Reductant-Free Cobalt Recovery from Similar Copper–Cobalt Oxide Ores via Synergistic Reductive-Acid Leaching
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Reagents
2.2. Leaching Process
2.3. Analysis and Characterization Methods
3. Results and Discussion
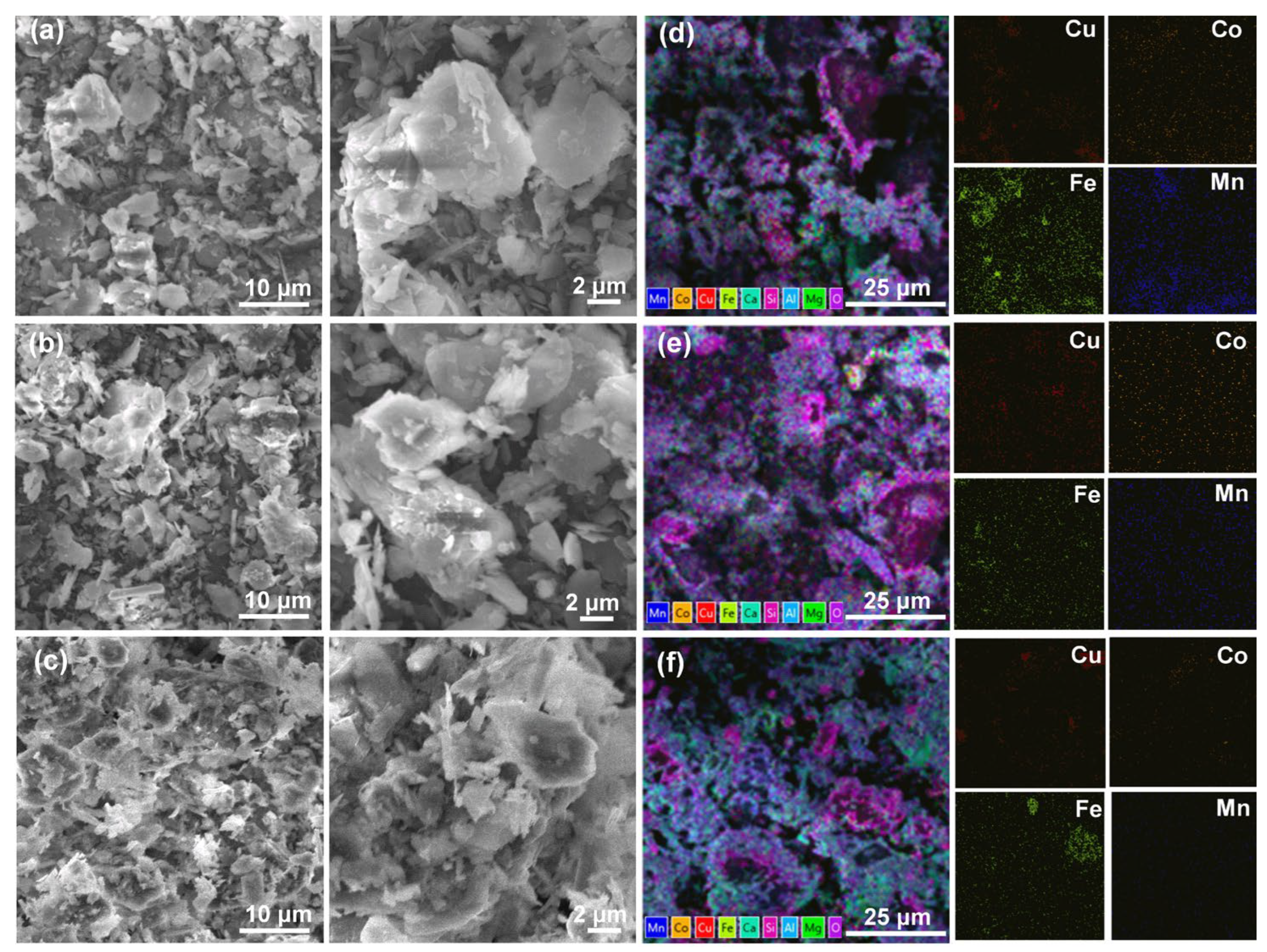
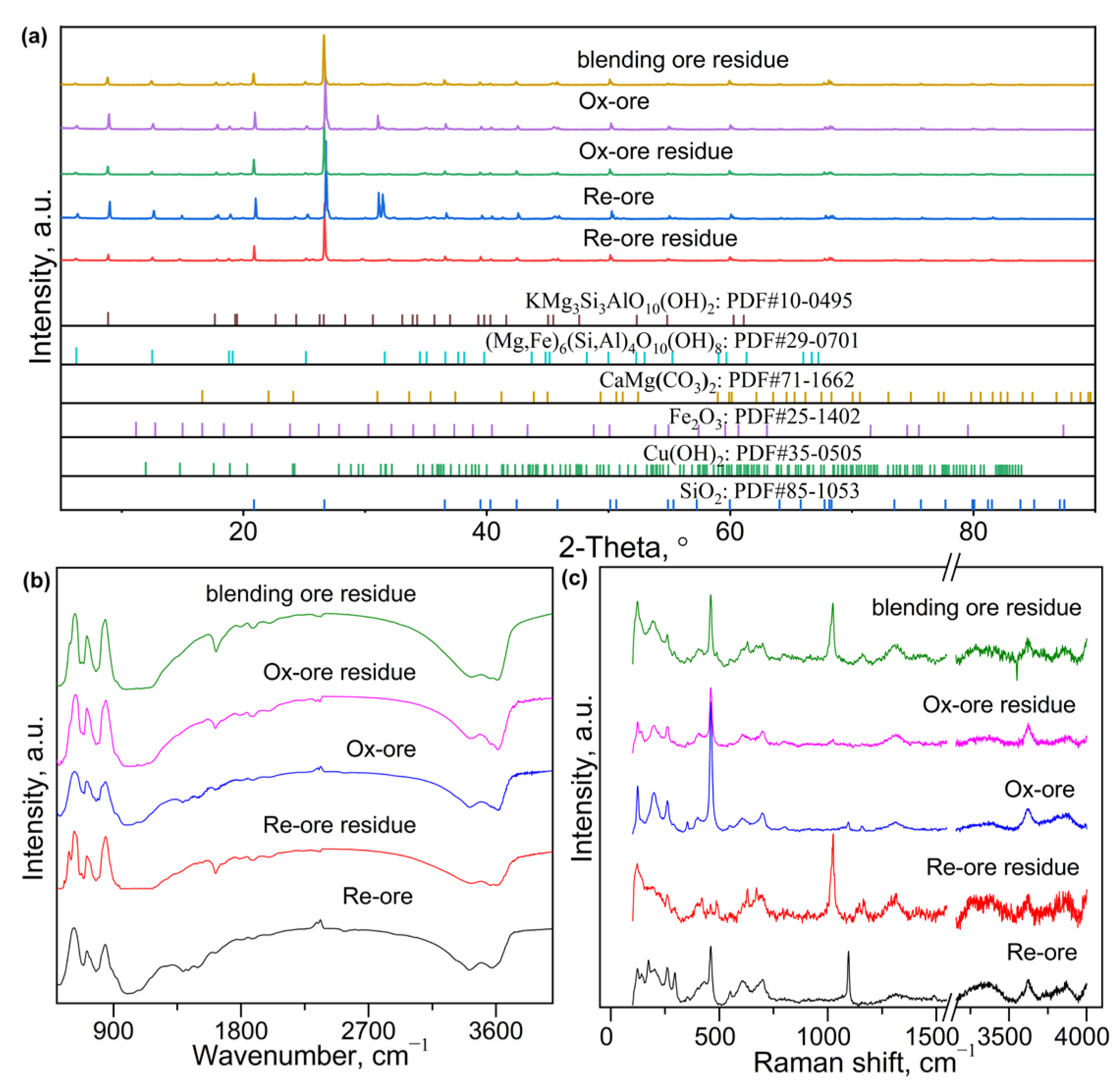
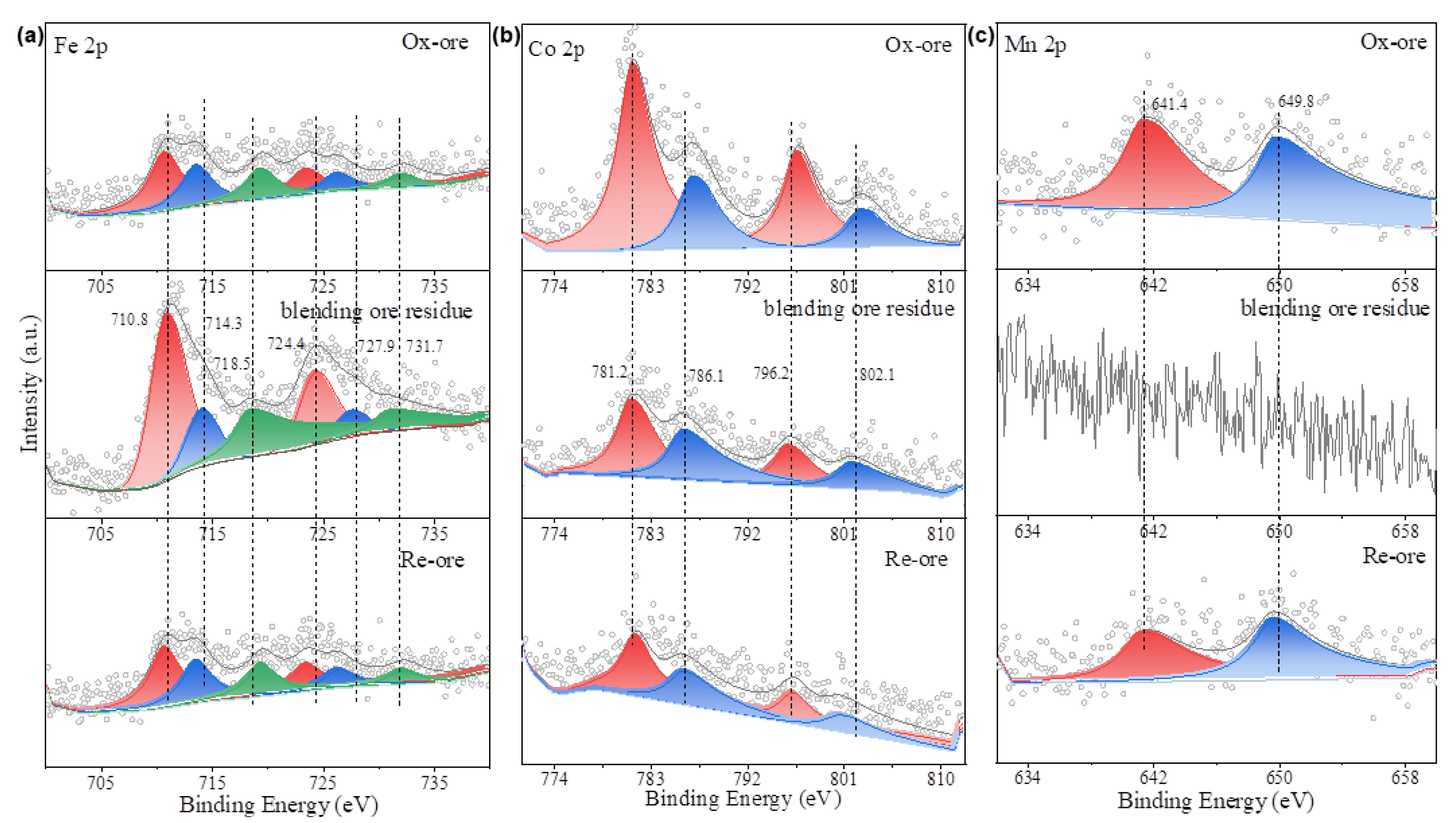
3.1. Chemical Analysis and Phase Composition
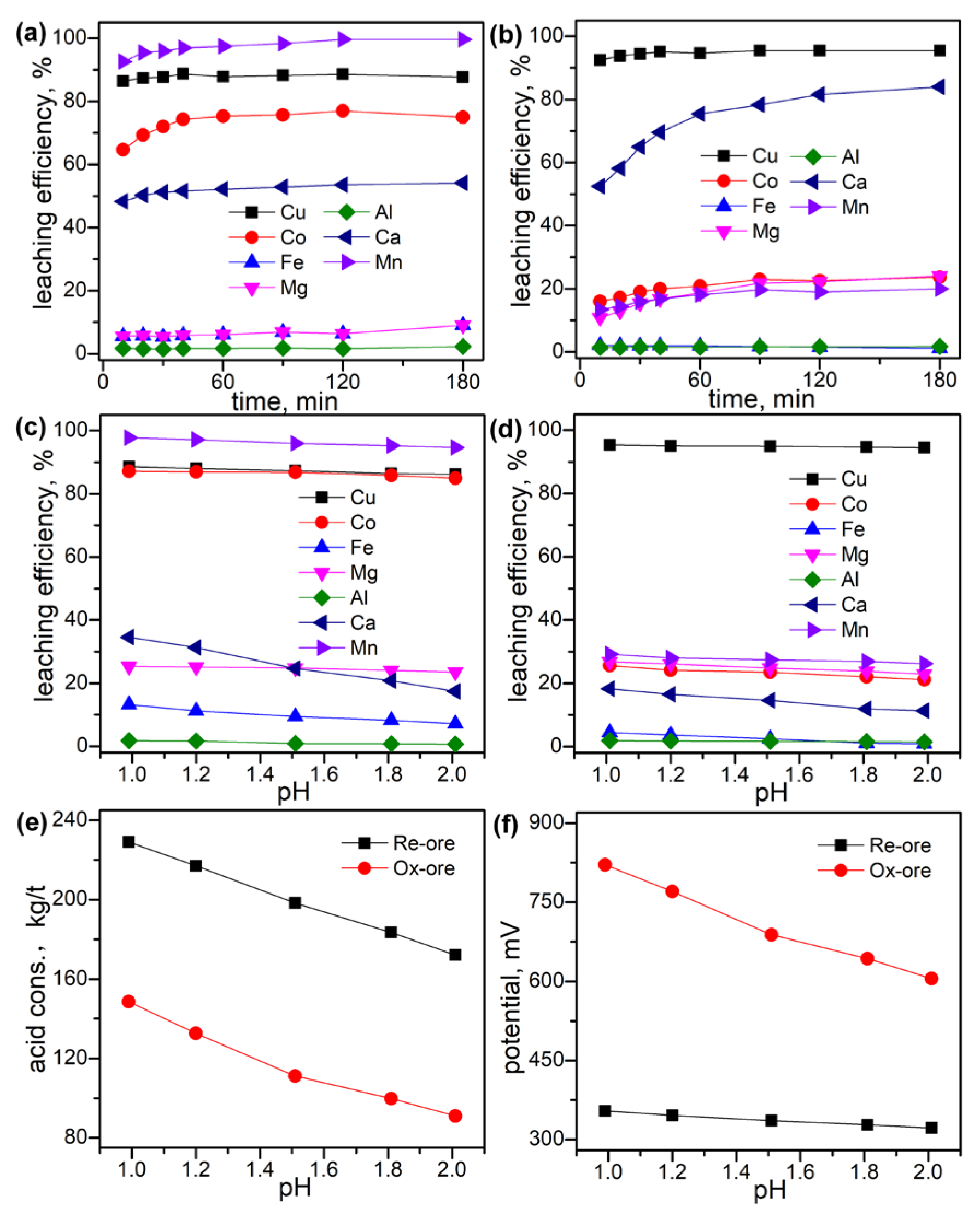
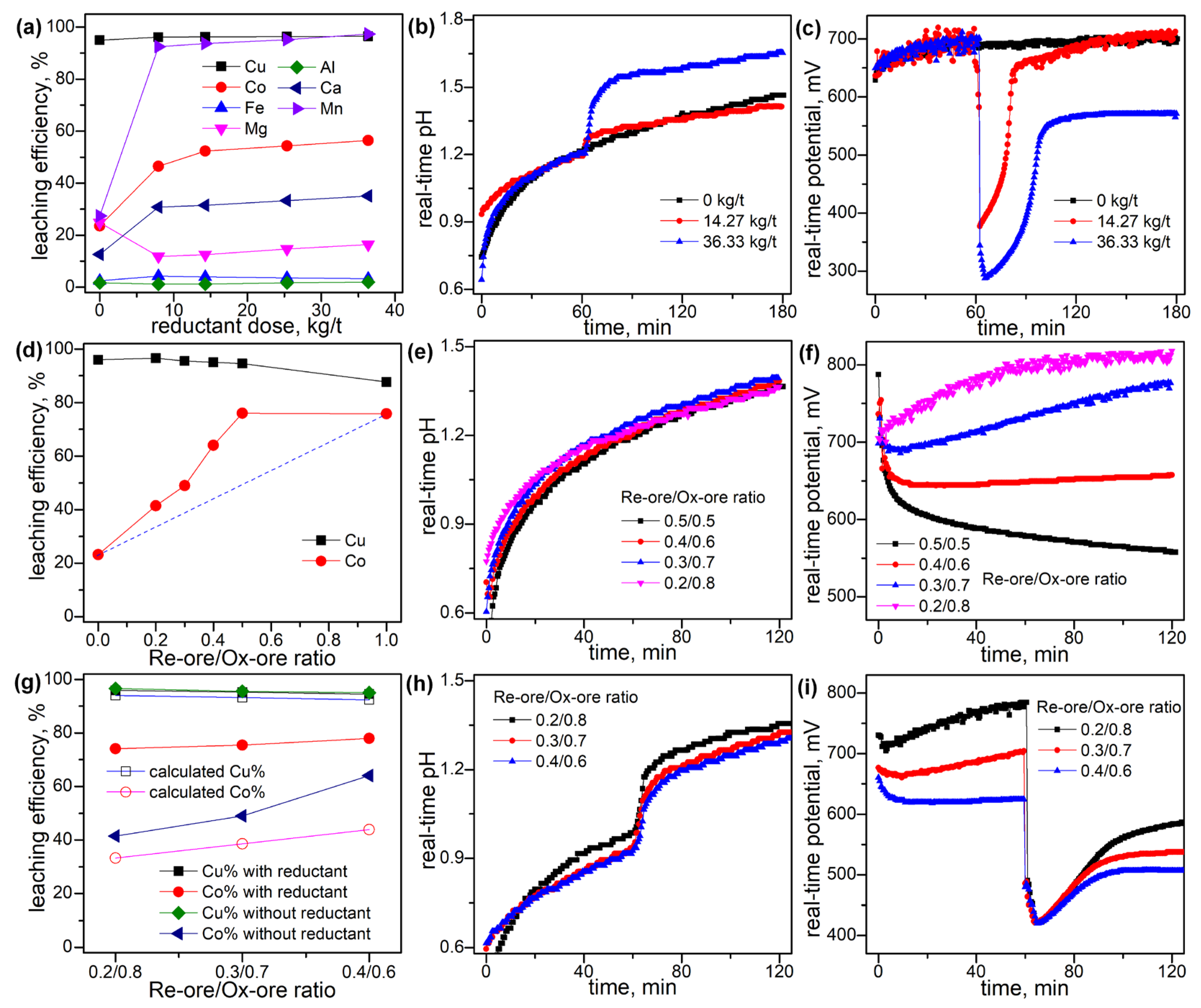
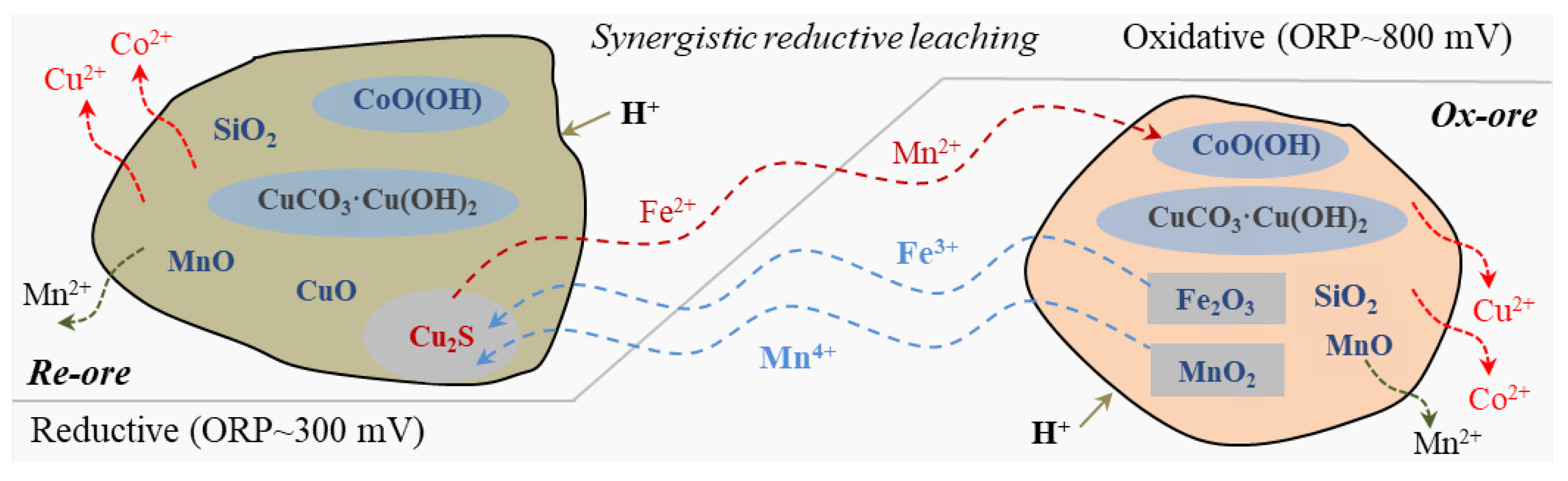
3.2. Leaching Behaviors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiquan, D.; Deyi, X. The security of critical mineral supply chains. Miner. Econ. 2023, 36, 401–412. [Google Scholar] [CrossRef]
- Machaca, D.M.C.; Botelho, A.B., Jr.; de Carvalho, T.C.; Tenório, J.A.S.; Espinosa, D.C.R. Hydrometallurgical processing of chalcopyrite: A review of leaching techniques. Int. J. Miner. Metall. Mater. 2024, 31, 2537–2555. [Google Scholar] [CrossRef]
- Nkulu, C.B.L.; Casas, L.; Haufroid, V.; De Putter, T.; Saenen, N.D.; Kayembe-Kitenge, T.; Obadia, P.M.; Mukoma, D.K.W.; Ilunga, J.M.L.; Nawrot, T.S. Sustainability of artisanal mining of cobalt in DR Congo. Nat. Sustain. 2018, 1, 495–504. [Google Scholar] [CrossRef]
- Crundwell, F.; Preez, N.D.; Knights, B. Production of cobalt from copper-cobalt ores on the African Copperbelt—An overview. Miner. Eng. 2020, 156, 106450. [Google Scholar] [CrossRef]
- Gulley, A.L. One hundred years of cobalt production in the Democratic Republic of the Congo. Resour. Policy 2022, 79, 103007. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Li, Y.; Gao, P. Recovering cobalt from cobalt oxide ore using suspension roasting and magnetic separation technique. J. Mater. Res. Technol. 2023, 27, 3005–3015. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Santoro, L.; Tshipeng, S.; Pirard, E.; Bouzahzah, H.; Kaniki, A. Herrington, Mineralogical reconciliation of cobalt recovery from the acid leaching of oxide ores from five deposits in Katanga (DRC). Miner. Eng. 2019, 137, 277–289. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals 2024, 14, 1119. [Google Scholar] [CrossRef]
- Mambwe, P.; Shengo, M.; Kidyanyama, T.; Muchez, P.; Chabu, M. Geometallurgy of cobalt black ores in the Katanga Copperbelt (Ruashi cu-co deposit): A new proposal for enhancing cobalt recovery. Minerals 2022, 12, 295. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Rao, M.; Chen, S.; Luo, J.; Li, G.; Jiang, T. Oxidative acid leaching behavior of Fe–Ni–Co alloy powder derived from a laterite ore. Int. J. Miner. Metall. Mater. 2025, 32, 825–834. [Google Scholar] [CrossRef]
- Stuurman, S.; Ndlovu, S.; Sibanda, V. Metallurgy, Comparing the extent of the dissolution of copper-cobalt ores from the DRC Region. J. S. Afr. Inst. Min. Metall. 2014, 114, 347–349. [Google Scholar]
- Mbuya, B.; Fosso-Kankeu, E.; Bongaerts, J.; Mulaba-Bafubiandi, A.F. Thermodynamic investigation on the impact of oxidized copper-cobalt and copper sulfide ores stream mixture toward the dissolution of Cu and Co. J. Sustain. Metall. 2025, 11, 1299–1318. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Shu, X.; Fu, B.; Peng, W.; Liu, J.; Cao, Y.; Zhu, X. Extraction and recycling technologies of cobalt from primary and secondary resources: A comprehensive review. Int. J. Miner. Metall. Mater. 2024, 31, 628–649. [Google Scholar] [CrossRef]
- Xu, J.; Qin, S.; Zheng, C.; Wang, J.; Yang, B.; Qiu, G.; Cui, S.; Ma, H. Characterization and Optimization of Copper-Cobalt Oxide Ores During Acid Leaching. JOM 2023, 75, 5785–5795. [Google Scholar] [CrossRef]
- Tshipeng, S.Y.; TshamalaKaniki, A.; Kime, M.B. Effects of the Addition Points of Reducing Agents on the Extraction of Copper and Cobalt from Oxidized Copper–Cobalt Ores. J. Sustain. Metall. 2017, 3, 823–828. [Google Scholar] [CrossRef]
- Ferron, C.J. Sulfur dioxide: A versatile reagent for the processing of cobaltic oxide minerals. JOM 2008, 60, 50–54. [Google Scholar] [CrossRef]
- Mwema, M.D.; Mpoyo, M.; Kafumbila, K. Use of sulphur dioxide as reducing agent in cobalt leaching at Shituru hydrometallurgical plant. J. South Afr. Inst. Min. Metall. 2002, 102, 1–4. [Google Scholar]
- Yaohua, C.; Wei, W.; Hongzhao, L.; Lin, L.; Bo, Z. A new process of leaching copper and cobalt from copper cobalt oxide ore in DR Congo. Multipurp. Util. Miner. Resour. 2024, 45, 135–138. [Google Scholar]
- Cao, Y.; Wang, W.; Liu, H.; Liu, L.; Zhang, B. Leaching of copper and cobalt from copper-cobalt oxide ore. Hydrometall. China 2020, 39, 478–482. [Google Scholar]
- Zuo, Y.; Zhang, W.; Che, J.; Feng, S.; Chen, Y.; Wang, C. Efficient extraction of cobalt and copper: Leveraging redox chemistry in oxide and sulfide copper-cobalt ores. Sep. Purif. Technol. 2025, 354, 128671. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, G.; Zhao, Y.; Feng, X. A study of recovery of copper and cobalt from copper–cobalt oxide ores by ammonium salt roasting. Hydrometallurgy 2012, 129, 140–144. [Google Scholar] [CrossRef]
- Mwanat, M.H.-M.; Kasongo, K.B.; Muliangala, M.F.; Kayembe, M.M.; Kapiamba, K.F.; Ngenda, B.R. Simulation of Simultaneous Leaching of Copper and Cobalt Minerals in Acid-Reductive Media: Sensitivity Analysis and Optimization. J. Sustain. Metall. 2022, 8, 837–850. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Wang, R.; Huang, T. Process of ammonium leaching oxidation ore of cobalt and copper at high pressure. Chin. J. Rare Met. 2012, 36, 149–153. [Google Scholar]
- Jie, X.; Zhang, Y.; Ruan, S.; Wang, Z. SO2 reduction leaching of low-grade copper-cobalt oxide ore in sulfuric acid system. China Resour. Compr. Util. 2018, 36, 4–8+11. [Google Scholar]
- Li, Q.; Yang, B.; Ruan, S.; Yin, F. Two stage countercurrent reduction leaching of complex low grade oxidized copper and cobalt ores. Non-Ferr. Met. 2016, 5, 1–4+32. [Google Scholar]
- Uahengo, F.D.; Hara, Y.R.; Bazhko, O. Leaching a complex copper-cobalt oxide ore from Zebesha Mine in Zambia, a novel method, Transactions of the Institutions of Mining and Metallurgy, Section C. Miner. Process. Extr. Metall. 2024, 133, 119–127. [Google Scholar]
- Liu, Y.Y.; Yang, H.Y.; Tong, L.L.; Jin, Z.N. Novel hydrometallurgical process of refractory copper-cobalt ores from Zambia. J. Northeast. Univ. 2017, 38, 1285. [Google Scholar]
- Yu, W. Direct reduction leaching of copper and cobalt in oxide ore from Congo (Kinshasa). Hydrometall. China 2019, 38, 88–91. [Google Scholar]
- Apua, M.C.; Mulaba-Bafubiandi, A.F. Dissolution of oxidised Co–Cu ores using hydrochloric acid in the presence of ferrous chloride. Hydrometallurgy 2011, 108, 233–236. [Google Scholar] [CrossRef]
- Mbuya, B.; Fosso-Kankeu, E.; Bongaerts, J.; Mulaba-Bafubiandi, A.F. Simultaneous acid Co and Cu leaching from oxidised and sulfide mixture ores: Optimisation and kinetic study. Can. Metall. Q. 2025, 64, 1–26. [Google Scholar] [CrossRef]
- Mbuya, B.; Zeka, L.; Mulaba-Bafubiandi, A.F. Fe3+-Fe2+ Redox Cycle Peculiarity in the Acid Dissolution of Copper–Cobalt Complex Ores. In Recovery of Values from Low-Grade and Complex Minerals: Development of Sustainable Processes; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 179–215. [Google Scholar]
- Mbuya, B.; Ntakamusthi, P.; Kime, M.B.; Zeka, L.; Nkulu, G.; Mwamba, A.; Mulaba-Bafubiandi, A.F. Metallurgical Evaluation of the Leaching Behavior of Copper–Cobalt-bearing Ores by the Principal Component Analysis Approach: Case Study of the DRC Copperbelt Ore Deposits. J. Sustain. Metall. 2021, 7, 985–994. [Google Scholar] [CrossRef]
- Apua, M.C.; Madiba, M.S. Leaching kinetics and predictive models for elements extraction from copper oxide ore in sulphuric acid. J. Taiwan Inst. Chem. Eng. 2021, 121, 313–320. [Google Scholar] [CrossRef]
- Mwamba, P.; Masinja, J.H.; Manchisi, J.; Kabondo, L. Sulphuric acid bake-leach process for the treatment of mixed copper-cobalt oxide ores. J. Miner. Mater. Charact. Eng. 2022, 10, 174–184. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Fu, C.; Li, S.; Huang, R.; He, J.; Zheng, C.; Liu, S.; Hu, J. Reductant-Free Cobalt Recovery from Similar Copper–Cobalt Oxide Ores via Synergistic Reductive-Acid Leaching. Minerals 2025, 15, 1022. https://doi.org/10.3390/min15101022
Yao X, Fu C, Li S, Huang R, He J, Zheng C, Liu S, Hu J. Reductant-Free Cobalt Recovery from Similar Copper–Cobalt Oxide Ores via Synergistic Reductive-Acid Leaching. Minerals. 2025; 15(10):1022. https://doi.org/10.3390/min15101022
Chicago/Turabian StyleYao, Xianzhao, Chenxiao Fu, Songjiang Li, Rongwei Huang, Jian He, Chaozhen Zheng, Sanping Liu, and Jiugang Hu. 2025. "Reductant-Free Cobalt Recovery from Similar Copper–Cobalt Oxide Ores via Synergistic Reductive-Acid Leaching" Minerals 15, no. 10: 1022. https://doi.org/10.3390/min15101022
APA StyleYao, X., Fu, C., Li, S., Huang, R., He, J., Zheng, C., Liu, S., & Hu, J. (2025). Reductant-Free Cobalt Recovery from Similar Copper–Cobalt Oxide Ores via Synergistic Reductive-Acid Leaching. Minerals, 15(10), 1022. https://doi.org/10.3390/min15101022





