Geochemical Characteristics and Health Risks of Coal Dust: An Integrated Review from Component-Dependent Toxicity to Emerging Oxidative Toxicity Indicators
Abstract
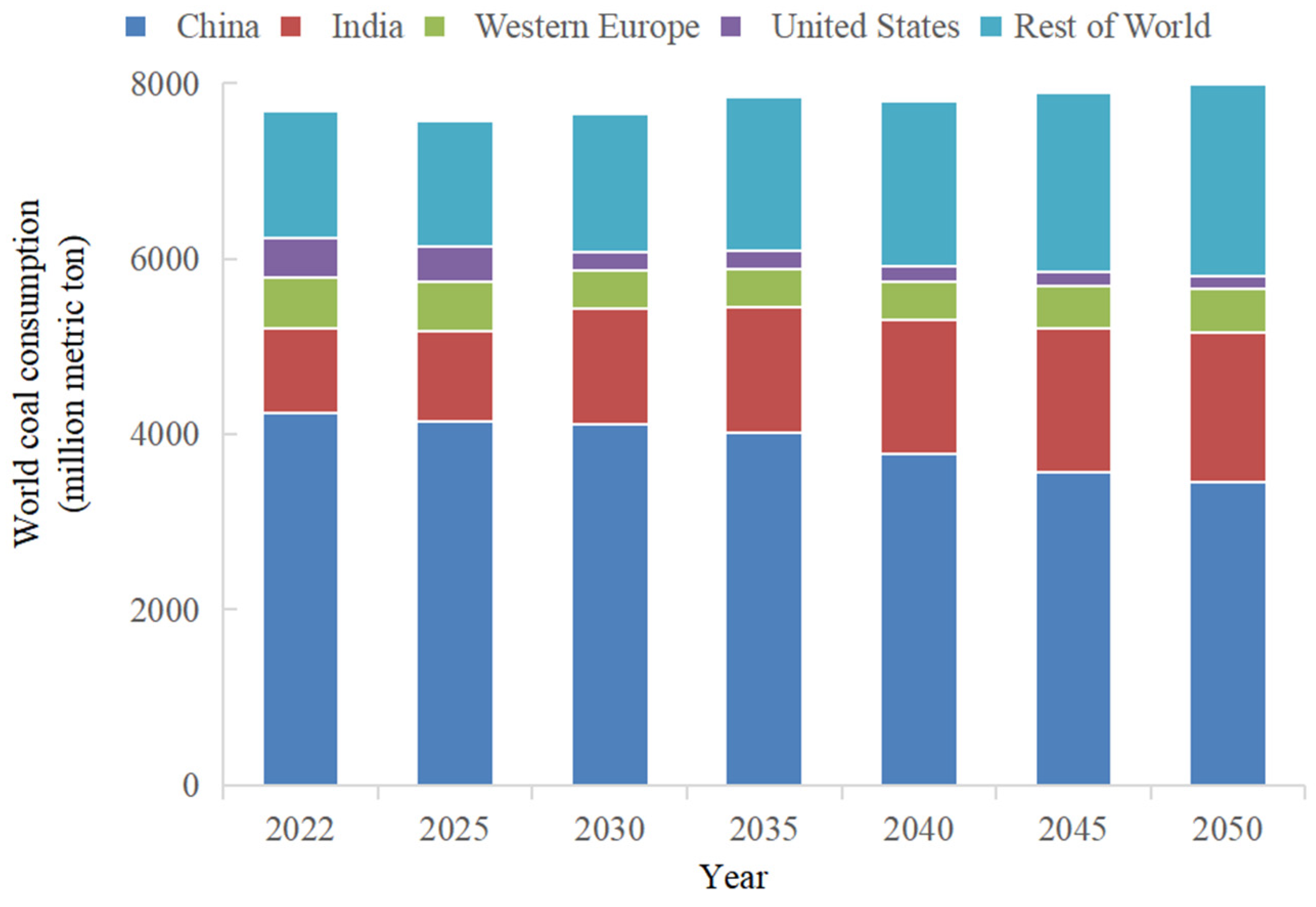
1. Introduction
2. Bibliometric Analysis
2.1. Data Sources and Methods
2.2. Analysis of Bibliometric Results
2.2.1. Literature Quantity Analysis
2.2.2. Country Co-Authorship Analysis
2.2.3. Keyword Burst Analysis
2.2.4. Document Co-Citation Analysis
3. Geochemical Characteristics of Coal Dust
4. Bioaccessibility of Substances in Coal Dust
4.1. Metals
4.2. Organic Compounds
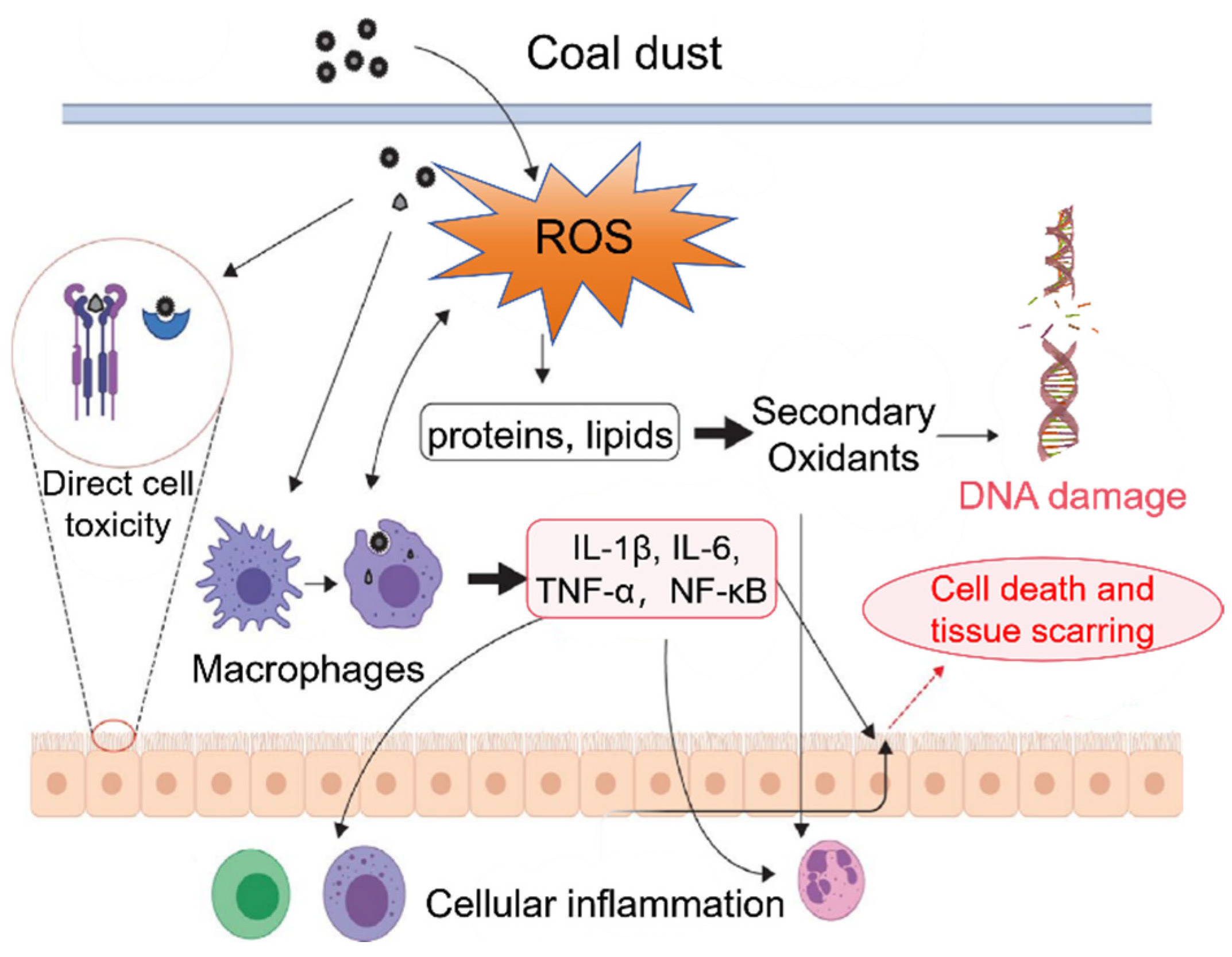
5. Coal Dust Oxidative Toxicity Assessment
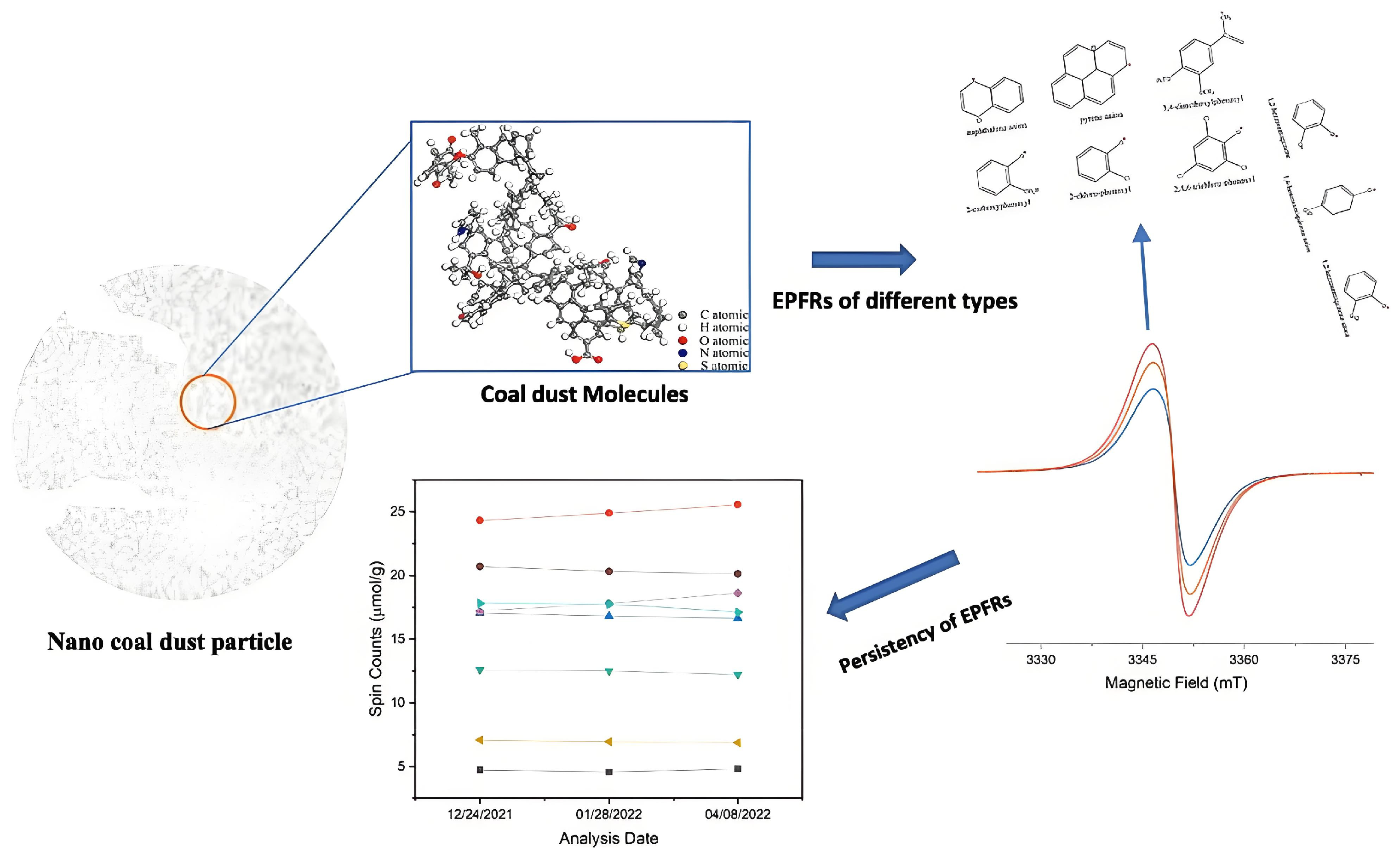
5.1. Environmentally Persistent Free Radicals (EPFRs)
5.2. Oxidative Potential (OP)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- China National Coal Association, China National Coal Association 2023 China Coal Industry Development Annual Report; China National Coal Association: Beijing, China, 2024.
- Wei, W.; Mushtaq, Z.; Sharif, M.; Zeng, X.; Wan-Li, Z.; Qaisrani, M.A. Evaluating the Coal Rebound Effect in Energy Intensive Industries of China. Energy 2020, 207, 118247. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2023; U.S. Energy Information Administration: Washington, DC, USA, 2023. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 1 June 2024).
- Sarver, E.; Keles, C.; Rezaee, M. Characteristics of Respirable Dust in Eight Appalachian Coal Mines: A Dataset Including Particle Size and Mineralogy Distributions, and Metal and Trace Element Mass Concentrations. Data Brief 2019, 25, 104032. [Google Scholar] [CrossRef]
- Eskanlou, A.; Arnold, B.J. An Evaluation of Pyrite as a Component of Respirable Coal Dust. J. Hazard. Mater. 2024, 477, 135340. [Google Scholar] [CrossRef]
- Das, M.; Salinas, V.; LeBoeuf, J.; Khan, R.; Jacquez, Q.; Camacho, A.; Hovingh, M.; Zychowski, K.; Rezaee, M.; Roghanchi, P.; et al. A Toxicological Study of the Respirable Coal Mine Dust: Assessment of Different Dust Sources within the Same Mine. Minerals 2023, 13, 433. [Google Scholar] [CrossRef]
- Sarver, E.; Keleş, Ç.; Afrouz, S.G. Particle Size and Mineralogy Distributions in Respirable Dust Samples from 25 US Underground Coal Mines. Int. J. Coal Geol. 2021, 247, 103851. [Google Scholar] [CrossRef]
- Graber, J.M.; Harris, G.; Almberg, K.S.; Rose, C.S.; Petsonk, E.L.; Cohen, R.A. Increasing Severity of Pneumoconiosis Among Younger Former US Coal Miners Working Exclusively Under Modern Dust-Control Regulations. J Occup Env. Med 2017, 59, e105–e111. [Google Scholar] [CrossRef]
- Fubini, B.; Fenoglio, I. Toxic Potential of Mineral Dusts. Elements 2007, 3, 407–414. [Google Scholar] [CrossRef]
- Sventeková, E.; Prievozník, P.; Mlčoch, J.; Vandlíčková, M. Assessment of the Dust in Underground Coal Mine. Appl. Sci. 2024, 14, 6038. [Google Scholar] [CrossRef]
- LaBranche, N.; Keles, C.; Sarver, E.; Johnstone, K.; Cliff, D. Characterization of Particulates from Australian Underground Coal Mines. Minerals 2021, 11, 447. [Google Scholar] [CrossRef]
- Chen, C.; Song, M. Visualizing a Field of Research: A Methodology of Systematic Scientometric Reviews. PLoS ONE 2019, 14, e0223994. [Google Scholar] [CrossRef]
- Petsonk, E.L.; Rose, C.; Cohen, R. Coal Mine Dust Lung Disease. New Lessons from an Old Exposure. Am. J. Respir. Crit. Care Med. 2013, 187, 1178–1185. [Google Scholar] [CrossRef]
- Laney, A.S.; Weissman, D.N. Respiratory Diseases Caused by Coal Mine Dust. J. Occup. Environ. Med. 2014, 56, S18–S22. [Google Scholar] [CrossRef]
- Kurth, L.M.; McCawley, M.; Hendryx, M.; Lusk, S. Atmospheric Particulate Matter Size Distribution and Concentration in West Virginia Coal Mining and Non-Mining Areas. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 405–411. [Google Scholar]
- Onder, M.; Onder, S. Evaluation of Occupational Exposures to Respirable Dust in Underground Coal Mines. Ind. Health 2009, 47, 43–49. [Google Scholar] [PubMed]
- Cohen, R.; Patel, A.; Green, F. Lung Disease Caused by Exposure to Coal Mine and Silica Dust. Semin. Respir. Crit. Care Med. 2009, 29, 651–661. [Google Scholar]
- Moreno, T.; Trechera, P.; Querol, X.; Lah, R.; Johnson, D.; Wrana, A.; Williamson, B. Trace Element Fractionation between PM10 and PM2.5 in Coal Mine Dust: Implications for Occupational Respiratory Health. Int. J. Coal Geol. 2019, 203, 52–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Shao, B.; Pang, D.; Han, G.; Lin, L. Effects of Abnormal Expression of Fusion and Fission Genes on the Morphology and Function of Lung Macrophage Mitochondria in SiO2-Induced Silicosis Fibrosis in Rats in Vivo. Toxicol. Lett. 2019, 312, 181–187. [Google Scholar] [CrossRef]
- Sarver, E.; Keles, C.; Rezaee, M. Beyond Conventional Metrics: Comprehensive Characterization of Respirable Coal Mine Dust. Int. J. Coal Geol. 2019, 207, 84–95. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Wirbisky-Hershberger, S.E.; Olivero-Verbel, J.; de la Rosa, J.; Freeman, J.L. Embryonic Exposure to an Aqueous Coal Dust Extract Results in Gene Expression Alterations Associated with the Development and Function of Connective Tissue and the Hematological System, Immunological and Inflammatory Disease, and Cancer in Zebrafish. Metallomics 2018, 10, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Johann-Essex, V.; Keles, C.; Sarver, E. A Computer-Controlled SEM-EDX Routine for Characterizing Respirable Coal Mine Dust. Minerals 2017, 7, 15. [Google Scholar] [CrossRef]
- Skubacz, K.; Wojtecki, Ł.; Urban, P. Aerosol Concentration and Particle Size Distributions in Underground Excavations of a Hard Coal Mine. Int. J. Occup. Saf. Ergon. 2017, 23, 318–327. [Google Scholar] [CrossRef]
- Hajizadehmotlagh, M.; Paprotny, I. Miniaturized Wearable Respirable Dust Monitor (WEARDM) for Underground Coal Mines: Designs and Experimental Evaluation. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October; IEEE: Montreal, QC, Canada, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Doney, B.C.; Blackley, D.; Hale, J.M.; Halldin, C.; Kurth, L.; Syamlal, G.; Laney, A.S. Respirable Coal Mine Dust in Underground Mines, United States, 1982–2017. Am. J. Ind. Med. 2019, 62, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Xue, S.; Zhou, Z.; Zhang, X.; Kang, Y.; Zhang, J.; Zhang, M. Exposure to Coal Dust Exacerbates Cognitive Impairment by Activating the IL6/ERK1/2/SP1 Signaling Pathway. Sci. Total Environ. 2024, 946, 174202. [Google Scholar] [CrossRef]
- Kamanzi, C.; Becker, M.; Von Holdt, J.; Hsu, N.-J.; Konečný, P.; Broadhurst, J.; Jacobs, M. Machine Learning Demonstrates Dominance of Physical Characteristics over Particle Composition in Coal Dust Toxicity. Environ. Sci. Technol. 2024, 58, 1636–1647. [Google Scholar] [CrossRef]
- Buitrago-Rodríguez, M.Y.; Rangel, N.; Vega-Valderrama, J.D.; Pulido-Medellín, M.; Rondón-Lagos, M. Unraveling Chromosomal and Genotoxic Damage in Individuals Occupationally Exposed to Coal from Underground Mining. Front. Genet. 2024, 15, 1422938. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Liu, Y.; Li, H.; Guan, S.; Zhu, L.; Jia, L.; Liu, Z.; Xu, H. The Role of Macrophage Polarization and Related Key Molecules in Pulmonary Inflammation and Fibrosis Induced by Coal Dust Dynamic Inhalation Exposure in Sprague-Dawley Rats. Cytokine 2024, 173, 156419. [Google Scholar] [CrossRef]
- Din, I.U.; Muhammad, S.; Faisal, S.; ur Rehman, I.; Ali, W. Heavy Metal(Loid)s Contamination and Ecotoxicological Hazards in Coal, Dust, and Soil Adjacent to Coal Mining Operations, Northwest Pakistan. J. Geochem. Explor. 2024, 256, 107332. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. The Impacts of Coal Dust on Miners’ Health: A Review. Env. Res 2020, 190, 109849. [Google Scholar] [CrossRef]
- Blackley, D.J.; Halldin, C.N.; Laney, A.S. Continued Increase in Prevalence of Coal Workers’ Pneumoconiosis in the United States, 1970–2017. Am J Public Health 2018, 108, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Johann-Essex, V.; Keles, C.; Rezaee, M.; Scaggs-Witte, M.; Sarver, E. Respirable Coal Mine Dust Characteristics in Samples Collected in Central and Northern Appalachia. Int. J. Coal Geol. 2017, 182, 85–93. [Google Scholar] [CrossRef]
- Trechera, P.; Moreno, T.; Córdoba, P.; Moreno, N.; Zhuang, X.; Li, B.; Li, J.; Shangguan, Y.; Kandler, K.; Dominguez, A.O.; et al. Mineralogy, Geochemistry and Toxicity of Size-Segregated Respirable Deposited Dust in Underground Coal Mines. J Hazard Mater 2020, 399, 122935. [Google Scholar] [CrossRef]
- Hall, N.B.; Blackley, D.J.; Halldin, C.N.; Laney, A.S. Current Review of Pneumoconiosis Among US Coal Miners. Curr. Environ. Health Rep. 2019, 6, 137–147. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Dehbandi, R.; Mohammadyan, M.; Aarabi, M.; Dominguez, A.O.; Kelly, F.J.; Khodabakhshloo, N.; Rahman, M.M.; Naidu, R. Physico-Chemical Properties and Reactive Oxygen Species Generation by Respirable Coal Dust: Implication for Human Health Risk Assessment. J Hazard Mater 2021, 405, 124185. [Google Scholar] [CrossRef]
- Cohen, R.A.; Petsonk, E.L.; Rose, C.; Young, B.; Regier, M.; Najmuddin, A.; Abraham, J.L.; Churg, A.; Green, F.H.Y. Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am. J. Respir. Crit. Care Med. 2016, 193, 673–680. [Google Scholar] [CrossRef]
- Su, X.; Ding, R.; Zhuang, X. Characteristics of Dust in Coal Mines in Central North China and Its Research Significance. ACS Omega 2020, 5, 9233–9250. [Google Scholar] [CrossRef]
- Trechera, P.; Querol, X.; Lah, R.; Johnson, D.; Wrana, A.; Williamson, B.; Moreno, T. Chemistry and Particle Size Distribution of Respirable Coal Dust in Underground Mines in Central Eastern Europe. Int. J. Coal Sci. Technol. 2022, 9, 3. [Google Scholar] [CrossRef]
- Salinas, V.; Das, M.; Jacquez, Q.; Camacho, A.; Zychowski, K.; Hovingh, M.; Medina, A.; Rubasinghege, G.; Rezaee, M.; Baltrusaitis, J.; et al. Characterization and Toxicity Analysis of Lab-Created Respirable Coal Mine Dust from the Appalachians and Rocky Mountains Regions. Minerals 2022, 12, 898. [Google Scholar] [CrossRef]
- Keles, C.; Taborda, M.J.; Sarver, E. Updating “Characteristics of Respirable Dust in Eight Appalachian Coal Mines: A Dataset Including Particle Size and Mineralogy Distributions, and Metal and Trace Element Mass Concentrations” with Expanded Data to Cover a Total of 25 US Mines. Data Brief 2022, 42, 108125. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; Zhuang, X.; Querol, X.; Li, B.; Li, J.; Moreno, N.; Trechera, P.; Sola, P.C.; Uzu, G. Mineralogical and Geochemical Variations from Coal to Deposited Dust and Toxicity of Size-Segregated Respirable Dust in a Blasting Mining Underground Coal Mine in Hunan Province, South China. Int. J. Coal Geol. 2021, 248, 103863. [Google Scholar] [CrossRef]
- Shangguan, Y.; Zhuang, X.; Querol, X.; Li, B.; Moreno, N.; Trechera, P.; Sola, P.C.; Uzu, G.; Li, J. Physicochemical Characteristics and Oxidative Potential of Size-Segregated Respirable Coal Mine Dust: Implications for Potentially Hazardous Agents and Health Risk Assessment. Int. J. Coal Geol. 2024, 282, 104433. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Xu, X.; Cao, X. In Vitro Bioaccessibility and Health Risk Assessment of Heavy Metals in Atmospheric Particulate Matters from Three Different Functional Areas of Shanghai, China. Sci. Total Environ. 2018, 610–611, 546–554. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Oroszlany, B.; Muzandu, K.; Choongo, K.; Kabalo, A.N.; Ntapisha, J.; et al. Lead Poisoning in Children from Townships in the Vicinity of a Lead–Zinc Mine in Kabwe, Zambia. Chemosphere 2015, 119, 941–947. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Yabe, J.; Makumba, J.; Schutzmeier, P.; Ericson, B.; Caravanos, J. Lead Intoxicated Children in Kabwe, Zambia. Environ. Res. 2018, 165, 420–424. [Google Scholar] [CrossRef]
- Ren, H.; Yu, Y.; An, T. Bioaccessibilities of Metal(Loid)s and Organic Contaminants in Particulates Measured in Simulated Human Lung Fluids: A Critical Review. Environ. Pollut. 2020, 265, 115070. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Cihlová, M.; Jarošíková, A.; Mihaljevič, M.; Drahota, P.; Kříbek, B.; Vaněk, A.; Penížek, V.; Sracek, O.; Klementová, M.; et al. Oral Bioaccessibility of Metal(Loid)s in Dust Materials from Mining Areas of Northern Namibia. Environ. Int. 2019, 124, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Limbeck, A. Comparison of the Extraction Efficiencies of Different Leaching Agents for Reliable Assessment of Bio-Accessible Trace Metal Fractions in Airborne Particulate Matter. E3S Web Conf. 2013, 1, 05001. [Google Scholar] [CrossRef]
- Zádrapová, D.; Titěra, A.; Száková, J.; Čadková, Z.; Cudlín, O.; Najmanová, J.; Tlustoš, P. Mobility and Bioaccessibility of Risk Elements in the Area Affected by the Long-Term Opencast Coal Mining. J. Environ. Sci. Health Part A 2019, 54, 1159–1169. [Google Scholar] [CrossRef]
- Sun, Y.; Kinsela, A.S.; Cen, X.; Sun, S.; Collins, R.N.; Cliff, D.I.; Wu, Y.; Waite, T.D. Impact of Reactive Iron in Coal Mine Dust on Oxidant Generation and Epithelial Lung Cell Viability. Sci. Total Environ. 2022, 810, 152277. [Google Scholar] [CrossRef]
- Bourliva, A.; Kelepertzis, E.; Papadopoulou, L.; Patinha, C.; Kantiranis, N. Enhanced Gastric/Lung Arsenic Bioaccessibility from Lignite Fly Ashes: Comparing Bioaccessibility Rates with Multiple Environmental Matrices. Toxics 2023, 11, 358. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Clough, R.; Foulkes, M.; Worsfold, P. Stability of Arsenic Species During Bioaccessibility Assessment Using the In Vitro UBM and HPLC-ICP-MS Detection. Biol. Trace Elem. Res. 2020, 198, 332–338. [Google Scholar] [CrossRef]
- Chen, X.; Singh, A.; Kitts, D.D. In-Vitro Bioaccessibility and Bioavailability of Heavy Metals in Mineral Clay Complex Used in Natural Health Products. Sci. Rep. 2020, 10, 8823. [Google Scholar] [CrossRef]
- Sun, Y.; Jones, K.; Sun, Z.; Shen, J.; Bu, F.; Ma, F.; Gu, Q. Can Arsenic Bioavailability Be Predicted in Soils Using in Vitro Gastro-Intestinal Simulation? Ecotoxicol. Environ. Saf. 2024, 275, 116235. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, D.; Baciocchi, R. Different Approaches for Incorporating Bioaccessibility of Inorganics in Human Health Risk Assessment of Contaminated Soils. Appl. Sci. 2021, 11, 3005. [Google Scholar] [CrossRef]
- Özlü, E. Investigation of Bioaccessibility and Sources of Elements in Road Dust: Implications for Ecological and Human Health Risks. Microchem. J. 2024, 205, 111374. [Google Scholar] [CrossRef]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van de Wiele, T.; Wragg, J.; Rompelberg, C.J.M.; et al. Comparison of Five In Vitro Digestion Models To Study the Bioaccessibility of Soil Contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Skála, J.; Boahen, F.; Száková, J.; Vácha, R.; Tlustoš, P. Arsenic and Lead in Soil: Impacts on Element Mobility and Bioaccessibility. Environ. Geochem. Health 2022, 44, 943–959. [Google Scholar] [CrossRef]
- Sun, L.; Sun, C.; Liu, F.; Bao, X. Health Risk Assessment of Oral Bioaccessibility of Heavy Metal in Soil from Coalfield in Huaibei City, China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 2045–2055. [Google Scholar] [CrossRef]
- Soares, M.; Oliveira, H.; Alves, C. Airborne Particulate Matter Inhalation Bioaccessibility: A Review of Methodological Aspects. Chem. Biol. Interact. 2025, 408, 111403. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zong, Z.-M.; Ding, M.-J.; Zhou, L.; Huang, Y.-G.; Zheng, Y.-X.; Jin, X.; Ma, Y.-M.; Wei, X.-Y. Separation and Analysis of Organic Compounds in an Erdos Coal. Fuel 2009, 88, 469–474. [Google Scholar] [CrossRef]
- Coronado-Posada, N.; Cabarcas-Montalvo, M.; Olivero-Verbel, J. Phytotoxicity Assessment of a Methanolic Coal Dust Extract in Lemna Minor. Ecotoxicol. Environ. Saf. 2013, 95, 27–32. [Google Scholar] [CrossRef]
- Tirado-Ballestas, I.P.; Alvarez-Ortega, N.; Maldonado-Rojas, W.; Olivero-Verbel, J.; Caballero-Gallardo, K. Oxidative Stress and Alterations in the Expression of Genes Related to Inflammation, DNA Damage, and Metal Exposure in Lung Cells Exposed to a Hydroethanolic Coal Dust Extract. Mol. Biol. Rep. 2022, 49, 4861–4871. [Google Scholar] [CrossRef]
- Schulz, H.-M. Coal Mine Workers’ Pneumoconiosis (CWP): In Vitro Study of the Release of Organic Compounds from Coal Mine Dust in the Presence of Physiological Fluids. Env. Res 1997, 74, 74–83. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Lao, J.-Y.; Wu, C.-C.; Bao, L.-J.; Zeng, E.Y. In Vitro Inhalation Bioaccessibility for Particle-Bound Hydrophobic Organic Chemicals: Method Development, Effects of Particle Size and Hydrophobicity, and Risk Assessment. Environ. Int. 2018, 120, 295–303. [Google Scholar] [CrossRef]
- Gao, P.; Guo, H.; Wang, S.; Guo, L.; Xing, Y.; Yao, C.; Jia, L.; Fan, Q.; Hang, J. In Vitro Investigations of High Molecular Weight Polycyclic Aromatic Hydrocarbons in Winter Airborne Particles Using Simulated Lung Fluids. Atmos. Environ. 2019, 201, 293–300. [Google Scholar] [CrossRef]
- Vanka, K.S.; Shukla, S.; Gomez, H.M.; James, C.; Palanisami, T.; Williams, K.; Chambers, D.C.; Britton, W.J.; Ilic, D.; Hansbro, P.M.; et al. Understanding the Pathogenesis of Occupational Coal and Silica Dust-Associated Lung Disease. Eur. Respir. Rev. 2022, 31, 210250. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Shi, X.; Dalal, N.S.; Irr, W.; Castranova, V. Generation of Free Radicals from Freshly Fractured Silica Dust: Potential Role in Acute Silica-Induced Lung Injury. Am. Rev. Respir. Dis. 1988, 138, 1213–1219. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Harrington, A.D.; Laffers, R.; Strongin, D.R. Role of Hydrogen Peroxide and Hydroxyl Radical in Pyrite Oxidation by Molecular Oxygen. Geochim. Cosmochim. Acta 2010, 74, 4971–4987. [Google Scholar] [CrossRef]
- Cohn, C.A.; Mueller, S.; Wimmer, E.; Leifer, N.; Greenbaum, S.; Strongin, D.R.; Schoonen, M.A. Pyrite-Induced Hydroxyl Radical Formation and Its Effect on Nucleic Acids. Geochem. Trans. 2006, 7, 3. [Google Scholar] [CrossRef]
- Perret, J.L.; Plush, B.; Lachapelle, P.; Hinks, T.S.C.; Walter, C.; Clarke, P.; Irving, L.; Brady, P.; Dharmage, S.C.; Stewart, A. Coal Mine Dust Lung Disease in the Modern Era. Respirology 2017, 22, 662–670. [Google Scholar] [CrossRef]
- Yao, L.; Zhou, Y.; Li, J.; Wickens, L.; Conforti, F.; Rattu, A.; Ibrahim, F.M.; Alzetani, A.; Marshall, B.G.; Fletcher, S.V.; et al. Bidirectional Epithelial–Mesenchymal Crosstalk Provides Self-Sustaining Profibrotic Signals in Pulmonary Fibrosis. J. Biol. Chem. 2021, 297, 101096. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Chemistry of ROS-Mediated Oxidation to the Guanine Base in DNA and Its Biological Consequences. Int. J. Radiat. Biol. 2022, 98, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Jarem, D.A.; Volle, C.B.; Yennie, C.J. Chemical and Biological Consequences of Oxidatively Damaged Guanine in DNA. Free Radic. Res. 2012, 46, 420–441. [Google Scholar] [CrossRef]
- Storr, S.J.; Woolston, C.M.; Zhang, Y.; Martin, S.G. Redox Environment, Free Radical, and Oxidative DNA Damage. Antioxid. Redox Signal. 2013, 18, 2399–2408. [Google Scholar] [CrossRef]
- Omari Shekaftik, S.; Nasirzadeh, N. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Biomarker of Oxidative DNA Damage Induced by Occupational Exposure to Nanomaterials: A Systematic Review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, S.; Sun, H.; Mu, Z.; Zhang, L.; Li, Y.; Chen, Q. Study on the Oxidation Potential of the Water-Soluble Components of Ambient PM2.5 over Xi’an, China: Pollution Levels, Source Apportionment and Transport Pathways. Environ. Int. 2020, 136, 105515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shen, G.; Shi, L.; Li, H.; Lang, D.; Zhang, L.; Pan, B.; Tao, S. Real-World Emission Characteristics of Environmentally Persistent Free Radicals in PM2.5 from Residential Solid Fuel Combustion. Environ. Sci. Technol. 2022, 56, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Wang, H.; Xiao, K.; Luo, Z.; Li, Y.; Xing, R.; Jiang, K.; Fu, D.; Liu, W.; et al. Environmental Persistent Free Radicals in Highly Polluted Soils and the Association with Polycyclic Aromatic Compounds. Environ. Res. 2024, 262, 119853. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, L.; Zhang, S.; Wang, J.; Czech, B.; Oleszczuk, P.; Minkina, T.; Gao, Y. Formation and Biotoxicity of Environmentally Persistent Free Radicals in Steelworks Soil under Thermal Treatment. J. Hazard. Mater. 2024, 467, 133697. [Google Scholar] [CrossRef]
- Xu, M.; Wu, T.; Tang, Y.-T.; Chen, T.; Khachatryan, L.; Iyer, P.R.; Guo, D.; Chen, A.; Lyu, M.; Li, J.; et al. Environmentally Persistent Free Radicals in PM2.5: A Review. Waste Dispos. Sustain. Energy 2019, 1, 177–197. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Su, G.; Chen, X.; Meng, J.; Li, Q.; Wang, C.; Shi, B. Scientific and Regulatory Challenges of Environmentally Persistent Free Radicals: From Formation Theory to Risk Prevention Strategies. J. Hazard. Mater. 2023, 456, 131674. [Google Scholar] [CrossRef]
- Guo, C.; Richmond-Bryant, J. A Critical Review of Environmentally Persistent Free Radical (EPFR) Solvent Extraction Methodology and Retrieval Efficiency. Chemosphere 2021, 284, 131353. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Wang, Y.; Zhang, L.; Xue, J.; Sun, H.; Mu, Z. Rapid Determination of Environmentally Persistent Free Radicals (EPFRs) in Atmospheric Particles with a Quartz Sheet-Based Approach Using Electron Paramagnetic Resonance (EPR) Spectroscopy. Atmos. Environ. 2018, 184, 140–145. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhu, Q.; Zhao, Y.; Wu, X.; Xu, Y. Highly Elevated Levels and Particle-Size Distributions of Environmentally Persistent Free Radicals in Haze-Associated Atmosphere. Environ. Sci. Technol. 2017, 51, 7936–7944. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Neyman, K.M.; Risse, T.; Sterrer, M.; Fischbach, E.; Freund, H.-J.; Nasluzov, V.A.; Pacchioni, G.; Rösch, N. Density-Functional Model Cluster Studies of EPR g Tensors of Fs+ Centers on the Surface of MgO. J. Chem. Phys. 2006, 124, 044708. [Google Scholar] [CrossRef] [PubMed]
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper Oxide-Based Model of Persistent Free Radical Formation on Combustion-Derived Particulate Matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Q.; Yun, T.; Yan, K. Density Functional Theory Investigation of Possible Structures of Radicals in Coal Undergoing O2 Chemisorption at Ambient Temperature. Energy Fuels 2017, 31, 953–958. [Google Scholar] [CrossRef]
- dela Cruz, A.L.N.; Cook, R.L.; Lomnicki, S.M.; Dellinger, B. Effect of Low Temperature Thermal Treatment on Soils Contaminated with Pentachlorophenol and Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2012, 46, 5971–5978. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, S.; Yang, W.; Tang, Z.; Hu, X.; Song, W.; Zhou, B.; Yang, K. Secondary Oxidation of Crushed Coal Based on Free Radicals and Active Groups. Fuel 2021, 290, 120051. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, Y.; Gao, S.; Yang, C.; Shi, X. Oxidation Characteristics of Functional Groups in Relation to Coal Spontaneous Combustion. ACS Omega 2021, 6, 7669–7679. [Google Scholar] [CrossRef]
- Azam, S.; Kurashov, V.; Golbeck, J.H.; Bhattacharyya, S.; Zheng, S.; Liu, S. Comparative 6+ Studies of Environmentally Persistent Free Radicals on Nano-Sized Coal Dusts. Sci. Total Environ. 2023, 878, 163163. [Google Scholar] [CrossRef] [PubMed]
- Taub, T.; Ruthstein, S.; Cohen, H. The Involvement of Carbon-Centered Radicals in the Aging Process of Coals under Atmospheric Conditions: An EPR Study. Phys. Chem. Chem. Phys. 2018, 20, 27025–27035. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Chemical Properties of Superfine Pulverized Coal Particles. Part 1. Electron Paramagnetic Resonance Analysis of Free Radical Characteristics. Adv. Powder Technol. 2014, 25, 916–925. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Qu, B.; Tian, Y. Photoformation of Environmentally Persistent Free Radicals on Particulate Organic Matter in Aqueous Solution: Role of Anthracene and Formation Mechanism. Chemosphere 2022, 291, 132815. [Google Scholar] [CrossRef]
- Dalal, N.S.; Jafari, B.; Petersen, M.; Green, F.H.Y.; Vallyathan, V. Presence of Stable Coal Radicals in Autopsied Coal Miners’ Lungs and Its Possible Correlation to Coal Workers’ Pneumoconiosis. Arch. Environ. Health Int. J. 1991, 46, 366–372. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Vaziri, N.D. Air Pollution and Circulating Biomarkers of Oxidative Stress. Air Qual. Atmosphere Health 2011, 4, 37–52. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the Toxicity of Airborne Particulate Matter and Nanoparticles by Measuring Oxidative Stress Potential—A Workshop Report and Consensus Statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef]
- Clemente, Á.; Gil-Moltó, J.; Yubero, E.; Juárez, N.; Nicolás, J.F.; Crespo, J.; Galindo, N. Sensitivity of PM10 Oxidative Potential to Aerosol Chemical Composition at a Mediterranean Urban Site: Ascorbic Acid versus Dithiothreitol Measurements. Air Qual. Atmosphere Health 2023, 16, 1165–1172. [Google Scholar] [CrossRef]
- Taghvaee, S.; Sowlat, M.H.; Diapouli, E.; Manousakas, M.I.; Vasilatou, V.; Eleftheriadis, K.; Sioutas, C. Source Apportionment of the Oxidative Potential of Fine Ambient Particulate Matter (PM2.5) in Athens, Greece. Sci. Total Environ. 2019, 653, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Naraki, H.; Keshavarzi, B.; Zarei, M.; Moore, F.; Abbasi, S.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Urban Street Dust in the Middle East Oldest Oil Refinery Zone: Oxidative Potential, Source Apportionment، and Health Risk Assessment of Potentially Toxic Elements. Chemosphere 2021, 268, 128825. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; Zhuang, X.; Querol, X.; Li, B.; Moreno, N.; Trechera, P.; Sola, P.C.; Uzu, G.; Li, J. Characterization of Deposited Dust and Its Respirable Fractions in Underground Coal Mines: Implications for Oxidative Potential-Driving Species and Source Apportionment. Int. J. Coal Geol. 2022, 258, 104017. [Google Scholar] [CrossRef]
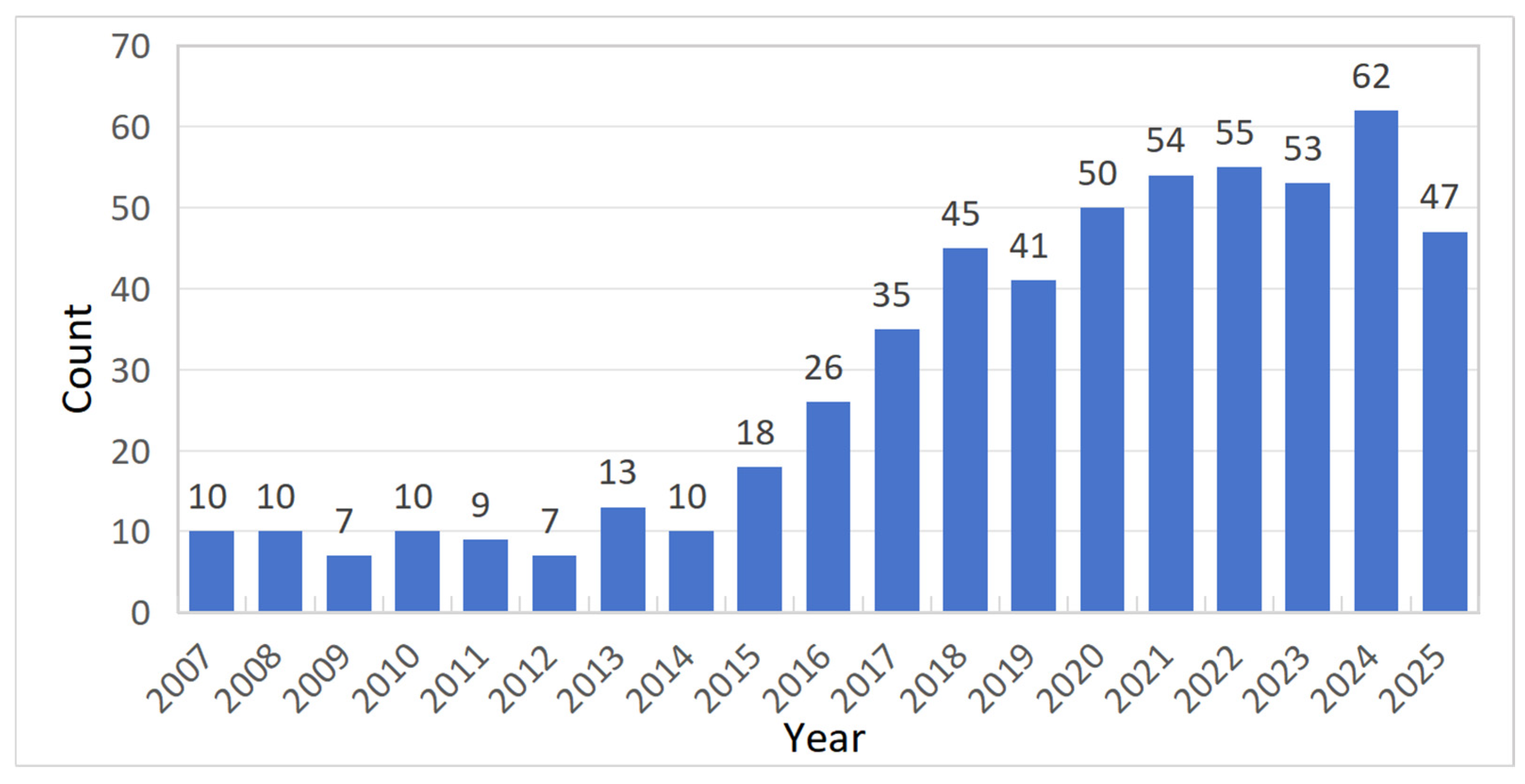

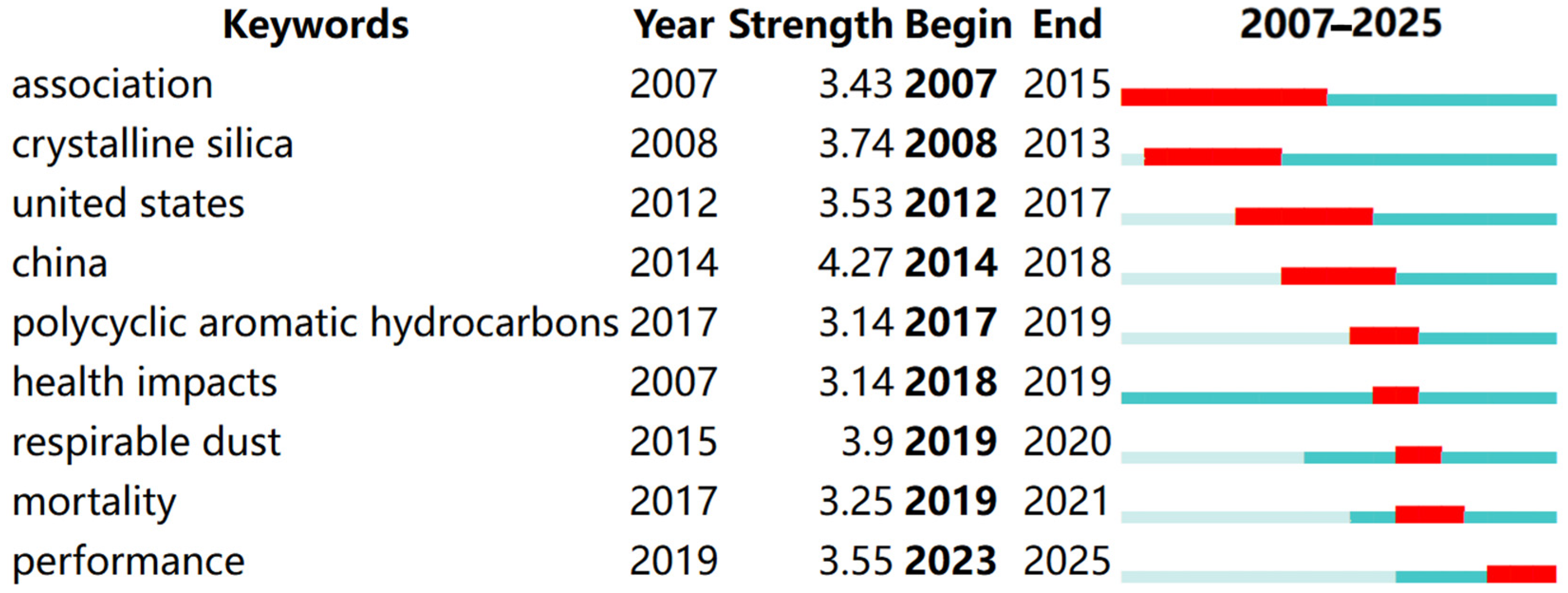
| Count | Year | Country | Author | Title |
|---|---|---|---|---|
| 36 | 2020 | USA | Liu T | The impacts of coal dust on miners’ health: A review [31] |
| 34 | 2019 | USA | Sarver E | Beyond conventional metrics: Comprehensive characterization of respirable coal mine dust [20] |
| 27 | 2018 | USA | Blackley DJ | Continued Increase in Prevalence of Coal Workers’ Pneumoconiosis in the United States, 1970–2017 [32] |
| 21 | 2019 | USA | Doney BC | Respirable coal mine dust in underground mines, United States, 1982–2017 [25] |
| 21 | 2017 | USA | Johann-Essex V | Respirable coal mine dust characteristics in samples collected in central and northern Appalachia [33] |
| 20 | 2020 | Spain | Trechera P | Mineralogy, geochemistry and toxicity of size-segregated respirable deposited dust in underground coal mines [34] |
| 19 | 2019 | USA | Hall NB | Current Review of Pneumoconiosis Among US Coal Miners [35] |
| 19 | 2021 | Iran | Zazouli MA | Physico-chemical properties and reactive oxygen species generation by respirable coal dust: Implication for human health risk assessment [36] |
| 18 | 2016 | USA | Cohen RA | Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates [37] |
| 18 | 2019 | Spain | Moreno T | Trace element fractionation between PM10 and PM2.5 in coal mine dust: Implications for occupational respiratory health [18] |
| Element | Iran Coal Dust [36] | Iran Coal [36] | Shanxi Coal Dust [43] | Hunan Coal Dust [42] | Hunan Coal [42] | Slovenia Coal Dust [18] | Slovenia Coal [18] | World Coal [36] |
|---|---|---|---|---|---|---|---|---|
| Be | 1.29 | 3.75 | 2.02 | 1.26 | 1.98 | <0.1 | - | 1.5 |
| V | 347 | 13.75 | 49.49 | 79.33 | 40.60 | 41 | 28 | 25 |
| Cr | 149 | 8 | 32.53 | 50.44 | 21.07 | 33 | - | 10 |
| Mn | 455 | 82 | 115.73 | 218.78 | 29.33 | 778 | 542 | 50 |
| Fe | 31,200 | 32,149 | 10,700 | 31,950 | 16,600 | 10,000 | ||
| Co | 21.46 | 10.1 | 9.33 | 9.24 | 20.80 | 5.85 | 2.6 | 5 |
| Cu | 69.16 | 13.5 | 23.96 | 30.90 | 24.33 | 33 | 8.6 | 15 |
| Zn | 425.93 | 12.5 | 81.57 | 77.63 | 58.67 | 111 | 22 | 50 |
| As | 26.21 | 3.8 | 16.82 | 80.47 | 7.82 | 19 | 8.2 | 26 |
| Sr | 55.44 | 354.75 | 225.02 | 44.33 | 28.33 | 121.5 | 94 | 130 |
| Ag | 0.27 | 0.07 | - | - | - | - | - | 0.08 |
| Cd | 0.86 | 0.03 | - | - | - | 0.25 | 0.2 | 0.3 |
| Sb | 5.97 | 0.68 | 9.34 | 8.68 | 4.30 | 5.75 | 0.4 | 3 |
| Ba | 163 | 274.5 | 333.55 | 38.86 | 9.57 | 158 | 78 | 120 |
| Pb | 45.21 | 8.52 | 35.97 | 34.33 | 45.00 | 35 | 8.7 | 25 |
| Al | 23,400 | 43,200 | 38,500 | - | - | 31,800 | 17,200 | 10,000 |
| Ca | 8140 | 229,000 | 12,300 | - | - | 36,900 | 25,600 | 10,000 |
| K | 21,400 | 4283 | 5400 | - | - | 6650 | 2700 | 1000 |
| Mg | 12,100 | 95,200 | 1900 | - | - | 5250 | 2700 | 2000 |
| Na | 29,200 | 4140 | 3200 | - | - | 3100 | 1000 | 2000 |
| Ti | - | - | 2284.22 | 1014.56 | 535.67 | 1068 | 640 | - |
| Category | Gamble’s Solution (GS) [40] | Artificial Lysosomal Fluid (ALF) [40] | ||
|---|---|---|---|---|
| Dust dosage (g) | 0.020 | 0.020 | ||
| Simulated liquid volume (mL) | 100 | 100 | ||
| Simulated liquid composition (g/L) | NaCl | 6.779 | NaCl | 3.210 |
| NaHCO3 | 2.268 | Na2HPO4 | 0.071 | |
| Sodium citrate | 0.055 | Sodium citrate | 0.077 | |
| NH4Cl | 0.535 | Glycine | 0.059 | |
| Glycine | 0.357 | NaOH | 6.000 | |
| NaH2PO4 | 1.872 | Citric acid | 20.80 | |
| L-cysteine | 0.121 | CaCl2.2H2O | 0.128 | |
| CaC12.2H2O | 0.026 | Na2SO4 | 0.039 | |
| MgCl2.6H2O | 0.050 | |||
| Disodium tartrate | 0.090 | |||
| Sodium lactate | 0.085 | |||
| Sodium pyruvate | 0.172 | |||
| Extraction steps | Stir at 1000 rpm for 24 h at 37 °C, centrifuge, and analyze the supernatant | Stir at 1000 rpm for 24 h at 37 °C, centrifuge, and analyze the supernatant | ||
| Category | UBM [53,54] | PBET [55] | SBET [56,57] |
|---|---|---|---|
| Mimic organ | Mouth–Gastric–Intestinal phase | Gastric–Intestinal phase | Gastric phase |
| Extraction steps | Oral phase: pH 6.5 ± 0.5 Add α-amylase(145 mg/L); Mucoprotein(50 mg/L); Uric Acid (15 mg/L) Oscillate at 37 °C for 5 min. | Gastric Phase Simulation: pH 2.5 Add pepsin (1.25 g/L), sodium citrate(0.5 g/L), sodium malate (0.5 g/L), lactic acid (420 μL/L), acetic acid (500 μL/L); Oscillate at 37 °C for 1 h. | Gastric Phase Simulation Add pepsin (1.25 g/L), Adjust pH to 1.5 ± 0.1 using HCl Oscillate at 37 °C for 1 h. |
| Gastric phase: pH 1.2 Add pepsin(1.0 g/L), mucoprotein (3.0 g/L), bovine serum albumin(1 g/L) shaken for 1 h. | Intestinal Phase Simulation:pH 7 Add pancreatin (0.5 g/L), porcine bile salts (1.75 g/L), Adjust pH to 7.0 using NaHCO3. Oscillating for 4 h | ||
| Intestinal Phase: pH 4 Duodenal fluid:pH 7.4 ± 0.2 Add CaCl2 (200 mg/L), bovine serum albumin(1 g/L) pancreatin (3 g/L), lipase (500 mg/L), Bile fluid: pH 8.0 ± 0.2 Add CaCl2 (222 mg/L), bovine serum albumin(1.8 g/L), bile (6 g/L), Oscillate at 37 °C for 4 h. | |||
| solid-to-liquid ratio | 1:15; 1:22.5; 1:60 | 1:100 | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Yang, J. Geochemical Characteristics and Health Risks of Coal Dust: An Integrated Review from Component-Dependent Toxicity to Emerging Oxidative Toxicity Indicators. Minerals 2025, 15, 1075. https://doi.org/10.3390/min15101075
Feng X, Yang J. Geochemical Characteristics and Health Risks of Coal Dust: An Integrated Review from Component-Dependent Toxicity to Emerging Oxidative Toxicity Indicators. Minerals. 2025; 15(10):1075. https://doi.org/10.3390/min15101075
Chicago/Turabian StyleFeng, Xiujuan, and Jing Yang. 2025. "Geochemical Characteristics and Health Risks of Coal Dust: An Integrated Review from Component-Dependent Toxicity to Emerging Oxidative Toxicity Indicators" Minerals 15, no. 10: 1075. https://doi.org/10.3390/min15101075
APA StyleFeng, X., & Yang, J. (2025). Geochemical Characteristics and Health Risks of Coal Dust: An Integrated Review from Component-Dependent Toxicity to Emerging Oxidative Toxicity Indicators. Minerals, 15(10), 1075. https://doi.org/10.3390/min15101075






