The Adsorption Characteristics of Calcium Ions on Spodumene with Different Colors and Their Associated Activation Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microflotation Tests
2.3. Zeta Potential Measurements
2.4. The Adsorption Amount Measurements
2.5. Contact Angle Measurement
2.6. Atomic Force Microscopy (AFM) Imaging
2.7. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.8. Quantum Chemical Simulation
3. Results
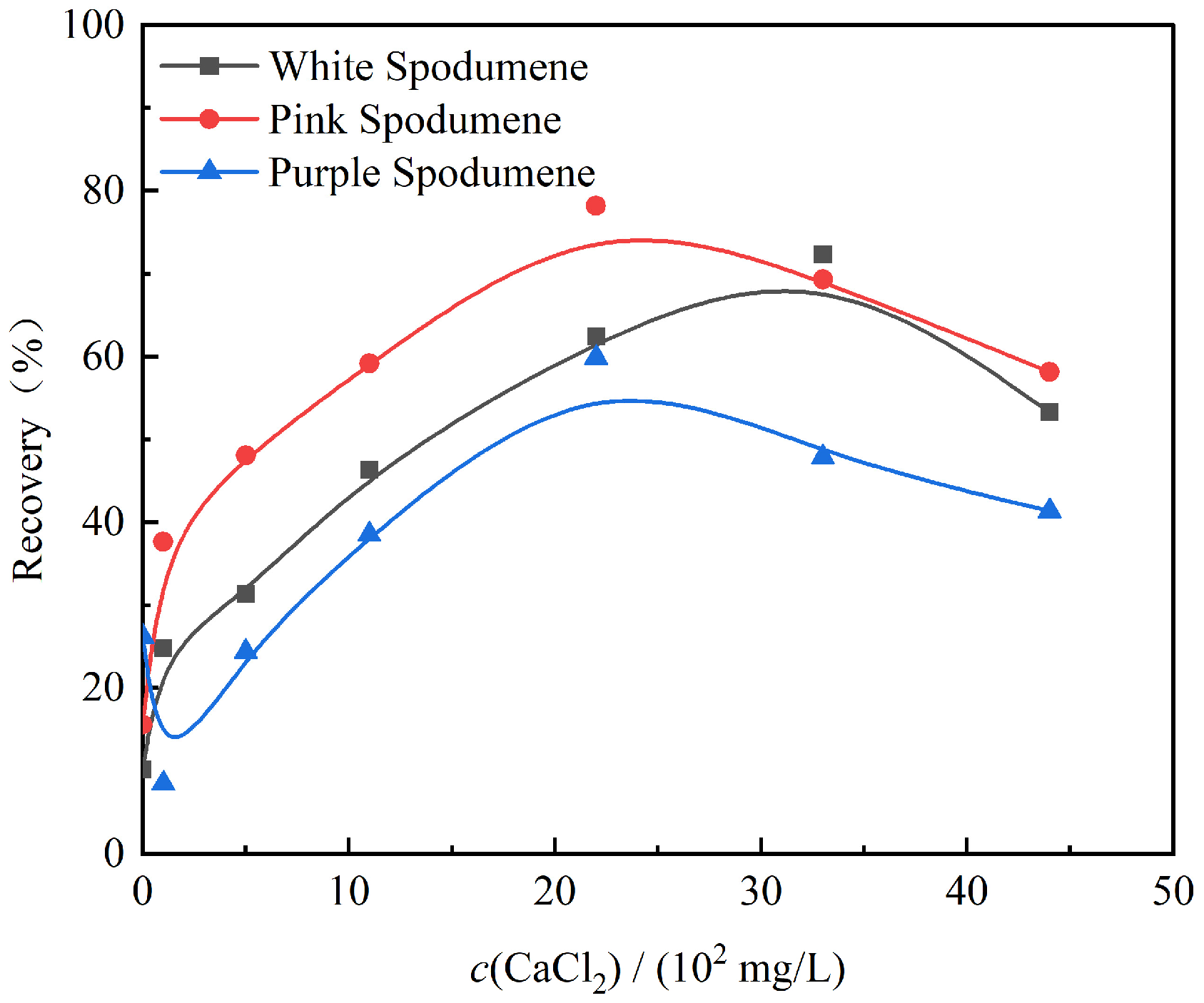
3.1. Microflotation Tests
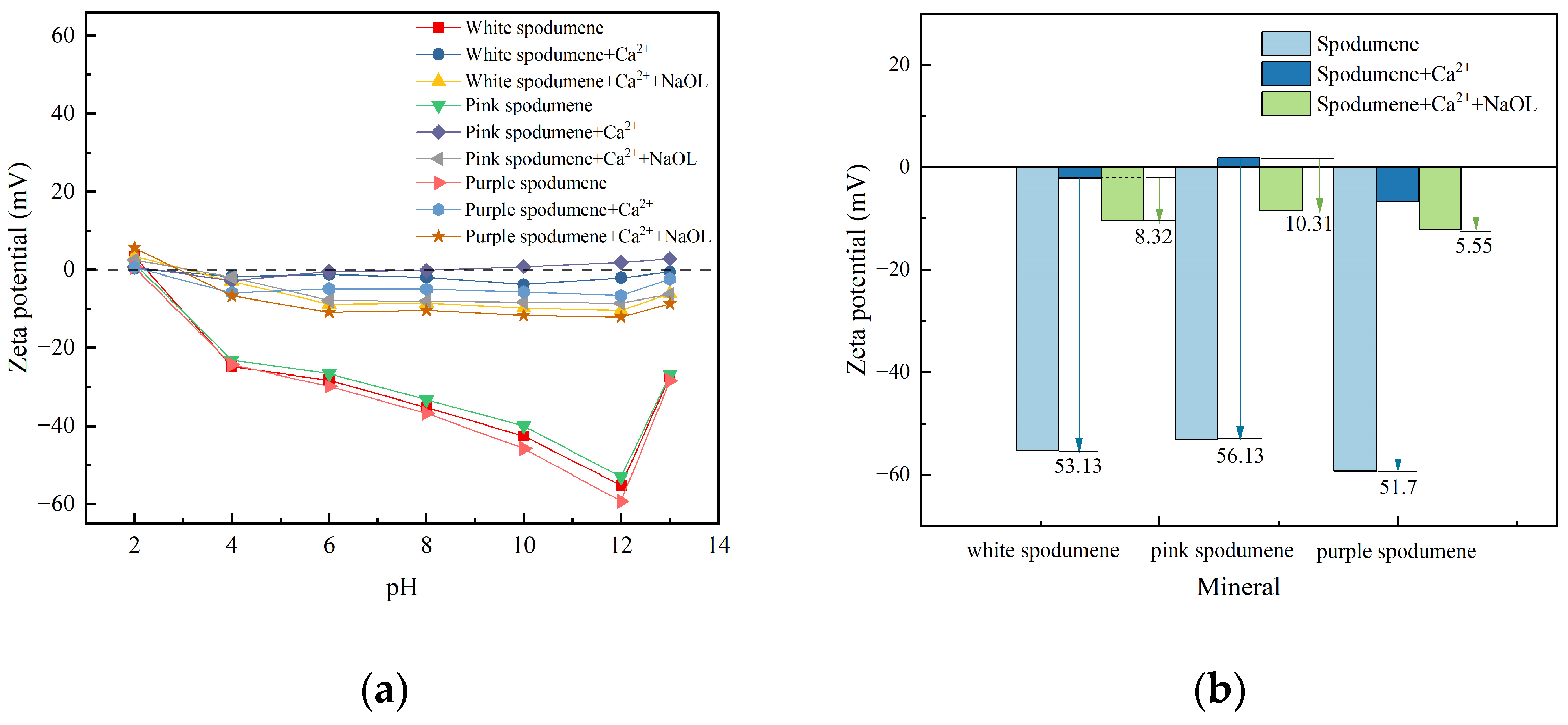
3.2. Zeta-Potential Analysis
3.3. Species Distribution Analysis
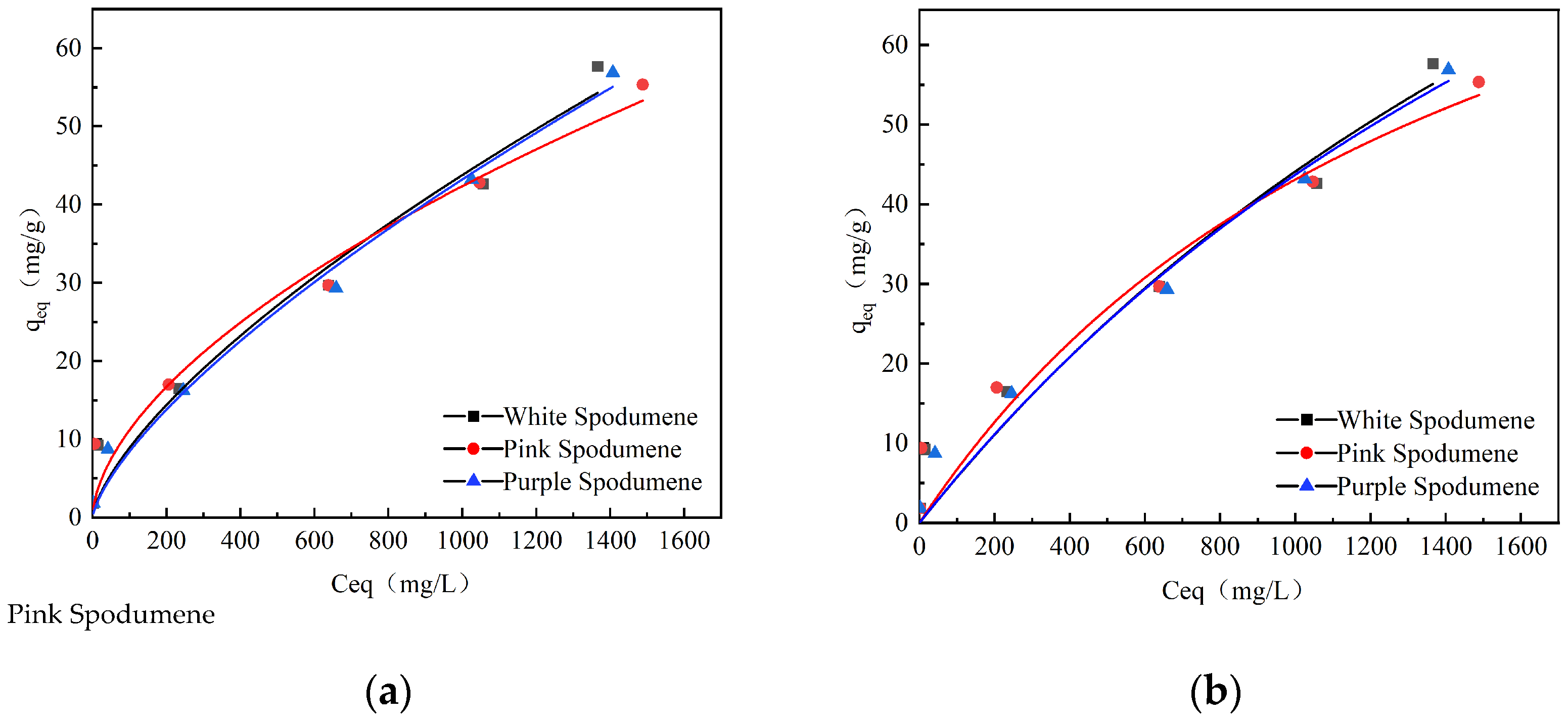
3.4. Adsorption Capacity Measurements
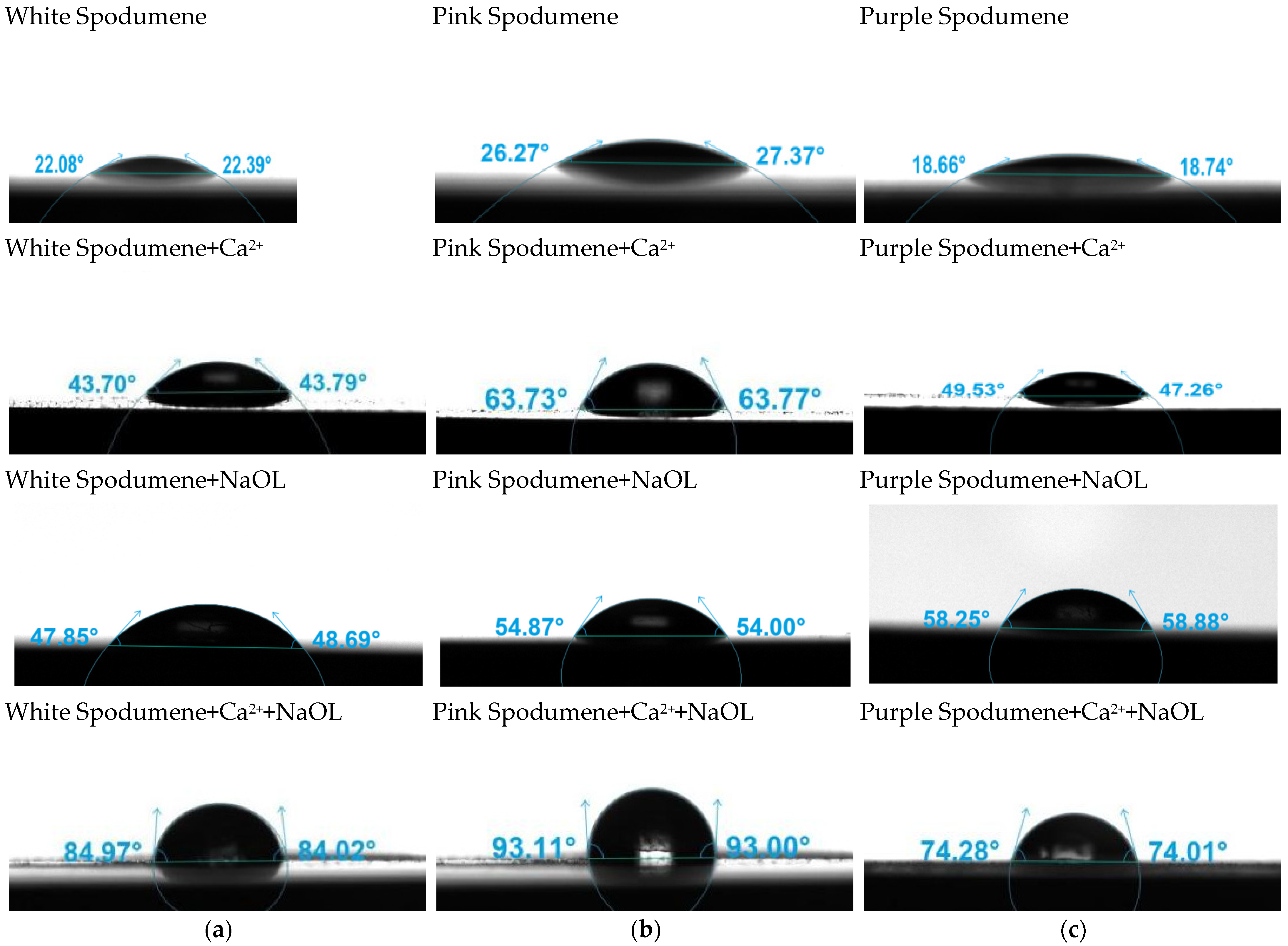
3.5. Contact Angle Analysis
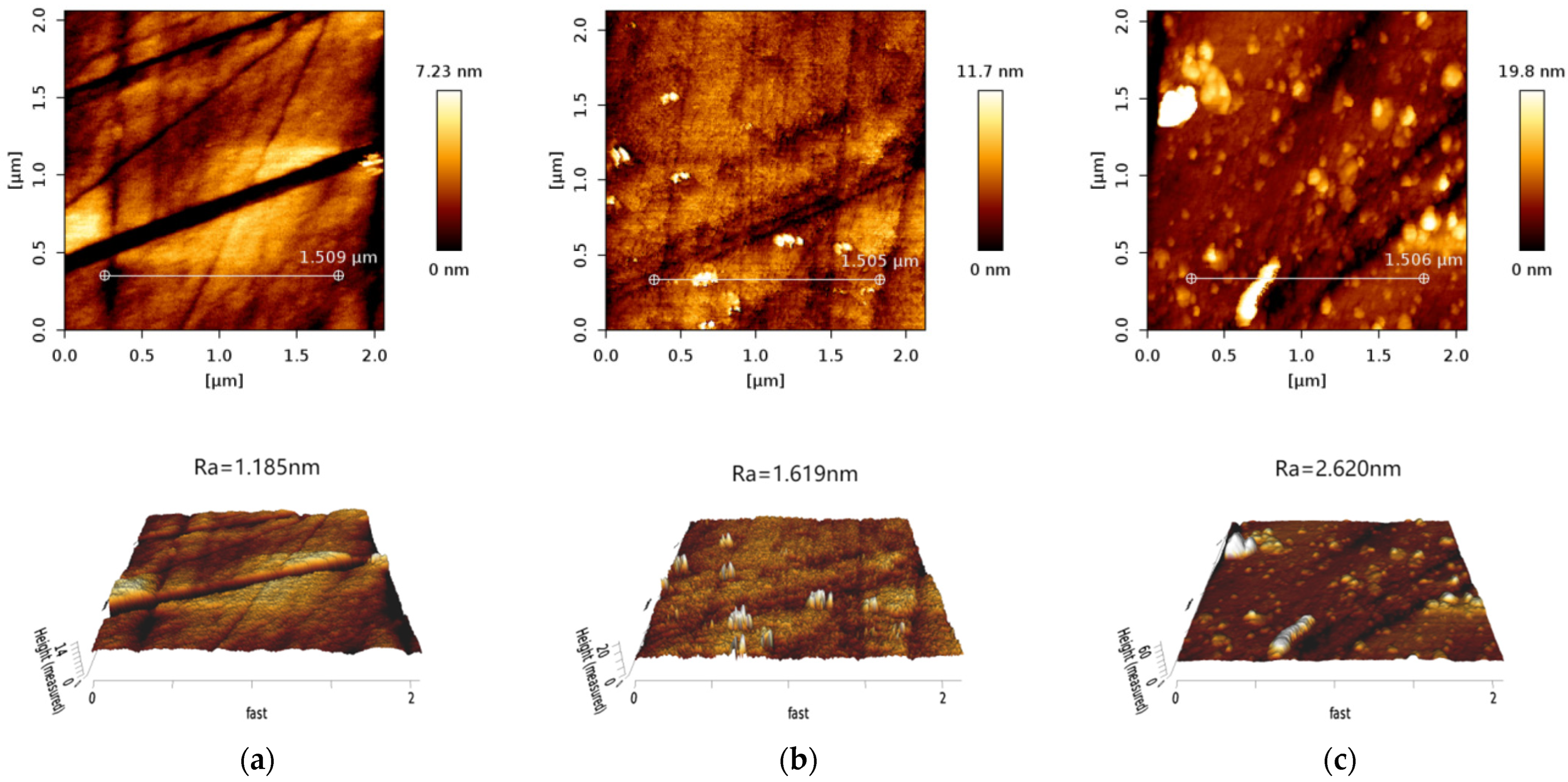
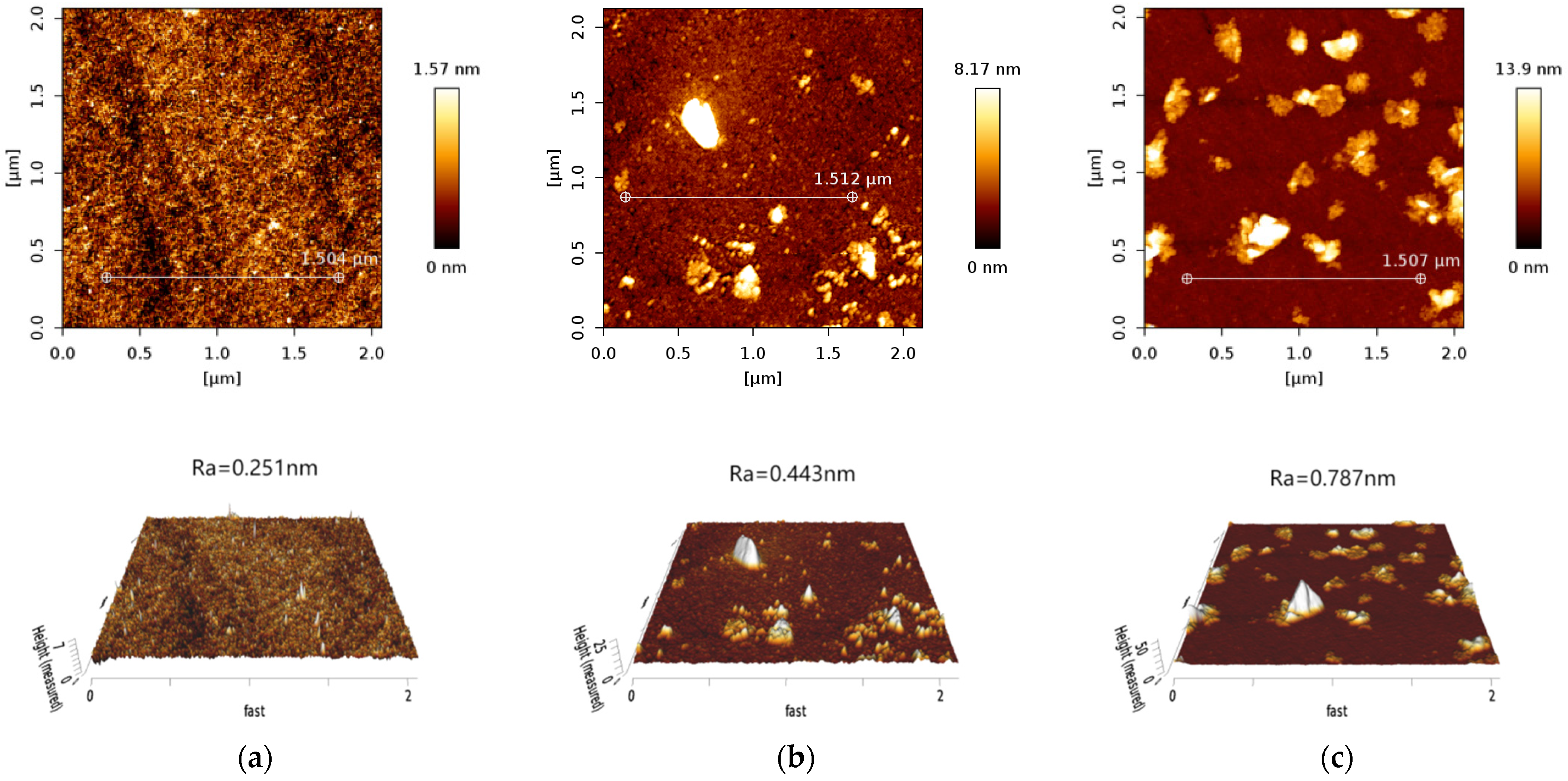
3.6. AFM Analysis
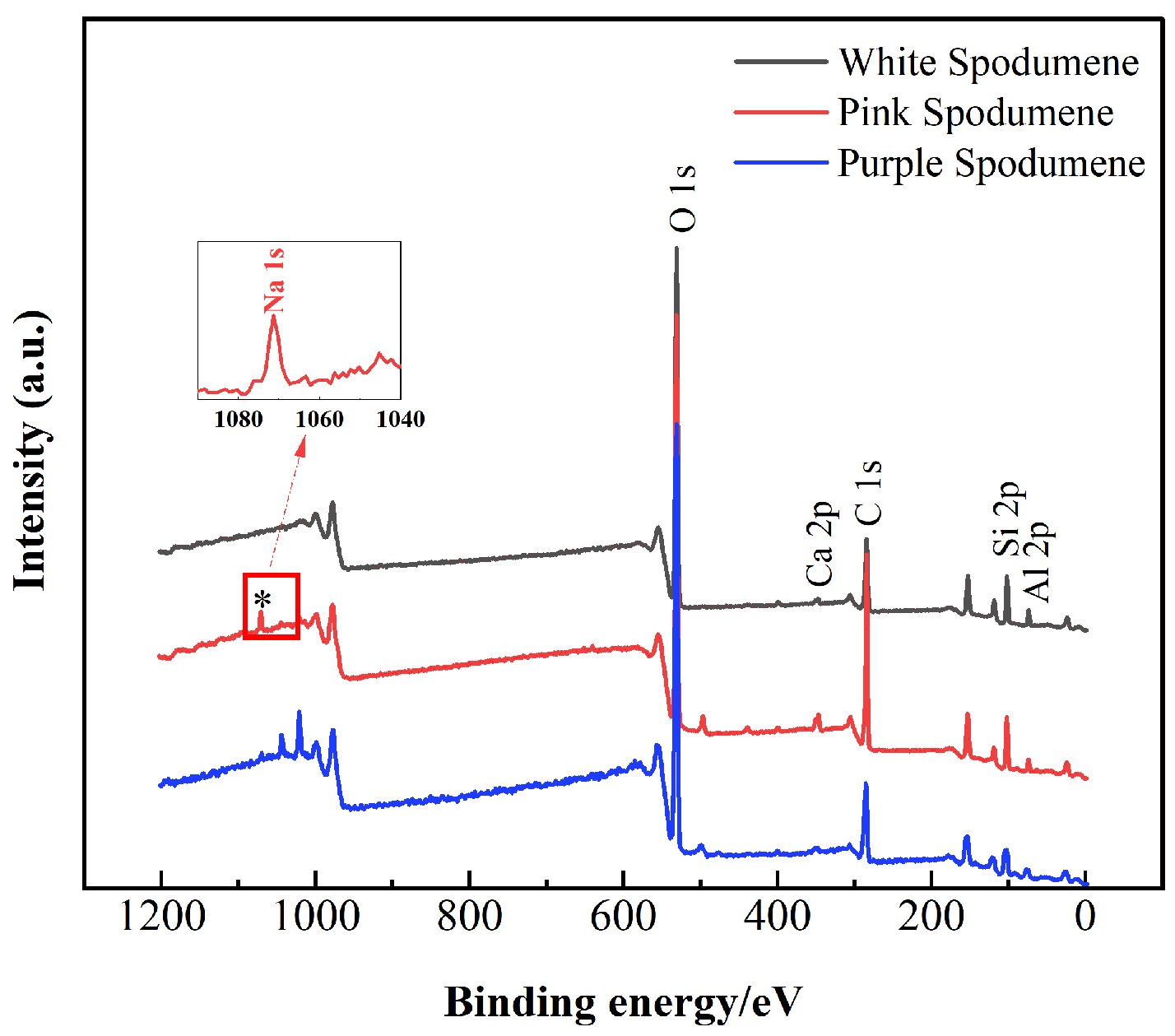
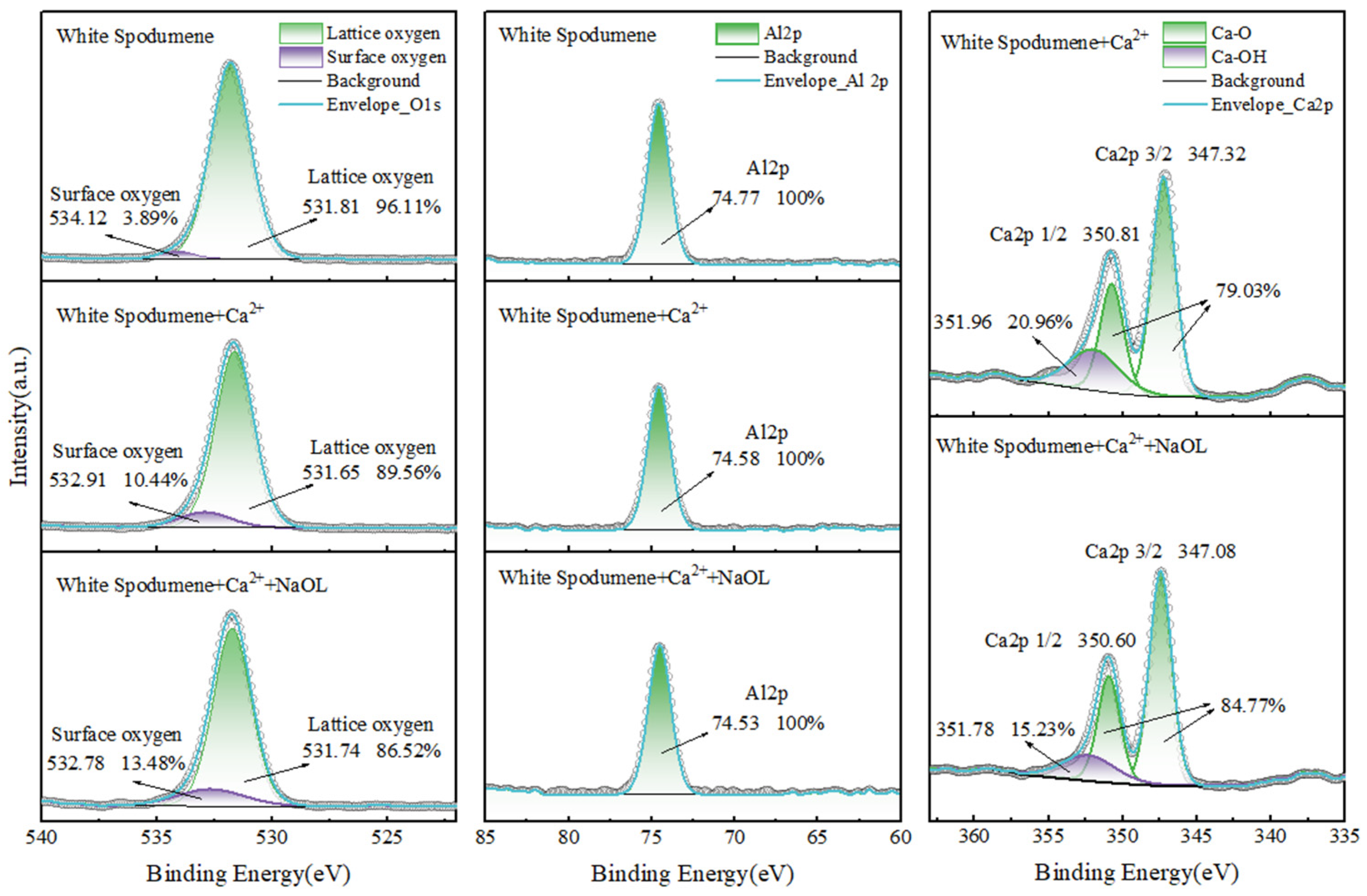
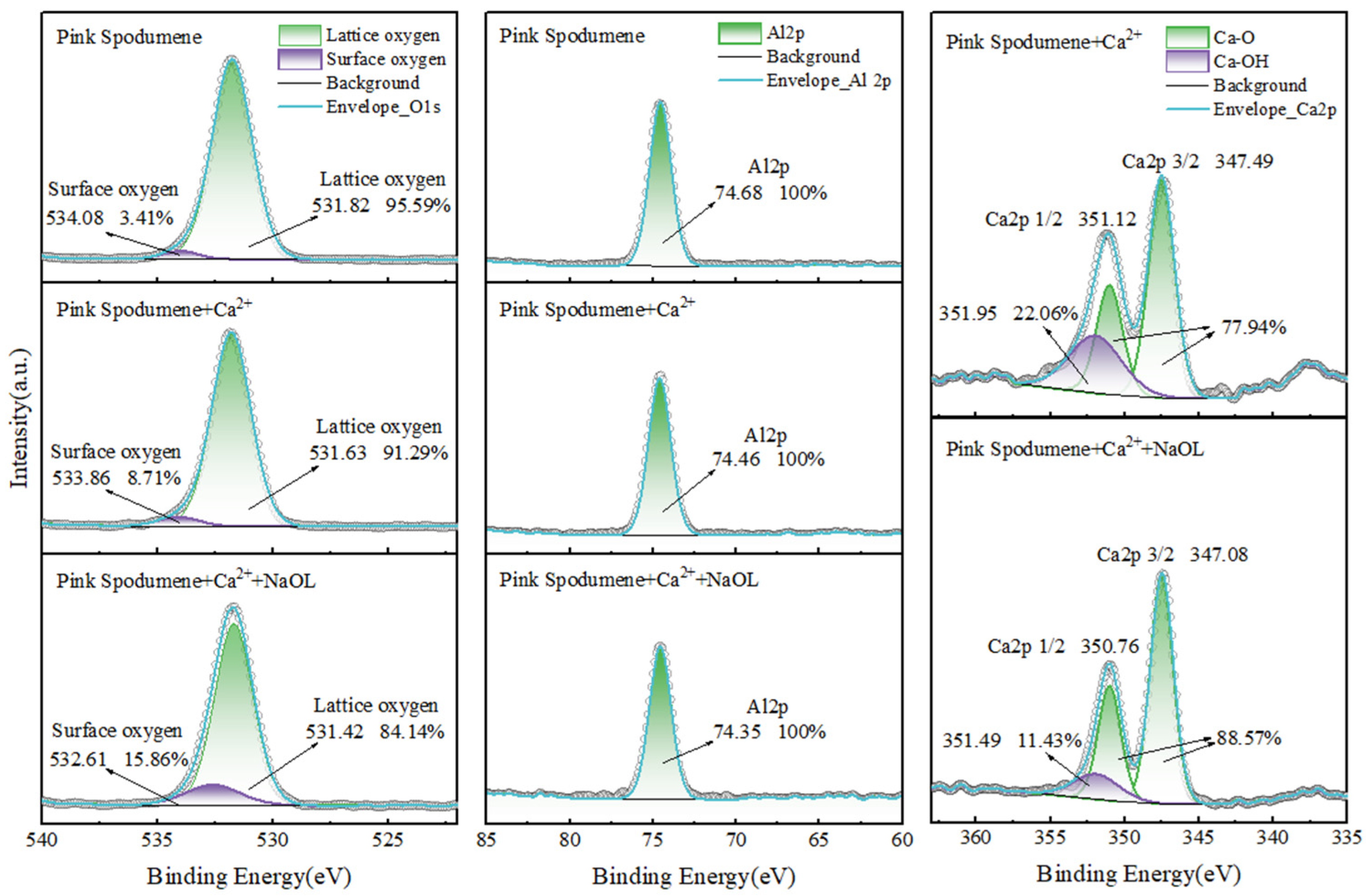
3.7. XPS and Calcium Ion Adsorption Analysis
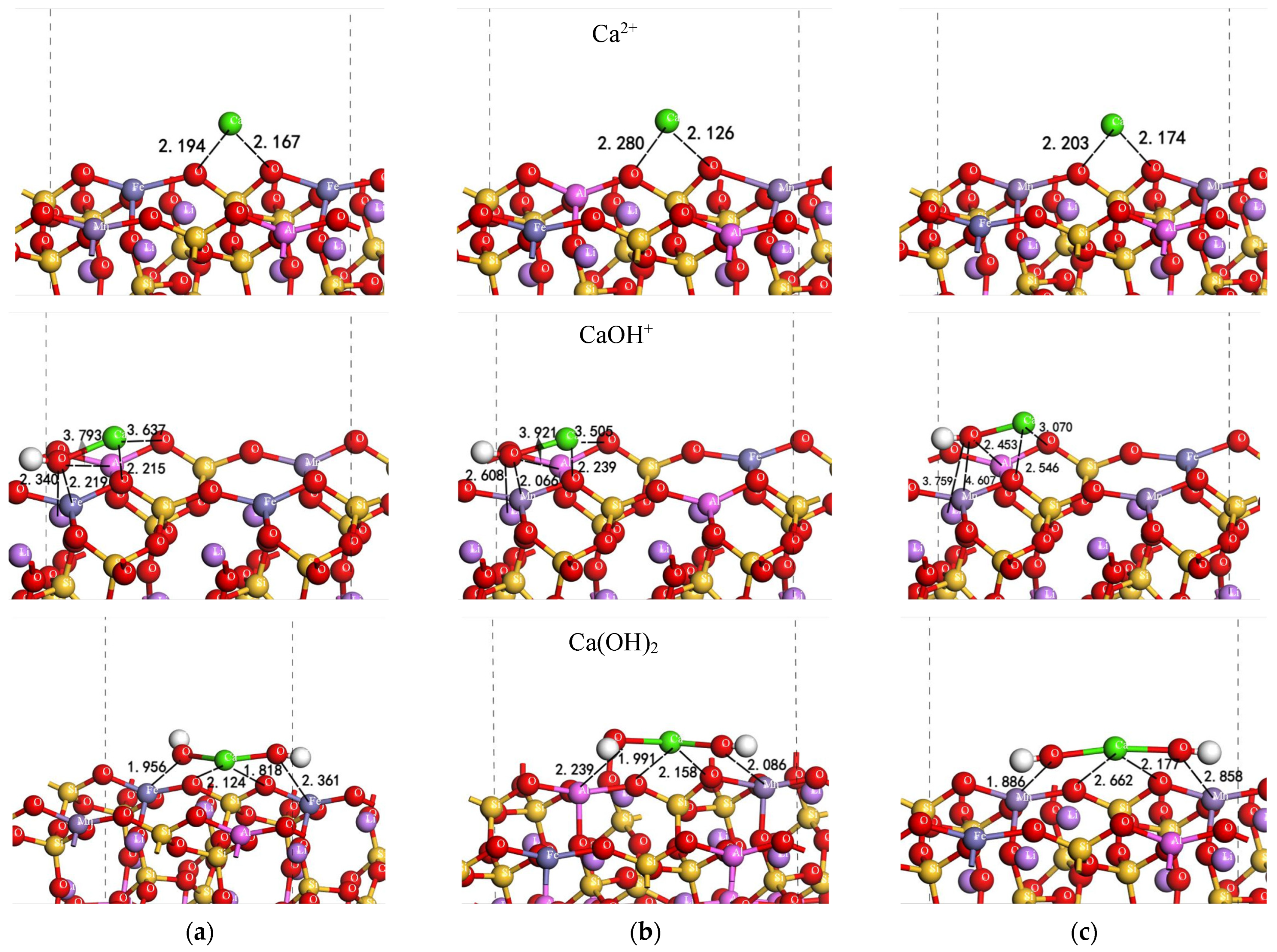
3.8. Quantum Chemical Simulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Gao, X.; An, H.; Qi, Y.; Sun, X.; Wang, Z. Supply and demand response trends of lithium resources driven by the demand of emerging renewable energy technologies in China. Resour. Conserv. Recycl. 2019, 145, 311–321. [Google Scholar] [CrossRef]
- Guo, Z.F.; Tan, C.G.; Dai, D. Lithium-the new focus of the world’s mining development. Chin. Nucl. Ind. 2017, 9, 28–29. [Google Scholar]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene Flotation Mechanism. Minerals 2019, 9, 6. [Google Scholar] [CrossRef]
- Burt, R.O.; Zhang, L. Denidae—A new type of low-iron spodumene. Miner. Prot. Util. 1989, 5, 52–55. [Google Scholar]
- James, S.B.; Barrett, H.C.; Pan, C.L. Flotation separation of spodumene and beryl. Min. Eng. 1963, 1, 28–33. [Google Scholar]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 6. [Google Scholar] [CrossRef]
- Zhu, G.; Cao, Y.; Wang, Y.; Wang, X.; Miller, J.D.; Lu, D. Surface chemistry features of spodumene with isomorphous substitution. Miner. Eng. 2020, 146, 5. [Google Scholar] [CrossRef]
- Yu, X. Mineralogical Characteristics and Geological Significance of Spodumene in Lijiagou Pegmatite Deposit in Sichuan. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2021. [Google Scholar]
- Qiu, C.Z. Study on the Mineralogical Characteristics of Spodumene in Altai Pegmatites in Xinjiang. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2014. [Google Scholar]
- Luo, X.P.; Zhang, Y.B.; Zhou, H.P.; Xie, F.X.; Yang, Z.Z.; Zhang, B.Y. Flotation separation of spodumene and albite with activation of calcium ion hydrolysate components. Rare Metals 2022, 41, 3919–3931. [Google Scholar] [CrossRef]
- Meng, J.P.; Xu, L.H.; Wang, D.H.; Xue, K.; Luo, L.P.; Shi, X.Z. The activation mechanism of metal ions on spodumene flotation from the perspective of in situ ATR-FTIR and ToF-SIMS. Miner. Eng. 2022, 182, 107567. [Google Scholar] [CrossRef]
- Zhang, Y.b.; Zhou, H.p.; Cao, Y.J.; Luo, X.p.; Xie, F.X.; Zhang, B.Y.; Yang, S.Q. Activation mechanism of calcium hydrolysate on the spodumene surface and its effect on the adsorption of collector. Miner. Eng. 2021, 174, 107221. [Google Scholar]
- Li, L.C.; Su, J.; Lu, T.J.; Shen, M.D.; Zhang, J. Luminescence characteristics of green spodumene. In Proceedings of the 2013 China Jewelry Academic Exchange Conference, Beijing, China, 30 October 2013; pp. 94–97. (In Chinese). [Google Scholar]
- Sun, C.Y. Differences in the floatiness of different colors of spodumene and crystal chemical analysis. Nonferrous Metal. 2000, 4, 107–110+103. [Google Scholar]
- Rehman, H.U.; Martens, G.; Tsai, Y.L.; Chankhantha, C.; Kidkhunthod, P.; Shen, A.H. An X-ray Absorption Near-Edge Structure (XANES) Study on the Oxidation State of Chromophores in Natural Kunzite Samples from Nuristan, Afghanistan. Minerals 2020, 10, 5. [Google Scholar] [CrossRef]
- Rossman, G.R. The Geochemistry of Gems and Its Relevance to Gemology: Different Traces, Different Prices. Elements 2009, 5, 159–162. [Google Scholar] [CrossRef]
- Ito, A.S.; Isotani, S. Heating effects on the optical absorption spectra of irradiated, natural spodumene. Radiat. Eff. Defects Solids 1991, 116, 307–314. [Google Scholar] [CrossRef]
- Sun, C.Y. Analysis of the difference in planktony of spodumene and neonite in the same ore body. J. China Univ. Min. Technol. 2001, 6, 5–10. [Google Scholar]
- Zhou, H.P.; Xie, F.X.; Zhang, Y.B.; Zhang, B.Y.; Yang, S.Q.; Luo, X.P. Insights into the floatability between spodumene and albite from crystal chemistry standpoint. Int. J. Min. Sci. Technol. 2022, 32, 1329–1339. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Kadziolka-Gawel, M.; Winiarski, A. Some Complementary Data about the Spectroscopic Properties of Manganese Ions in Spodumene Crystals. Minerals 2020, 10, 554. [Google Scholar] [CrossRef]
- Han, B. Spectroscopic Analysis and Color Optimization of Kunzite in Endeavan. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2021. [Google Scholar]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2008, 164, 161–171. [Google Scholar] [CrossRef]
- Cui, L.; Guo, Y.; Tang, J.; Yang, Y. Spectroscopy Characteristics and Color-Influencing Factors of Green Iron-Bearing Elbaite. Crystals 2023, 13, 1461. [Google Scholar] [CrossRef]
- Pasetti, L.; Borromeo, L.; Bersani, D.; Andò, S.; Schnellrath, J.; Hennebois, U. Identification of Some Gem Quality Blue to Green Li-Tourmalines. Minerals 2024, 14, 44. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Słowakiewicz, M.; Natkaniec-Nowak, L.; Proniewicz, L.M. Raman microspectroscopy of organic inclusions in spodumenes from Nilaw. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, R.P.; Zhu, G.L.; Li, G.S.; Cao, Y.J. A DFT study on the effect of lattice defects on the electronic structures and floatability of spodumene. Phys. B Condens. Matter 2024, 676, 415657. [Google Scholar] [CrossRef]
- Isotani, S.; Watari, K.; Mizukami, A.; Bonventi, W.; Ito, A.S. UV optical absorption spectra analysis of spodumene crystals from Brazil. Phys. B Condens. Matter 2007, 391, 322–330. [Google Scholar] [CrossRef]
- Hu, Y. Effects of Organic Inhibitors in Different Collector Systems on the Flotation Behavior of Spodumene and Gangue Minerals. Ph.D. Thesis, Central South University, Changsha, China, 2023. [Google Scholar]
- Jiang, W. Kinetic Simulation of Spodumene Adsorbent Molecules. Ph.D. Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2015. [Google Scholar]
- Yu, F.S.; Yan, P.K.; Jiang, M.; Wang, J.L.; Li, J.; An, F.W. Genetic characteristics of spodumene and potassium feldspar minerals and their floatability. Met. Mines 2020, 06, 75–80. [Google Scholar]
- Yu, F.S.; Sun, Y.F.; Jiang, M.; Xu, G.D.; Wang, J.L.; Li, J. Activation behavior and mechanism of metal cations in spodumene flotation. Chin. J. Nonferrous Met. 2021, 31, 203–210. [Google Scholar]
- Li, S.Y.; Liu, J.; Han, Y.X.; Zhang, S.M. A review of pegmatite-type lithium and beryllium rare metal ores and their flotation separation in China. Met. Mines 2022, 558, 129–138. [Google Scholar] [CrossRef]
- Xie, R.Q.; Zhu, Y.M.; Liu, J.; Li, Y.J. Study on spodumene crystal structure and chemical gene characteristics of (110) surface surface based on density functional theory. Met. Mines 2020, 6, 68–74. [Google Scholar]
- Xu, L.H.; Dong, Q.F.; Liu, J.; Wang, Z.; Wang, J.M. Separation of surface crystal chemistry of diaspore monohydrate and kaolinite by anionic collector flotation. Miner. J. 2016, 36, 265–270. [Google Scholar]
- Zheng, H.T.; Wang, Y.H.; Zhao, Y.H.; Zhu, G.L.; Lu, D.F.; Zheng, X.Y. The influence of NaOH and mechanical stirring on the surface of spodumene and its flotation behavior. Nonferrous Metall. Eng. 2019, 9, 61–68. [Google Scholar]
- Xi, Y.; Dai, S.J.; Zhang, Z.J. Effect of Ca2+ on magnesite flotation behavior. Met. Mines 2019, 9, 98–101. [Google Scholar]
- Zhang, G.F.; Tang, P.H.; Zhu, Y.G.; Ding, P.; Feng, Y. Solution chemistry of flotation separation of apatite and quartz in Ca2+-Na2CO3 system. Ind. Miner. Process. 2011, 40, 1–4+19. [Google Scholar]
- Zeng, W.W.; Liu, X. Effect of calcium and magnesium ions on flotation of feldspar and epidote and its mechanism. Min. Metall. Eng. 2022, 42, 59–63. [Google Scholar]
- Liu, S.; Xie, L.; Liu, J.; Liu, G.Y.; Zhong, H.; Wang, Y.X.; Zeng, H.B. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation. Chem. Eng. J. 2019, 374, 123–132. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Ultrasonic-assisted preparation of plasmonic ZnO/Ag/Ag2WO4 nanocomposites with high visible-light photocatalytic performance for degradation of organic pollutants. J. Colloid Interface Sci. 2017, 491, 216–229. [Google Scholar] [CrossRef]
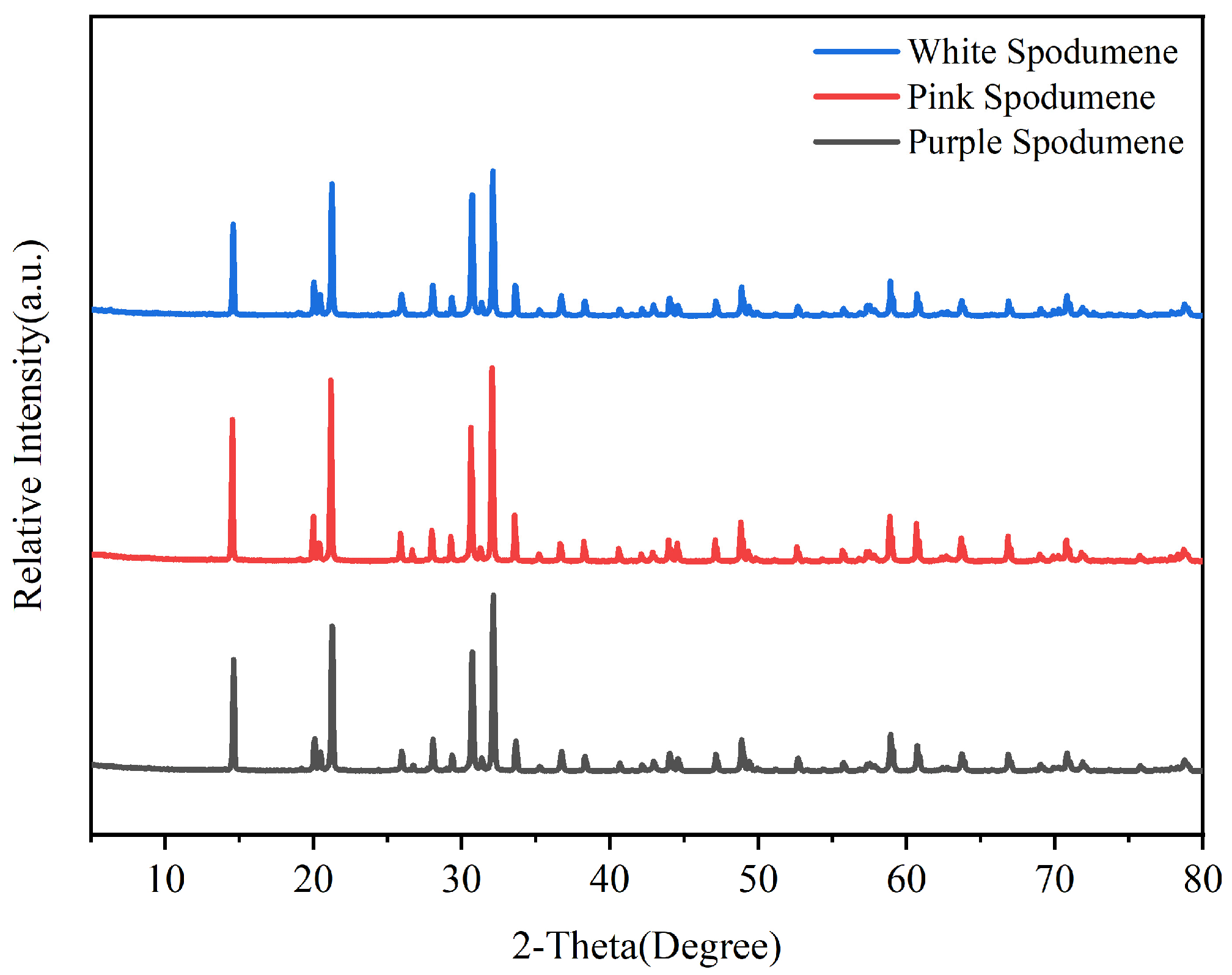








| Chemical Element | Li2O | SiO2 | Al2O3 | Na2O | MnO | CaO | K2O | P2O5 | MgO | Fe2O3 | TiO2 | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White spodumene | 7.62 | 63.81 | 26.67 | 0.804 | 0.165 | 0.318 | 0.074 | 0.060 | 0.047 | 0.468 | 0.013 | 0.0005 |
| Pink spodumene | 7.37 | 63.78 | 26.48 | 1.174 | 0.180 | 0.484 | 0.133 | 0.081 | 0.247 | 0.130 | 0.005 | 0.0003 |
| Purple spodumene | 7.43 | 63.58 | 26.86 | 1.140 | 0.179 | 0.609 | 0.122 | 0.067 | 0.033 | 0.062 | 0.014 | 0.0002 |
| Colors | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | b (L/mg) | R2 | KF | 1/n | R2 | |
| White | 173.19 | 3.42 | 0.94 | 0.37 | 0.69 | 0.96 |
| Pink | 108.15 | 6.63 | 0.93 | 0.78 | 0.58 | 0.96 |
| Purple | 163.52 | 3.65 | 0.97 | 0.32 | 0.71 | 0.98 |
| Minerals | Ra (nm) | ||
|---|---|---|---|
| Spodumene | Spodumene + Ca2+ | Spodumene + Ca2+ + NaOL | |
| White spodumene | 0.209 | 0.485 | 0.910 |
| Pink spodumene | 1.185 | 1.619 | 2.620 |
| Purple spodumene | 0.251 | 0.443 | 0.787 |
| Sample | Relative Content (%) | ||||
|---|---|---|---|---|---|
| C | O | Ca | Si | Al | |
| White spodumene | 24.63 | 50.26 | 0.64 | 16.88 | 7.59 |
| White spodumene + Ca2+ | 28.68 | 45.82 | 0.72 | 16.20 | 8.58 |
| White spodumene + Ca2+ + NaOL | 40.08 | 38.39 | 0.97 | 13.54 | 7.01 |
| Pink spodumene | 33.98 | 42.19 | 0.42 | 15.51 | 7.91 |
| Pink spodumene + Ca2+ | 34.96 | 41.37 | 0.75 | 15.71 | 7.21 |
| Pink spodumene + Ca2+ + NaOL | 51.70 | 30.41 | 1.39 | 11.12 | 5.38 |
| Purple spodumene | 31.15 | 45.31 | 0.38 | 14.79 | 8.37 |
| Purple spodumene + Ca2+ | 32.29 | 43.81 | 0.88 | 15.12 | 7.90 |
| Purple spodumene + Ca2+ + NaOL | 55.65 | 28.43 | 1.11 | 9.42 | 5.39 |
| Acting Components | Mineral Color | Esurface+adsorbate | Eadsorbate | Esurface | Eads |
|---|---|---|---|---|---|
| Ca2+ | White | −24,113.77600 | −676.79725 | −23,436.70830 | −7.360 |
| Pink | −23,092.71119 | −676.79725 | −22,415.63322 | −7.639 | |
| Purple | −24,001.04950 | −676.79725 | −23,323.99613 | −6.969 | |
| Ca(OH)+ | White | −24,189.78006 | −752.956724 | −23,436.67971 | −3.908 |
| Pink | −23,168.77055 | −752.957945 | −22,415.65643 | −4.250 | |
| Purple | −24,077.08097 | −752.989768 | −23,323.99575 | −2.598 | |
| Ca(OH)2 | White | −24,265.84491 | −829.02002 | −23,436.67051 | −4.201 |
| Pink | −23,244.79134 | −829.01777 | −22,415.60910 | −4.476 | |
| Purple | −24,153.13202 | −829.03372 | −23,323.99575 | −2.791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, R.; Zheng, Y.; Zhang, X.; Zhang, Y.; Li, C.; Li, G.; Cao, Y. The Adsorption Characteristics of Calcium Ions on Spodumene with Different Colors and Their Associated Activation Mechanism. Minerals 2025, 15, 48. https://doi.org/10.3390/min15010048
Zhu G, Wang R, Zheng Y, Zhang X, Zhang Y, Li C, Li G, Cao Y. The Adsorption Characteristics of Calcium Ions on Spodumene with Different Colors and Their Associated Activation Mechanism. Minerals. 2025; 15(1):48. https://doi.org/10.3390/min15010048
Chicago/Turabian StyleZhu, Guangli, Ruping Wang, Yan Zheng, Xu Zhang, Yuzhe Zhang, Chao Li, Guosheng Li, and Yijun Cao. 2025. "The Adsorption Characteristics of Calcium Ions on Spodumene with Different Colors and Their Associated Activation Mechanism" Minerals 15, no. 1: 48. https://doi.org/10.3390/min15010048
APA StyleZhu, G., Wang, R., Zheng, Y., Zhang, X., Zhang, Y., Li, C., Li, G., & Cao, Y. (2025). The Adsorption Characteristics of Calcium Ions on Spodumene with Different Colors and Their Associated Activation Mechanism. Minerals, 15(1), 48. https://doi.org/10.3390/min15010048









