Limestone-Based Hybrid Passive Treatment for Copper-Rich Acid Mine Drainage: From Laboratory to Field
Abstract
1. Introduction
2. Materials and Methods
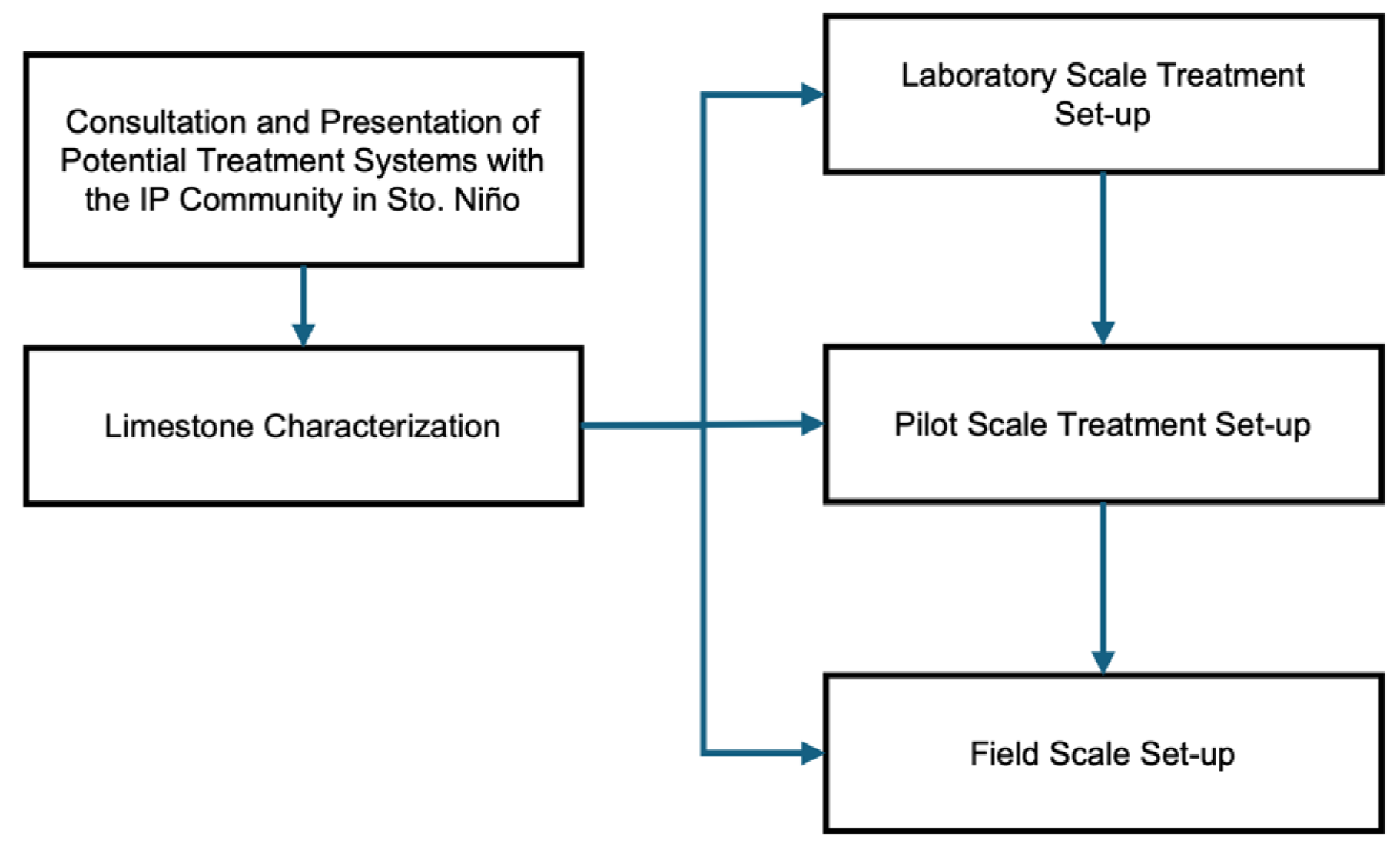
2.1. Case Study: Identification of Acid Mine Drainage in Legacy Mine in Sto. Niño, Tublay, Benguet, and Development of the Limestone-Based Hybrid Passive Treatment System
2.2. Laboratory-Scale Setup
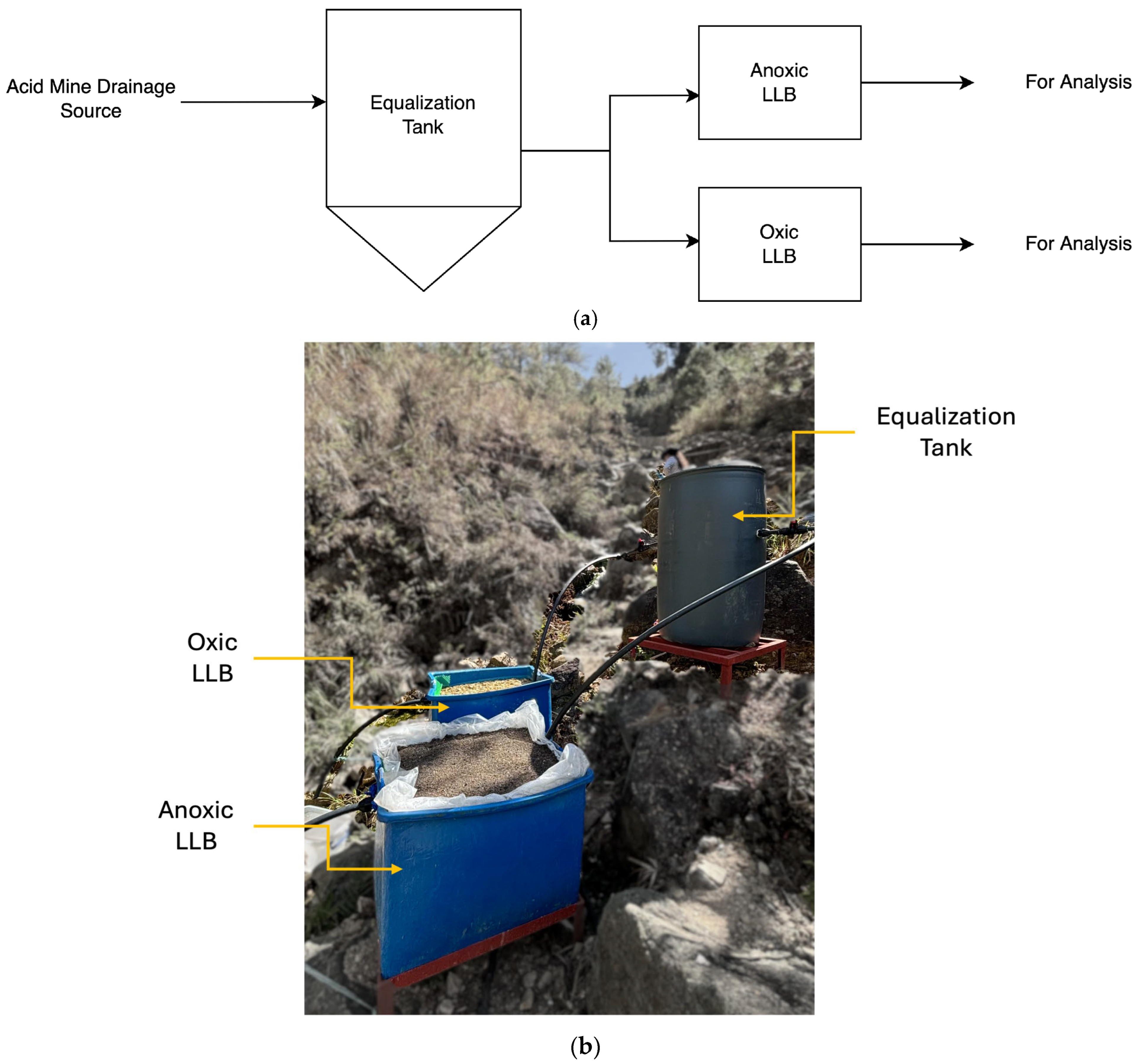
2.3. Pilot-Scale Setup
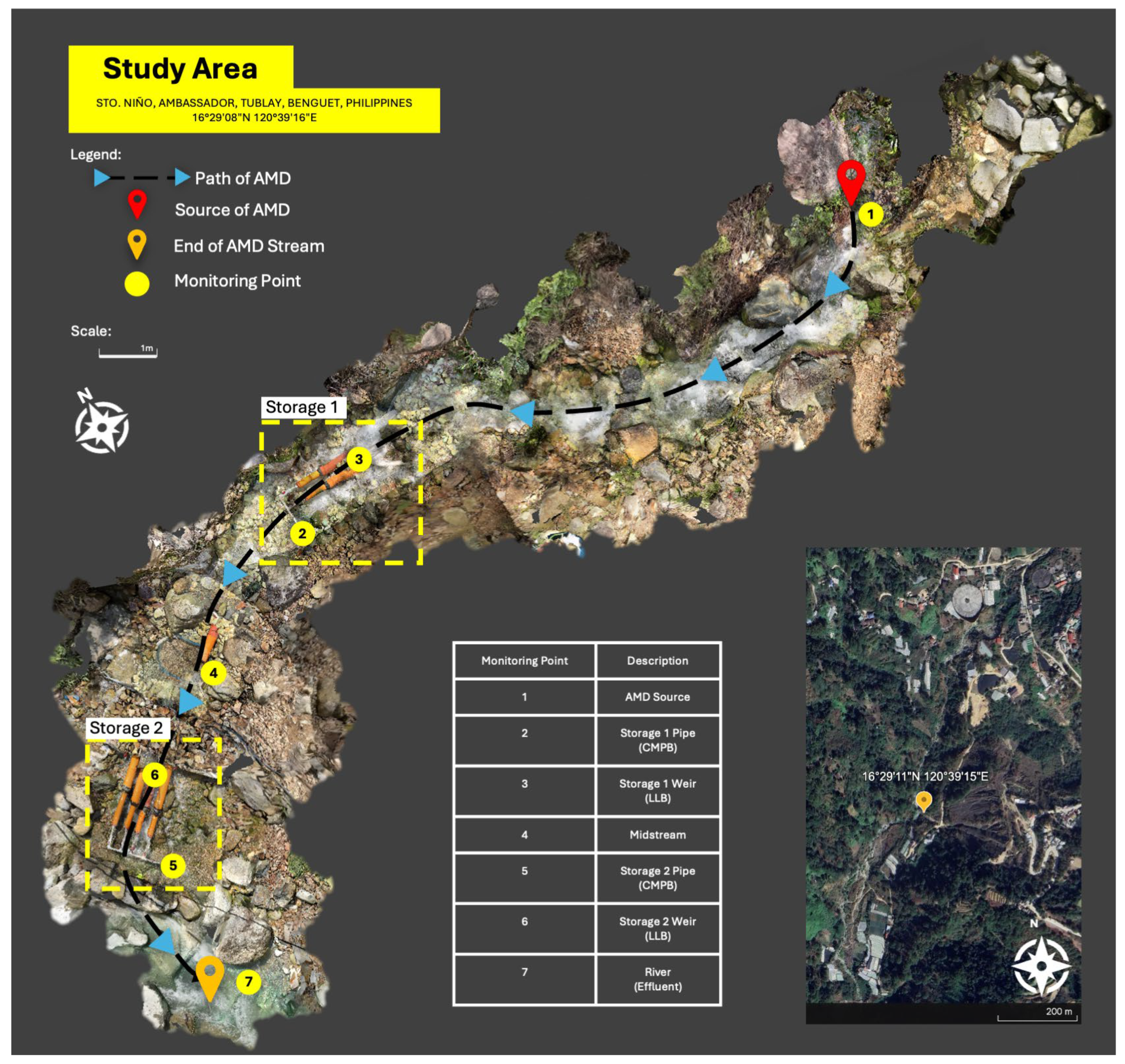
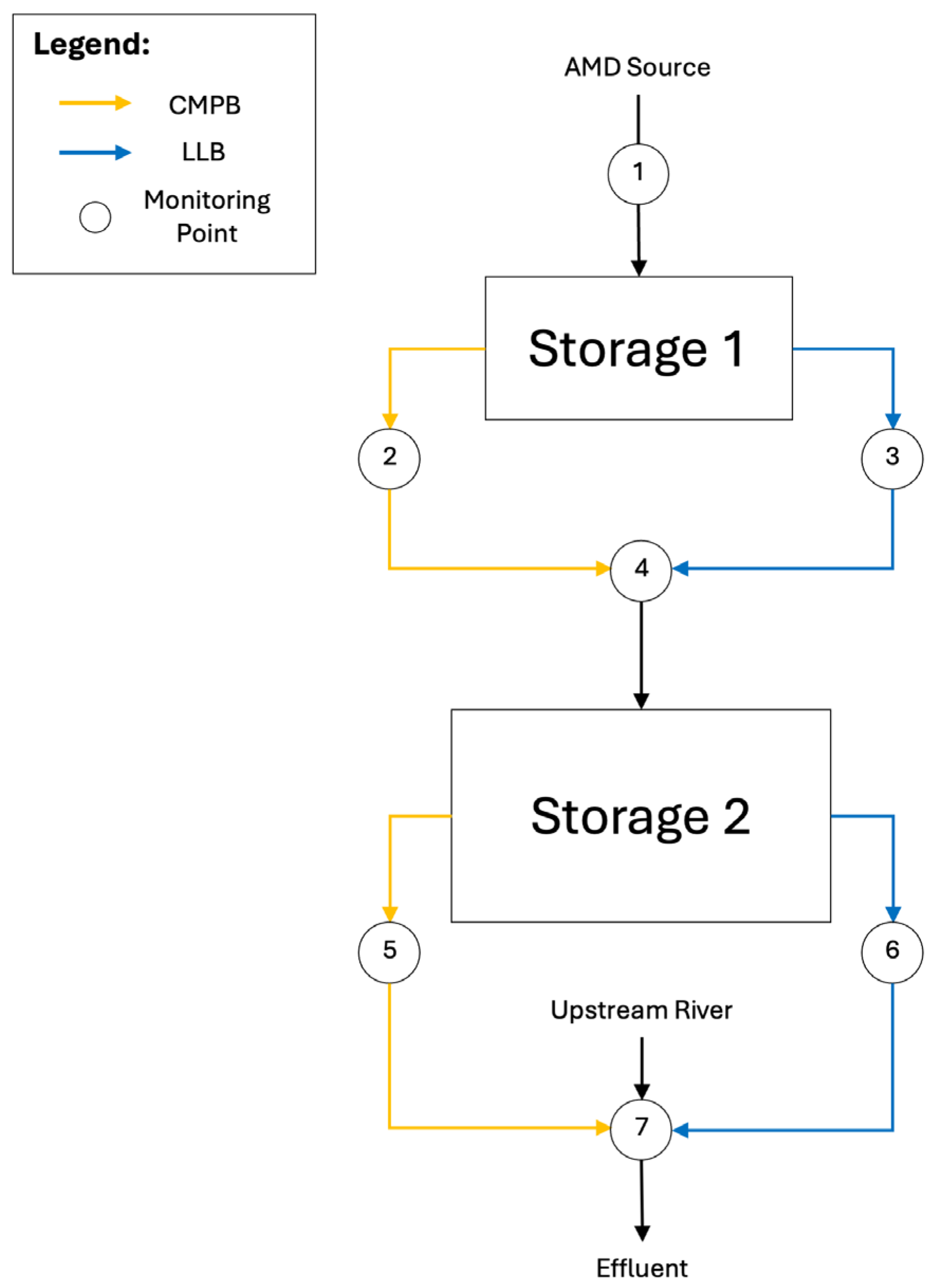
2.4. Field-Scale Setup
2.5. Performance Assessment
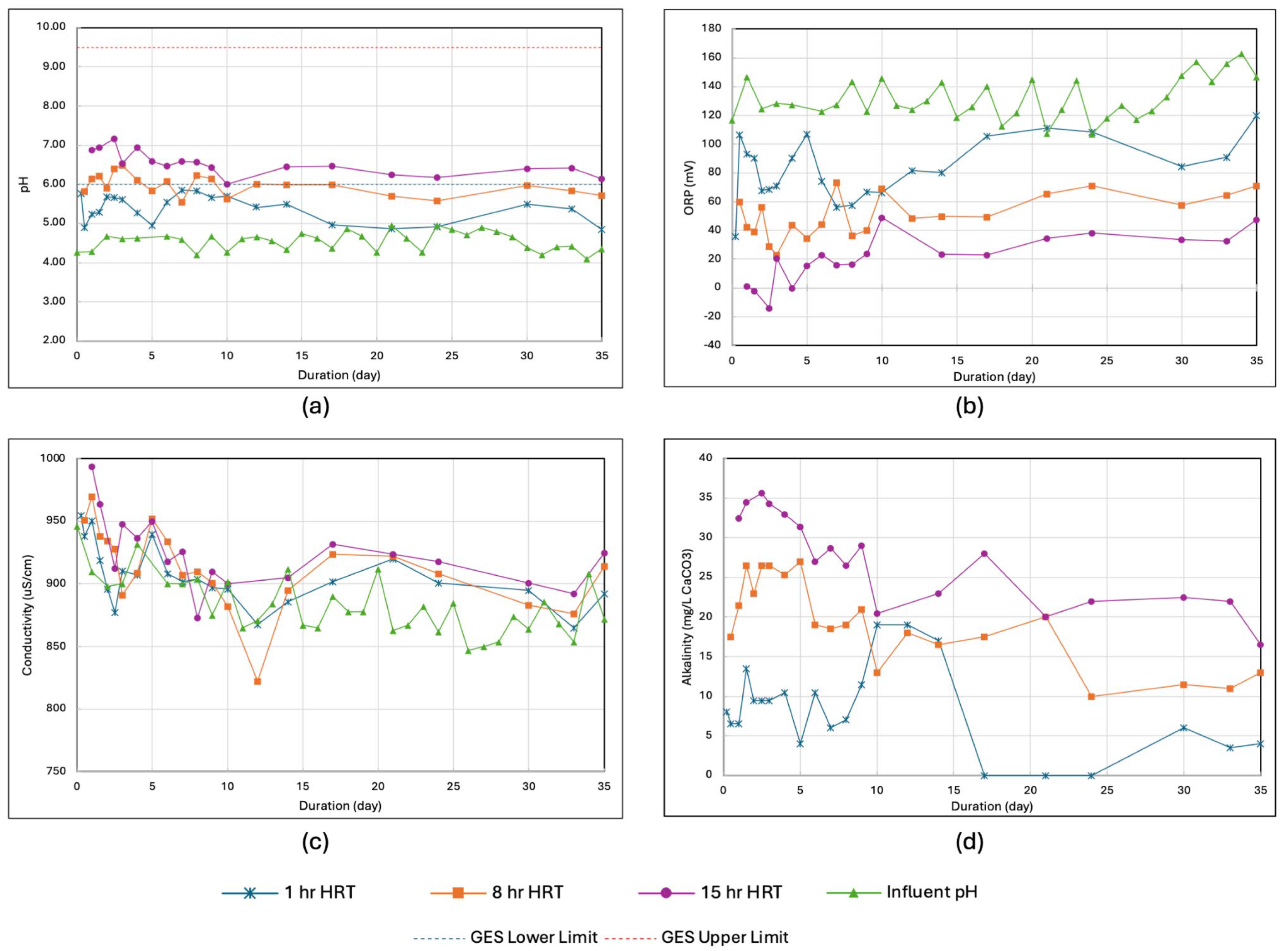
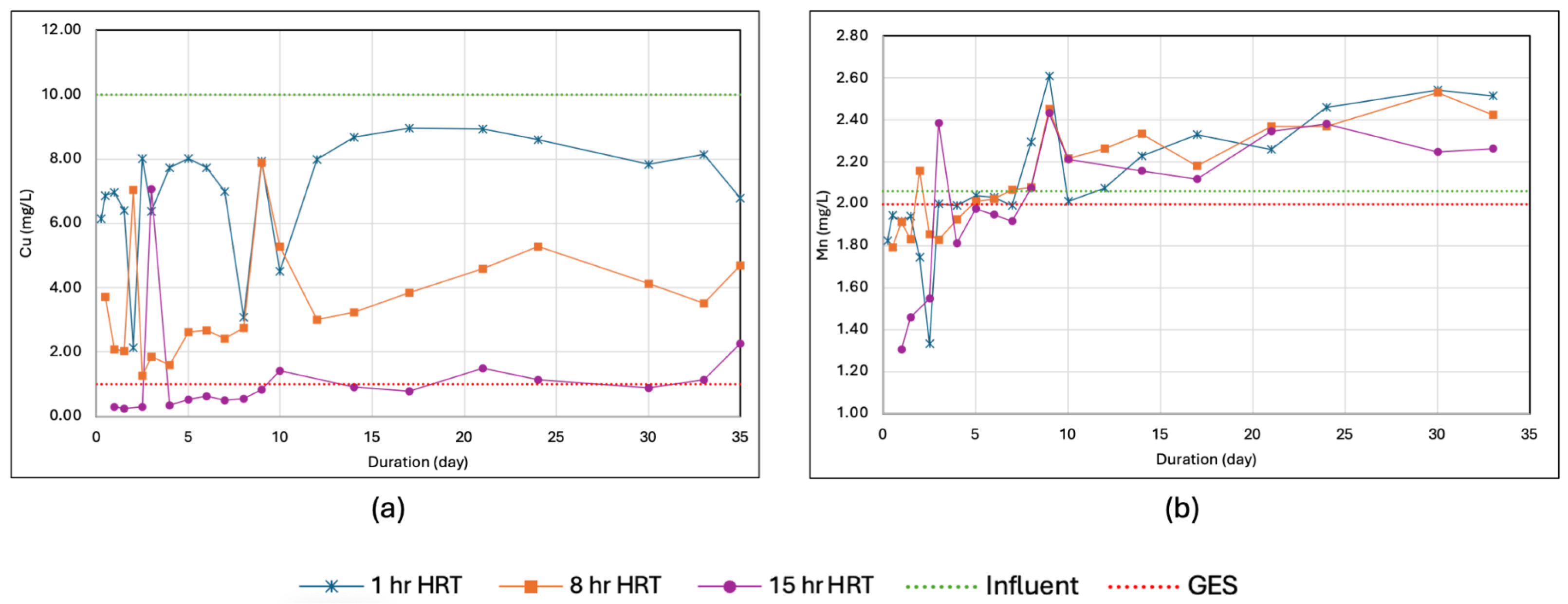
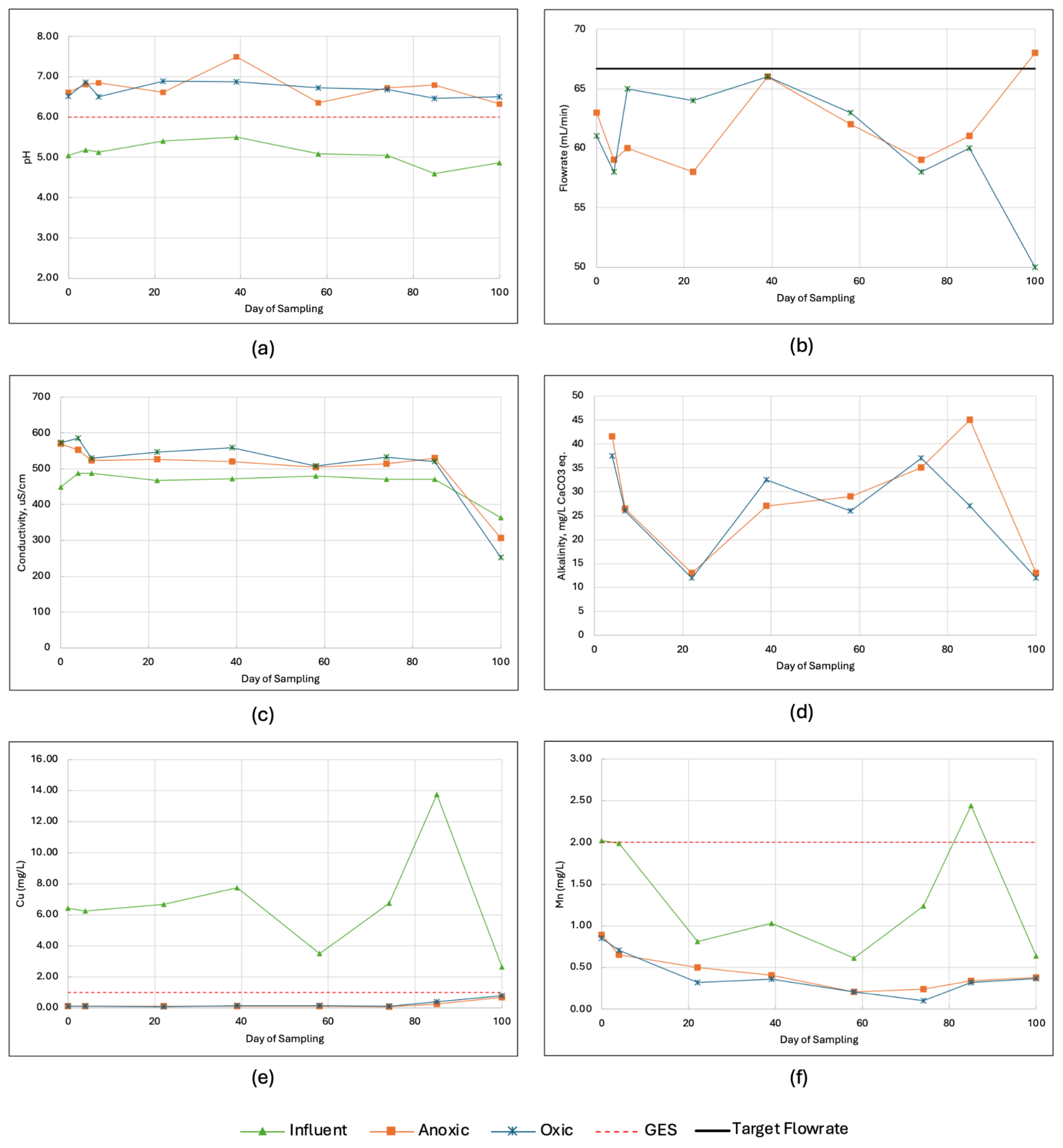
3. Results and Discussion
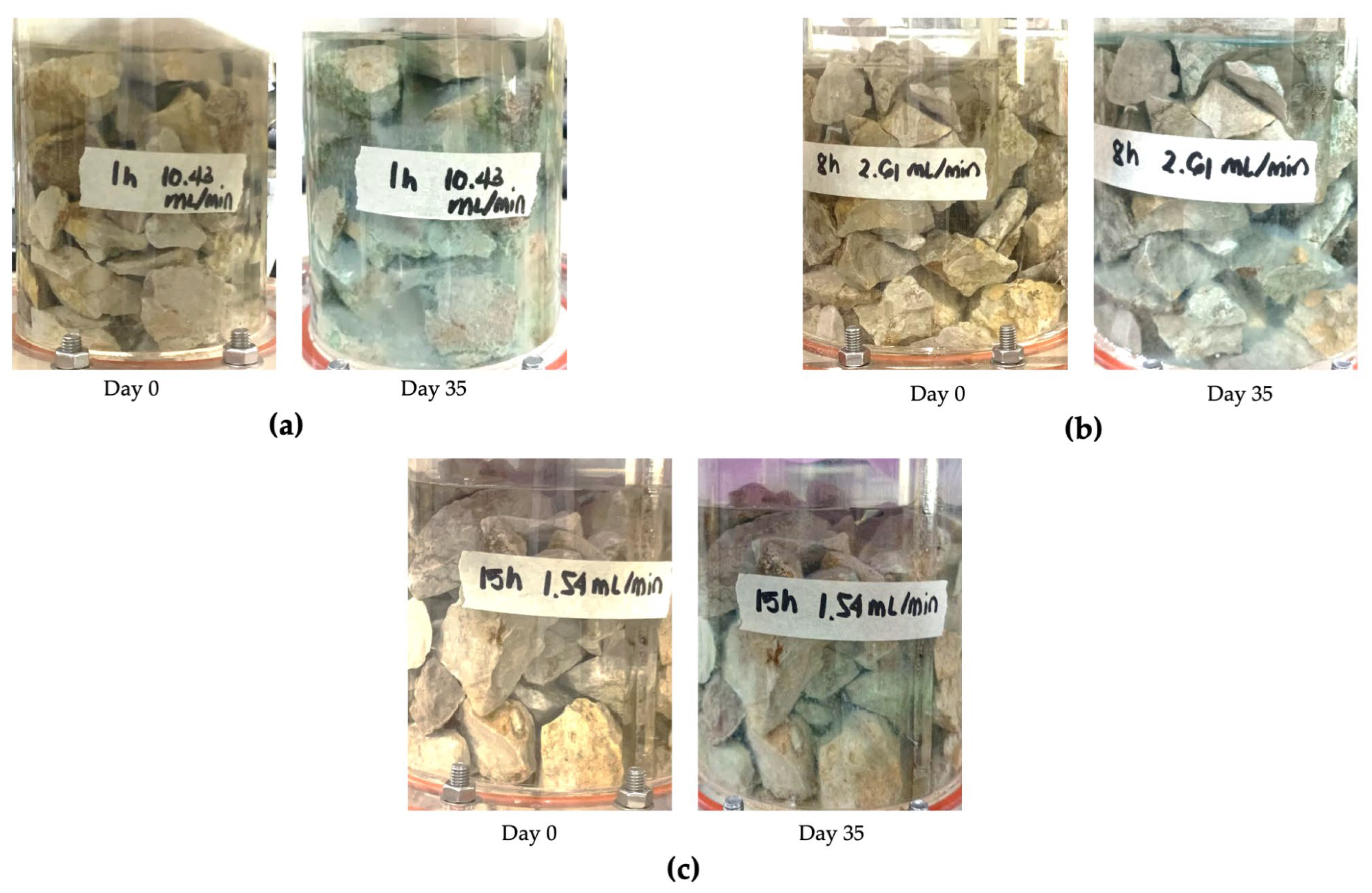
3.1. Limestone Characterization
3.2. Determination of HRT from the Laboratory-Scale Setup
3.3. Comparison of Anoxic and Oxic Conditions in a Pilot-Scale Treatment
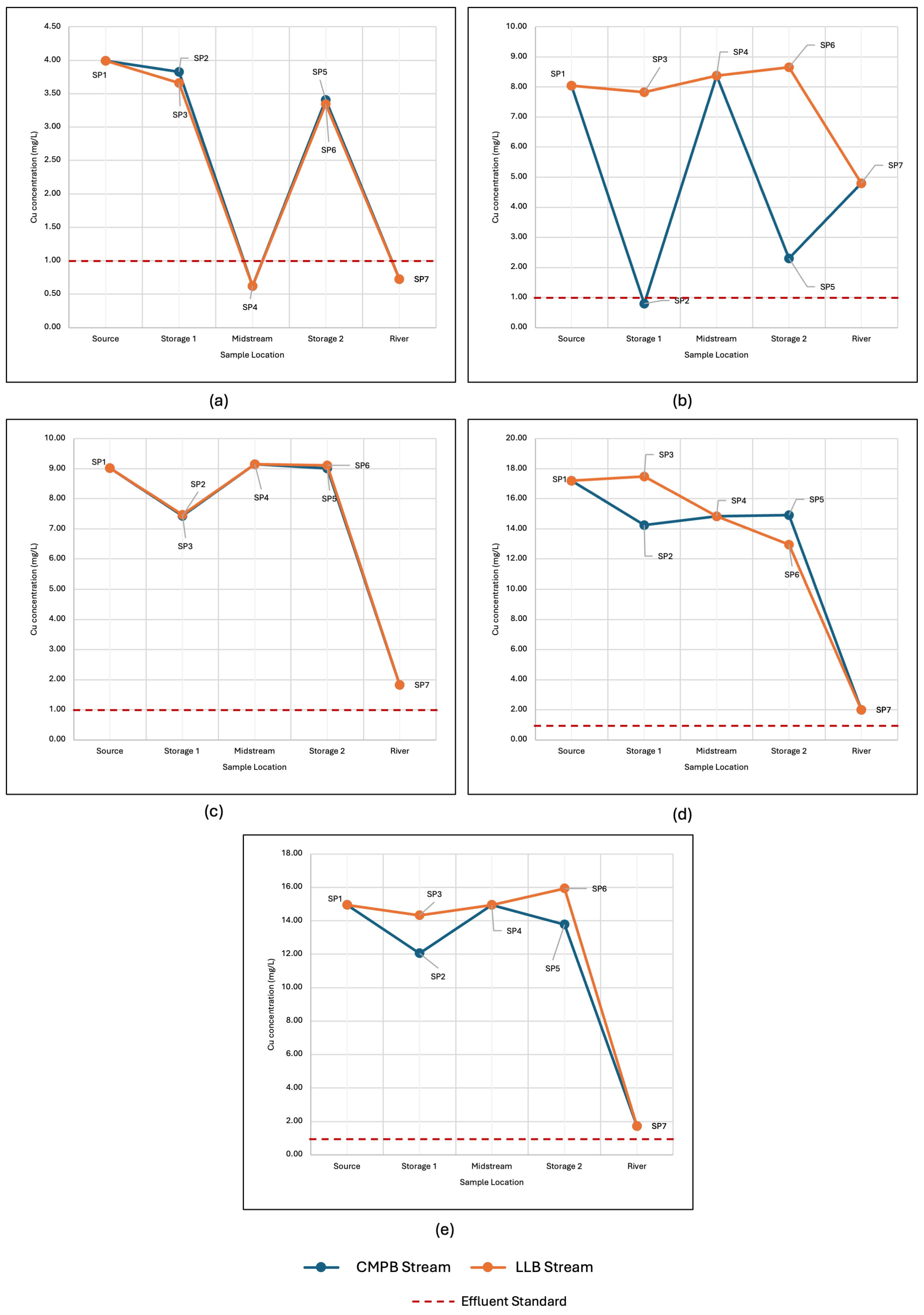
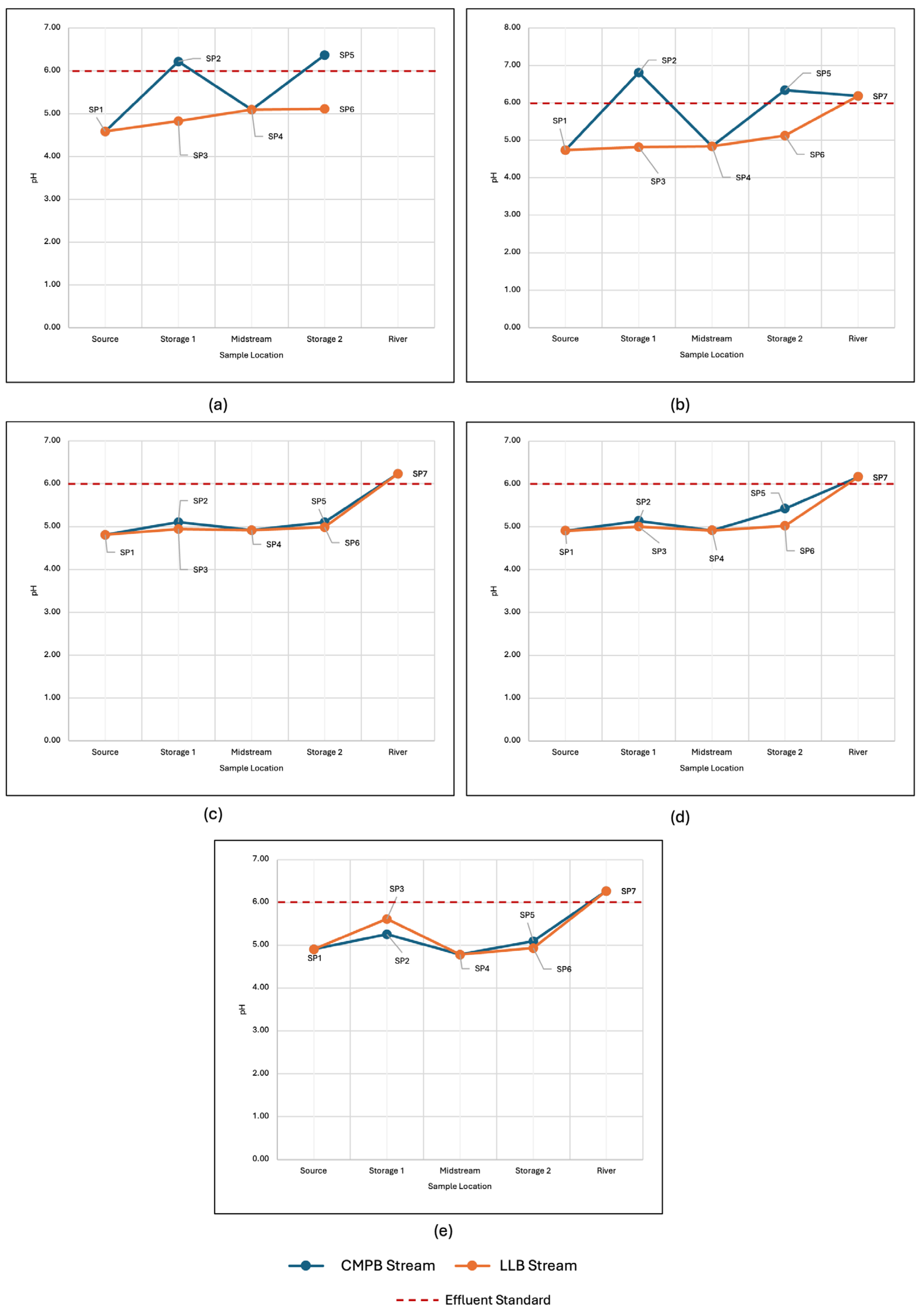
3.4. Implementation of Field-Scale System: Treatment Performance and Challenges
- Limited land area—Achieving an HRT of greater than 15 h requires a large land area, which the site does not allow due to it being situated on a slope. Using a CMPB allowed for the stream to be stored longer and be in contact with the limestone.
- High flow rate of the stream—The AMD stream in the area can reach more than 3 L/s, implying that the system developed must handle at least 162 m3 to achieve the desired HRT. Thus, including an LLB in the treatment system allowed the stream to be in contact with the limestone and neutralize the AMD.
- Minimal access to manpower and energy—Given that the limestone required further processing to reach the desired particle size, both 50–100 mm and 5–25 mm limestones were used in the LLB and CMPB, respectively. Additionally, the implementation of the passive treatment allowed for less frequent maintenance compared to its active counterpart.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic Absorption Spectroscopy |
| AMD | Acid Mine Drainage |
| CMPB | Controlled Modular Packed Bed |
| DAO | Department of Environment and Natural Resources Administrative Order |
| EC | Electrical Conductivity |
| PP | Polypropylene |
| HDPE | High-Density Polyethylene |
| HRT | Hydraulic Retention Time |
| IP | Indigenous People |
| IPLC | Indigenous People Local Community |
| LiDAR | Light Detection and Ranging |
| LLB | Limestone Leach Bed |
| ORP | Oxidation Reduction Potential |
| PES | Polyethersulfone |
| SMEWW | Standard Methods for the Examination of Water and Wastewater |
| XRD | X-Ray Diffraction |
| XRF | X-Ray Fluorescence |
References
- Alonzo, D.; Tabelin, C.B.; Dalona, I.M.; Abril, J.M.V.; Beltran, A.; Orbecido, A.; Villacorte-Tabelin, M.; Resabal, V.J.; Promentilla, M.A.; Suelto, M.; et al. Working with the Community for the Rehabilitation of Legacy Mines: Approaches and Lessons Learned from the Literature. Resour. Policy 2024, 98, 105351. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid Mine Drainage: Prevention, Treatment Options, and Resource Recovery: A Review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Betrie, G.D.; Sadiq, R.; Morin, K.A.; Tesfamariam, S. Selection of Remedial Alternatives for Mine Sites: A Multicriteria Decision Analysis Approach. J. Environ. Manag. 2013, 119, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid Mine Drainage Formation, Control and Treatment: Approaches and Strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid Mine Drainage Sources and Hydrogeochemistry at the Yatani Mine, Yamagata, Japan: A Geochemical and Isotopic Study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Canovas, C.R.; Olias, M.; Ayora, C. Acid Mine Drainage in the Iberian Pyrite Belt: 1. Hydrochemical Characteristics and Pollutant Load of the Tinto and Odiel Rivers. Environ. Sci. Pollut. Res. 2013, 20, 7509–7519. [Google Scholar] [CrossRef]
- Tao, X.; Wu, P.; Tang, C.; Liu, H.; Sun, J. Effect of Acid Mine Drainage on a Karst Basin: A Case Study on the High-As Coal Mining Area in Guizhou Province, China. Environ. Earth Sci. 2012, 65, 631–638. [Google Scholar] [CrossRef]
- Bálintová, M.; Singovszká, E.; Holub, M.; Demčák, Š. Influence of Acid Mine Drainage on Surface Water Quality. In Water Resources in Slovakia: Part I: Assessment and Development; Negm, A.M., Zeleňáková, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 239–258. ISBN 978-3-319-92853-1. [Google Scholar]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Rajabpoor, S.; Schat, H. Acid Mine Drainage (AMD) Endangers Pomegranate Trees Nearby a Copper Mine. Sci. Total Environ. 2023, 889, 164269. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Makungo, R.; Mathivha, F.; Rivers, N.; Volenzo, T.; Odiyo, J.O. Chapter 5-Influence of Global Climate Change on Water Resources in South Africa: Toward an Adaptive Management Approach. In Water Conservation and Wastewater Treatment in BRICS Nations; Singh, P., Milshina, Y., Tian, K., Gusain, D., Bassin, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 83–115. ISBN 978-0-12-818339-7. [Google Scholar]
- Alonzo, D.; Tabelin, C.B.; Dalona, I.M.; Beltran, A.; Orbecido, A.; Villacorte-Tabelin, M.; Resabal, V.J.; Promentilla, M.A.; Brito-Parada, P.; Plancherel, Y.; et al. Bio+Mine Project: Empowering the Community to Develop a Site-Specific System for the Rehabilitation of a Legacy Mine. Int. J. Qual. Methods 2023, 22, 1–8. [Google Scholar] [CrossRef]
- Promentilla, M.A.; Beltran, A.; Orbecido, A.; Bernardo-Arugay, I.; Resabal, V.J.; Villacorte-Tabelin, M.; Dalona, I.M.; Opiso, E.; Alloro, R.; Alonzo, D.; et al. Systems Approach toward a Greener Eco-Efficient Mineral Extraction and Sustainable Land Use Management in the Philippines. Chem. Eng. Trans. 2021, 88, 1171–1176. [Google Scholar] [CrossRef]
- Nairn, R.W.; LaBar, J.A.; Strevett, K.A. Passive Treatment Opportunities in a Drastically Disturbed Watershed: Reversing the Irreversible? Proc. Am. Soc. Min. Reclam. 2011, 450–468. [Google Scholar] [CrossRef]
- Araujo, F.; Taborda-Llano, I.; Nunes, E.; Santos, R. Recycling and Reuse of Mine Tailings: A Review of Advancements and Their Implications. Geosciences 2022, 12, 319. [Google Scholar] [CrossRef]
- Larsson, M.; Nosrati, A.; Kaur, S.; Wagner, J.; Baus, U.; Nydén, M. Copper Removal from Acid Mine Drainage-Polluted Water Using Glutaraldehyde-Polyethyleneimine Modified Diatomaceous Earth Particles. Heliyon 2018, 4, e00520. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Lundås, M.; Siriyappagouder, P.; Kristensen, T.; Olsvik, P.A. Ecotoxicological Assessment of Cu-Rich Acid Mine Drainage of Sulitjelma Mine Using Zebrafish Larvae as an Animal Model. Ecotoxicol. Environ. Saf. 2024, 269, 115796. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Pape, S.; Murphy, N. A Summary of Passive and Active Treatment Technologies for Acid and Metalliferous Drainage (AMD). In Proceedings of the Fifth Australian Workshop on Acid Drainage, Fremantle, Australia, 29–31 August 2005; pp. 1–49. [Google Scholar]
- Wang, Y.; Wang, C.; Feng, R.; Li, Y.; Zhang, Z.; Guo, S. A Review of Passive Acid Mine Drainage Treatment by PRB and LPB: From Design, Testing, to Construction. Environ. Res. 2024, 251, 118545. [Google Scholar] [CrossRef] [PubMed]
- Pocaan, J.; Turingan, C.O.A.; Tabelin, C.B.; Zoleta, J.B.; Arima, T.; Park, I.; Ito, M.; Orbecido, A.H. A Mixed Media Approach Using Locally Available Neutralizing Materials for the Passive Treatment of Acid Mine Drainage. Heliyon 2025, e41984. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Skousen, J.; Ziemkiewicz, P. Performance of 116 Passive Treatment Systems for Acid Mine Drainage. In Proceedings of the 22nd American Society of Mining and Reclamation Annual National Conference 2005, Breckenridge, CO, USA, 19–23 June 2005; Volume 2, pp. 1100–1133. [Google Scholar] [CrossRef]
- Skousen, J. Anoxic Limestone Drains for Acid Mine Drainage Treatment. Green Lands 1991, 21, 30–35. [Google Scholar]
- Cravotta, C.A.; Ward, S.J. Downflow Limestone Beds for Treatment of Net-Acidic, Oxic, Iron-Laden Drainage from a Flooded Anthracite Mine, Pennsylvania, USA: 1. Field Evaluation. Mine Water Environ. 2008, 27, 67–85. [Google Scholar] [CrossRef]
- Turingan, C.; Singson, G.; Melchor, B.; Alorro, R.; Beltran, A.; Orbecido, A. A Comparison of the Acid Mine Drainage (AMD) Neutralization Potential of Low Grade Nickel Laterite and Other Alkaline-Generating Materials. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012142. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Wang, R.; Yang, L.; Cao, Y.; Wang, H. A Novel Approach for Treating Acid Mine Drainage by Forming Schwertmannite Driven by a Combination of Biooxidation and Electroreduction before Lime Neutralization. Water Res. 2022, 221, 118748. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, B.B.; Skousen, J.G. Treatment of Acid Mine Drainage by Passive Treatment Systems. Proc. Am. Soc. Min. Reclam. 1994, 250–257. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Skousen, J.G.; Brant, D.L.; Sterner, P.L.; Lovett, R.J. Acid Mine Drainage Treatment with Armored Limestone in Open Limestone Channels. J. Environ. Qual. 1997, 26, 1017–1024. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Sibrell, P.L.; Belkin, H.E. Characterization of Limestone Reacted with Acid-Mine Drainage in a Pulsed Limestone Bed Treatment System at the Friendship Hill National Historical Site, Pennsylvania, USA. Appl. Geochem. 2003, 18, 1705–1721. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and Avenues for Acid Mine Drainage Treatment, Beneficiation, and Valorisation in Circular Economy: A Review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Clyde, E.J.; Champagne, P.; Jamieson, H.E.; Gorman, C.; Sourial, J. The Use of a Passive Treatment System for the Mitigation of Acid Mine Drainage at the Williams Brothers Mine (California): Pilot-Scale Study. J. Clean. Prod. 2016, 130, 116–125. [Google Scholar] [CrossRef]
- Soler, J.M.; Boi, M.; Mogollón, J.L.; Cama, J.; Ayora, C.; Nico, P.S.; Tamura, N.; Kunz, M. The Passivation of Calcite by Acid Mine Water. Column Experiments with Ferric Sulfate and Ferric Chloride Solutions at pH 2. Appl. Geochem. 2008, 23, 3579–3588. [Google Scholar] [CrossRef]
- Department of Environment and Natural Resources. Water Quality Guidelines and General Effluent Standards of 2016; Environmental Management Bureau: Quezon City, Philippines, 2016; pp. 1–34. Available online: https://pab.emb.gov.ph/wp-content/uploads/2017/07/DAO-2016-08-WQG-and-GES.pdf (accessed on 9 June 2025).
- Department of Environment and Natural Resources. Updated Water Quality Guidelines (WQG) and General Effluent Standards (GES) for Selected Parameters; Environmental Management Bureau: Quezon City, Philippines, 2021; pp. 1–5. Available online: https://water.emb.gov.ph/wp-content/uploads/2022/09/DAO-2021-19_1627862906.pdf (accessed on 9 June 2025).
- Alonzo, D.; Abril, J.M.V.; Villonez, G.; Armstrong, R.; Dalona, I.M.; Beltran, A.; Orbecido, A.; Tabelin, C.B.; Villacorte-Tabelin, M.; Promentilla, M.A.; et al. Integrating Indigenous Knowledge and Skills in Mining Operations: A Systematic Literature Review. Extr. Ind. Soc. 2025, 24, 101706. [Google Scholar] [CrossRef]
- Balboa, C.J.P.; Pocaan, J.P.; Baute, R.; Orbecido, A.; Beltran, A.B.; Santos, A.L.; Jungblut, A.; Plancherel, Y.; Brito-Parada, P.; Tabelin, C.B.; et al. A Novel Water Pollution Index for Domestic Water Quality Assessment in Acid Mine Drainage-Impacted Mining Areas. Miner. Eng. 2025, 234, 109730. [Google Scholar] [CrossRef]
- Aghili, S.; Vaezihir, A.; Hosseinzadeh, M. Distribution and Modeling of Heavy Metal Pollution in the Sediment and Water Mediums of Pakhir River, at the Downstream of Sungun Mine Tailing Dump, Iran. Environ. Earth Sci. 2018, 77, 128. [Google Scholar] [CrossRef]
- Santos, A.L.; Johnson, D.B. Design and Application of a Low pH Upflow Biofilm Sulfidogenic Bioreactor for Recovering Transition Metals From Synthetic Waste Water at a Brazilian Copper Mine. Front. Microbiol. 2018, 9, 2051. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Uyama, A.; Tomiyama, S.; Villacorte-Tabelin, M.; Phengsaart, T.; Silwamba, M.; Jeon, S.; Park, I.; Arima, T.; Igarashi, T. Geochemical Audit of a Historical Tailings Storage Facility in Japan: Acid Mine Drainage Formation, Zinc Migration and Mitigation Strategies. J. Hazard. Mater. 2022, 438, 129453. [Google Scholar] [CrossRef]
- Turingan, C.O.A.; Cordero, K.S.; Santos, A.L.; Tan, G.S.L.; Tabelin, C.B.; Alorro, R.D.; Orbecido, A.H. Acid Mine Drainage Treatment Using a Process Train with Laterite Mine Waste, Concrete Waste, and Limestone as Treatment Media. Water 2022, 14, 1070. [Google Scholar] [CrossRef]
- Turingan, C.O.A.; Singson, G.B.; Melchor, B.T.; Alorro, R.D.; Beltran, A.B.; Orbecido, A.H. Evaluation of Efficiencies of Locally Available Neutralizing Agents for Passive Treatment of Acid Mine Drainage. Minerals 2020, 10, 845. [Google Scholar] [CrossRef]
- Turingan, C.; Fabella, D.; Sadol, K.A.; Beltran, A.; Alorro, R.; Orbecido, A. Comparing the Performance of Low-Grade Nickel Ore and Limestone for Treatment of Synthetic Acid Mine Drainage. Asia-Pac. J. Chem. Eng. 2020, 15, e2457. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 0-87553-287-X. [Google Scholar]
- Fathy, A.T.; Moneim, M.A.; Ahmed, E.A.; El-Ayaat, A.M.; Dardir, F.M. Effective Removal of Heavy Metal Ions (Pb, Cu, and Cd) from Contaminated Water by Limestone Mine Wastes. Sci. Rep. 2025, 15, 1680. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. Available online: https://www.geo.arizona.edu/xtal/group/pdf/am88_247.pdf (accessed on 14 June 2025).
- Cravotta, C.A. Dissolved Metals and Associated Constituents in Abandoned Coal-Mine Discharges, Pennsylvania, USA. Part 2: Geochemical Controls on Constituent Concentrations. Appl. Geochem. 2008, 23, 203–226. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of Metal Sulphide Precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Le Bourre, B.; Neculita, C.M.; Coudert, L.; Rosa, E. Manganese Removal Processes and Geochemical Behavior in Residues from Passive Treatment of Mine Drainage. Chemosphere 2020, 259, 127424. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Chang, X.; Du, W.; Zhang, F.; Kuruc, M.; Slaný, M.; Chen, G. Use of Highly Dispersed Mixed Metal Hydroxide Gel Compared to Bentonite Based Gel for Application in Drilling Fluid under Ultra-High Temperatures. Gels 2023, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A Review of Recent Strategies for Acid Mine Drainage Prevention and Mine Tailings Recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.; Sasowsky, I.D. The Interaction of Acid Mine Drainage with a Carbonate Terrane: Evidence from the Obey River, North-Central Tennessee. J. Hydrol. 1994, 161, 327–346. [Google Scholar] [CrossRef]
- Khani, Y.; Pyo, S.; Bahadoran, F.; Cho, K.; Jeong, K.; Park, Y. Synthesis of Coke-Resistant Catalyst Using NiAl2O4 Support for Hydrogen Production via Autothermal Dry Reforming of Methane. ChemCatChem 2025, 17, e202401015. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Liang, B.; Gu, F.; Xu, Z. Degradation of Decabromodiphenyl Ether by Nano Zero-Valent Iron Immobilized in Mesoporous Silica Microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef]
- Cervi, E.C.; Clark, S.; Boye, K.E.; Gustafsson, J.P.; Baken, S.; Burton, G.A. Copper Transformation, Speciation, and Detoxification in Anoxic and Suboxic Freshwater Sediments. Chemosphere 2021, 282, 131063. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Portales, R.L.; Amabilis-Sosa, L.E.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Nyquist, J.; Greger, M. A Field Study of Constructed Wetlands for Preventing and Treating Acid Mine Drainage. Ecol. Eng. 2009, 35, 630–642. [Google Scholar] [CrossRef]
- Murray-Gulde, C.L.; Huddleston, G.M.; Garber, K.V.; Rodgers, J.H. Contributions of Schoenoplectus Californicus in a Constructed Wetland System Receiving Copper Contaminated Wastewater. Water. Air. Soil Pollut. 2005, 163, 355–378. [Google Scholar] [CrossRef]








| Parameter | Average Values | Extreme Values | Effluent Limits [33,34] |
|---|---|---|---|
| pH | 4.76 ± 0.16 | 4.57 | 6.00 to 9.50 |
| Metals (mg/L) | |||
| Al | 2.39 ± 1.04 | 3.64 | - |
| Ca | 71.22 ± 11.67 | 85.46 | - |
| Cu | 8.90 ± 2.53 | 10.75 | 1.00 |
| Fe | 0.11 ± 0.06 | 0.18 | 7.50 |
| Mg | 16.41 ± 6.61 | 24.04 | - |
| Mn | 1.53 ± 0.37 | 2.06 | 2.00 |
| Ni | 0.01 ± 0.11 | 0.23 | 1.00 |
| Zn | 0.75 ± 0.17 | 0.97 | 4.00 |
| Si | - | 17.94 | - |
| SO42− | 292.03 ± 173.37 | 333.00 | 550 |
| Treatment System | Effective Volume (m3) |
|---|---|
| Storage 1 | |
| LLB | 0.364 |
| CMPB | 0.0472 |
| Storage 2 | |
| LLB | 0.561 |
| CMPB | 0.0944 |
| Parameter | Method of Analysis/Equipment Used |
|---|---|
| pH | Electrode; Hach, HQ4300 and PHC 101 Probe, Loveland, CO, USA |
| Conductivity | Electrode; Hach, HQ4300 and CDC 401 Probe, Loveland, CO, USA |
| ORP | Electrode; Hach, HQ4300 and MTC 101 Probe, Loveland, CO, USA |
| Metals (Cu, Mn) | Atomic Absorption Spectroscopy; Shimadzu, AA-6300, Kyoto, Japan |
| Element | Concentration (wt %) |
|---|---|
| Ca | 91.30 |
| Fe | 3.02 |
| Si | 3.01 |
| Al | 1.14 |
| Cl | 0.48 |
| Ti | 0.27 |
| Others (Cu, K, Mn, Zn, Rh) | 0.78 |
| Total | 100.00 |
| Element | 1 h HRT | 8 h HRT | 15 h HRT |
|---|---|---|---|
| in wt% | |||
| Cu | 32.5 | 42.2 | 49.5 |
| Si | 28.24 | 23.3 | 20.1 |
| Al | 21.0 | 16.2 | 12.7 |
| Fe | 5.80 | 8.97 | 10.8 |
| S | 5.03 | 2.52 | 1.46 |
| Ca | 4.14 | 3.97 | 2.13 |
| Cl | 1.83 | 1.32 | 0.78 |
| Zn | 0.65 | 1.20 | 1.95 |
| Mn | 0.07 | 0.12 | 0.20 |
| Others | 0.79 | 0.24 | 0.41 |
| Time, Days | Weather Condition Prior to Sampling | AMD Source/LLB (L/s) | Storage 1 CMPB (L/s) | Storage 2 CMPB (L/s) |
|---|---|---|---|---|
| 0 | Clear, no precipitation observed | 2.43 | - | - |
| 15 | Clear, no precipitation observed | 3.07 | 9.78 × 10−3 | 9.02 × 10−3 |
| 39 | Scattered rain | - | - | - |
| 59 | Heavy thunderstorm | 3.69 | 5.81 × 10−3 | 16.17 × 10−3 |
| 78 | Heavy thunderstorm | (overflowing) | 8.22 × 10−3 | 30.31 × 10−3 |
| Element | wt% |
|---|---|
| Cu | 65.43 |
| Si | 18.43 |
| Al | 10.76 |
| Ca | 1.75 |
| Fe | 1.38 |
| Zn | 1.15 |
| Others (S, K, Mn) | 1.10 |
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocaan, J.P.; Bueno, B.G.; Pagaduan, J.M.; Capingian, J.; Pablo, M.A.N.; Paulo, J.L.R.W.; Beltran, A.B.; Orbecido, A.H.; Tanhueco, R.M.; Tabelin, C.B.; et al. Limestone-Based Hybrid Passive Treatment for Copper-Rich Acid Mine Drainage: From Laboratory to Field. Minerals 2025, 15, 1043. https://doi.org/10.3390/min15101043
Pocaan JP, Bueno BG, Pagaduan JM, Capingian J, Pablo MAN, Paulo JLRW, Beltran AB, Orbecido AH, Tanhueco RM, Tabelin CB, et al. Limestone-Based Hybrid Passive Treatment for Copper-Rich Acid Mine Drainage: From Laboratory to Field. Minerals. 2025; 15(10):1043. https://doi.org/10.3390/min15101043
Chicago/Turabian StylePocaan, Joshua Pascual, Brian Gerald Bueno, Jaica Mae Pagaduan, Johara Capingian, Michelle Airah N. Pablo, Jacob Louies Rohi W. Paulo, Arnel B. Beltran, Aileen H. Orbecido, Renan Ma. Tanhueco, Carlito Baltazar Tabelin, and et al. 2025. "Limestone-Based Hybrid Passive Treatment for Copper-Rich Acid Mine Drainage: From Laboratory to Field" Minerals 15, no. 10: 1043. https://doi.org/10.3390/min15101043
APA StylePocaan, J. P., Bueno, B. G., Pagaduan, J. M., Capingian, J., Pablo, M. A. N., Paulo, J. L. R. W., Beltran, A. B., Orbecido, A. H., Tanhueco, R. M., Tabelin, C. B., Villacorte-Tabelin, M., Resabal, V. J. T., Dalona, I. M., Alonzo, D., Brito-Parada, P., Plancherel, Y., Armstrong, R., Jungblut, A. D., Santos, A., ... Promentilla, M. A. B. (2025). Limestone-Based Hybrid Passive Treatment for Copper-Rich Acid Mine Drainage: From Laboratory to Field. Minerals, 15(10), 1043. https://doi.org/10.3390/min15101043









