Effect of Manganese Oxide Mineralogy and Surface Mo Coverage on Mo Isotope Fractionation During the Adsorption Process
Abstract
1. Introduction
2. Materials and Methods
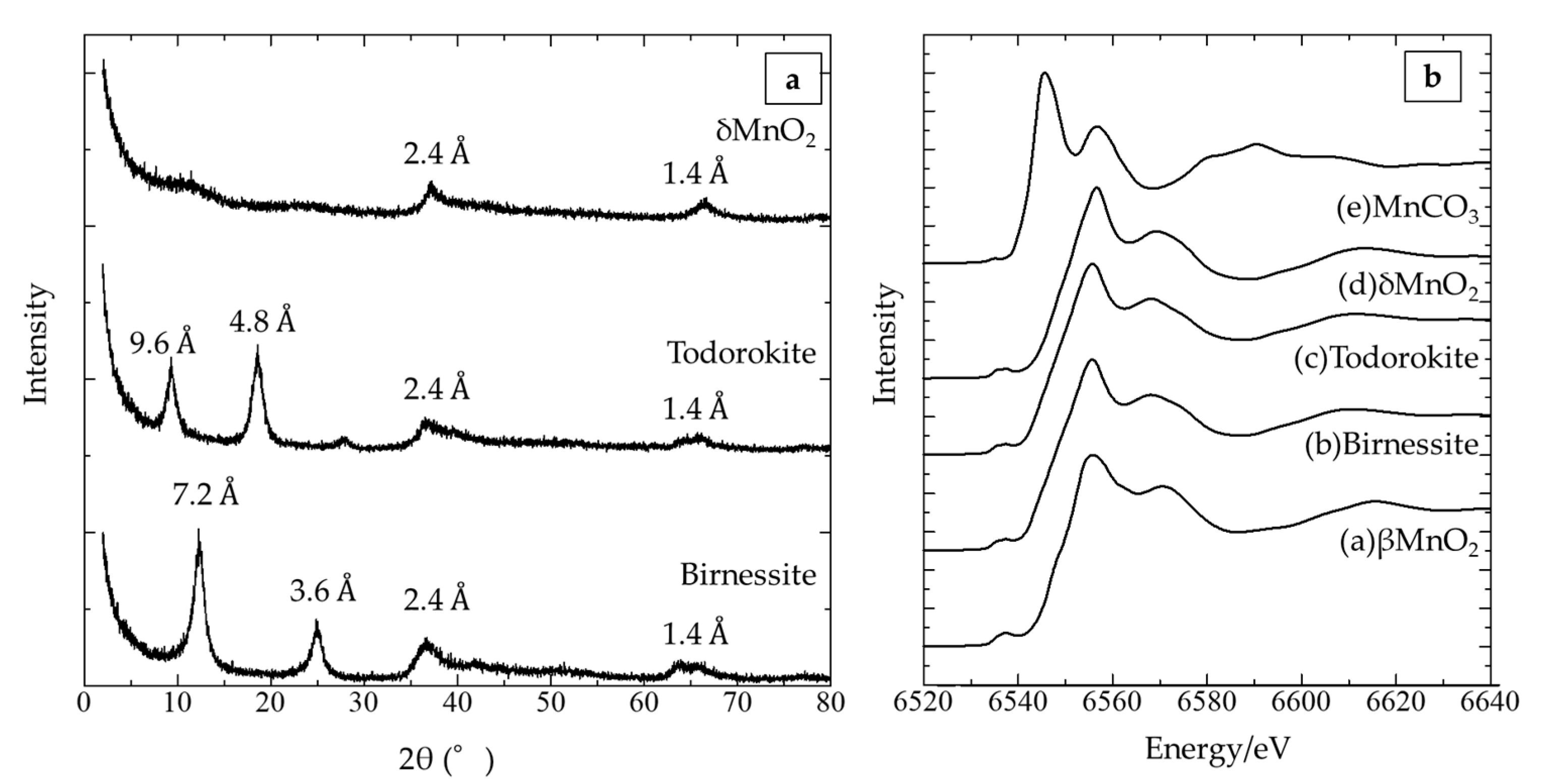
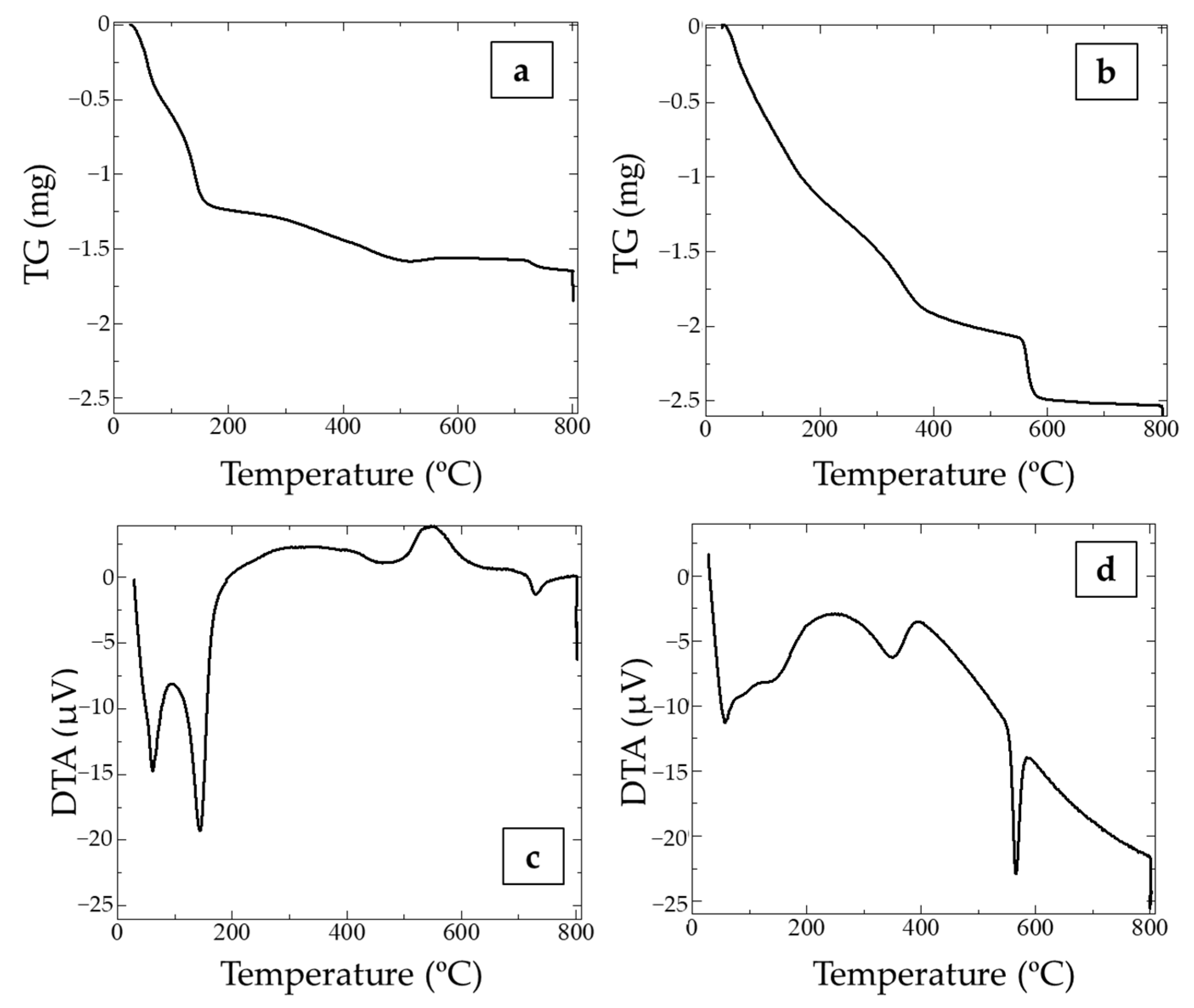
2.1. Manganese Oxide Synthesis and Characterization
2.2. Adsorption Experiments
2.3. Sample Preparation for Isotope Measurements
2.4. Mo Isotope Measurements
3. Results
3.1. Mineralogical Characterization of Synthetic Manganese Oxides
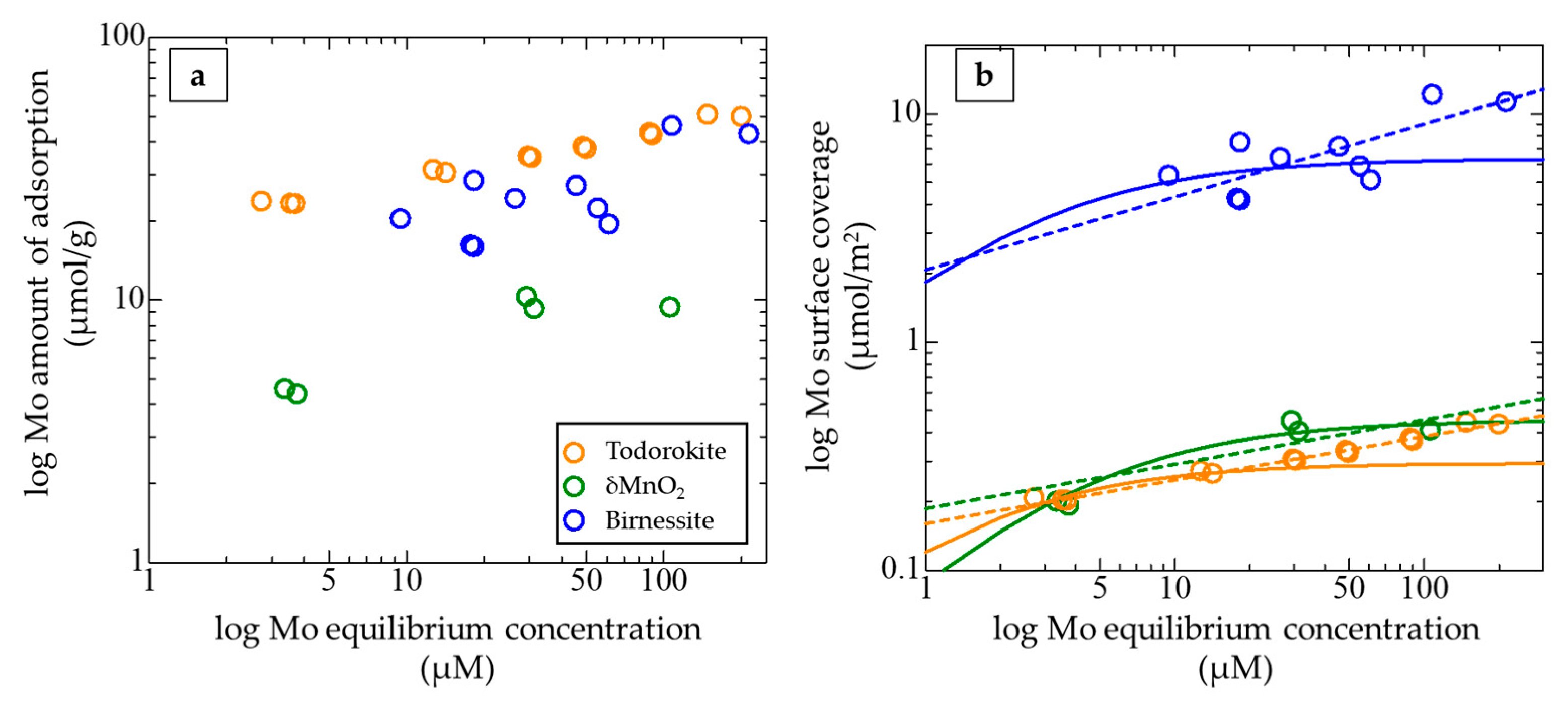
3.2. Adsorption Behavior of Mo on Manganese Oxides
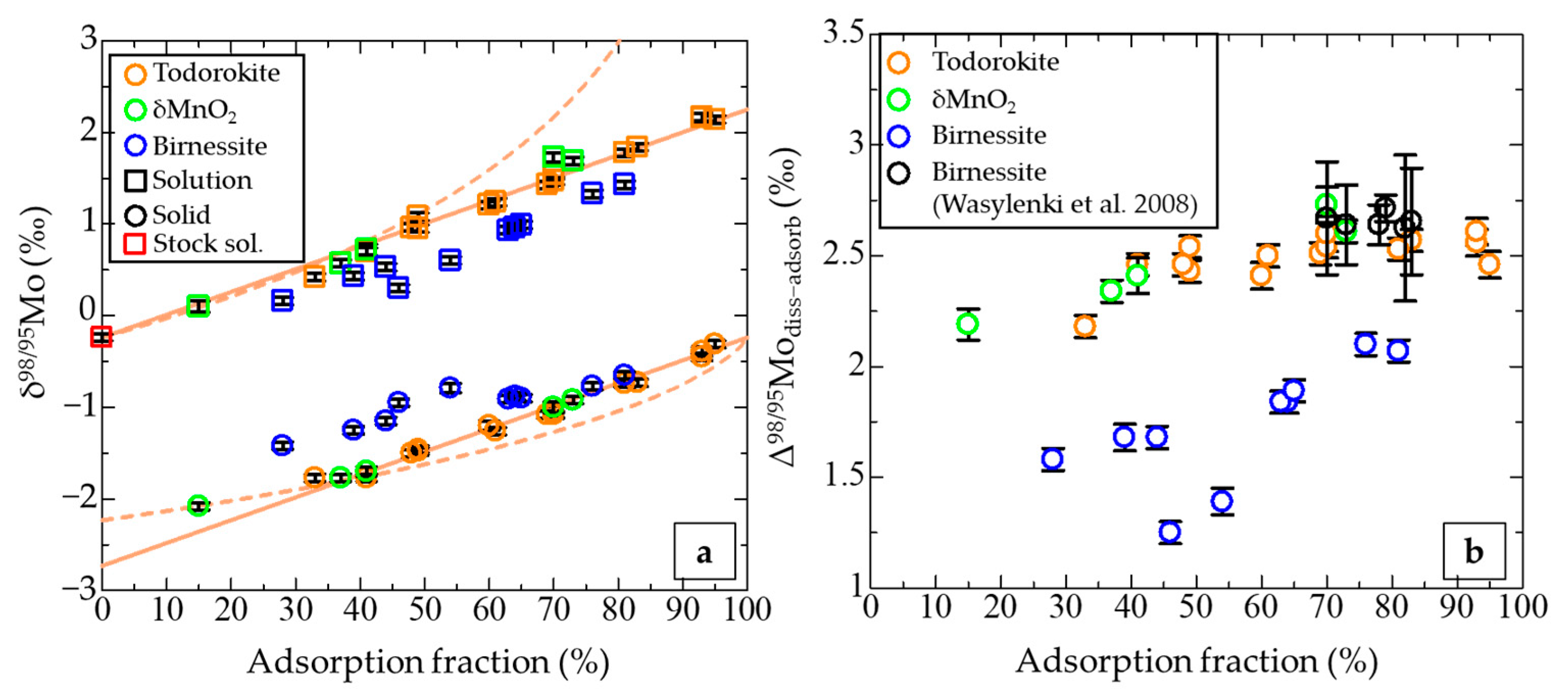
3.3. Isotope Measurements of Mo Adsorbed on Manganese Oxides
4. Discussion
4.1. Dependence of Mo Isotope Fractionation on Surface Coverage
4.2. Why Does Mo Fractionation on Manganese Oxides Decrease with Surface Coverage?
4.3. Implications for the Mo Isotope Fractionation of Natural Ferromanganese Oxides
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barling, J.; Arnold, G.L.; Anbar, A.D. Natural Mass-Dependent Variations in the Isotopic Composition of Molybdenum. Earth Planet. Sci. Lett. 2001, 193, 447–457. [Google Scholar] [CrossRef]
- Siebert, C.; Nägler, T.F.; von Blanckenburg, F.; Kramers, J.D. Molybdenum Isotope Records as a Potential New Proxy for Paleoceanography. Earth Planet. Sci. Lett. 2003, 211, 159–171. [Google Scholar] [CrossRef]
- Barling, J.; Anbar, A.D. Molybdenum Isotope Fractionation during Adsorption by Manganese Oxides. Earth Planet. Sci. Lett. 2004, 217, 315–329. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Rolfe, B.A.; Weeks, C.L.; Spiro, T.G.; Anbar, A.D. Experimental Investigation of the Effects of Temperature and Ionic Strength on Mo Isotope Fractionation during Adsorption to Manganese Oxides. Geochim. Cosmochim. Acta 2008, 72, 5997–6005. [Google Scholar] [CrossRef]
- Goldberg, T.; Archer, C.; Vance, D.; Poulton, S.W. Mo Isotope Fractionation during Adsorption to Fe (Oxyhydr)Oxides. Geochim. Cosmochim. Acta 2009, 73, 6502–6516. [Google Scholar] [CrossRef]
- Tossell, J.A. Calculating the Partitioning of the Isotopes of Mo between Oxidic and Sulfidic Species in Aqueous Solution. Geochim. Cosmochim. Acta 2005, 69, 2981–2993. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Tanimizu, M. A XAFS Study on the Mechanism of Isotopic Fractionation of Molybdenum during Its Adsorption on Ferromanganese Oxides. Geochem. J. 2009, 43, e31–e36. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Tanimizu, M.; Usui, A. Molecular-Scale Mechanisms of Distribution and Isotopic Fractionation of Molybdenum between Seawater and Ferromanganese Oxides. Geochim. Cosmochim. Acta 2011, 75, 5762–5784. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Weeks, C.L.; Bargar, J.R.; Spiro, T.G.; Hein, J.R.; Anbar, A.D. The Molecular Mechanism of Mo Isotope Fractionation during Adsorption to Birnessite. Geochim. Cosmochim. Acta 2011, 75, 5019–5031. [Google Scholar] [CrossRef]
- Tanaka, M.; Ariga, D.; Kashiwabara, T.; Takahashi, Y. Adsorption Mechanism of Molybdenum(VI) on Manganese Oxides Causing a Large Isotope Fractionation. ACS Earth Space Chem. 2018, 2, 1187–1195. [Google Scholar] [CrossRef]
- Wang, X.; Sherman, D.M. Molecular Speciation of Mo (VI) on Goethite and Its Implications for Molybdenum and Its Isotopic Cycle in Ocean. Geochim. Cosmochim. Acta 2021, 313, 116–132. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-Ocean Mineral Deposits as a Source of Critical Metals for High- and Green-Technology Applications: Comparison with Land-Based Resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Min, S.; Kim, Y. Physicochemical Characteristics of the Birnessite and Todorokite Synthesized Using Various Methods. Minerals 2020, 10, 884. [Google Scholar] [CrossRef]
- Foster, A.L.; Brown, G.E.; Parks, G.A. X-Ray Absorption Fine Structure Study of As(V) and Se(IV) Sorption Complexes on Hydrous Mn Oxides. Geochim. et Cosmochim. Acta 2003, 67, 1937–1953. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Fukami, Y.; Kubo, S.; Watakabe, A.; Kurisu, M.; Tokeshi, S.; Iizuka, T.; Suzuki, K. High-Precision Stable Isotope Measurements of Tungsten and Molybdenum in Single Sample Aliquots Combined with Optimized Separation for Mixed Double Spikes. J. Anal. At. Spectrom. 2024, 39, 1759–1777. [Google Scholar] [CrossRef]
- Rudge, J.F.; Reynolds, B.C.; Bourdon, B. The Double Spike Toolbox. Chem. Geol. 2009, 265, 420–431. [Google Scholar] [CrossRef]
- Feng, Q.; Yanagisawa, K.; Yamasaki, N. Hydrothermal Soft Chemical Process for Synthesis of Manganese Oxides with Tunnel Structures; Springer: Berlin/Heidelberg, Germany, 1998; Volume 18. [Google Scholar]
- Feng, Q.; Kanoh, H.; Miyai, Y.; Ooi, K. Hydrothermal Synthesis of Lithium and Sodium Manganese Oxides and Their Metal Ion Extraction/Insertion Reactions; ACS Publications: Washington, DC, USA, 1995; Volume 7. [Google Scholar]
- Liu, L.; Feng, Q.; Yanagisawa, K.; Wang, Y. Characterization of Birnessite-Type Sodium Manganese Oxides Prepared by Hydrothermal Reaction Process. J. Mater. Sci. Lett. 2000, 19, 2047–2050. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; Wiley: Hoboken, NJ, USA, 1992. [Google Scholar] [CrossRef][Green Version]
- Rayleigh, L. L. Theoretical Considerations Respecting the Separation of Gases by Diffusion and Similar Processes. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 42, 493–498. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry: Sixth Edition; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–285. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Kubo, S.; Tanaka, M.; Senda, R.; Iizuka, T.; Tanimizu, M.; Takahashi, Y. Stable Isotope Fractionation of Tungsten during Adsorption on Fe and Mn (Oxyhydr) Oxides. Geochim. Cosmochim. Acta 2017, 204, 52–67. [Google Scholar] [CrossRef]
- Noack, J.; Rosowski, F.; Schlögl, R.; Trunschke, A. Speciation of Molybdates under Hydrothermal Conditions. Z. Anorg. Allg. Chem. 2014, 640, 2730–2736. [Google Scholar] [CrossRef]
- Davantes, A.; Costa, D.; Sallman, B.; Rakshit, S.; Lefevre, G. Surface Polymerization of Mo(VI) and W(VI) Anions on Hematite Revealed by in Situ Infrared Spectroscopy and DFT+U Theoretical Study. J. Phys. Chem. C 2017, 121, 324–332. [Google Scholar] [CrossRef]

| Mineral | Initial Conc. (μM) | Ads. Fraction (%) *1 | Amount of Adsorption (μmol/g) | Coverage (μmol/m2) | δ98/95Mo in Reacted Solutions (‰) | 2σ *2 | δ98/95Mo in Reacted Solids (‰) | 2σ *2 | Δ98/95Mo (‰) | 2σ *3 |
|---|---|---|---|---|---|---|---|---|---|---|
| δMnO2 | 12.5 | 73 | 4.56 | 0.20 | 1.69 | 0.04 | −0.92 | 0.04 | 2.61 | 0.05 |
| 12.5 | 70 | 4.37 | 0.19 | 1.73 | 0.05 | −1.00 | 0.06 | 2.73 | 0.08 | |
| 50 | 37 | 9.24 | 0.40 | 0.57 | 0.04 | −1.77 | 0.04 | 2.34 | 0.05 | |
| 50 | 41 | 10.25 | 0.45 | 0.72 | 0.06 | −1.70 | 0.05 | 2.41 | 0.08 | |
| 125 | 15 | 9.35 | 0.41 | 0.10 | 0.06 | −2.08 | 0.04 | 2.19 | 0.07 | |
| Todorokite | 50 | 93 | 23.22 | 0.20 | 2.17 | 0.04 | −0.39 | 0.05 | 2.56 | 0.06 |
| 50 | 95 | 23.63 | 0.21 | 2.14 | 0.04 | −0.31 | 0.04 | 2.46 | 0.06 | |
| 50 | 93 | 23.15 | 0.20 | 2.16 | 0.05 | −0.45 | 0.04 | 2.61 | 0.06 | |
| 75 | 83 | 31.13 | 0.27 | 1.84 | 0.04 | −0.73 | 0.04 | 2.57 | 0.05 | |
| 75 | 81 | 30.38 | 0.26 | 1.78 | 0.04 | −0.74 | 0.04 | 2.53 | 0.05 | |
| 100 | 69 | 34.61 | 0.30 | 1.43 | 0.04 | −1.08 | 0.04 | 2.51 | 0.05 | |
| 100 | 70 | 34.97 | 0.30 | 1.47 | 0.04 | −1.08 | 0.04 | 2.54 | 0.05 | |
| 100 | 70 | 35.05 | 0.30 | 1.54 | 0.05 | −1.05 | 0.06 | 2.60 | 0.08 | |
| 125 | 60 | 37.46 | 0.33 | 1.21 | 0.04 | −1.20 | 0.05 | 2.41 | 0.06 | |
| 125 | 61 | 38.17 | 0.33 | 1.24 | 0.04 | −1.26 | 0.04 | 2.50 | 0.05 | |
| 175 | 49 | 43.23 | 0.38 | 0.95 | 0.04 | −1.48 | 0.04 | 2.43 | 0.05 | |
| 175 | 48 | 42.26 | 0.37 | 0.96 | 0.04 | −1.50 | 0.04 | 2.46 | 0.05 | |
| 175 | 49 | 42.96 | 0.37 | 1.08 | 0.04 | −1.46 | 0.04 | 2.54 | 0.05 | |
| 250 | 41 | 50.70 | 0.44 | 0.70 | 0.04 | −1.77 | 0.04 | 2.46 | 0.05 | |
| 300 | 33 | 49.76 | 0.43 | 0.42 | 0.04 | −1.77 | 0.04 | 2.18 | 0.05 | |
| Birnessite | 50 | 81 | 20.25 | 5.33 | 1.43 | 0.04 | −0.65 | 0.04 | 2.07 | 0.05 |
| 50 | 64 | 16.08 | 4.23 | 0.96 | 0.04 | −0.88 | 0.04 | 1.84 | 0.05 | |
| 50 | 63 | 15.82 | 4.16 | 0.93 | 0.04 | −0.91 | 0.04 | 1.84 | 0.05 | |
| 75 | 76 | 28.32 | 7.45 | 1.33 | 0.04 | −0.77 | 0.04 | 2.10 | 0.05 | |
| 75 | 65 | 24.21 | 6.37 | 0.99 | 0.04 | −0.90 | 0.04 | 1.89 | 0.05 | |
| 100 | 54 | 27.12 | 7.14 | 0.60 | 0.04 | −0.79 | 0.05 | 1.39 | 0.06 | |
| 100 | 39 | 19.33 | 5.09 | 0.43 | 0.04 | −1.25 | 0.04 | 1.68 | 0.06 | |
| 100 | 44 | 22.24 | 5.85 | 0.53 | 0.04 | −1.15 | 0.04 | 1.68 | 0.05 | |
| 200 | 46 | 45.91 | 12.08 | 0.30 | 0.04 | −0.95 | 0.04 | 1.25 | 0.05 | |
| 300 | 28 | 42.66 | 11.23 | 0.16 | 0.04 | −1.42 | 0.04 | 1.58 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, A.; Kashiwabara, T.; Kurisu, M.; Takahashi, Y.; Fukushi, K. Effect of Manganese Oxide Mineralogy and Surface Mo Coverage on Mo Isotope Fractionation During the Adsorption Process. Minerals 2025, 15, 79. https://doi.org/10.3390/min15010079
Okuyama A, Kashiwabara T, Kurisu M, Takahashi Y, Fukushi K. Effect of Manganese Oxide Mineralogy and Surface Mo Coverage on Mo Isotope Fractionation During the Adsorption Process. Minerals. 2025; 15(1):79. https://doi.org/10.3390/min15010079
Chicago/Turabian StyleOkuyama, Akihiro, Teruhiko Kashiwabara, Minako Kurisu, Yoshio Takahashi, and Keisuke Fukushi. 2025. "Effect of Manganese Oxide Mineralogy and Surface Mo Coverage on Mo Isotope Fractionation During the Adsorption Process" Minerals 15, no. 1: 79. https://doi.org/10.3390/min15010079
APA StyleOkuyama, A., Kashiwabara, T., Kurisu, M., Takahashi, Y., & Fukushi, K. (2025). Effect of Manganese Oxide Mineralogy and Surface Mo Coverage on Mo Isotope Fractionation During the Adsorption Process. Minerals, 15(1), 79. https://doi.org/10.3390/min15010079







