The Study of Gold Mineralization at the Polymetallic Dapingzhang VMS-Type Copper–Gold Deposit, Yunnan Province, China
Abstract
1. Introduction
2. Geological Setting
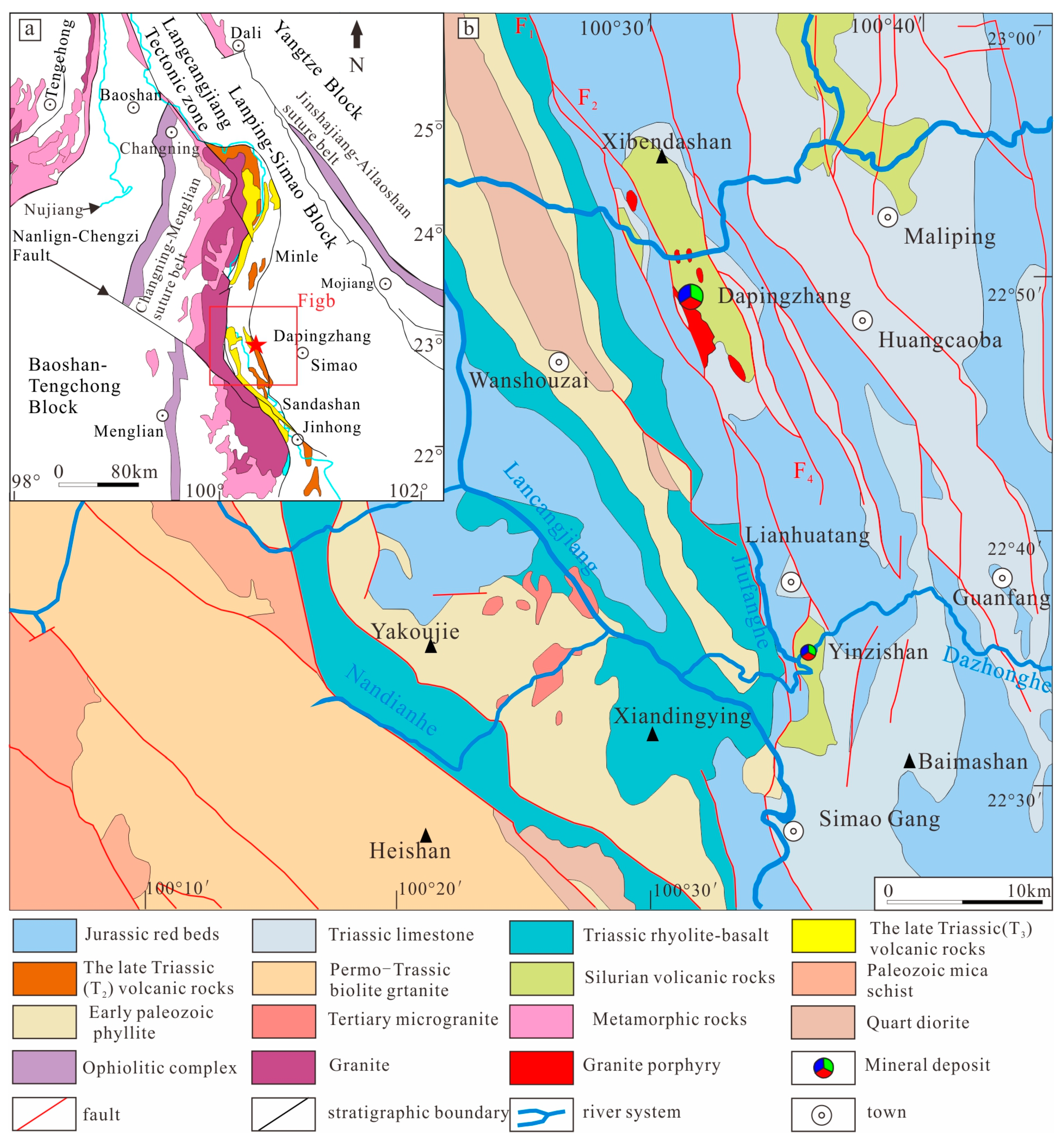
2.1. Regional Geology
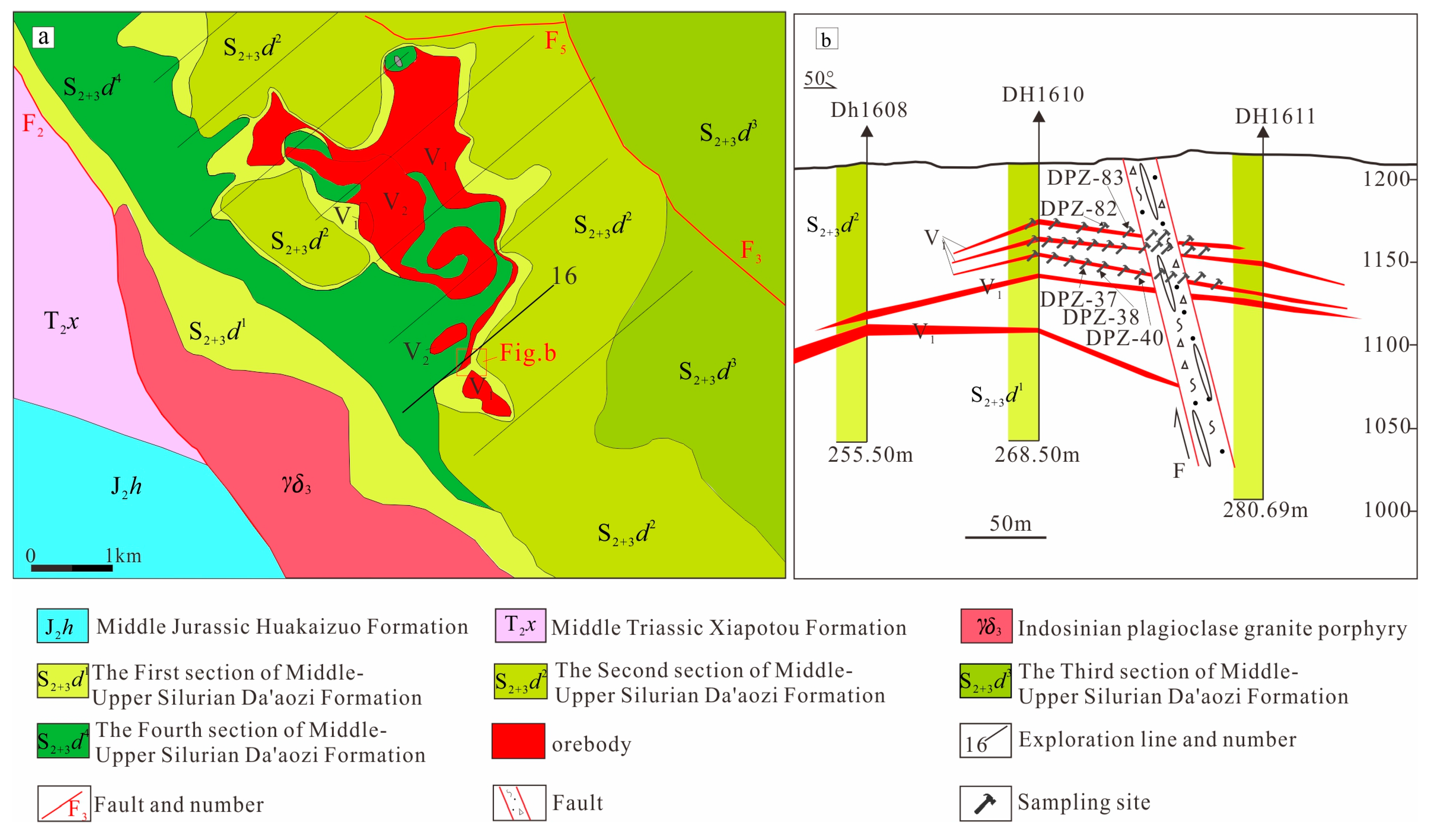
2.2. Deposit Geology
2.3. Orebody
3. Sampling and Analytical Methods
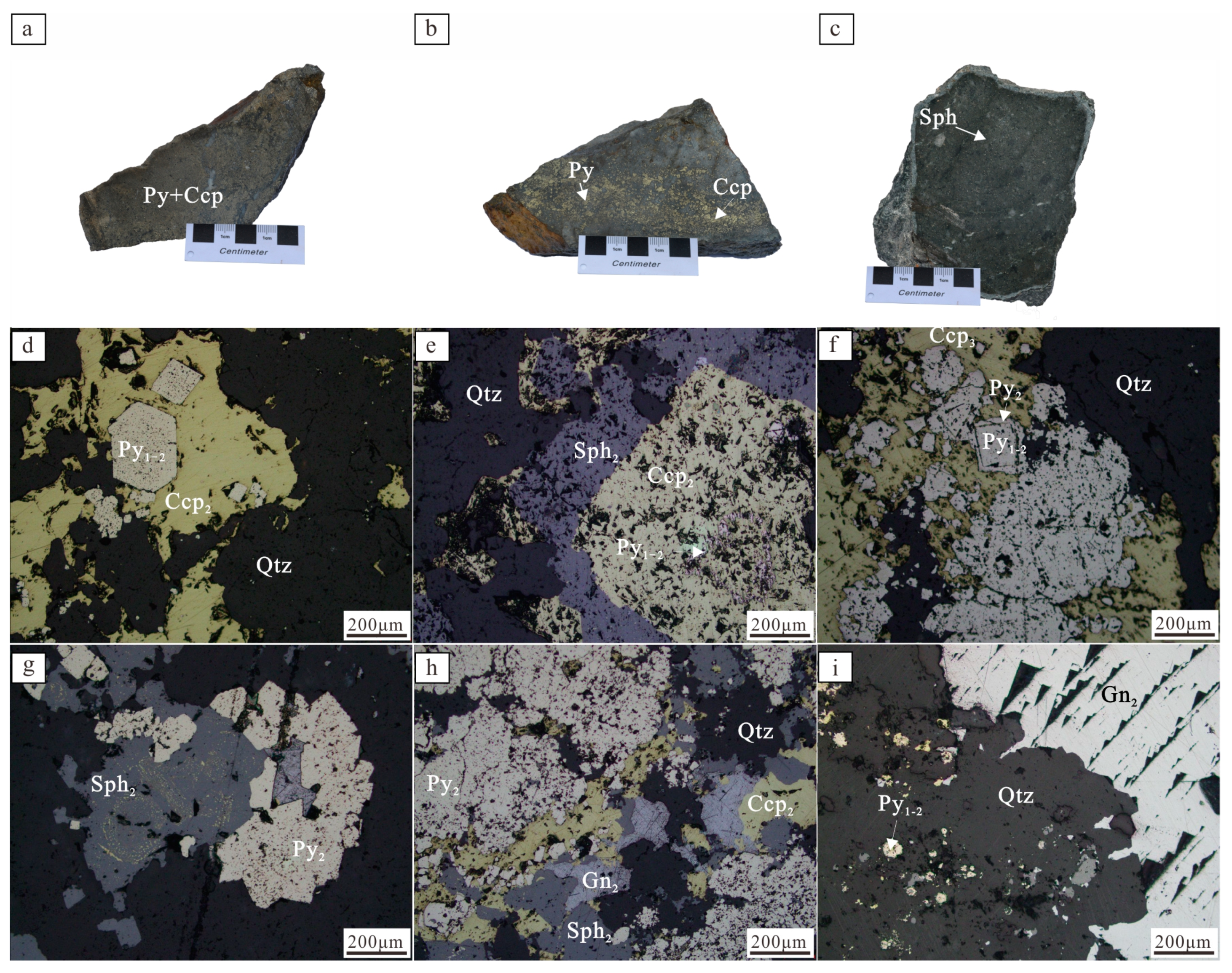
3.1. Mineralogy and Microstructure
3.2. In Situ Main and Trace Element Analysis of Minerals
4. Results
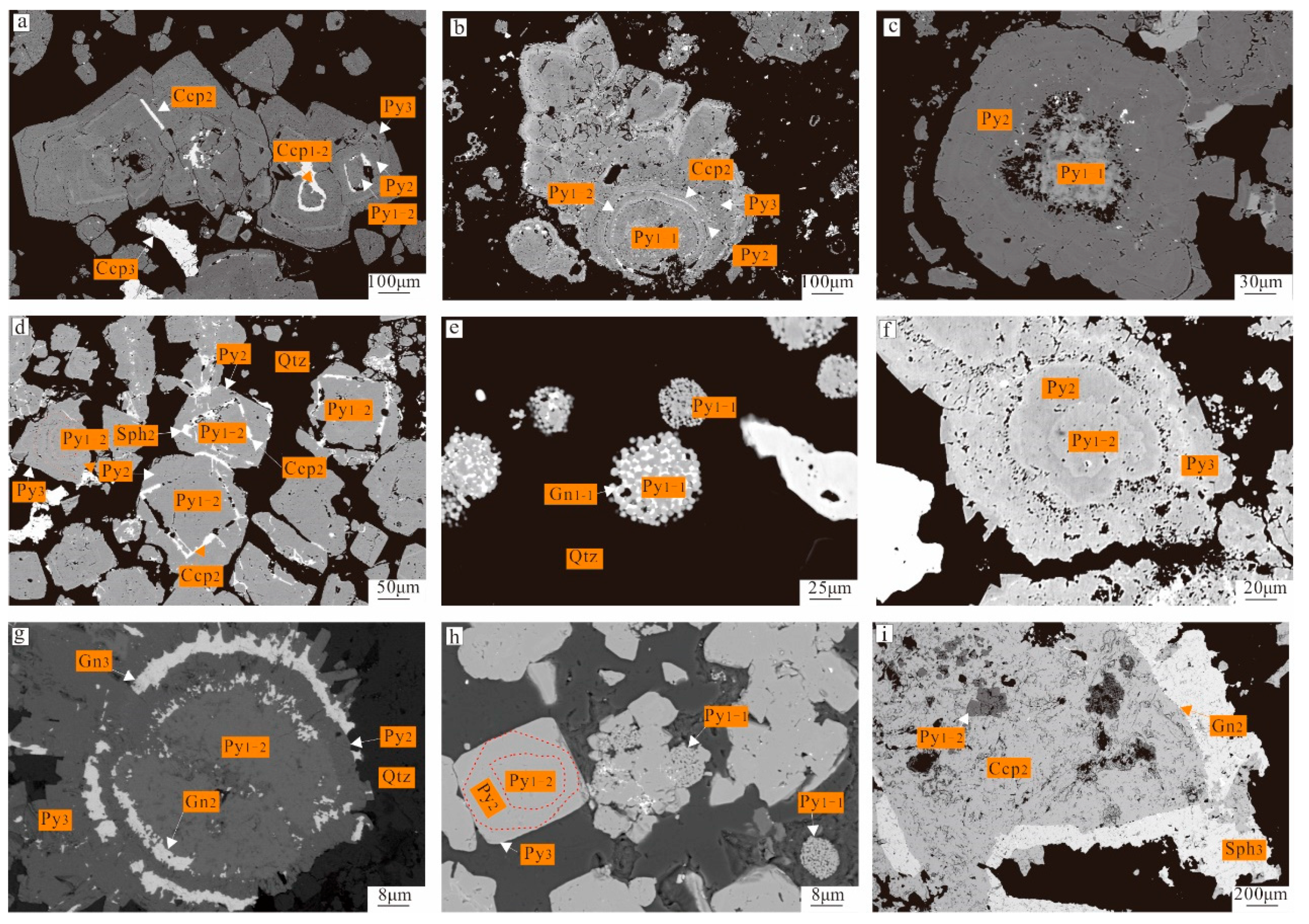
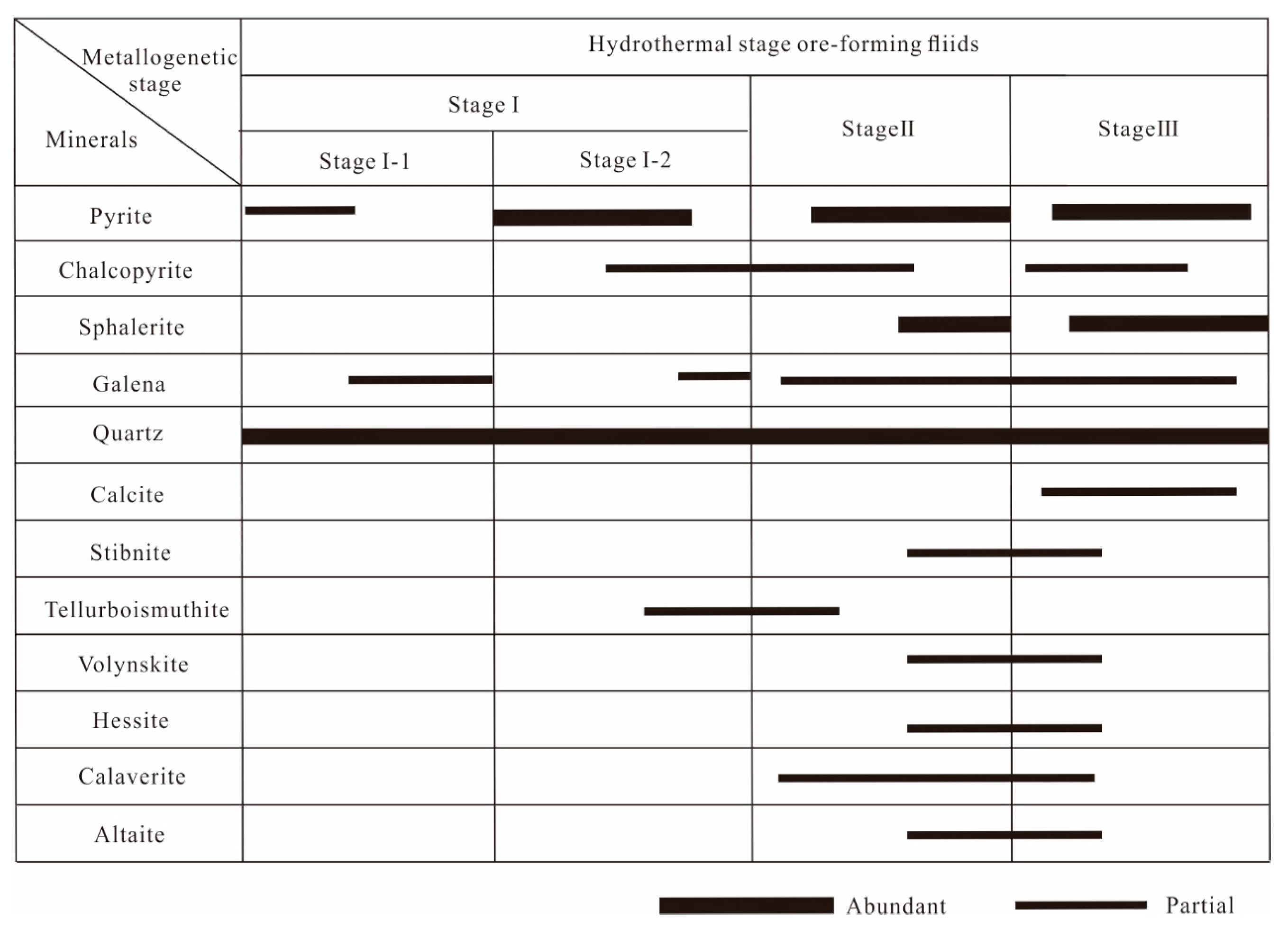
4.1. Mineral Microstructure and Sequence
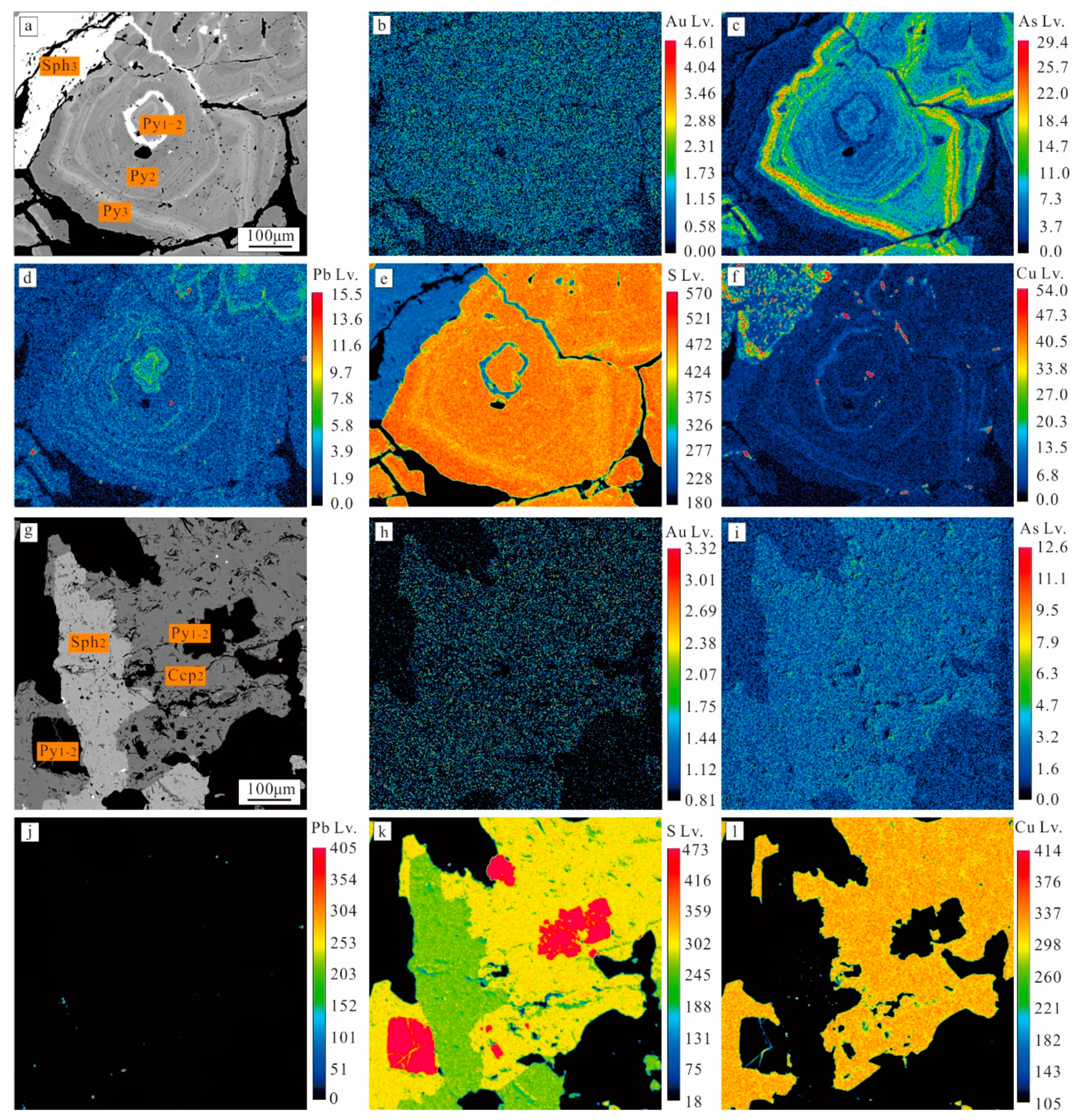
4.2. Result of BSE
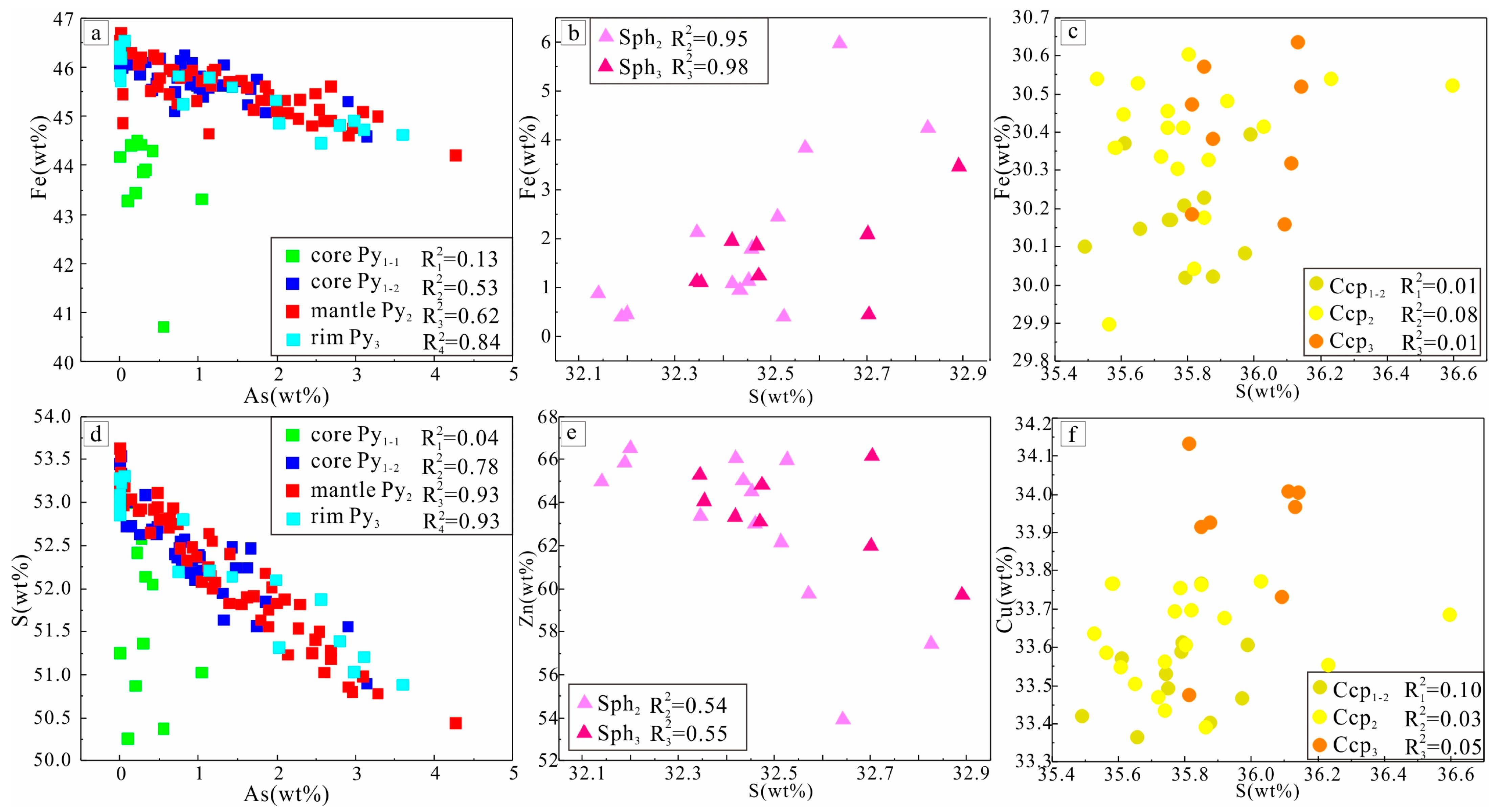
4.3. Result of EPMA
4.3.1. Main Element Content and Correlation of Au-Bearing Minerals
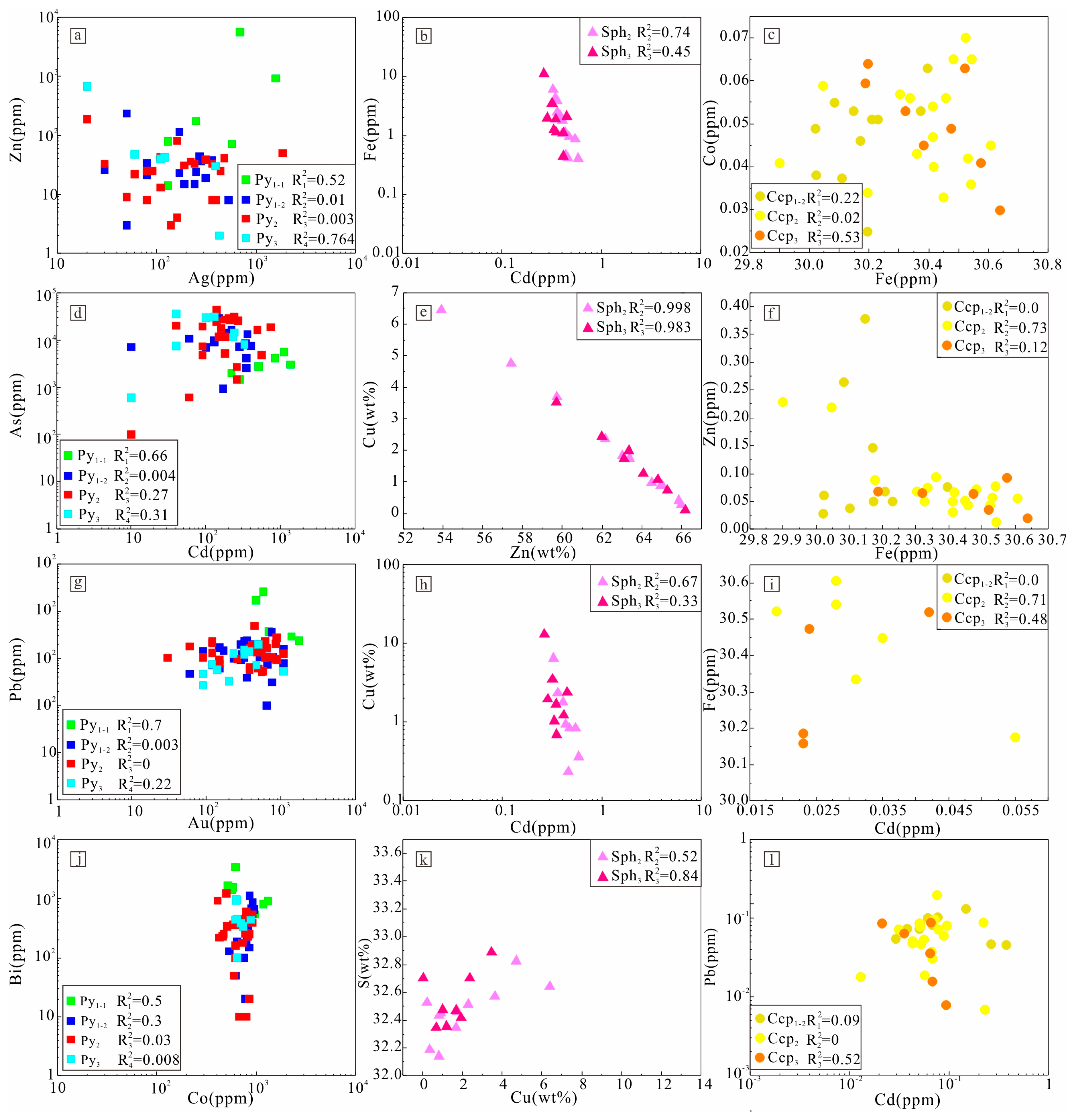
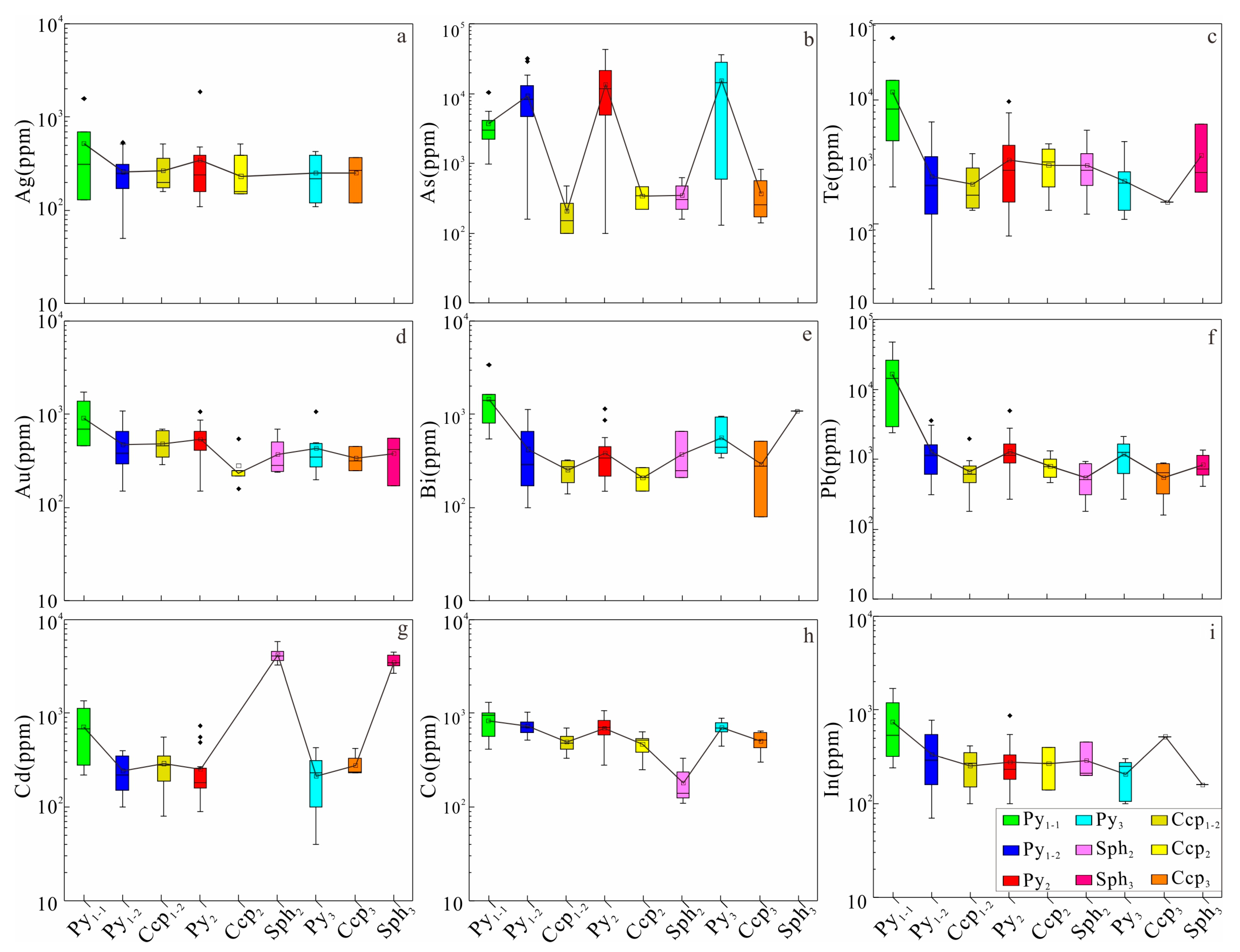
4.3.2. Trace Element Content and Correlation of Au-Bearing Minerals
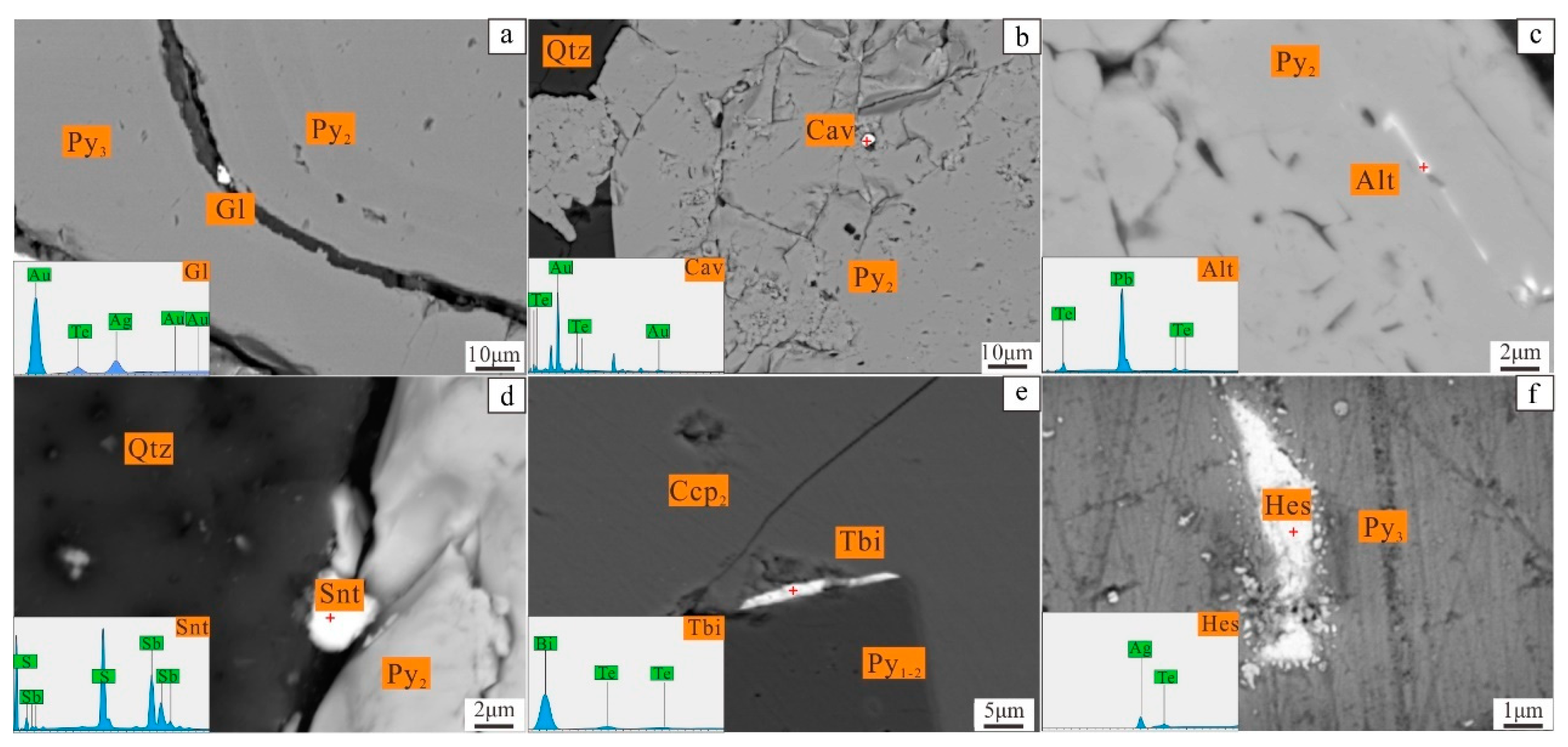
4.3.3. Result of EPMA Mapping
5. Discussion
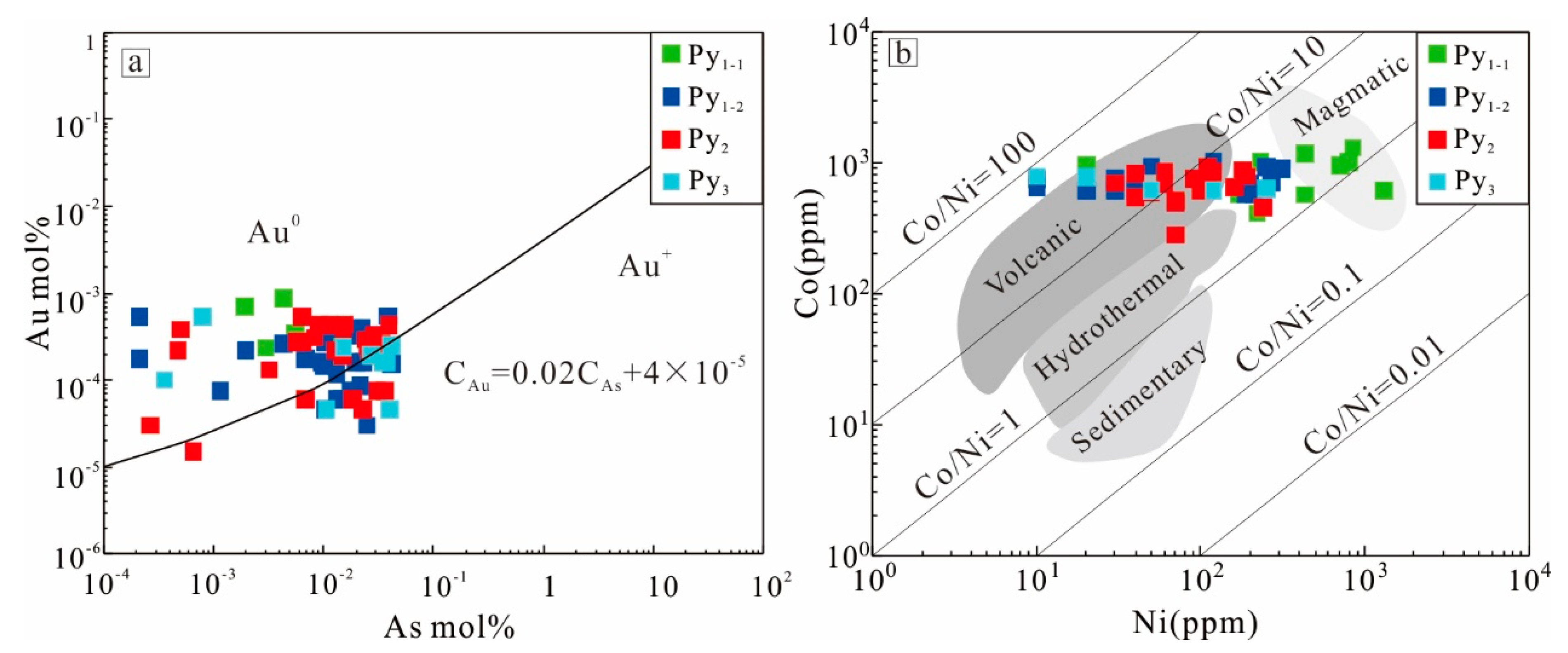
5.1. The Occurrence of Gold
5.2. Correlation of Trace Element in Sulfides
5.3. Possible Precipitation Enrichment Mechanism of Gold
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simon, G.; Kesler, S.E.; Chryssoulis, S. Geochemistry and textures of gold-bearing arsenian pyrite, Twin Creeks, Nevada: Implications for deposition of gold in carlin-type deposits. Econ. Geol. 1999, 94, 405–421. [Google Scholar] [CrossRef]
- Müller, D.; Franz, L.; Petersen, S.; Herzig, P.M.; Hannington, M.D. Comparison between magmatic activity and gold mineralization at Conical Seamount and Lihir Island, Papua New Guinea. Mineral. Petrol. 2003, 79, 259–283. [Google Scholar] [CrossRef]
- Petersen, S.; Herzig, P.M.; Kuhn, T.; Franz, L.; Hannington, M.D.; Monecke, T.; Gemmell, J.B. Shallow Drilling of Seafloor Hydrothermal Systems Using the BGS Rockdrill: Conical Seamount (New Ireland Fore-Arc) and PACMANUS (Eastern Manus Basin), Papua New Guinea. Mar. Georesour. Geotechnol. 2005, 23, 175–193. [Google Scholar] [CrossRef]
- Franklin, J.M.; Gibson, H.L.; Jonasson, I.R.; Galley, A.G. Volcanogenic massive sulfide deposits. Econ. Geol. 2005, 1, 523–560. [Google Scholar] [CrossRef]
- Galley, A.G.; Hannington, M.D.; Jonasson, I.R. Volcanogenic massive sulfide deposits, in Goodfellow, W.D., ed., Mineral deposits of Canada: A synthesis of major deposit types, district metallogeny, the evolution of geological provinces, and exploration methods: Geological Association Canada. Miner. Depos. Div. 2007, 3, 141–161. [Google Scholar] [CrossRef]
- Patrick, M.L.; Mark, D.H.; Benoît, D. The gold content of volcanogenic massive sulfide deposits. Min. Depos. 2011, 46, 509–539. [Google Scholar] [CrossRef]
- Vikentyev, I.V. Precious metal and telluride mineralogy of large volcanic-hosted massive sulfide deposits in the urals. Mineral. Petrol. 2006, 87, 305–326. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Geng, X.X.; Yang, F.Q.; Zhang, Z.X.; Xu, Q.F. Gold enrichment and the Au–Te association in the Huangtan Au–Cu–Zn volcanogenic massive sulfide deposit, Kalatag, East Tianshan, China: An in-situ LA–ICP–MS analysis. Ore Geol. Rev. 2021, 138, 104407. [Google Scholar] [CrossRef]
- Zhu, W.G.; Zhong, H.; Yang, Y.J.; Ren, T. The origin of the Dapingzhang volcanogenic Cu–Pb–Zn ore deposit, Yunnan province, SW China: Constraints from host rock geochemistry and ore Os–Pb–S–C–O–H isotopes. Ore Geol. Rev. 2016, 75, 327–344. [Google Scholar] [CrossRef]
- Li, F.; Ru, S.S.; Wu, J. Regional tectonic and Cu-poly metallic metallogenic evolution in Lanping-Simao basin. J. Yunnan Univ. (Nat. Sci.) 2012, 2, 134–142. [Google Scholar]
- Ru, S.S. Mineralization Model for the Dapingzhang Cu Polymetallic Deposits in Simao Basin, China. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2014; pp. 36–42. [Google Scholar]
- Ru, S.S.; Li, F.; Wu, J. Metallogenic characteristics and mineralization model of the Dapingzhang copper polymetallic deposit in Puer, Yunnan Province. Geol. Explor. 2014, 50, 48–57. [Google Scholar]
- Wang, X.Y.; Lü, Q.T.; Cao, D.H.; Guo, W.G.; Peng, R. Geochronology, geochemistry and zircon Hf-O isotopic composition of ore-bearing volcanic rocks at Dapingzhang VMS Cu-Zn deposit, SW China: Petrogenetic, metallogenic and tectonic implications. Ore Geol. Rev. 2021, 133, 104040. [Google Scholar] [CrossRef]
- Wang, X.Y. Tectonic Settings, Deep Geological Process and Metallogeny of VMS Deposit in Dapingzhang, Yunnan. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2021; pp. 25–43. [Google Scholar]
- Ji, X.; Liu, S.S.; Wu, S.Y.; Luo, Z.X.; Zhang, Y.L.; Wang, G.; Yang, Y.F.; Leng, Q.F.; Wang, K.Y. Occurrence of gold in Dapingzhang copper-gold polymetallic deposit, Simao basin, Yunnan. Miner. Depos. 2024, 42, 170–191. [Google Scholar]
- Zhong, H.; Hu, R.Z.; Ye, Z.J. Sufer, lead, hydrogen and oxygen isotopic geochemistry of the Dapingzhang copper-polymetallic deposit, Yunnan Province. Geochimica 2000, 29, 136–142. [Google Scholar]
- Ru, S.S.; Li, F.; Wu, J.; Li, J.B.; Wang, D.W.; Huang, Y.C. Geochemistry and chronology of granodiorite porphyry in the Dapingzhang Cu polymetallic deposit. Acta Petrol. Mineral. 2012, 31, 531–540. [Google Scholar]
- Zhang, D.; Chen, Y.; Mao, Q.; Su, B.; Jia, L.H.; Guo, S. Progress and challenge of electron probe microanalysis technique. Acta Petrol. Sin. 2019, 35, 261–274. [Google Scholar]
- Cook, N.J.; Chryssoulis, S.L. Concentrations of “invisible gold” in the common sulfides. Can. Mineral. 1990, 28, 1–16. [Google Scholar]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Bralia, A.; Sabatini, G.; Troja, F. A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis problems. Miner. Depos. 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emso, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Gopon, P.; Douglas, J.O.; Auger, M.A.; Hansen, L.; Wade, J.; Cline, J.S.; Robb, L.J.; Moddy, M.P. A nanoscale investigation of carlin-type gold deposits: An atom-scale elemental and isotopic perspective. Econ. Geol. 2019, 114, 1123–1133. [Google Scholar] [CrossRef]
- Fougerouse, D.; Reddy, S.M.; Saxey, D.W.; Rickard, W.D.A.; Riessen, A.; Micklethwaite, S. Nanoscale gold clusters in arsenopyrite controlled by growth rate not concentration: Evidence from atom probe microscopy. Am. Mineral. 2016, 101, 1916–1919. [Google Scholar] [CrossRef]
- Zhang, B.; Li, N.; Chen, Y.J. Occurrence state of gold in hydrothermal deposits and related research methods. Earth Sci. Front. 2018, 25, 251–265. [Google Scholar]
- Li, Y.; Deng, X.H.; Wu, Y.; Yue, S.; Lin, Z.; Yang, L.; Chen, X.; Xu, J. A study of the occurrences of gold and silver in the massive sulfide deposit in the Kalatag region, NW China. Earth Sci. Front. 2018, 25, 69–82. [Google Scholar]
- Friedl, J.; Wagner, F.E.; Wang, N. On the chemical state of combined gold in sulfidic ores: Conclusions from Mssbauer source experiments. Neues Jahrb. Mineral.-Abh. 1995, 169, 279–290. [Google Scholar]
- Simon, G.; Huang, H.; Penner-Hahn, J.E.; Kesler, S.E.; Kao, L.S. Oxidation state of gold and arsenic in gold-bearing arsenian pyrite. Am. Mineral. 1999, 84, 1071–1079. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from carlin trend gold deposits and laboratory synthesis. Am. Mineral. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Ashley, P.M.; Creagh, C.J.; Ryan, C.G. Invisible gold in ore and mineral concentrates from the hill grove gold-antimony deposits, NSW, Australia. Miner. Depos. 2000, 35, 285–301. [Google Scholar] [CrossRef]
- Pals, D.W.; Spry, P.G.; Chryssoulis, S. Invisible gold and tellurium in arsenic-rich pyrite from the Emperor gold deposit, Fiji: Implications for gold distribution and deposition. Econ. Geol. 2003, 98, 479–493. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Doyle, K.; Holwell, D.A.; Jenkin, G.R.T.; Barry, T.L.; Becker, J.; Rampe, J. Pyrite chemistry: A new window into Au–Te ore-forming processes in alkaline epithermal districts, Cripple Creek, Colorado. Geochim. Cosmochim. Acta 2020, 274, 172–191. [Google Scholar] [CrossRef]
- Chen, L.; Ding, D.; Jian, W. Gold occurrence and pyrite trace elements in the Xiejiagou gold deposit, Jiaodong Peninsula, China: Implications for the mineralization process. J. Earth Syst. Sci. 2024, 133, 125. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Morishita, Y.; Shimada, N.; Shimada, K. Invisible gold in arsenian pyrite from the high-grade Hishikari gold deposit, Japan: Significance of variation and distribution of Au/As ratios in pyrite. Ore Geol. Rev. 2018, 95, 79–93. [Google Scholar] [CrossRef]
- Augustin, J.; Gaboury, D. Multi-stage and multi-sourced fluid and gold in the formation of orogenic gold deposits in the world class Mana district of Burkina Faso-Revealed by LA-ICP-MS analysis of pyrites and arsenopyrites. Ore Geol. Rev. 2019, 104, 495–521. [Google Scholar] [CrossRef]
- Bajwah, Z.U.; Seccombe, P.K.; Offier, R. Trace element distribution, Co/Ni ratios and genesis of the Big Cadia iron-copper deposit, New South Wales, Australia. Miner. Depos. 1987, 22, 292–300. [Google Scholar] [CrossRef]
- Stephanie, E.M.; Andrew, G.T.; Roberto, F.W.; Fan, H.R. Implications of pyrite geochemistry for gold mineralization and remobilization in the Jiaodong gold district, northeast China. Ore Geol. Rev. 2015, 71, 150–168. [Google Scholar]
- Lisard, T.; Diego, B.; Jean, V.; Laurent, O.; Ortiz-Benavente, B.A.; Chelle-Michou, C.; Proenza, J.A.; Fontboté, L. Trace element geochemistry of sphalerite and chalcopyrite in arc-hosted VMS deposits. J. Geochem. Explor. 2022, 232, 121–152. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Hu, Y.S.; Huang, Z.L.; Danyushevsky, L.; Wang, H.Y. LA-ICP-MS analyses of trace elements in base metal sulfides from carbonate-hosted Zn-Pb deposits, South China: A case study of the Maoping deposit. Ore Geol. Rev. 2021, 130, 145–178. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.J.; Ulrich, T.; Zhang, J.; Wang, J. Trace element compositions of sulfides from Pb-Zn deposits in the Northeast Yunnan and northwest Guizhou Provinces, SW China: Insights from LA-ICP-MS analyses of sphalerite and pyrite. Ore Geol. Rev. 2022, 141, 278–315. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, C.Q.; Yu, H.J.; Yang, Y.M.; Zhao, Y.X.; Zhu, C.C.; Ding, Q.F.; Zhou, Y.B.; Yang, J.C.; Xu, Y. Element enrichment characteristics: Insights from element geochemistry of sphalerite in Daliangzi Pb–Zn deposit, Sichuan, Southwest China. J. Geochem. Explor. 2018, 186, 187–201. [Google Scholar] [CrossRef]
- Donnay, G.; Corliss, L.M.; Donnay, J.D.H.; Elliott, N.; Hastings, J.M. Symmetry of magnetic structures: Magnetic structure of chalcopyrite. Phys. Rev. 1958, 112, 1917–1923. [Google Scholar] [CrossRef]
- Hall, S.R.; Stewart, J.M. The crystal structure refinement of chalcopyrite, CuFeS2. Acta Crystallogr. Sect. B 1973, 29, 579–585. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- George, L.L.; Cook, N.J.; Crowe, B.B.P. Trace elements in hydrothermal chalcopyrite. Mineral. Mag. 2018, 82, 59–88. [Google Scholar] [CrossRef]
- Moss, R.; Scott, S.D.; Binns, R.A. Gold content of eastern Manus basin volcanic rocks implications for enrichment in associated hydrothermal precipitates. Econ. Geol. 2001, 96, 91–107. [Google Scholar] [CrossRef]
- Gruen, G.; Weis, P.; Driesner, T.; Heinrich, C.A.; de Ronde, C.E.J. Hydrodynamic modeling of magmatic–hydrothermal activity at submarine arc volcanoes, with implications for ore formation. Earth Planet. Sci. Lett. 2014, 404, 307–318. [Google Scholar] [CrossRef]
- Martin, A.J.; Jamieson, J.W.; de Ronde, C.E.J.; Layne, G.D.; Piercey, G.; Brandl, P.A. Constraining temporal variations in metal and sulfur sources using high-resolution mineral-scale analysis of pyrite: Evidence from the Brothers volcano, Kermadec arc, New Zealand. Min. Depos. 2023, 58, 1237–1262. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, H.Y.; Feng, Y.Z.; Pan, Y.M. Episodic magmatism contributes to sub-seafloor copper mineralization: Insights from textures and geochemistry of zoned pyrite in the Ashele VMS deposit. American Mineralogist. 2024, 9, 23–59. [Google Scholar] [CrossRef]
- Li, R.H.; Yang, L.Q.; Yun, M.H. Genetic Relationship between Micro-Deformation and Gold Enrichment of Gold-Bearing Pyrite in Jiaodong: Constraint from EBSD Fabrics and Geochemistry. Miner. Depos. 2019, 38, 303–318. [Google Scholar]
- Li, R.H.; Wang, X.Q.; Yang, L.Q.; Zhang, B.M.; Liu, Q.Q.; Liu, D.S. The Characteristic of Microstructural Deformation of Gold Bearing Pyrite in Jiaodong: The Links between Nanoscale Gold Enrichment and Crystal Distortion. Ore Geol. Rev. 2020, 122, 367–392. [Google Scholar] [CrossRef]
- Yang, M.Z.; Li, J.W.; Zhao, X.F. Electron Back-Scattered Diffraction and LA-ICP-MS Analysis of Pyrite from the DahuLode gold Deposit, Southern North China Craton: Insights into Geochemistry and Distribution of Trace Element Connection to Microstructure of Pyrite. Ore Geol. Rev. 2019, 115, 148–190. [Google Scholar] [CrossRef]
- Lehmann, B.; Zhao, X.F.; Zhou, M.F.; Du, A.D.; Mao, J.W.; Zeng, P.S.; Kunst, F.H.; Heppe, K. Mid-Silurian back-arc spreading at the northeastern margin of Gondwana: The Dapingzhang dacite-hosted massive sulfide deposit, Lancangjiang zone, southwestern Yunnan, China. Gondwana Res. 2013, 24, 648–663. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, D.H. Mechanisms of Gold Transportation and Deposition in Hydrothermal Gold Deposits. Geol. Sci. Technol. Inf. 2013, 32, 162–170. [Google Scholar]
- Chung, S.Y.; Kim, Y.M.; Kim, J.G. Multiphase transformation and Ostwald’s rule of stages during crystallization of a metal phosphate. Nat. Phys. 2009, 5, 68–73. [Google Scholar] [CrossRef]
- Hadjittofis, E.; Vargas, S.M.; Litster, J.D. Exploring the role of crystal habit in the Ostwald rule of stages. Proceedings of the Royal Society A: Mathematical. Phys. Eng. Sci. 2022, 478, 22–58. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. How Do Crystals Nucleate and Grow: Ostwald’s Rule of Stages and Beyond. How Do Crystals Nucleate and Grow: Ostwald’s Rule of Stages and Beyond. Therm. Phys. Therm. Anal. 2017, 11, 195–211. [Google Scholar]
- Li, J.J.; Deepak, F.L. In Situ Kinetic Observations on Crystal Nucleation and Growth. Chem. Rev. 2022, 122, 16911–16982. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kajimura, T. The hydrothermal mineralization at Suiyo Seamount. Izu-Ogasawara Arc Resour. Geol. 1994, 44, 133–140. [Google Scholar]
- Moss, R.; Scott, S.D. Geochemistry and mineralogy of gold-rich hydrothermal precipitates from the eastern Manus basin, Papua New Guinea. Can. Miner. 2001, 39, 957–978. [Google Scholar] [CrossRef]
- Hannington, M.D.; De Ronde, C.E.J.; Petersen, S. Sea-floor tectonics and submarine hydrothermal systems. Econ. Geol. 2005, 111–141. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanic-hosted massive sulfide deposit (southern Urals, Russia) using laser ablation-inductively coupled plasma mass spectrometry (LA-ICPMS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Christo, N. Thermodynamic and molecular-kinetic considerations of the initial growth of newly born crystals; crystal size distribution; Dissolution of small crystals during Ostwald ripening due to temperature changes. Prog. Cryst. Growth Charact. Mater. 2023, 69, 363–401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, S.; Li, G.; Xue, C.; Li, F.; Zou, S.; Wang, W.; Zhou, H. The Study of Gold Mineralization at the Polymetallic Dapingzhang VMS-Type Copper–Gold Deposit, Yunnan Province, China. Minerals 2025, 15, 54. https://doi.org/10.3390/min15010054
Ru S, Li G, Xue C, Li F, Zou S, Wang W, Zhou H. The Study of Gold Mineralization at the Polymetallic Dapingzhang VMS-Type Copper–Gold Deposit, Yunnan Province, China. Minerals. 2025; 15(1):54. https://doi.org/10.3390/min15010054
Chicago/Turabian StyleRu, Shanshan, Guo Li, Chuandong Xue, Feng Li, Shunhong Zou, Wei Wang, and Honglin Zhou. 2025. "The Study of Gold Mineralization at the Polymetallic Dapingzhang VMS-Type Copper–Gold Deposit, Yunnan Province, China" Minerals 15, no. 1: 54. https://doi.org/10.3390/min15010054
APA StyleRu, S., Li, G., Xue, C., Li, F., Zou, S., Wang, W., & Zhou, H. (2025). The Study of Gold Mineralization at the Polymetallic Dapingzhang VMS-Type Copper–Gold Deposit, Yunnan Province, China. Minerals, 15(1), 54. https://doi.org/10.3390/min15010054