Effect of Acidified Water Glass on Flotation Separation of Fluorite and Calcite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation Tests
2.2.2. Contact Angle Measurements
2.2.3. Zeta Potential Tests
2.2.4. FT-IR Detection
2.2.5. X-Ray Photoelectron Spectroscopy Measurement
3. Results and Discussion
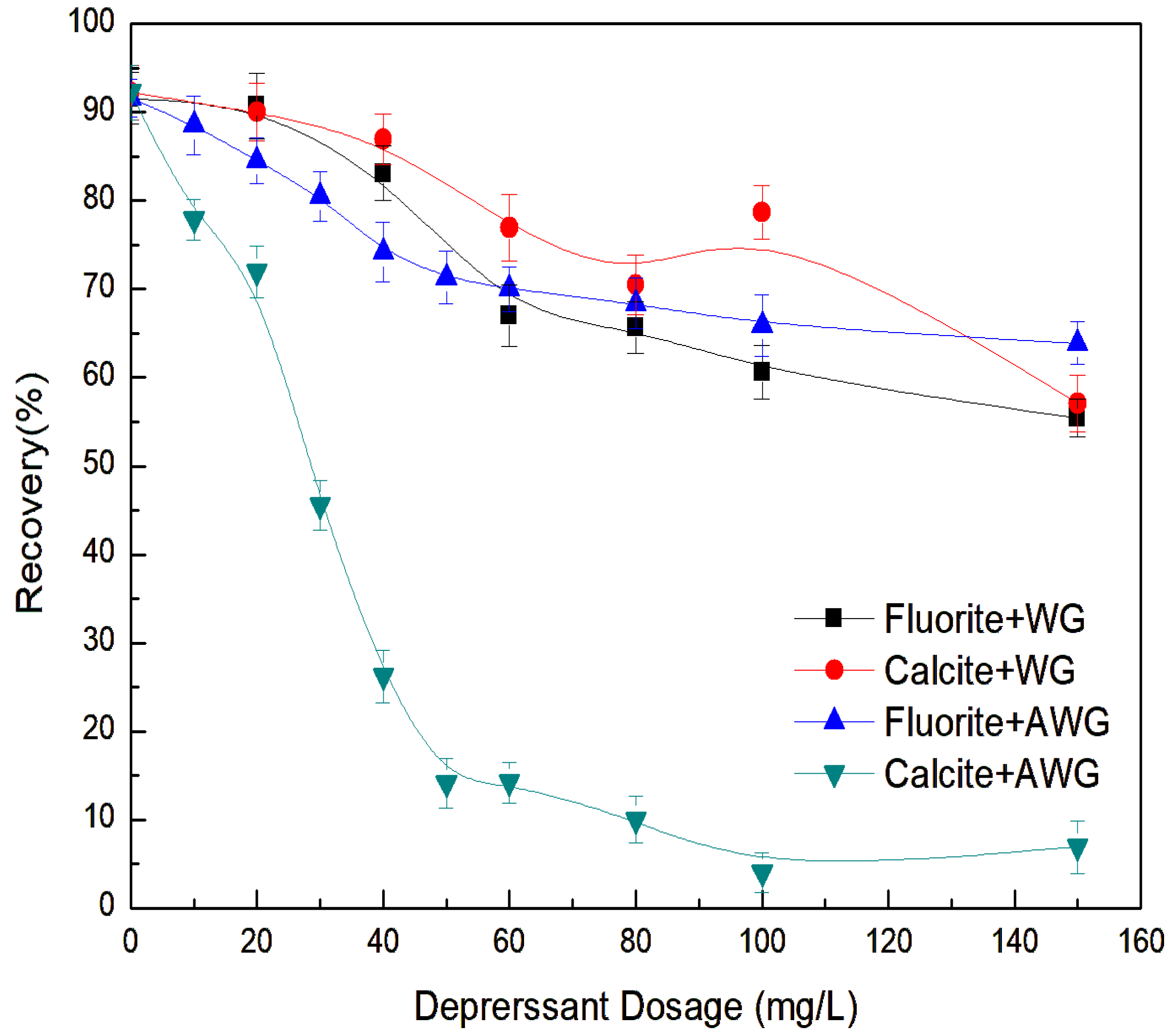
3.1. Flotation Studies
3.2. Contact Angle Analysis
3.3. Computational Analysis of Solution Chemistry
3.4. Zeta Potential Analysis
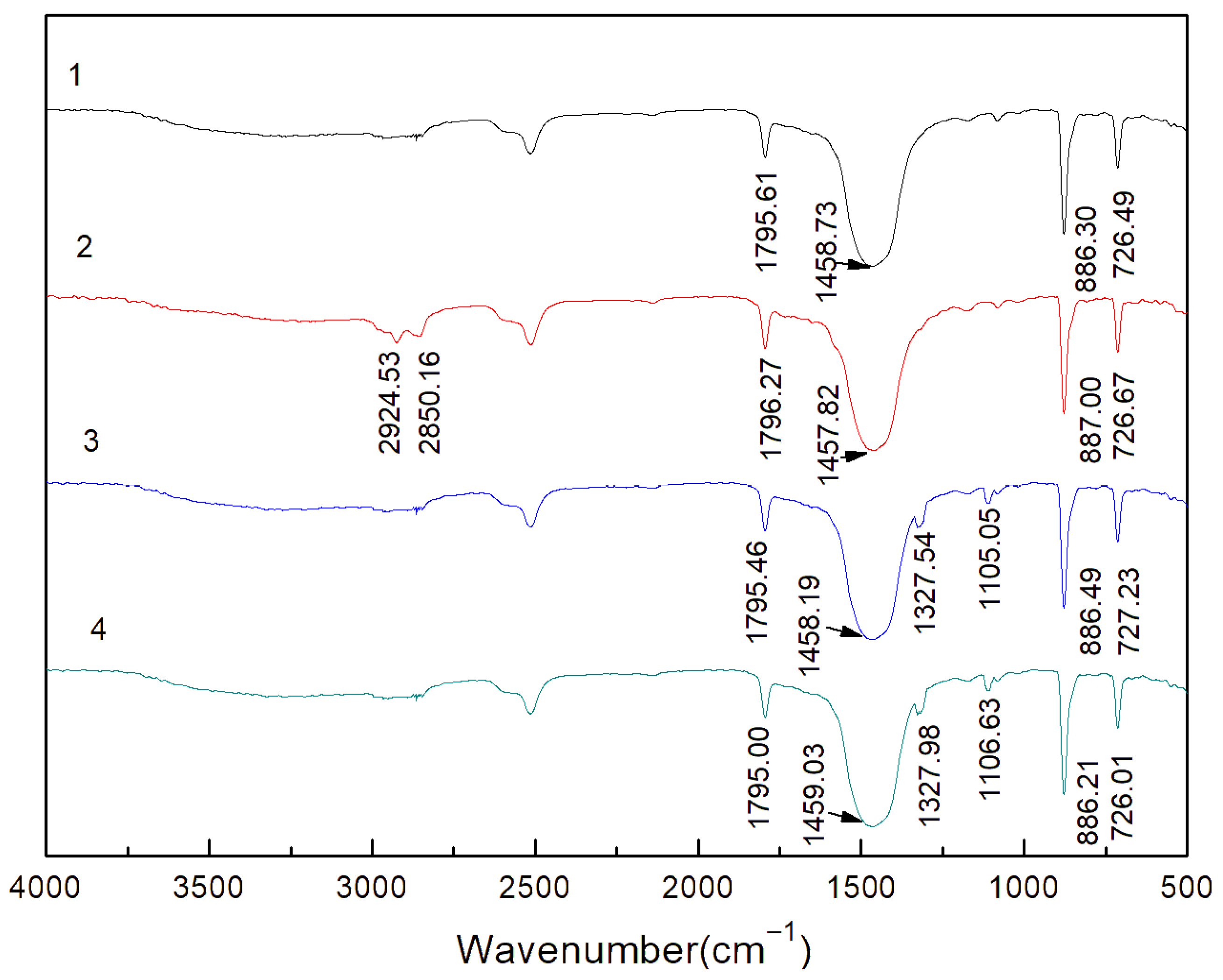
3.5. FT-IR Spectra Analysis
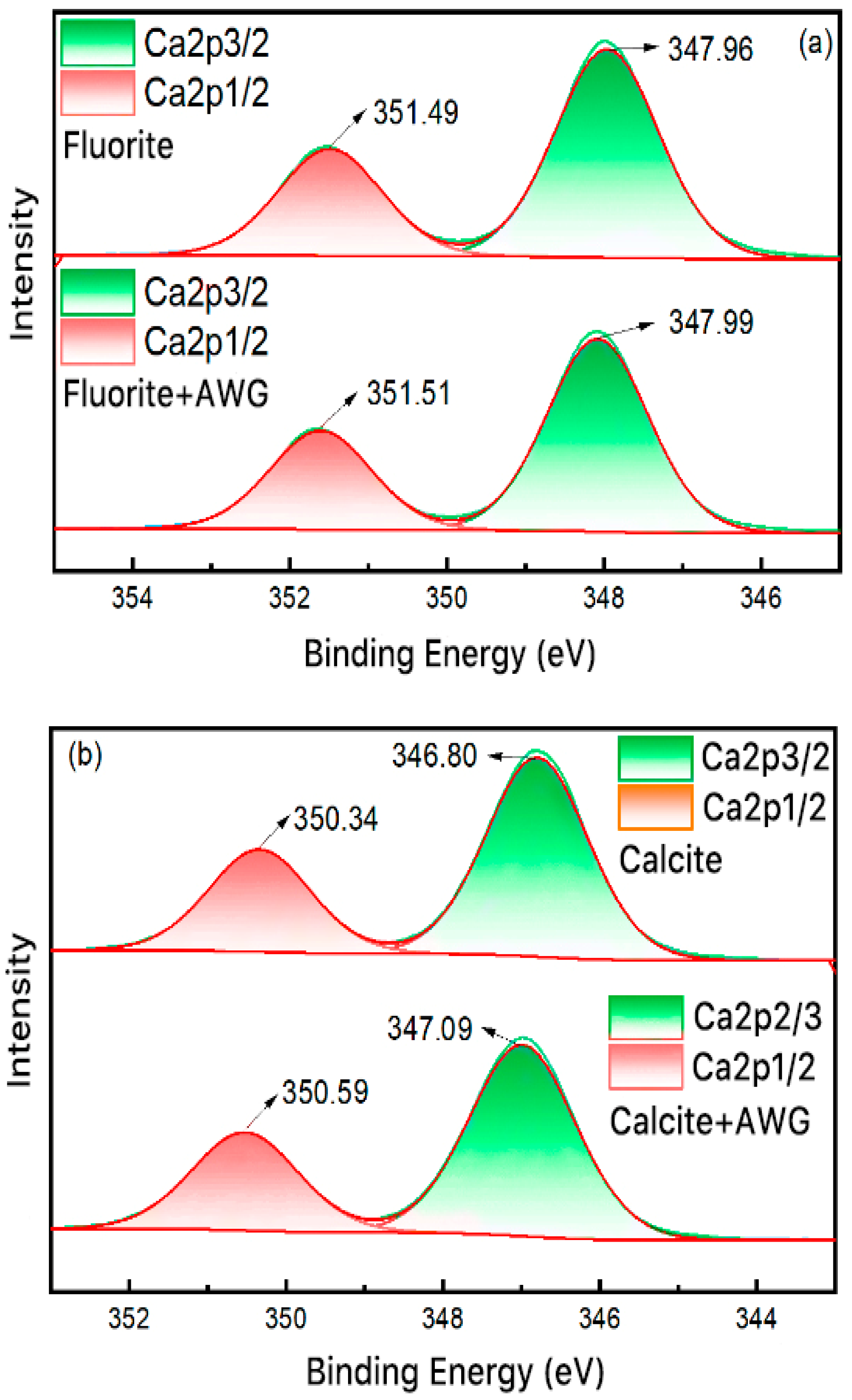
3.6. XPS Result Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, W.J.; Zhang, L.Y.; Bai, L.L.; Qiu, Y.S. Flotation research of a fluorite ore. Appl. Mech. Mater. 2014, 539, 781–784. [Google Scholar] [CrossRef]
- Zhou, W.B.; Moreno, J.; Torres, R.; Valle, H.; Song, S.X. Flotation of fluorite from ores by using acidized water glass as depressant. Miner. Eng. 2013, 45, 142–145. [Google Scholar] [CrossRef]
- Liu, C.; Song, S.; Li, H. Selective flotation of fluorite from barite using trisodium phosphate as a depressant. Miner. Eng. 2019, 134, 390–393. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Tune surface physicochemical property of fluorite particles by regulating the exposure degree of crystal surfaces. Miner. Eng. 2018, 128, 123–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S. Beneflciation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Kienko, L.A.; Samatova, L.A.; Voronova, O.V.; Kondrat’ev, S.A. Lower temperature flotation of carbonate-fluorite ores. J. Min. Sci. 2010, 46, 317–323. [Google Scholar] [CrossRef]
- Aliaga, W.; Sampaio, C.H.; Brum, I.A.S.; Ferreira, K.R.S.; Batistella, M.A. Flotation of high-grade fluorite in a short column under negative bias regime. Miner. Eng. 2006, 19, 1393–1396. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Chen, Z.; Qian, Y.; Li, Y.J. Effects of crystal chemistry on sodium oleate adsorption on fluorite surface investigated by molecular dynamics simulation. Miner. Eng. 2018, 124, 77–85. [Google Scholar] [CrossRef]
- Sun, R.F.; Liu, D.; Liu, Y.B.; Wang, D.Q.; Wen, S.M. Pb-water glass as a depressant in the flotation separation of fluorite from calcite. Miner. Eng. 2021, 628, 127447. [Google Scholar] [CrossRef]
- Deng, J.; Liu, C.; Yang, S.Y.; Li, H.Q.; Liu, Y. Flotation separation of barite from calcite using acidified water glass as the depressant. Colloid. Surf. A 2019, 579, 123605. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, S.J. Acidized sodium silicate—An effective modifier in fluorite flotation. Miner. Eng. 1992, 5, 435–444. [Google Scholar] [CrossRef]
- Ding, K.; Laskowski, J.S. Application of a modified water glass in a cationic flotation of calcite and dolomite. J. Can. Metall. Q. 2006, 45, 199–206. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effect of Zn2+ and its addition sequence on flotation separation of scheelite from calcite using water glass. Colloid. Surf. A 2020, 588, 124394. [Google Scholar] [CrossRef]
- Jin, S.Z.; Ou, L.M.; Ma, X.Q.; Zhou, H.; Zhang, Z.J. Activation mechanisms of sodium silicate-inhibited fluorite in flotation under neutral and slightly alkaline conditions. Miner. Eng. 2021, 161, 106738. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.P.; Wang, J.P.; Wang, P.C. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Sun, W.; Zeng, X.; Fang, S.; Han, H.; Hong, K.; Hu, Y. Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, Z.; Wang, J.L.; Cao, Y.D. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earths 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.M.; Forssberg, E. Mechanism of oleate interaction on salttype minerals, part II. Adsorption and electrokinetic studies of apatite in the presence of sodium oleate and sodium metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Cui, Y.F.; Jiao, F.; Wei, Q.; Wang, X.; Dong, L.Y. Flotation separation of fluorite from calcite using sulfonated lignite as depressant. Sep. Purif. Technol. 2020, 242, 116698. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Qi, W.G.; Klauber, C.; Warren, L.J. Mechanism of action of sodium silicate in the flotation of apatite from hematite. Int. J. Miner. Process. 1993, 39, 3–4. [Google Scholar] [CrossRef]
- Wang, D.Z.; Hu, Y.H. Chemistry of Flotation Solution; Hunan Science and Technology Press: Changsha, China, 1988; pp. 336–337. (In Chinese) [Google Scholar]
- Foucaud, Y.; Badawi, M.; Filippov, L.O.; Barres, O.; Filippova, I.V.; Lebègue, S. Synergistic adsorption of Na2CO3 and Na2SiO3 on calcium minerals revealed by spectroscopic and ab initio molecular dynamics studies. Chem. Sci. 2019, 10, 9928–9940. [Google Scholar] [CrossRef]
- Yang, S.Y.; Xu, Y.L.; Liu, C. The anionic flotation of fluorite from barite using gelatinized starch as the depressant. Colloid. Surf. A 2020, 597, 124794. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.H.; Huang, K.H. Studies of benzyl hydroxamic acid/calcium lignosulphonate addition order in the flotation separation of smithsonite from calcite. Int. J. Min. Sci. Technol. 2021, 31, 1153–1158. [Google Scholar] [CrossRef]
- Shi, J.Y.; Lv, J.; Wang, J.L.; Cao, Z. Hydrolytic polymaleic acid: An environmentally friendly inhibitor for the flotation separation of parisite from calcite and fluorite. Appl. Surf. Sci. 2025, 680, 161396. [Google Scholar] [CrossRef]
- Luo, N.; Shi, J.Y.; Yan, B.B.; Wang, X.P. Flotation separation of magnesite from dolomite using sodium silicate modified with zinc sulfate as a selective depressant. Minerals 2024, 14, 355. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G. Electrokinetic and flotation behaviors of hemimorphite in the presence of sodium oleate. Miner. Eng. 2015, 84, 74–76. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- He, J.; Chen, H.; Zhang, M.; Chen, L.; Yao, Q.; Dai, Y.; Zhu, L.; Liu, C. Combined inhibitors of Fe3+, Cu2+ or Al3+ and sodium silicate on the flotation of fluorite and quartz. Colloids Surf. Physicochem. Eng. Asp. 2022, 643, 128702. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Selective flotation separation of fluorite from calcite by using sesbania gum as depressant. Miner. Eng. 2021, 174, 107239. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Zhu, Y.; Gong, G.; Han, Y. Mechanism of HCA and CEPPA in flotation separation of cassiterite and fluorite. Miner. Eng. 2022, 187, 107773. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, Y.M.; Li, Y.J. Adsorption and depression mechanism of an eco-friendly depressant PBTCA on fluorite surface for the efficient separation of cassiterite from fluorite. Miner. Eng. 2021, 171, 107124. [Google Scholar] [CrossRef]









| Samples | CaF2 | CaCO3 | SiO2 | Others | Total |
|---|---|---|---|---|---|
| Fluorite | 99.10 | 0.25 | 0.32 | 0.33 | 100 |
| Calcite | / | 98.30 | 0.51 | 1.19 | 100 |
| AWG Dosage (mg/L) | Product | Productivity (%) | Fluorite | Calcite | ||
|---|---|---|---|---|---|---|
| Grade (%) | Recovery (%) | Grade (%) | Recovery (%) | |||
| 0 | concentrate | 95.33 | 81.89 | 95.53 | 18.11 | 94.44 |
| tailing | 4.67 | 78.17 | 4.47 | 21.83 | 5.56 | |
| raw ore | 100 | 81.72 | 100 | 18.28 | 100 | |
| 100 | concentrate | 76.34 | 90.10 | 86.67 | 9.90 | 36.62 |
| tailing | 23.66 | 44.71 | 13.33 | 55.29 | 63.38 | |
| raw ore | 100 | 79.36 | 100 | 20.64 | 100 | |
| 150 | concentrate | 65.85 | 93.27 | 75.93 | 6.73 | 23.19 |
| tailing | 34.15 | 57.02 | 24.07 | 42.98 | 76.81 | |
| raw ore | 100 | 80.89 | 100 | 19.11 | 100 | |
| NaOL Concentration (×10−4 mol/L) | Fluorite Contact Angle | Calcite Contact Angle |
|---|---|---|
| 0 | 39.17° | 26.54° |
| 1.0 | 68.80° | 60.23° |
| 1.5 | 97.01° | 84.92° |
| 2.0 | 96.38° | 84.47° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Yan, B.; Li, X.; Li, D. Effect of Acidified Water Glass on Flotation Separation of Fluorite and Calcite. Minerals 2025, 15, 1020. https://doi.org/10.3390/min15101020
Luo N, Yan B, Li X, Li D. Effect of Acidified Water Glass on Flotation Separation of Fluorite and Calcite. Minerals. 2025; 15(10):1020. https://doi.org/10.3390/min15101020
Chicago/Turabian StyleLuo, Na, Baobao Yan, Xia Li, and Dahu Li. 2025. "Effect of Acidified Water Glass on Flotation Separation of Fluorite and Calcite" Minerals 15, no. 10: 1020. https://doi.org/10.3390/min15101020
APA StyleLuo, N., Yan, B., Li, X., & Li, D. (2025). Effect of Acidified Water Glass on Flotation Separation of Fluorite and Calcite. Minerals, 15(10), 1020. https://doi.org/10.3390/min15101020





