First Report of Fluorescent Sodalite from the Ditrău Alkaline Massif, Romania: A Mineralogical and Spectroscopic Investigation
Abstract
1. Introduction
- To document and describe the macroscopic (visible light and UV fluorescence) and microscopic features of newly discovered fluorescent sodalite samples.
- To confirm the mineral’s identity and characterize its vibrational properties using Raman and Fourier-Transform Infrared (FT-IR) spectroscopy.
- To interpret the origin of the observed fluorescence, infer the likely luminescence activators, and discuss their implications for the Ditrău Massif’s geological evolution.
2. Materials and Methods
2.1. Geological Setting and Samples
2.2. Macroscopic and Microscopic Analysis
2.3. Vibrational Spectroscopy
3. Results
3.1. Macroscopic Features
3.2. Petrographic Description
3.3. Raman Spectroscopy
3.4. FT-IR Spectroscopy
4. Discussion
4.1. Vibrational Characteristics and Confirmation of Sodalite
| Observed Raman Peak (cm−1) | Observed FT-IR Peak (cm−1) | Assignment (Vibrational Mode) | Reference(s) |
|---|---|---|---|
| 263, 270, 293 | - | Framework and [ClNa4]3+ cluster bending modes | [1,9] |
| 411 | 409 | T-O-T/O-T-O framework bending | [15,38] |
| - | 437 | T-O-T/O-T-O framework bending | [35,39] |
| 465 | 465 | Symmetric T-O-T bending; [ClNa4]3+ cluster stretching | [9,36] |
| - | 575, 623 | Cancrinite-like O-T-O bending; SO42−/S3− vibrations | [35,40] |
| - | 668, 688 | Symmetric νs(T-O-T) stretching (fingerprint region) | [35,41] |
| - | 712 | Symmetric νs(T-O-T) stretching (fingerprint region) | [39,42] |
| 736 | 736, 763 | Symmetric νs(T-O-T) stretching (fingerprint region) | [9,40] |
| 968 | 966 | Asymmetric/Symmetric T-O-T stretching | [9,15] |
| 987 | 979 | Symmetric ν1(T-O)/Asymmetric νas(T-O-T) stretching | [35,36] |
| 1056, 1061 | 1028, 1034 | Asymmetric T-O stretching; possible CO32− contribution | [9,43] |
4.2. Interpretation of Spectral Variations
4.3. The Origin and Variation of Fluorescence
4.4. Comparison with Global Occurrences
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAM | Ditrău Alkaline Massif |
| FT-IR | Fourier-Transform Infrared |
| LW-UV | Long-Wave Ultraviolet |
| UV | Ultraviolet |
Appendix A
| Sample | 3D Sample URL 1 | Whole Thin Section Scan URL 1 |
|---|---|---|
| Sample D1 | https://skfb.ly/p7NQT | https://macrockscopic.ro/public/ditrau/D1/ |
| Sample D1bis | https://skfb.ly/pqAFH | https://macrockscopic.ro/public/ditrau/D1bis/ |
| Sample D2 | https://skfb.ly/p7NRD | https://macrockscopic.ro/public/ditrau/D2/ |
| Sample D3 | https://skfb.ly/p7NRM | https://macrockscopic.ro/public/ditrau/D3/ |
References
- Chukanov, N.V.; Aksenov, S.M. Structural features, chemical diversity, and physical properties of microporous sodalite-type materials: A review. Int. J. Mol. Sci. 2024, 25, 10218. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites; The Geological Society: London, UK, 2004. [Google Scholar]
- Zahoransky, T.; Friis, H.; Marks, M.A.W. Luminescence and tenebrescence of natural sodalites: A chemical and structural study. Phys. Chem. Miner. 2016, 43, 459–480. [Google Scholar] [CrossRef]
- Lezhnina, M.M.; Kynast, U.H. NIR- and upconverted luminescence from rare earth sodalites. Phys. Solid State 2005, 47, 1485–1488. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K. Structural chemistry, IR spectroscopy, properties, and genesis of natural and synthetic microporous cancrinite- and sodalite-related materials: A review. Microporous Mesoporous Mater. 2021, 323, 111098. [Google Scholar] [CrossRef]
- Kaiheriman, M.; Maimaitinaisier, A.; Rehiman, A.; Aierken, S. Photoluminescence properties of green and red luminescence from natural and heat-treated sodalite. Phys. Chem. Miner. 2013, 41, 227–235. [Google Scholar] [CrossRef]
- Finch, A.A.; Friis, H.; Maghrabi, M. Defects in sodalite-group minerals determined from X-ray-induced luminescence. Phys. Chem. Miner. 2016, 43, 481–491. [Google Scholar] [CrossRef]
- Blumentritt, F.; Fritsch, E. Photochromism and Photochromic Gems: A Review and Some New Data (Part 1). J. Gemmol. 2021, 37, 780–800. [Google Scholar] [CrossRef]
- Gritsenko, Y.D.; Eremina, E.N.; Vigasina, M.F.; Vyatkina, S.V.; Ogorodov, L.P.; Maltseva, V.V.; Melchakova, L.V. Sodalite: Spectroscopic and thermochemical investigations. Geochem. Int. 2023, 61, 735–743. [Google Scholar] [CrossRef]
- Honour, V.C.; Goodenough, K.M.; Shaw, R.A.; Gabudianu, I.; Hîrtopanu, P. REE mineralisation within the Ditrău Alkaline Complex, Romania: Interplay of magmatic and hydrothermal processes. Lithos 2018, 314–315, 360–381. [Google Scholar] [CrossRef]
- Morogan, V.; Upton, B.G.J.; Fitton, J.G. The petrology of the Ditrău alkaline complex, Eastern Carpathians. Mineral. Petrol. 2000, 69, 227–265. [Google Scholar] [CrossRef]
- Balassone, G.; Mormone, A.; Petti, C.; Bernardi, A.M.; Ghiara, M.R. Sodalite-group minerals from Somma-Vesuvius. Period. Mineral. 2015, 84, 427–440. [Google Scholar]
- Agamah, C.; Vuori, S.; Colinet, P.; Norrbo, I.; de Carvalho, J.M.; Okada Nakamura, L.K.; Lindblom, J.; van Goethem, L.; Emmermann, A.; Saarinen, T.; et al. Hackmanite—The natural glow-in-the-dark material. Chem. Mater. 2020, 32, 8895–8905. [Google Scholar] [CrossRef]
- Peterson, R.C. The structure of hackmanite, a variety of sodalite from Mont St-Hilaire, Quebec. Can. Mineral. 1983, 21, 549–552. [Google Scholar]
- Song, C.; Guo, Q.; Liu, Y.; Rao, Y.; Liao, L. Photochromism, UV-Vis, vibrational and fluorescence spectroscopy of differently colored hackmanite. Crystals 2023, 13, 1607. [Google Scholar] [CrossRef]
- Liebisch, T. Über die Fluoreszenz der Sodalith-und Willemitgruppe im ultravioletten Licht; Sitzungberichte der Königlich Preussischen Akademie der Wiessenschaften: Berlin, Germany, 1912; pp. 229–240. [Google Scholar]
- Sidike, A.; Sawuti, A.; Wang, X.M.; Zhu, H.J.; Kobayashi, S.; Kusachi, I.; Yamashita, N. Fine structure in photoluminescence spectrum of S2− center in sodalite. Phys. Chem. Miner. 2007, 34, 477–484. [Google Scholar] [CrossRef]
- Kirk, R.D. The luminescence and tenebrescence of natural and synthetic sodalite. Am. Mineral. 1955, 40, 22–31. [Google Scholar]
- Pizani, P.S.; Terrile, M.C.; Farach, H.A.; Poole, C.P. Color centers in sodalite. Am. Mineral. 1985, 70, 1186–1192. [Google Scholar]
- Ogihara, S. The origin of Yooperlite fluorescence. J. Gemmol. Soc. Jpn. 2019, 34, 21–26. [Google Scholar]
- Laughlin, R.; Carlson, S.; Dice, D. A new find of fluorescent sodalite from Michigan’s Upper Peninsula. Miner. News 2018, 34, 1–6. [Google Scholar]
- Jakab, G. Geneza Masivului Alcalin Ditrau; Editura Mark House: Gheorgheni, Romania, 2017. [Google Scholar]
- Koch, A. A ditrói Syenittömzs Kőzettani és Hegyszerkezeti Viszonyairól; Magyar Tudományos Akadémia Könyvkiadó Hivatala: Budapest, Hungary, 1879. [Google Scholar]
- Brana, V. Zăcămintele Nemetalifere din România [Non-Metallic Deposits in Romania]; Editura Tehnică: Bucharest, Romania, 1967; p. 266. [Google Scholar]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Batki, A.; Pál-Molnár, E.; Dobosi, G.; Skelton, A. Petrogenetic significance of ocellar camptonite dykes in the Ditrău Alkaline Massif, Romania. Lithos 2014, 200–201, 181–196. [Google Scholar] [CrossRef]
- Pál-Molnár, E.; Kiri, L.; Lukács, R.; Dunkl, I.; Batki, A.; Szemerédi, M.; Almási, E.E.; Sogrik, E.; Harangi, S. Timing of magmatism of the Ditrău Alkaline Massif, Romania—A review based on new U–Pb and K/Ar data. Cent. Eur. Geol. 2021, 64, 18–37. [Google Scholar] [CrossRef]
- Klötzli, U.; Burda, J.; Li, Q.L.; Liu, Y.; Jakab, G.; Ionescu, L.; Tibuleac, P. Petrochronological Evidence for a Three-Stage Magmatic Evolution of the Youngest Nepheline Syenites from the Ditrău Alkaline Massif, Romania. Minerals 2022, 12, 657. [Google Scholar] [CrossRef]
- Ódri, Á.; Harris, C.; Le Roux, P. The role of crustal contamination in the petrogenesis of nepheline syenite to granite magmas in the Ditrău Complex, Romania: Evidence from O-, Nd-, Sr- and Pb-isotopes. Contrib. Mineral. Petrol. 2020, 175, 100. [Google Scholar] [CrossRef]
- Kräutner, H.; Bindea, G. Timing of the Ditrău alkaline intrusive complex (Eastern Carpathians, Romania). Slovak Geol. Mag. 1998, 4, 213–221. [Google Scholar]
- Fall, A.; Bodnar, R.J.; Szabó, C.; Pál-Molnár, E. Fluid evolution in the nepheline syenites of the Ditrău Alkaline Massif, Transylvania, Romania. Lithos 2007, 95, 331–345. [Google Scholar] [CrossRef]
- Apopei, A.I. Towards Mineralogy 4.0? Atlas of 3D Rocks and Minerals: Digitally Archiving Interactive and Immersive 3D Data of Rocks and Minerals. Minerals 2024, 14, 1196. [Google Scholar] [CrossRef]
- Apopei, A.I.; Buzgar, N.; Buzatu, A.; Maftei, A.E.; Apostoae, L. Digital 3D Models of Minerals and Rocks in a Nutshell: Enhancing Scientific, Learning, and Cultural Heritage Environments in Geosciences by Using Cross-Polarized Light Photogrammetry. Carpathian J. Earth Environ. Sci. 2021, 16, 237–249. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. 1. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; De Gruyter (O): Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Hettmann, K.; Wenzel, T.; Marks, M.; Markl, G. The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals. Am. Mineral. 2012, 97, 1653–1661. [Google Scholar] [CrossRef]
- Farsang, S.; Caracas, R.; Adachi, T.B.M.; Schnyder, C.; Zajacz, Z. S2− and S3− radicals and the S42− polysulfide ion in lazurite, haüyne, and synthetic ultramarine blue revealed by resonance Raman spectroscopy. Am. Mineral. 2023, 108, 2234–2243. [Google Scholar] [CrossRef]
- Porotnikova, T.P.; Derevyankin, V.A. Infrared absorption spectra of equilibrium solid phases of the Na2O-Al2O3-SiO2-H2O system. Sov. J. Non-Ferr. Met. 1975, 8, 38–41. [Google Scholar]
- Addai-Mensah, J.; Gerson, A.R.; Smart, R.S.C. The chemistry, crystallization, and physicochemical properties of sodium aluminosilicate solid phases. Ind. Eng. Chem. Res. 2002, 41, 3326–3334. [Google Scholar]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V. Infrared spectroscopy as a tool for the analysis of framework topology and extra-framework components in microporous cancrinite- and sodalite-related aluminosilicates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 121993. [Google Scholar] [CrossRef]
- Bellatreccia, F.; Della Ventura, G.; Di Muro, A.; Cavallo, A. H2O and CO2 in haüyne-sodalite solid solution. Period. Di Mineral. 2009, 78, 27–40. [Google Scholar]
- Hermeler, G.; Buhl, J.C.; Hoffman, W. Influence of carbonate on the synthesis of an intermediate phase between sodalite and cancrinite. Zeolites 1991, 11, 424–426. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Pekov, I.V.; Sapozhnikov, A.N.; Shcherbakov, V.D.; Varlamov, D.A. Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals 2022, 12, 887. [Google Scholar] [CrossRef]
- Lilova, K.; Pierce, E.M.; Wu, L.; Jubb, A.M.; Subramani, T.; Navrotsky, A. Energetics of Salt-Bearing Sodalites, Na8Al6Si6O24X2 (X = SO4, ReO4, Cl, I): A Treatment Option for Pertechnetate-Enriched Nuclear Waste Streams. ACS Earth Space Chem. 2020, 4, 2153–2161. [Google Scholar] [CrossRef]
- Gaft, M.; Panczer, G.; Nagli, L.; Yeates, H. Laser-induced time-resolved luminescence of tugtupite, sodalite and hackmanite. Phys. Chem. Miner. 2008, 36, 127–141. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4-NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Williams, E.R.; Simmonds, A.; Armstrong, J.A.; Weller, M.T. Compositional and structural control of tenebrescence. J. Mater. Chem. 2010, 20, 10883–10887. [Google Scholar] [CrossRef]
- Curutchet, A.; Le Bahers, T. Modeling the Photochromism of S-Doped Sodalites Using DFT, TD-DFT, and SAC-CI Methods. Inorg. Chem. 2017, 56, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V.; Sapozhnikov, A.N.; Shendrik, R.Y.; Vigasina, M.F.; Steudel, R. Spectroscopic and Crystal-Chemical Features of Sodalite-Group Minerals from Gem Lazurite Deposits. Minerals 2020, 10, 1042. [Google Scholar] [CrossRef]
- Kondo, D.; Beaton, D. Hackmanite/Sodalite from Myanmar and Afghanistan. Gems Gemol. 2009, 45, 38–43. [Google Scholar] [CrossRef]

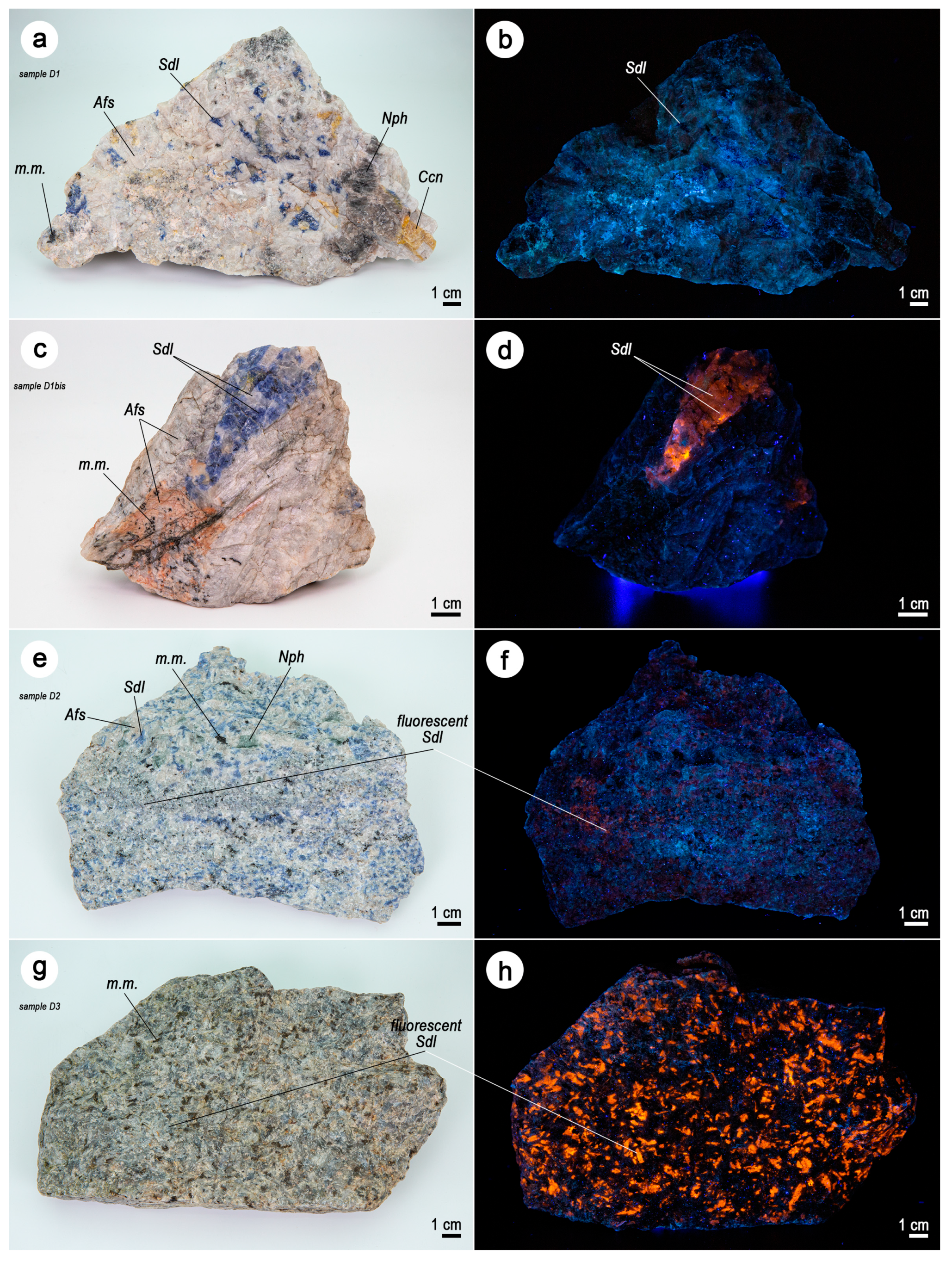
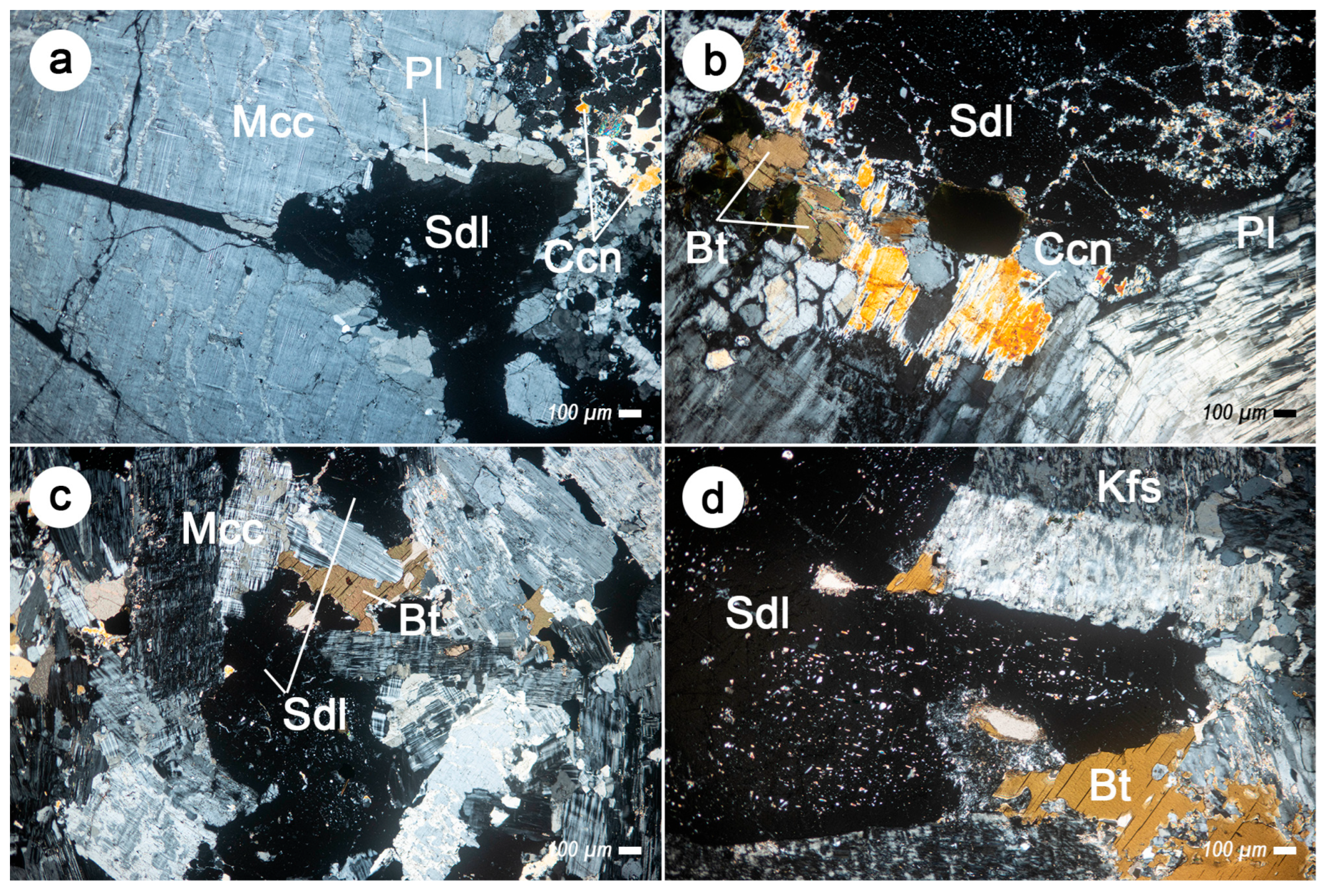
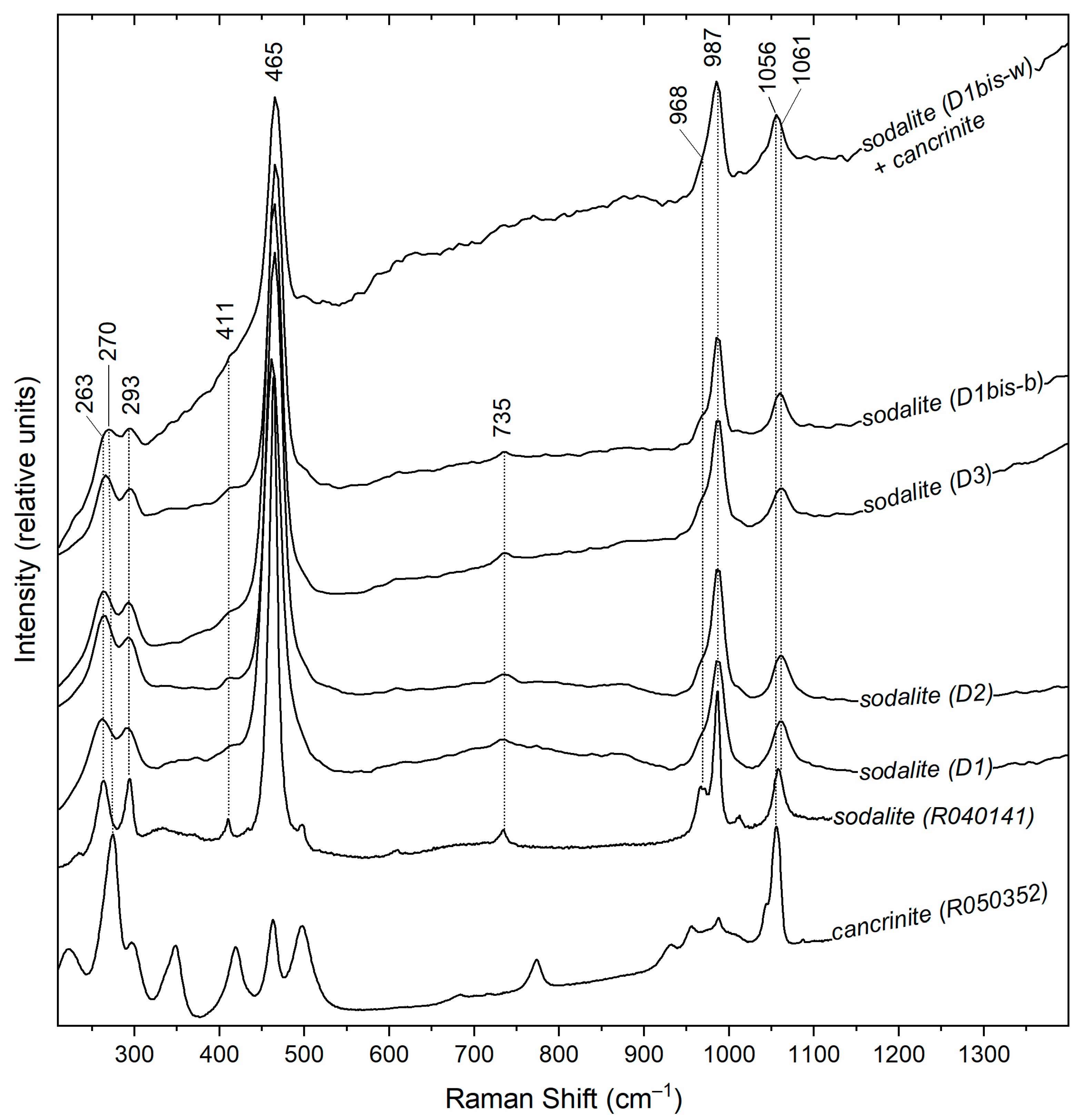

| Sample ID | Perimeter | Locality |
|---|---|---|
| D1 | Prișca (Piricske) | Ditrău (Ditró) valley |
| D1bis | Prișca (Piricske) | Ditrău (Ditró) valley |
| D2 | Jolotca (Orotva) | Teasc (Tászok) creek |
| D3 | Aurora (Hajnal) | Belcina/Belchia (Békény) valley |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apopei, A.I.; Aștefanei, D. First Report of Fluorescent Sodalite from the Ditrău Alkaline Massif, Romania: A Mineralogical and Spectroscopic Investigation. Minerals 2025, 15, 1006. https://doi.org/10.3390/min15101006
Apopei AI, Aștefanei D. First Report of Fluorescent Sodalite from the Ditrău Alkaline Massif, Romania: A Mineralogical and Spectroscopic Investigation. Minerals. 2025; 15(10):1006. https://doi.org/10.3390/min15101006
Chicago/Turabian StyleApopei, Andrei Ionuț, and Dan Aștefanei. 2025. "First Report of Fluorescent Sodalite from the Ditrău Alkaline Massif, Romania: A Mineralogical and Spectroscopic Investigation" Minerals 15, no. 10: 1006. https://doi.org/10.3390/min15101006
APA StyleApopei, A. I., & Aștefanei, D. (2025). First Report of Fluorescent Sodalite from the Ditrău Alkaline Massif, Romania: A Mineralogical and Spectroscopic Investigation. Minerals, 15(10), 1006. https://doi.org/10.3390/min15101006







