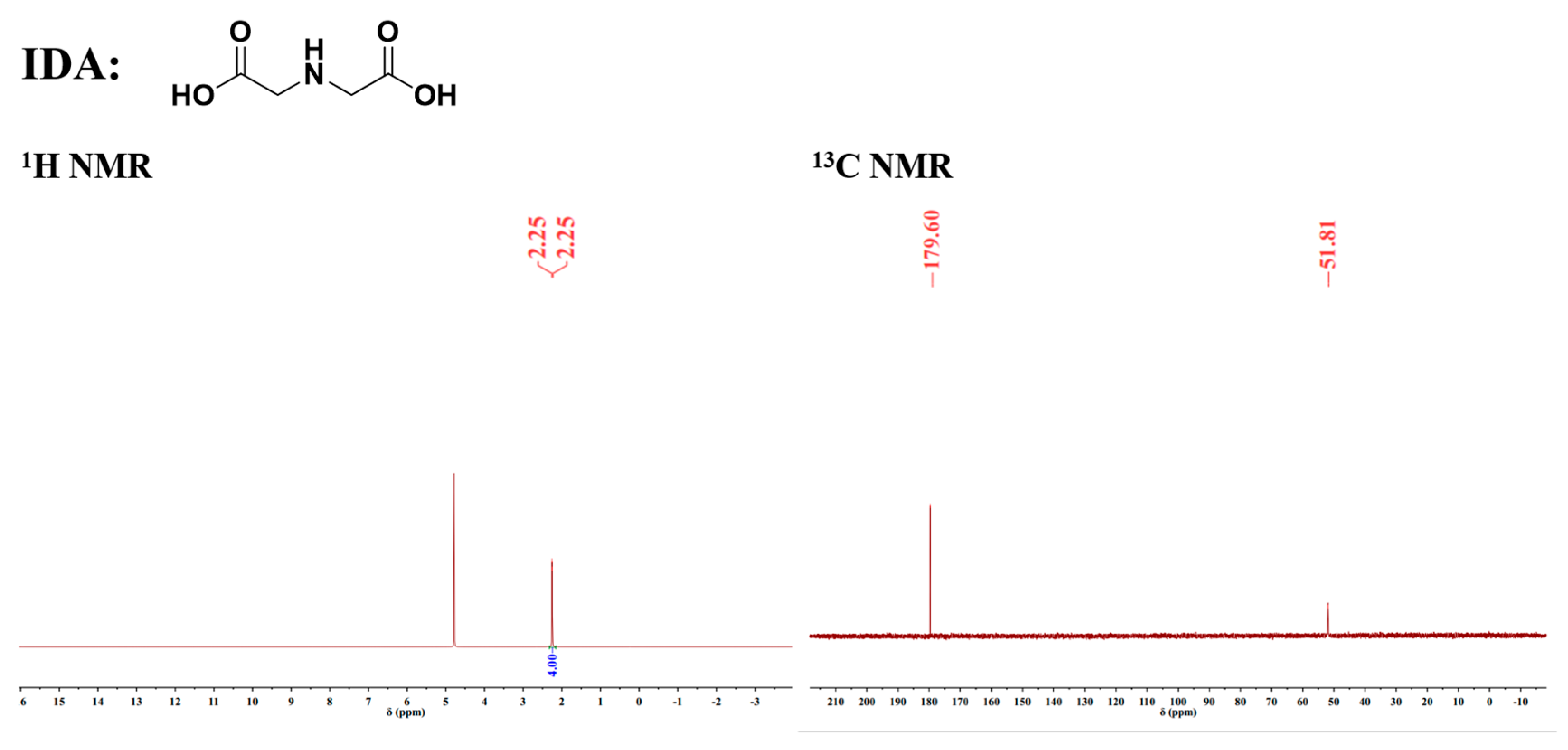


| Sample | SiO2 | MgO | CaO | Al2O3 | Fe | Ni | S |
|---|---|---|---|---|---|---|---|
| Serpentine | 41.77 | 36.06 | 0.23 | 0.88 | 5.41 | - | - |
| Pentlandite | - | - | - | - | 27.15 | 29.28 | 30.59 |
| Depressants | Products | Yields (%) | Grades (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| Ni | Cu | Ni | Cu | |||
| No Depressants | Concentrate | 18.07 | 3.75 | 2.15 | 49.91 | 34.26 |
| Tailing | 81.93 | 0.83 | 0.91 | 50.09 | 65.74 | |
| Feed | 100 | 1.36 | 1.13 | 100 | 100 | |
| IDA | Concentrate | 11.39 | 7.18 | 4.11 | 59.43 | 44.84 |
| Tailing | 88.61 | 0.63 | 0.65 | 40.57 | 55.16 | |
| Feed | 100 | 1.38 | 1.04 | 100 | 100 | |
| NTA | Concentrate | 11.33 | 7.22 | 4.19 | 59.81 | 45.94 |
| Tailing | 88.67 | 0.62 | 0.63 | 40.19 | 54.06 | |
| Feed | 100 | 1.37 | 1.03 | 100 | 100 | |
| GLY | Concentrate | 11.19 | 7.39 | 4.32 | 60.42 | 46.75 |
| Tailing | 88.81 | 0.61 | 0.62 | 39.58 | 53.25 | |
| Feed | 100 | 1.37 | 1.03 | 100 | 100 | |
| PMIDA | Concentrate | 11.13 | 7.45 | 4.39 | 61.26 | 47.82 |
| Tailing | 88.87 | 0.59 | 0.6 | 38.74 | 52.18 | |
| Feed | 100 | 1.35 | 1.02 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Sun, W.; Gao, Z.; Lu, B.; Su, X.; Luo, C.; Peng, X.; Cao, J. The Depression Effect of Micromolecular Depressant Containing Amino and Phosphonic Acid Group on Serpentine in the Flotation of Low-Grade Nickel Sulphide Ore. Minerals 2025, 15, 1116. https://doi.org/10.3390/min15111116
Zhang C, Sun W, Gao Z, Lu B, Su X, Luo C, Peng X, Cao J. The Depression Effect of Micromolecular Depressant Containing Amino and Phosphonic Acid Group on Serpentine in the Flotation of Low-Grade Nickel Sulphide Ore. Minerals. 2025; 15(11):1116. https://doi.org/10.3390/min15111116
Chicago/Turabian StyleZhang, Chenxu, Wei Sun, Zhiyong Gao, Bingang Lu, Xiaohui Su, Chunhua Luo, Xiangan Peng, and Jian Cao. 2025. "The Depression Effect of Micromolecular Depressant Containing Amino and Phosphonic Acid Group on Serpentine in the Flotation of Low-Grade Nickel Sulphide Ore" Minerals 15, no. 11: 1116. https://doi.org/10.3390/min15111116
APA StyleZhang, C., Sun, W., Gao, Z., Lu, B., Su, X., Luo, C., Peng, X., & Cao, J. (2025). The Depression Effect of Micromolecular Depressant Containing Amino and Phosphonic Acid Group on Serpentine in the Flotation of Low-Grade Nickel Sulphide Ore. Minerals, 15(11), 1116. https://doi.org/10.3390/min15111116









