Biological Control Versus Environmental Influence in Serpulid Tube Calcification
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Photograph and Image Acquisition
2.3. Electron Probe Microanalysis
2.4. Raman Spectroscopy Analysis
3. Results
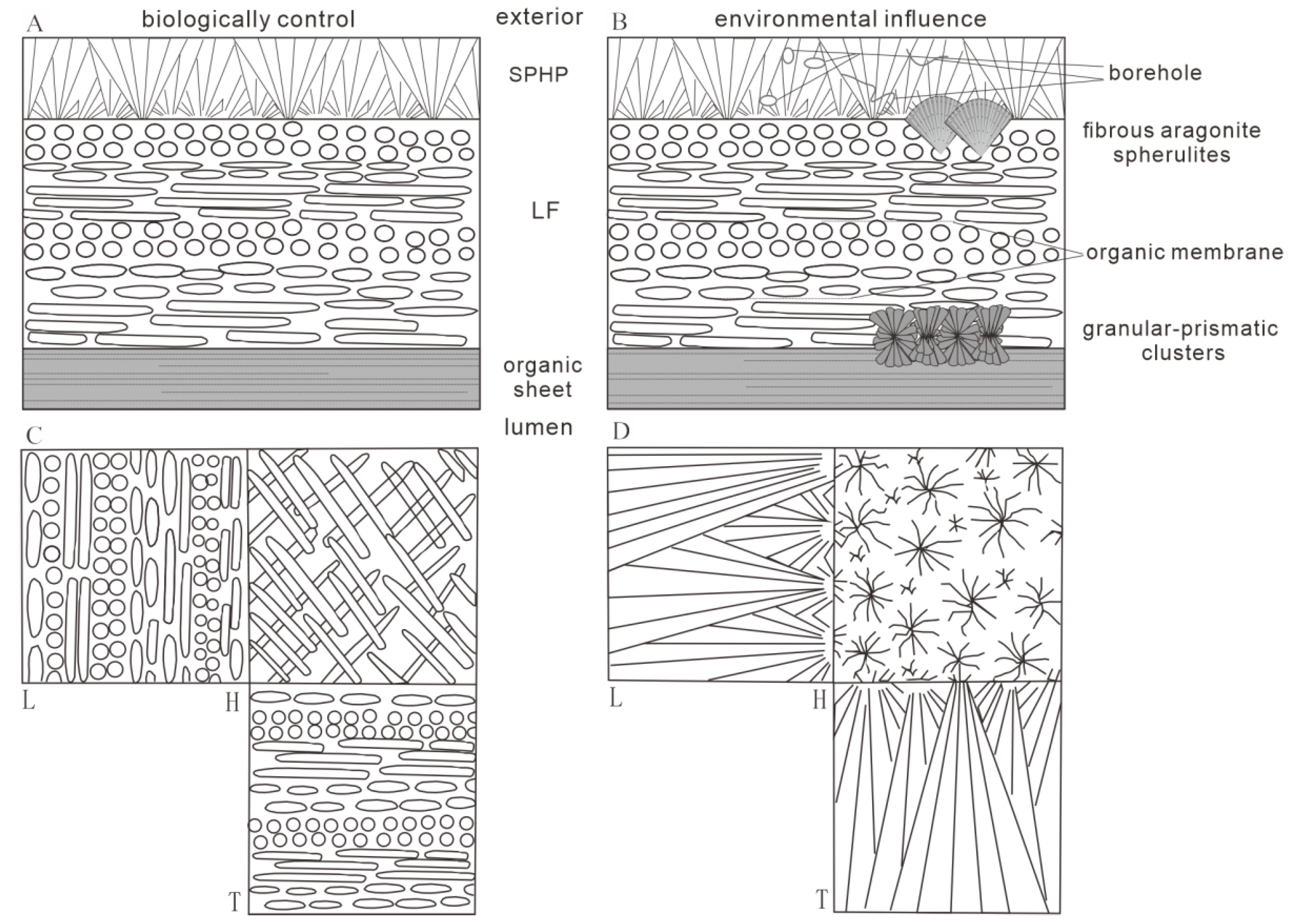
3.1. Ultrastructure and Morphology
3.2. Bioerosion and Secondary Infillings
3.3. Mineral Composition and Elemental Distribution
4. Discussion
4.1. Environmental Influence and Biological Control
4.2. Biomineralization Mode in Serpulid Tubes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinn, O.; Ten Hove, H.A.; Mutvei, H.; Kirsimäe, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Vinn, O.; Ten Hove, H.A.; Mutvei, H. On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta). Palaeontology 2008, 51, 295–301. [Google Scholar] [CrossRef]
- Vinn, O. The ultrastructure of calcareous cirratulid (Polychaeta, Annelida) tubes. Est. J. Earth Sci. 2009, 58, 153–156. [Google Scholar]
- Vinn, O.; Mutvei, H. Calcareous tubeworms of the Phanerozoic. Est. J. Earth Sci. 2009, 58, 286–296. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Reitano, A.; Insacco, G. First record of sabellid and serpulid polychaetes from the Permian of Sicily. Acta Palaeontol. Pol. 2017, 62, 25–38. [Google Scholar] [CrossRef]
- Vinn, O. The role of an internal organic tube lining in the biomineralization of serpulid tubes. Carnets Géologie 2011, 1, 13–16. [Google Scholar] [CrossRef]
- Smith, A.M.; Riedi, M.A.; Winter, D.J. Temperate reefs in a changing ocean: Skeletal carbonate mineralogy of serpulids. Mar. Biol. 2013, 160, 2281–2294. [Google Scholar] [CrossRef]
- Vinn, O.; Kupriyanova, E.K. Evolution of a dense outer protective tube layer in serpulids (Polychaeta, Annelida). Carnets Géol. 2011, 2011, 137–147. [Google Scholar] [CrossRef]
- Vinn, O. The role of aragonite in producing the microstructural diversity of serpulid skeletons. Minerals 2021, 11, 1435. [Google Scholar] [CrossRef]
- Vinn, O. Occurrence, formation and function of organic sheets in the mineral tube structures of Serpulidae (Polychaeta, Annelida). PLoS ONE 2013, 8, e75330. [Google Scholar] [CrossRef]
- Weedon, M.J. Tube microstructure of Recent and Jurassic serpulid polychaetes and the question of the Palaeozoic ‘spirorbids’. Acta Palaeontol. Pol. 1994, 39, 1–15. [Google Scholar]
- Vinn, O.; Alkahtane, A.A.; Al Farraj, S.; El Hedeny, M. New type of SIOP structure in Serpulidae: Formation and evolutionary implications. Minerals 2024, 14, 291. [Google Scholar] [CrossRef]
- Vinn, O.; Mutvei, H.; ten Hove, H.A.; Kirsimäe, K. Unique Mg-calcite skeletal ultrastructure in the tube of the serpulid polychaete Ditrupa. Neues Jahrb. Geol. Paläontol. Abh. 2008, 248, 79–89. [Google Scholar] [CrossRef]
- Vinn, O.; Kirsimäe, K.; ten Hove, H.A. Tube ultrastructure of Pomatoceros americanus (Polychaeta, Serpulidae): Implications for the tube formation of serpulids. Est. J. Earth Sci. 2009, 58, 148–152. [Google Scholar] [CrossRef]
- Vinn, O. On the unique isotropic aragonitic tube microstructure of some serpulids (Polychaeta, Annelida). J. Morphol. 2013, 274, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Vinn, O. SEM study of semi-oriented tube microstructures of Serpulidae (Polychaeta, Annelida): Implications for the evolution of complex oriented microstructures. Microsc. Res. Tech. 2013, 76, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Vinn, O.; Alkahtane, A.A.; El Hedeny, M.; Al Farraj, S. Discovery of rod-type crossed lamellar structure in Spiraserpula (Serpulidae, Polychaeta) from the Middle Miocene of Austria. Neues Jahrb. Geol. Paläontol. Abh. 2023, 310, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Vinn, O. Distribution of growth lines in the tube wall of serpulids (Polychaeta, Annelida). J. Mar. Biol. Assoc. UK 2024, 104, e24. [Google Scholar] [CrossRef]
- Słowiński, J.; Vinn, O.; Zatoń, M. Ultrastructure of the Jurassic serpulid tubes—Phylogenetic and paleoecological implications. PeerJ 2024, 12, e17389. [Google Scholar] [CrossRef]
- Tambutté, E.; Allemand, D.; Zoccola, D. Coral biomineralization: A focus on intra-skeletal organic matrix and calcification. Minerals 2021, 11, 523. [Google Scholar]
- Cuif, J.P.; Dauphin, Y. Organic matrix and biomineralization of scleractinian corals. Minerals 2020, 10, 189. [Google Scholar]
- Sommerdijk, N.A.J.M.; de With, G. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem. Rev. 2008, 108, 4499–4550. [Google Scholar] [CrossRef]
- Buckman, J.O. An overview of the tube fabric of Pomatoceros (Polychaeta, Serpulidae), illustrated by examples from the British Isles. Zool. Anz. 2015, 259, 54–60. [Google Scholar] [CrossRef]
- Grenier, C.; Berent, K.; Rodríguez-Navarro, A.; Vinn, O.; Checa, A.G. Microstructures, crystallography and growth patterns of serpulid tubeworms (Class Polychaeta). Mar. Biol. 2024, 171, 231. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Burke, E.A.J. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Hedley, R.H. Tube formation by Pomatoceros triqueter (Polychaeta). J. Mar. Biol. Assoc. UK 1958, 37, 315–322. [Google Scholar] [CrossRef]
- Hedley, R.H. Studies on serpulid tube formation. I. The secretion of the calcareous and organic components of the tube by Pomatoceros triqueter. Q. J. Microsc. Sci. 1956, 97, 411–419. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Crystallization pathways in biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.B. Effect of ambient Mg/Ca ratio on Mg fractionation in calcareous marine invertebrates: A record of the oceanic Mg/Ca ratio over the Phanerozoic. Geology 2004, 32, 981–984. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Vinn, O.; Taylor, P.D.; Schopf, J.W.; Kudryavtsev, A.; Bailey-Brock, J. Serpulids living deep: Calcareous tubeworms beyond the abyss. Deep Sea Res. I 2014, 90, 91–104. [Google Scholar] [CrossRef]
- Simkiss, K.; Wilbur, K.M. Biomineralization: Cell Biology and Mineral Deposition; Academic Press: New York, NY, USA, 1989. [Google Scholar]
- Neff, J.M. Mineral regeneration by serpulid polychaete worms. Biol. Bull. 1969, 136, 1–15. [Google Scholar] [CrossRef]
- Vinn, O.; Jäger, M.; Kirsimäe, K. Microscopic evidence of serpulid affinities of the problematic fossil tube ‘Serpula’ etalensis from the Lower Jurassic of Germany. Lethaia 2008, 41, 417–421. [Google Scholar] [CrossRef]
- Bubel, A. Electron microscope studies on the operculum of Spirorbis spirorbis (L.). In Proceedings of the 3rd International Biodegradation Symposium; Sharpley, J.M., Kaplan, A.M., Eds.; Applied Science Publishers: London, UK, 1976; pp. 495–514. [Google Scholar]
- Bubel, A.; Thorp, C.H.; Fitzsimmons, C.A.L. A histological and electron microscopical study of opercular regeneration in the serpulid Pileolaria (P.) granulata, with particular reference to the formation of the calcareous opercular plate. In Proceedings of the 4th International Congress on Marine Corrosion and Fouling, Juan-les-Pins, Antibes, France, 14–18 June 1977; pp. 85–96. [Google Scholar]
- Bubel, A.; Thorp, C.H.; Moore, M.N. A histological, histochemical and ultrastructural study of the operculum of the serpulid Pomatoceros triqueter L., with particular reference to the formation of the calcareous opercular plate during opercular regeneration. In Proceedings of the 4th International Biodegradation Symposium; Sharpley, J.M., Kaplan, A.M., Eds.; Applied Science Publishers: London, UK, 1980; pp. 275–290. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, C.; Li, L. Biological Control Versus Environmental Influence in Serpulid Tube Calcification. Minerals 2025, 15, 1034. https://doi.org/10.3390/min15101034
Xin C, Li L. Biological Control Versus Environmental Influence in Serpulid Tube Calcification. Minerals. 2025; 15(10):1034. https://doi.org/10.3390/min15101034
Chicago/Turabian StyleXin, Chunmei, and Luoyang Li. 2025. "Biological Control Versus Environmental Influence in Serpulid Tube Calcification" Minerals 15, no. 10: 1034. https://doi.org/10.3390/min15101034
APA StyleXin, C., & Li, L. (2025). Biological Control Versus Environmental Influence in Serpulid Tube Calcification. Minerals, 15(10), 1034. https://doi.org/10.3390/min15101034




